Abstract
Purpose
In this study, we examine whether an excitatory repetitive transcranial magnetic stimulation (rTMS) protocol called intermittent theta burst stimulation (iTBS) applied to the affected left hemisphere leads to changes in white matter fractional anisotropy (FA).
Methods
Diffusion tensor imaging (DTI) data were collected in 8 aphasic stroke patients before and after 10 daily iTBS treatments. Alignment of structural and DTI data and derivation of diffusion index maps were performed using Analysis of Functional NeuroImages software followed by Tract-Based Spatial Statistics using FMRIB Software Library. Paired t-tests were performed to compare pre- to post-rTMS changes in FA.
Results
There were significant (p<0.001) left-hemispheric FA increases near the inferior and superior frontal gyri and anterior corpus callosum. FA also increased in the right midbrain and bilaterally near temporal, parietal and posterior cingulate regions. FA decreased bilaterally near the fusiform gyrus and in left cerebellum.
Conclusions
Overall, left-hemispheric regions that showed increased FA corresponded to areas previously shown to have increases in fMRI language activation after iTBS. The increased white matter integrity near the stimulation sites may reflect improvements in cortical function mediated by excitatory rTMS through its ability to facilitate synaptic connections.
Keywords: DTI, fractional anisotropy, TBSS, white matter, excitatory rTMS, post-stroke aphasia
Introduction
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive technique used to modulate cortical excitability (Fitzgerald et al., 2006; Hoogendam et al., 2010; Pascual-Leone et al., 1998). Cortical excitation is achieved with high frequency trains of rTMS (e.g., >5 Hz), while low frequency trains (e.g., 1 Hz) elicit cortical inhibition (Chen & Seitz, 2001; Huang et al., 2007). Recently, rTMS was shown to be useful in post-stroke rehabilitation of aphasia by facilitating improvements in language function via stimulating cortical plasticity (Barwood et al., 2011a; Barwood et al., 2011b; Hamilton et al., 2010; Hamilton et al., 2011; Kakuda et al., 2010a; Kakuda et al., 2010b; Martin et al., 2009; Naeser et al., 2005; Naeser et al., 2010; Szaflarski et al., 2011; Weiduschat et al., 2011).
Aphasia is one of the devastating consequences of stroke in the territory of the left middle cerebral artery (LMCA) (Alexander, 1997). Since the 1861 postmortem study by Broca who described language impairment from damage to the left frontal lobe, the left hemisphere has been regarded as dominant for language. A number of theories to account for this leftward laterality have been proposed including anatomical asymmetries (Foundas et al., 1994) and stronger interhemispheric connectivity (Westerhausen et al., 2006) or increasing head/brain size (Josse et al., 2006; Ringo et al., 1994). One of them, the theory of callosal inhibition postulates that the dominant-for-language hemisphere inhibits the language functions in the non-dominant hemisphere via transcallosal connections (Heiss & Thiel, 2006; van der Knaap & van der Ham, 2011). Thus, based on this theory, damage to the dominant-for-language hemisphere will release inhibition of the right hemisphere causing function transfer to the intact (non-dominant up to this point) hemisphere (van der Knaap & van der Ham, 2011). Right hemispheric involvement in the acute stages of left hemisphere stroke has been suggested to play an important role in supporting early recovery after initial damage (Fernandez et al., 2004). However, increased recruitment of the right hemisphere in chronic post-stroke aphasia may be a maladaptive strategy that hinders language recovery (Anderson et al., 2011; Martin et al., 2004; Naeser et al., 2004; Price & Crinion, 2005; Rosen et al., 2000). Therefore, rTMS inhibition of the right-hemispheric language regions in stroke patients (i.e., homologues of Broca’s and Wernicke’s areas) is hypothesized to result in the forced re-utilization and increased activation of the lesioned left hemisphere to achieve corresponding improvements in language abilities (Heiss & Thiel, 2006). In line with the theory of hemispheric inhibition, the majority of rTMS treatment studies in post-stroke aphasia have applied low frequency (1 Hz) rTMS to the right-hemispheric language homologues in order to force activation and utilization reversal with switch of language-related activities to the previously dominant-for-language lesioned left hemisphere (see (Hamilton et al., 2011) for review).
Recently, our group reported application of the high frequency excitatory rTMS protocol to the left hemisphere for treatment of post-stroke aphasia (Szaflarski et al., 2011). This differential approach takes advantage of the capability of rTMS to mediate long-term potentiation (LTP) in neurons (Hoogendam et al., 2010). LTP is a form of neuronal plasticity that can facilitate long-lasting synaptic connections. Application of excitatory rTMS to the affected left hemisphere resulted in post-treatment improvements in language function and corresponding increases in the left frontal, temporal and parietal activation and right-hemispheric decreases during language fMRI (Szaflarski et al., 2011). The rTMS protocol utilized in that study was an intermittent theta burst stimulation (iTBS) paradigm that was previously shown to facilitate motor evoked potentials (MEPs) in a hand muscle after stimulating the hand area of the motor cortex (Huang et al., 2005; Szaflarski et al., 2011). The iTBS paradigm is also similar to stimulation paradigms used in animal studies to induce LTP in stimulated neurons (Capocchi et al., 1992; Heynen & Bear, 2001). Therefore, the objective of this study was to examine whether neuro-navigated iTBS in patients with post-stroke aphasia facilitates measurable improvements in the structural integrity and organization in the white matter underlying the cortical language regions that were previously reported to have significant pre- to post-treatment blood oxygenation-level dependent (BOLD) signal increases (Szaflarski et al., 2011).
Diffusion tensor imaging (DTI) is a technique that allows non-invasive examination of white matter microstructure by measuring the restricted diffusion of water molecules, which varies among different tissue types (Basser & Pierpaoli, 1996). For example, in white matter, axon bundles surrounded by myelin facilitate diffusion of water preferentially along their main direction (Basser & Pierpaoli, 1996; Eliassen et al., 2008; Pierpaoli et al., 1996); this diffusion can be characterized using scalar indices including fractional anisotropy (FA), axial diffusivity (AD), radial diffusivity (RD) and mean diffusivity (MD). FA provides a measure for the directionality of water diffusion in a voxel, with values ranging from 0 to 1 (Mori & Zhang, 2006; Pierpaoli et al., 1996). Higher FA values suggest more restricted (i.e., anisotropic) diffusion and greater fiber organization indicating higher white matter integrity (Bennett et al., 2010; Klingberg et al., 1999; Madden et al., 2009). AD and RD represent the movement of water along the length of and perpendicular to axons, respectively, while MD is a measure of average diffusion. Animal studies correlating DTI and immunohistochemistry found that decreased AD is indicative of axonal damage (Budde et al., 2008; Budde et al., 2009; Song et al., 2003) while increased RD is associated with demyelination (Song et al., 2002; Song et al., 2003), phenomena that both impact conduction velocities and neuronal signal transduction (Gutierrez et al., 1995; Waxman, 1980; Waxman et al., 1992). Thus, taken together, these diffusion indices can help characterize the white matter changes that may have been facilitated by rTMS treatment. We hypothesized that an excitatory rTMS treatment applied to the functionally active language regions in the affected left hemisphere would facilitate structural changes in the underlying white matter near the sites of stimulation. Specifically, we expected the left hemisphere FA in frontal (stimulated) language regions to increase after excitatory rTMS treatment.
Methods
Patients
Eight stroke patients (4 females), ages 32 to 78 (mean ± s.d. = 54.4 ± 12.7), participated in a previously published fMRI study of a two-week excitatory rTMS treatment for post-stroke aphasia (Fig. 1). In addition to the previously reported MRI, fMRI and language performance data (Szaflarski et al., 2011), DTI data were collected during the pre-/post-rTMS study visits. All subjects suffered from left middle cerebral artery stroke, were more than one year post-stroke (mean ± s.d. = 5.25 ± 3.62) and were aphasic at time of study. A linguistics expert determined aphasia type for each patient using results from language testing, and a trained neuroanatomist manually traced the lesion on each patient’s T1-weighted anatomical MRI to determine the residual volume of the stroke (Table 1). Patients had no contraindications to MRI, had no history of seizures and were right-handed prior to stroke as determined by a score of ≥50 on the Edinburgh Handedness Inventory (Oldfield, 1971). The study was approved by the University of Cincinnati Institutional Review Board, and it adhered to the Declaration of Helsinki regarding the use of human subjects in research. All patients signed informed consent prior to study participation.
Fig. 1.
Timeline of the study procedures. All subjects underwent language testing and MRI scanning within one week prior to and one week after the excitatory repetitive transcranial magnetic stimulation (rTMS) treatment (intermittent theta burst stimulation (iTBS)).
Table 1.
Description of aphasia type and lesion size/localization for the enrolled stroke subjects.
| Patient | Aphasia diagnosis | Left hemishere lesion location | Lesion volume (mm3) |
|---|---|---|---|
| 1 | Anomic, mild dysarthria | Inferior frontal and temporal with posterior fronto/parietal extension | 111,758 |
| 2 | Nonfluent (Broca-type) | Anterior/lateral temporal with extension to fronto- temporal junction | 79,633 |
| 3 | Anomic, mild dysarthria | Insular cortex with extension to subcortical white matter | 4,170 |
| 4 | Anomic | Anterior temporal and inferior/middle frontal gyri | 52,943 |
| 5 | Nonfluent (Broca-type) | Superior/posterior frontla and temporo-parieto-occipital junction | 191,188 |
| 6 | Anomic, conduction | Inferior frontal and insular with extension into fronto/parietal regions | 61,809 |
| 7 | Anomic | Temporo/parietal junction | 70,936 |
| 8 | Broca’s (non-fluent, poor comprehension) | Inferior frontal gyrus and temporo/parietal junction | 28,308 |
Excitatory Repetitive Transcranial Magnetic Stimulation
The details of the excitatory rTMS protocol have been previously published including determination of stimulation intensities for each subject (Szaflarski et al., 2011) and are only briefly described here. Stimulation intensities for iTBS were set at 80% of the active motor threshold that was determined with single-pulse TMS on the primary motor cortex in the right hemisphere using a Magstim 200® stimulator connected to a figure-8 coil by a Bistim® module (Magstim Co., Wales, UK). Each day for two weeks (5 days/week; Fig. 1), subjects received 200 seconds of iTBS consisting of two-second trains of three 50-Hz pulses given every 200 milliseconds and repeated every 10 seconds for a total of 600 pulses. BrainSightTM2 (Rogue Research Inc., Montreal, Canada) was used for neuronavigation to locate the site for stimulation in each subject, and the Magstim Rapid2® (Magstim Co., Wales, UK) was used to perform iTBS. The site of stimulation varied for each subject as it corresponded to the left hemispheric region that showed significant activation during a functional MRI language task (Szaflarski et al., 2011).
Magnetic Resonance Imaging
MRI scanning procedures were performed using a Varian 4 Tesla Unity INOVA whole body MRI/MRS scanner (Varian, Inc., Palo Alto, CA). Briefly, patients were positioned in the scanner and their head aligned to position the anterior to posterior commissure (AC-PC) reference line as close to the vertical axis of the scanner as possible. A 3-dimentional (3D) high resolution anatomical scan was acquired using a T1-weighted Modified Driven Equilibrium Fourier Transform (MDEFT) sequence (TMD = 1.1 s, TR= 13.1 ms, TE = 6 ms, FOV = 25.6 x 19.2 x 19.2 cm, 256 x 192 x 96 matrix, flip angle = 22 degrees, 1 mm3 reconstructed resolution) (Lee et al., 1995). A midsagittal localizer scan was performed in order to ensure that slice acquisition encompassed the entire brain. A multi-echo reference scan was obtained to correct for ghost and geometric distortion artifacts at high field MRI (Schmithorst et al., 2001). DTI was performed using a spin-echo echo planar image sequence modeled after the Jones30 scheme (Jones et al., 1999; Landman et al., 2007; Skare et al., 2000) to acquire diffusion-weighted images (DWIs) in 30 distinct directions (b = 1000.65 s/mm2) and six images with no diffusion weighting (b = 0 s/mm2) with the following parameters: TR = 10 s, TE = 96.1 ms, FOV = 25.6 cm x 25.6 cm, matrix = 256 x 256, flip angle = 90 degrees, slice thickness = 4 mm. Patients were scanned during the week preceding rTMS treatment and again during the week following the last treatment (Fig. 1; (Szaflarski et al., 2011)).
MRI Data Pre-processing
The anatomical MDEFT and DTI scans were reconstructed using an in-house program developed in the Interactive Data Language software environment (IDL 6.4; Research Systems Inc., Boulder, CO). MRI data alignment for each subject was performed using tools in the Analysis of Functional NeuroImages (AFNI) software package (Cox, 1996). First the post-rTMS anatomical scan was aligned to the pre-rTMS scan using the 3dAllineate program in AFNI. Then, for each scan session (pre- and post-rTMS) the DTI images with no diffusion weighting were motion-corrected and then averaged for alignment with the anatomical scan using the local Pearson correlation method (Saad et al., 2009). This was followed by motion-correction and eddy current distortion correction of the 30 DWIs by using three-dimensional affine registration to the first acquired DWI. Polar decomposition was performed using Octave (www.octave.org) to obtain the rigid-body component of the corrections that were applied to the diffusion gradients to account for the combined alignments of DTI to MDEFT and motion correction steps (Rohde et al., 2004). All intermediate spatial transformations were then combined and applied to the original DTI data such that only a single interpolation was performed to align the DTI data to the MDEFT and to correct for gradient distortions and head motion.
Quantification of Diffusion Indices
Derivation of FA from the pre-processed DTI data was performed using the 3dDWItoDT program in AFNI. The eigenvalues λ1, λ2, and λ3 (i.e., diffusion magnitudes) were also derived and used to determine AD and RD, where AD = λ1 and RD = (λ2 +λ3)/2. We then used FMRIB Software Library (Smith et al., 2004) to perform whole-brain voxelwise analysis of FA using the Tract-Based Spatial Statistics (TBSS, v1.2) method (Smith et al., 2006). Prior to spatial normalization, each FA image from each subject and each session (pre- and post-rTMS) is aligned to every other FA image in order to identify a group-specific target image. The target image then underwent affine transformation into 1 mm3 MNI152 standard space. The other FA images were also spatially normalized by combining the nonlinear transformation to the target image and the affine transformation of the target image to MNI152 space into a single transformation. Spatial normalization was followed by calculation of the mean FA image and its skeletonization to represent the center of all tracts common to the group. The aligned FA data for each subject were then projected onto this skeleton and the FA threshold set to ≥0.2 to restrict analysis to white matter. Voxelwise analysis using paired t-tests were performed to examine changes between pre-and post-rTMS white matter FA. Regions showing pre- to post-rTMS increases or decreases in FA at uncorrected p<0.001 (|t-score| > 5.41, degrees of freedom = 7) were considered significant and defined as white matter clusters of interest (Fig. 2). To further characterize the pre- to post-rTMS changes in the white matter clusters, we examined relative changes in AD and RD in those regions identified to have significant pre- to post-rTMS FA changes. This was achieved by applying the transformations used to derive the FA skeleton to AD and RD and then performing paired t-tests using the skeletonized AD and RD maps. In white matter clusters of interest that had significant FA differences at p<0.001, pre- to post-rTMS comparisons of AD and RD were performed at p<0.35 (|t-score| > 1, degrees of freedom = 7) to determine if these indices also showed relative increases or decreases. A permissive threshold for AD and RD was provided to allow for the examination of relative changes in these DTI indices with respect to the significant changes observed in FA.
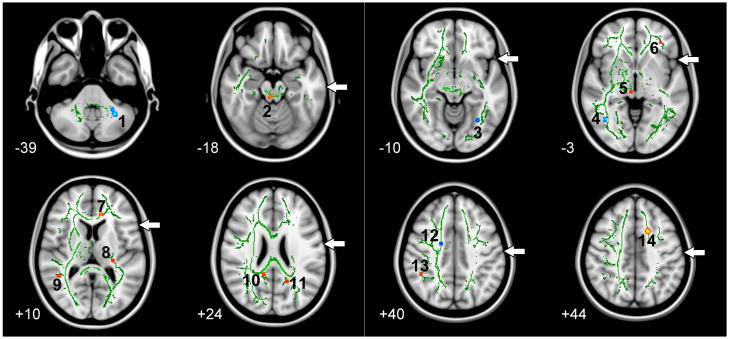
Fig. 2.
Tract-Based Spatial Statistics (TBSS) analysis of the pre- to post-rTMS changes in fractional anisotropy (FA). Clusters in blue represent decreases, while those in red/yellow represent increases in FA (only changes significant at uncorrected p<0.001 are shown). The numbers (1–14) correspond to the clusters described in Table 2. The green skeleton represents the center of white matter tracts common to the group with FA ≥ 0.2 and is superimposed on the standard MNI152 T1-weighted anatomical image. The white arrows indicate areas with breaks in the green skeleton that represent damaged white matter not included in TBSS analysis. The axial slices are displayed in in radiological convention (left on the picture is right in the brain) and range in MNI coordinates from z = -39 (top left) to z = +44 (bottom right).
Language Testing
Subjects performed a battery of language assessments pre- and post-rTMS, which has been described previously (Szaflarski et al., 2011). Briefly, testing consisted of the Boston Naming Test to assess semantic retrieval and word-finding abilities (Kaplan et al., 2001), the Peabody Picture Vocabulary Test to assess receptive vocabulary (Dunn & Dunn, 2007), the Semantic Fluency Test and Controlled Oral Word Association Test to assess verbal fluency (Kozora & Cullum, 1995; Lezak, 1995), and the Complex Ideation subtest of the Boston Diagnostic Aphasia Examination to assess comprehension of factual material presented in a story (Goodglass & Kaplan, 1972). Different versions of the assessments were used for pre- and post-rTMS language testing to minimize learning effects. Subjects also completed the mini-Communicative Abilities Log, a subjective measure of progress in everyday verbal communication (Pulvermuller et al., 2001; Szaflarski et al., 2008), pre- and post-rTMS.
Relating Changes in FA with Lesion Size and Language Performance
We performed exploratory analysis to examine the relationship between pre- to post-rTMS changes in FA with lesion size and with changes in language performance. Given the small sample size of the current study, we limited our analysis to the Semantic Fluency Test (SFT) in which patients had shown improvement following rTMS treatment (Szaflarski et al., 2011) and examined only regions near target stimulation sites showing significant pre- to post-rTMS increases in FA.
Results
Regions showing significant pre- to post-rTMS white matter changes in FA are shown in Fig. 2. The figure shows a green skeleton representing the center of white matter tracts common to all stroke patients. It should be noted that the skeleton shows breaks in the left hemisphere (right in the image is left in the brain) depicting regions of damaged tissue that were not included in the TBSS analysis (white arrows in Fig. 2). The location, MNI coordinates for the voxel showing the peak change in FA, and the corresponding relative changes in AD and RD for each cluster are summarized in Table 2. Significant decreases in FA were observed in the left cerebellum (Cluster 1), bilaterally in the occipital lobe near the fusiform gyrus (Clusters 3 and 4), and in the right superior corona radiata near the cingulate gyrus (Cluster 12). There was a corresponding FA increase in the superior region of left frontal lobe near the superior frontal gyrus (Cluster 14). A number of other regions showed pre- to post-rTMS increases in FA including in the left frontal lobe near the inferior frontal gyrus (Cluster 6) and near the anterior cingulate (Cluster 7), as well as a region in the left internal capsule near the posterior insula (Cluster 8). Increases in FA were also observed in the right temporal lobe near the superior temporal gyrus (Cluster 9), in the right parietal lobe near the supramarginal gyrus (Cluster 13), and bilaterally near the posterior cingulate (Clusters 10 and 11). Clusters 6, 7, 8, and 14 that showed increased pre- to post-rTMS FA were located near the left hemisphere target stimulation sites, and they roughly correspond to the changes in BOLD signal observed in previously published report (Fig. 3 in (Szaflarski et al., 2011)).
Table 2.
Location and corresponding t-values for regions showing significant pre- to post-rTMS changes in fractional anisotropy in Figure 2.
| MNI coordinates
|
t-values
|
||||||
|---|---|---|---|---|---|---|---|
| Cluster | Location | x | y | z | FA | AD | RD |
| Regions of decreased FA pre- to post-rTMS | |||||||
| 1 | L. Cerebellum (Middle Cerebellar Peduncle) | −29 | −53 | −39 | −7.66 | −2.31 | −1.38 |
| 3 | L. Occipital Lobe (Fusiform Gyrus) | −30 | −62 | −10 | −10.30 | 0.27 | 2.37 |
| 4 | R. Occipital Lobe (Fusiform Gyrus) | 40 | −60 | −5 | −8.09 | −0.44 | 1.11 |
| 12 | R. Corona Radiata (Cingulate Gyrus) | 18 | −5 | 39 | −5.47 | −2.01 | 1.88 |
| Regions of increased FA pre- to post-rTMS | |||||||
| 2 | R. Midbrain | 4 | −31 | −18 | 7.47 | −1.88 | −4.26 |
| 5 | R. Midbrain | 6 | −25 | −6 | 7.77 | −1.19 | −1.49 |
| 6 | L. Frontal Lobe (Inferior Frontal Gyrus) | −33 | 40 | −2 | 6.01 | −0.86 | −1.54 |
| 7 | L. Corpus Callosum (Anterior Cingulate) | −11 | 33 | 8 | 8.04 | −2.25 | −5.15 |
| 8 | L. Internal Capsule (Insula/Superior Temporal Gyrus) | −26 | −27 | 12 | 5.90 | −1.91 | −2.19 |
| 9 | R. Temporal Lobe (Superior Temporal Gyrus) | 44 | −47 | 11 | 5.60 | 0.16 | −3.38 |
| 10 | R. Corpus Callosum (Posterior Cingulate) | 13 | −45 | 23 | 6.43 | 1.13 | −6.42a |
| 11 | L. Parietal Lobe (Posterior Cingulate) | −17 | −54 | 25 | 5.57 | 0.20 | −1.07 |
| 13 | R. Parietal Lobe (Supramarginal Gyrus) | 45 | −45 | 40 | 6.24 | −1.32 | −3.07 |
| 14 | L. Frontal Lobe (Superior Frontal Gyrus) | −16 | 12 | 44 | 7.76 | 0.79 | −2.85 |
Note: Pre- to post-rTMS changes in fractional anisotropy (FA) are significant at p<0.001 (uncorrected).
Corresponding t-values reflecting relative changes in axial (AD) or radial (RD) diffusivity are also provided for each cluster that showed significant FA changes. Negative t-values indicate a decrease from pre- to post-rTMS, while positive t-values indicate a increase. L = left; R = right.
Decrease in RD pre- to post-rTMS is significant at p<0.001 (uncorrected).
Performance on the language tests have been reported previously, with patients showing a significant improvement in performance on the SFT following excitatory rTMS treatment (Szaflarski et al., 2011). There were no significant relationships between lesion volume and changes in FA for Cluster 6 (r=−0.132, p=0.755), Cluster 7 (r=−0.323, p=0.435), Cluster 8 (r=−0.563, p=0.147) and Cluster 14 (r=0.098, p=0.817). There were also no significant relationships between pre- to post-rTMS changes in SFT performance and changes in FA for Cluster 6 (r=0.289, p=0.488), Cluster 7 (r=−0.485, p=0.223), Cluster 8 (r=0.017, p=0.967) and Cluster 14 (r=0.234, p=0.577).
Discussion
The aim of this pilot study was to investigate using DTI whether excitatory rTMS is able to stimulate changes in white matter structural integrity in language regions of aphasic stroke patients that were targets of the excitatory rTMS intervention. We observed left hemispheric increases in FA (Clusters 6, 7, 8 and 14) near target stimulation sites for excitatory rTMS and near the regions that showed increased language fMRI activation in the previously reported fMRI and behavioral study (Szaflarski et al., 2011) supporting our hypothesis that rTMS may stimulate improvements in the integrity of the white matter tracts underlying residual left hemispheric language areas. Since the iTBS paradigm used in the current study has previously induced LTP and facilitated synaptic connections in stimulated neurons (Capocchi et al., 1992; Heynen & Bear, 2001), we can infer that the changes in cortical excitability induced by excitatory rTMS may have improved neuronal function at the stimulation site and, in turn, improved cognitive performance. The increased integrity of the underlying white matter may reflect this improved cortical function.
From previous DTI studies of healthy aging we have learned that decreased FA and increased MD are associated with cognitive decline (Bendlin et al., 2010; Madden et al., 2009; Stamatakis et al., 2011). Thus, it is possible that the increases in white matter FA observed in the current study may be related to improved language functions since a significant improvement in semantic fluency performance and positive trends for other measures were previously reported in these patients (Szaflarski et al., 2011). However, exploratory analyses did not show significant associations between pre- to post-rTMS changes in FA in the four clusters near the target stimulation sites and changes in SFT performance or lesion volume. Interestingly, FA changes in the left frontal regions (Clusters 6 and 14) that exhibited positive trends with changes in SFT performance (r=0.289 and r=0.234, respectively) were also the least affected by lesion size/distribution. A recent study in aphasic LMCA stroke patients found that fMRI activation in a similar left frontal region was positively correlated with in-scanner verbal fluency, an association that did not exist in healthy control subjects despite similar activation in that region (Allendorfer et al., in print). Again, one could postulate that increased white matter integrity underlying these left frontal regions may be associated with increased function. Unfortunately, our ability to adequately examine these types of structure-function relationships in the current study is hindered by the lack of statistically significant associations, likely due to the small sample size. Despite this limitation, our results provide preliminary evidence in support of the notion that excitatory rTMS may promote increased white matter structural integrity in stimulated language regions, and whether or not these changes correspond to improved performance on linguistic tasks deserves further study.
In parallel to the observed changes in FA, relative increases and decreases in RD and AD may provide additional guidance in interpreting the findings of our present study. Except for Cluster 1 in the left cerebellum, the degree of RD change in the clusters of interest was similar (e.g., Cluster 12 in the right corona radiate) or greater than the degree of change in AD. Radial diffusivity is increased in myelin-deficient mice (Nair et al., 2005; Song et al., 2002), and damage or loss of myelin has been shown to occur naturally during aging (Peters, 2002). Thus, the increase in FA combined with the respective changes in RD and AD suggests possible increases in the degree of myelination (Song et al., 2002; Song et al., 2003), and in turn, thicker myelin is associated with faster conduction velocities which could improve neuronal signal transduction (Gutierrez et al., 1995; Waxman, 1980). To our surprise, the degree of relative AD reduction in the left cerebellum was greater than the degree of RD reduction suggesting the possibility of axonal damage (Budde et al., 2008; Budde et al., 2009; Song et al., 2003), which can hinder the conduction of action potentials (Waxman et al., 1992). It must also be noted that the source of these FA changes may not necessarily be at the microscopic level of myelin and axons (Mori & Zhang, 2006). For example, the middle cerebellar peduncle consists of non-homogenous white matter, and the reduction in FA may be related more to fiber reorganization at a macroscopic level (Mori & Zhang, 2006), particularly since this change is occurring in the damaged hemisphere in an area associated with motor control. These findings, however, should be considered preliminary and interpreted with caution given the small sample size and unblended approach in this study. Furthermore, a permissive threshold for AD and RD was used to describe changes in these indices relative to the significant changes observed in FA; while this limits our interpretation of the changes in AD and RD, it provides additional perspective in the interpretation of FA changes.
The results of our study may also be considered in the context of brain plasticity. Structural changes in gray and white matter have been shown to occur after therapy and training (Draganski & May, 2008). For example, Schlaug et al. (2009) showed an increase in white matter volume in the right arcuate fasciculus after 75–80 days of intonation-based speech therapy for aphasic patients (Schlaug et al., 2009), while May et al. (2007) demonstrated that a 5-day treatment of low frequency (1Hz) rTMS applied to the left superior temporal cortex was enough to induce an increase in gray matter volume at the site of stimulation (May et al., 2007). Findings of these studies are consistent with the observed increases in white matter FA in our study near the target sites after 10 days of excitatory rTMS. Application of rTMS also induced bilateral increases and decreases in thalamic and temporal gray matter volume away from the site of stimulation (May et al., 2007), similar to the bilateral increases (Clusters 10 and 11) and decreases (Clusters 3 and 4) we observed with FA. There are a number of white matter tracts that connect the left frontal lobe, a location of rTMS stimulation, to the majority of regions where we also found significant changes in FA. For example, within each hemisphere the arcuate fasciculus forms connections between the frontal, parietal and temporal regions (Catani et al., 2005; Catani & Mesulam, 2008). There are also the inferior and superior fronto-occipital fasciculus that connects frontal and occipital regions within each hemisphere (Catani & Mesulam, 2008; Wakana et al., 2004) as well as transcallosal fibers including the corpus callosum (Wakana et al., 2004) to bridge regions in the left and right hemispheres. We hypothesize that these inter- and intra-hemispheric connections have facilitated the widespread FA changes that were observed far from the target stimulation site and are consistent with previous work showing rTMS-induced changes both proximal and distal to the site of stimulation (May et al., 2007). We also hypothesize that similar to the BOLD signal changes previously observed in this cohort, stimulation of one area of the network may induce changes at the local and global (i.e., network) level. Thus, our resulst support the data from the literature demonstrating that unilateral stimulation can lead to long-lasting bilateral changes, e.g., in the network that underlies language production (Kim et al., 2011), and may be reflective of the various connections between brain regions.
Repetitive TMS has been shown to elicit effects beyond the period of stimulation including increases and decreases in cortical excitability (Pascual-Leone et al., 1998). A PET study showed bilateral increases in glucose metabolism in the primary and supplementary motor cortices up to 10 minutes after 5 Hz rTMS to the hand area of the left motor strip (Siebner et al., 2000). The iTBS paradigm similar to the one used for excitatory rTMS treatment in the current study had been shown to increase the motor evoked potential in the hand muscle for about 15 minutes following simulation of the hand area in the motor cortex (Huang et al., 2005). The phenomenon of observable effects outlasting the stimulation period is similar to the training-induced changes that have been observed previously. For example, white matter FA was shown to increase after a 6-week training period for juggling, and this effect persisted after a subsequent 4-week period of no training (Scholz et al., 2009). While there was no long-term follow-up MRI in the current study, it is possible that excitatory rTMS treatment would have similar lasting effects on FA. Evaluation of the long-term effects of excitatory rTMS treatment should be incorporated into larger future studies.
It is believed that small to moderate sample sizes, particularly in neuroimaging studies, contribute to false positive findings (Harrington & Farias, 2008). Thus, the small sample size in this study needs to be taken into account when interpreting the current results. For example, a number of the brain regions that showed increases and decreases in FA were located beyond the site of stimulation that we hypothesized would show significant changes. It is possible that these may be spurious findings that could be resolved with a greater number of subjects. However, rTMS treatment has been shown to elicit non-specific structural changes (i.e., increases and decreases in gray matter volume) in brain regions such as the cerebellum and thalamus, away from the site of stimulation in a treatment group relative to a control group (May et al., 2007). Although results from a control group would help to determine whether or not the FA changes observed in the current study were specific to rTMS treatment or to other factors independent of the treatment, such a control was not available due to the preliminary nature of the investigation. A blinded design and the addition of a sham treatment group in a study with a larger number of subjects is necessary to parcel out study confounds. Therefore, caution should be taken in interpreting these results until they are replicated in a larger controlled study. Despite these limitations, however, the current study provides preliminary evidence for excitatory rTMS-induced changes in white matter integrity that supports and extends the findings of May et al. (2007) and others of a rapidly adjusting neuronal system. Further investigation is warranted to replicate these results and determine how changes in white matter relate to corresponding changes in fMRI activation and language function.
Acknowledgments
Support for this study was provided by grants from the University of Cincinnati Research Council (to JPS) and the National Institute of Neurological Disorders and Stroke (NINDS; NIH R01 NS04828 to JPS).
References
- Alexander MP. Aphasia: Clinical and anatomical aspects. In: feinberg TE, Farah MJ, editors. Behavioral neurology and neuropsychology. New York: McGraw-Hill; 1997. [Google Scholar]
- Allendorfer JB, Kissela BM, Holland SK, Szaflarski JP. Different patterns of language activation in post-stroke aphasia are detected by overt and covert versions of the verb generation fMRI task. Med Sci Monit. in print doi: 10.12659/MSM.882518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson V, Spencer-Smith M, Wood A. Do children really recover better? neurobehavioural plasticity after early brain insult. Brain. 2011;134(Pt 8):2197–2221. doi: 10.1093/brain/awr103. [DOI] [PubMed] [Google Scholar]
- Barwood CH, Murdoch BE, Whelan BM, Lloyd D, Riek S, O'Sullivan JD, Coulthard A, Wong A. Improved language performance subsequent to low-frequency rTMS in patients with chronic non-fluent aphasia post-stroke. Eur J Neurol. 2011a;18(7):935–943. doi: 10.1111/j.1468-1331.2010.03284.x. [DOI] [PubMed] [Google Scholar]
- Barwood CH, Murdoch BE, Whelan BM, Lloyd D, Riek S, O'Sullivan J, Coulthard A, Wong A, Aitken P, Hall G. The effects of low frequency repetitive transcranial magnetic stimulation (rTMS) and sham condition rTMS on behavioural language in chronic non-fluent aphasia: Short term outcomes. NeuroRehabilitation. 2011b;28(2):113–128. doi: 10.3233/NRE-2011-0640. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111(3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Bendlin BB, Fitzgerald ME, Ries ML, Xu G, Kastman EK, Thiel BW, Rowley HA, Lazar M, Alexander AL, Johnson SC. White matter in aging and cognition: A cross-sectional study of microstructure in adults aged eighteen to eighty-three. Dev Neuropsychol. 2010;35(3):257–277. doi: 10.1080/87565641003696775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett IJ, Madden DJ, Vaidya CJ, Howard DV, Howard JH., Jr Age-related differences in multiple measures of white matter integrity: A diffusion tensor imaging study of healthy aging. Hum Brain Mapp. 2010;31(3):378–390. doi: 10.1002/hbm.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde MD, Xie M, Cross AH, Song SK. Axial diffusivity is the primary correlate of axonal injury in the experimental autoimmune encephalomyelitis spinal cord: A quantitative pixelwise analysis. J Neurosci. 2009;29(9):2805–2813. doi: 10.1523/JNEUROSCI.4605-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde MD, Kim JH, Liang HF, Russell JH, Cross AH, Song SK. Axonal injury detected by in vivo diffusion tensor imaging correlates with neurological disability in a mouse model of multiple sclerosis. NMR Biomed. 2008;21(6):589–597. doi: 10.1002/nbm.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capocchi G, Zampolini M, Larson J. Theta burst stimulation is optimal for induction of LTP at both apical and basal dendritic synapses on hippocampal CA1 neurons. Brain Res. 1992;591(2):332–336. doi: 10.1016/0006-8993(92)91715-q. [DOI] [PubMed] [Google Scholar]
- Catani M, Mesulam M. The arcuate fasciculus and the disconnection theme in language and aphasia: History and current state. Cortex. 2008;44(8):953–961. doi: 10.1016/j.cortex.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Jones DK, ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57(1):8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Chen R, Seitz RJ. Changing cortical excitability with low-frequency magnetic stimulation. Neurology. 2001;57(3):379–380. doi: 10.1212/wnl.57.3.379. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Draganski B, May A. Training-induced structural changes in the adult human brain. Behav Brain Res. 2008;192(1):137–142. doi: 10.1016/j.bbr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn DM. Peabody picture vocabulary test. 4. Minneapolis, MN: NCS Pearson, Inc; 2007. [Google Scholar]
- Eliassen JC, Boespflug EL, Lamy M, Allendorfer J, Chu WJ, Szaflarski JP. Brain-mapping techniques for evaluating poststroke recovery and rehabilitation: A review. Top Stroke Rehabil. 2008;15(5):427–450. doi: 10.1310/tsr1505-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez B, Cardebat D, Demonet JF, Joseph PA, Mazaux JM, Barat M, Allard M. Functional MRI follow-up study of language processes in healthy subjects and during recovery in a case of aphasia. Stroke. 2004;35(9):2171–2176. doi: 10.1161/01.STR.0000139323.76769.b0. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117(12):2584–2596. doi: 10.1016/j.clinph.2006.06.712. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Leonard CM, Gilmore R, Fennell E, Heilman KM. Planum temporale asymmetry and language dominance. Neuropsychologia. 1994;32(10):1225–1231. doi: 10.1016/0028-3932(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. The assessment of aphasia and related disorders. Philadelphia, PA: Lea & Febiger; 1972. [Google Scholar]
- Gutierrez R, Boison D, Heinemann U, Stoffel W. Decompaction of CNS myelin leads to a reduction of the conduction velocity of action potentials in optic nerve. Neurosci Lett. 1995;195(2):93–96. doi: 10.1016/0304-3940(94)11789-l. [DOI] [PubMed] [Google Scholar]
- Hamilton RH, Chrysikou EG, Coslett B. Mechanisms of aphasia recovery after stroke and the role of noninvasive brain stimulation. Brain Lang. 2011;118(1–2):40–50. doi: 10.1016/j.bandl.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton RH, Sanders L, Benson J, Faseyitan O, Norise C, Naeser M, Martin P, Coslett HB. Stimulating conversation: Enhancement of elicited propositional speech in a patient with chronic non-fluent aphasia following transcranial magnetic stimulation. Brain Lang. 2010;113(1):45–50. doi: 10.1016/j.bandl.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington GS, Farias ST. Sex differences in language processing: Functional MRI methodological considerations. J Magn Reson Imaging. 2008;27(6):1221–1228. doi: 10.1002/jmri.21374. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Thiel A. A proposed regional hierarchy in recovery of post-stroke aphasia. Brain Lang. 2006;98(1):118–123. doi: 10.1016/j.bandl.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Heynen AJ, Bear MF. Long-term potentiation of thalamocortical transmission in the adult visual cortex in vivo. J Neurosci. 2001;21(24):9801–9813. doi: 10.1523/JNEUROSCI.21-24-09801.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogendam JM, Ramakers GM, Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 2010;3(2):95–118. doi: 10.1016/j.brs.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Chen RS, Rothwell JC, Wen HY. The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin Neurophysiol. 2007;118(5):1028–1032. doi: 10.1016/j.clinph.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45(2):201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Jones DK, Horsfield MA, Simmons A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn Reson Med. 1999;42(3):515–525. [PubMed] [Google Scholar]
- Josse G, Herve PY, Crivello F, Mazoyer B, Tzourio-Mazoyer N. Hemispheric specialization for language: Brain volume matters. Brain Res. 2006;1068(1):184–193. doi: 10.1016/j.brainres.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Kakuda W, Abo M, Uruma G, Kaito N, Watanabe M. Low-frequency rTMS with language therapy over a 3-month period for sensory-dominant aphasia: Case series of two post-stroke japanese patients. Brain Inj. 2010a;24(9):1113–1117. doi: 10.3109/02699052.2010.494587. [DOI] [PubMed] [Google Scholar]
- Kakuda W, Abo M, Kaito N, Watanabe M, Senoo A. Functional MRI-based therapeutic rTMS strategy for aphasic stroke patients: A case series pilot study. Int J Neurosci. 2010b;120(1):60–66. doi: 10.3109/00207450903445628. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston naming test. 2. Baltimore, MD: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- Kim KK, Karunanayaka P, Privitera MD, Holland SK, Szaflarski JP. Semantic association investigated with functional MRI and independent component analysis. Epilepsy Behav. 2011;20(4):613–622. doi: 10.1016/j.yebeh.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T, Vaidya CJ, Gabrieli JD, Moseley ME, Hedehus M. Myelination and organization of the frontal white matter in children: A diffusion tensor MRI study. Neuroreport. 1999;10(13):2817–2821. doi: 10.1097/00001756-199909090-00022. [DOI] [PubMed] [Google Scholar]
- Kozora E, Cullum CM. Generative naming in normal aging: Total output and qualitative changes using phonemic and semantic constraints. The Clinical Neuropsychologist. 1995;9(4):313–320. [Google Scholar]
- Landman BA, Farrell JA, Jones CK, Smith SA, Prince JL, Mori S. Effects of diffusion weighting schemes on the reproducibility of DTI-derived fractional anisotropy, mean diffusivity, and principal eigenvector measurements at 1.5T. Neuroimage. 2007;36(4):1123–1138. doi: 10.1016/j.neuroimage.2007.02.056. [DOI] [PubMed] [Google Scholar]
- Lee JH, Garwood M, Menon R, Adriany G, Andersen P, Truwit CL, Ugurbil K. High contrast and fast three-dimensional magnetic resonance imaging at high fields. Magn Reson Med. 1995;34(3):308–312. doi: 10.1002/mrm.1910340305. [DOI] [PubMed] [Google Scholar]
- Lezak M. Neuropsychological assessment. New York, NY: Oxford University Press; 1995. [Google Scholar]
- Madden DJ, Bennett IJ, Song AW. Cerebral white matter integrity and cognitive aging: Contributions from diffusion tensor imaging. Neuropsychol Rev. 2009;19(4):415–435. doi: 10.1007/s11065-009-9113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PI, Naeser MA, Theoret H, Tormos JM, Nicholas M, Kurland J, Fregni F, Seekins H, Doron K, Pascual-Leone A. Transcranial magnetic stimulation as a complementary treatment for aphasia. Semin Speech Lang. 2004;25(2):181–191. doi: 10.1055/s-2004-825654. [DOI] [PubMed] [Google Scholar]
- Martin PI, Naeser MA, Ho M, Doron KW, Kurland J, Kaplan J, Wang Y, Nicholas M, Baker EH, Alonso M, Fregni F, Pascual-Leone A. Overt naming fMRI pre- and post-TMS: Two nonfluent aphasia patients, with and without improved naming post-TMS. Brain Lang. 2009;111(1):20–35. doi: 10.1016/j.bandl.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A, Hajak G, Ganssbauer S, Steffens T, Langguth B, Kleinjung T, Eichhammer P. Structural brain alterations following 5 days of intervention: Dynamic aspects of neuroplasticity. Cereb Cortex. 2007;17(1):205–210. doi: 10.1093/cercor/bhj138. [DOI] [PubMed] [Google Scholar]
- Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51(5):527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Lundgren K, Klein R, Kaplan J, Treglia E, Ho M, Nicholas M, Alonso M, Pascual-Leone A. Improved language in a chronic nonfluent aphasia patient after treatment with CPAP and TMS. Cogn Behav Neurol. 2010;23(1):29–38. doi: 10.1097/WNN.0b013e3181bf2d20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Kobayashi M, Theoret H, Fregni F, Maria-Tormos J, Kurland J, Doron KW, Pascual-Leone A. Improved picture naming in chronic aphasia after TMS to part of right broca's area: An open-protocol study. Brain Lang. 2005;93(1):95–105. doi: 10.1016/j.bandl.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Baker EH, Hodge SM, Sczerzenie SE, Nicholas M, Palumbo CL, Goodglass H, Wingfield A, Samaraweera R, Harris G, Baird A, Renshaw P, Yurgelun-Todd D. Overt propositional speech in chronic nonfluent aphasia studied with the dynamic susceptibility contrast fMRI method. Neuroimage. 2004;22(1):29–41. doi: 10.1016/j.neuroimage.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Nair G, Tanahashi Y, Low HP, Billings-Gagliardi S, Schwartz WJ, Duong TQ. Myelination and long diffusion times alter diffusion-tensor-imaging contrast in myelin-deficient shiverer mice. Neuroimage. 2005;28(1):165–174. doi: 10.1016/j.neuroimage.2005.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Canete C, Catala MD. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol. 1998;15(4):333–343. doi: 10.1097/00004691-199807000-00005. [DOI] [PubMed] [Google Scholar]
- Peters A. The effects of normal aging on myelin and nerve fibers: A review. J Neurocytol. 2002;31(8–9):581–593. doi: 10.1023/a:1025731309829. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201(3):637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- Price CJ, Crinion J. The latest on functional imaging studies of aphasic stroke. Curr Opin Neurol. 2005;18(4):429–434. doi: 10.1097/01.wco.0000168081.76859.c1. [DOI] [PubMed] [Google Scholar]
- Pulvermuller F, Neininger B, Elbert T, Mohr B, Rockstroh B, Koebbel P, Taub E. Constraint-induced therapy of chronic aphasia after stroke. Stroke. 2001;32(7):1621–1626. doi: 10.1161/01.str.32.7.1621. [DOI] [PubMed] [Google Scholar]
- Ringo JL, Doty RW, Demeter S, Simard PY. Time is of the essence: A conjecture that hemispheric specialization arises from interhemispheric conduction delay. Cereb Cortex. 1994;4(4):331–343. doi: 10.1093/cercor/4.4.331. [DOI] [PubMed] [Google Scholar]
- Rohde GK, Barnett AS, Basser PJ, Marenco S, Pierpaoli C. Comprehensive approach for correction of motion and distortion in diffusion-weighted MRI. Magn Reson Med. 2004;51(1):103–114. doi: 10.1002/mrm.10677. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Petersen SE, Linenweber MR, Snyder AZ, White DA, Chapman L, Dromerick AW, Fiez JA, Corbetta MD. Neural correlates of recovery from aphasia after damage to left inferior frontal cortex. Neurology. 2000;55(12):1883–1894. doi: 10.1212/wnl.55.12.1883. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Glen DR, Chen G, Beauchamp MS, Desai R, Cox RW. A new method for improving functional-to-structural MRI alignment using local pearson correlation. Neuroimage. 2009;44(3):839–848. doi: 10.1016/j.neuroimage.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaug G, Marchina S, Norton A. Evidence for plasticity in white-matter tracts of patients with chronic broca's aphasia undergoing intense intonation-based speech therapy. Ann N Y Acad Sci. 2009:1169385–394. doi: 10.1111/j.1749-6632.2009.04587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Dardzinski BJ, Holland SK. Simultaneous correction of ghost and geometric distortion artifacts in EPI using a multiecho reference scan. IEEE Trans Med Imaging. 2001;20(6):535–539. doi: 10.1109/42.929619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Klein MC, Behrens TE, Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci. 2009;12(11):1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebner HR, Peller M, Willoch F, Minoshima S, Boecker H, Auer C, Drzezga A, Conrad B, Bartenstein P. Lasting cortical activation after repetitive TMS of the motor cortex: A glucose metabolic study. Neurology. 2000;54(4):956–963. doi: 10.1212/wnl.54.4.956. [DOI] [PubMed] [Google Scholar]
- Skare S, Hedehus M, Moseley ME, Li TQ. Condition number as a measure of noise performance of diffusion tensor data acquisition schemes with MRI. J Magn Reson. 2000;147(2):340–352. doi: 10.1006/jmre.2000.2209. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1S):208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20(3):1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17(3):1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Stamatakis EA, Shafto MA, Williams G, Tam P, Tyler LK. White matter changes and word finding failures with increasing age. PLoS One. 2011;6(1):e14496. doi: 10.1371/journal.pone.0014496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, Vannest J, Wu SW, Difrancesco MW, Banks C, Gilbert DL. Excitatory repetitive transcranial magnetic stimulation induces improvements in chronic post-stroke aphasia. Med Sci Monit. 2011;17(3):CR132–139. doi: 10.12659/MSM.881446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, Ball A, Grether S, Al-Fwaress F, Griffith NM, Neils-Strunjas J, Newmeyer A, Reichhardt R. Constraint-induced aphasia therapy stimulates language recovery in patients with chronic aphasia after ischemic stroke. Med Sci Monit. 2008;14(5):CR243–250. [PMC free article] [PubMed] [Google Scholar]
- van der Knaap LJ, van der Ham IJ. How does the corpus callosum mediate interhemispheric transfer? A review. Behav Brain Res. 2011;223(1):211–221. doi: 10.1016/j.bbr.2011.04.018. [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230(1):77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Waxman SG. Determinants of conduction velocity in myelinated nerve fibers. Muscle Nerve. 1980;3(2):141–150. doi: 10.1002/mus.880030207. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Black JA, Stys PK, Ransom BR. Ultrastructural concomitants of anoxic injury and early post-anoxic recovery in rat optic nerve. Brain Res. 1992;574(1–2):105–119. doi: 10.1016/0006-8993(92)90806-k. [DOI] [PubMed] [Google Scholar]
- Weiduschat N, Thiel A, Rubi-Fessen I, Hartmann A, Kessler J, Merl P, Kracht L, Rommel T, Heiss WD. Effects of repetitive transcranial magnetic stimulation in aphasic stroke: A randomized controlled pilot study. Stroke. 2011;42(2):409–415. doi: 10.1161/STROKEAHA.110.597864. [DOI] [PubMed] [Google Scholar]
- Westerhausen R, Kreuder F, Dos Santos Sequeira S, Walter C, Woerner W, Wittling RA, Schweiger E, Wittling W. The association of macro- and microstructure of the corpus callosum and language lateralisation. Brain Lang. 2006;97(1):80–90. doi: 10.1016/j.bandl.2005.07.133. [DOI] [PubMed] [Google Scholar]