The Surveillance, Epidemiology, and End Results–Medicare linked database was used to describe the extent of nonadherence to anthracycline-based chemotherapy regimens in older patients with early breast cancer and to explore factors associated with nonadherence.
Keywords: Adherence to chemotherapy, Anthracyclines, Early breast cancer, Elderly patients with breast cancer, SEER–Medicare database
Learning Objectives
After completing this course, the reader will be able to:
Describe rates of adherence to anthracycline-based chemotherapy in elderly patients with early breast cancer, using a population-based database.
Identify a subset of early breast cancer patients with a higher likelihood of non-adherence to the course of chemotherapy treatment.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Background.
Rates of anthracycline adherence in breast cancer (BC) patients are unknown, but noncompletion of chemotherapy is associated with worse outcomes.
Methods.
Using the Surveillance, Epidemiology, and End Results–Medicare database, we obtained demographics, comorbidities, tumor characteristics, and treatment and hospitalization data from stage I–III BC patients diagnosed at age ≥66 years in 1996–2005 treated with surgery who had anthracycline claims. We compared variables between patients with claims for less than four cycles, considered nonadherent cases, and those with claims for four or more cycles using logistic regression analyses.
Results.
The sample included 7,399 patients, of whom 1,222 (16.5%) were nonadherent cases. Two hundred forty-three (3.3%) patients had one claim, 298 (4.0%) had two claims, and 681 (9.2%) had three claims. The multivariate regression model showed statistically significant associations between nonadherence and older age, black race, unmarried status, diagnosis before the year 2001, and hospitalizations.
Conclusions.
Eighty-three percent of older patients with early-stage BC completed at least four cycles of an anthracycline-based chemotherapy regimen. We identified a subset of patients with a higher likelihood of not adhering to the course of treatment. Further research is warranted to develop interventions to enhance adherence.
Introduction
In 2010, an estimated 207,090 women in the U.S. were newly diagnosed with breast cancer, and 39,840 died as a result of this disease [1]. The treatment of breast cancer usually entails a multidisciplinary approach, with the specific treatment depending on several factors, such as the stage at diagnosis, histology, hormonal receptor status, and human epidermal growth factor receptor 2/neu status. The current standard of care can include surgery, chemotherapy, radiation therapy, hormonal therapy, targeted therapy, or various combinations of these. Other factors that can influence therapy are the patient's age, comorbidities, and the patient's personal preference [2].
The benefits of adjuvant chemotherapy in effectively reducing the risk for recurrence and death from breast cancer have been shown by multiple randomized control trials and meta-analysis [3]. Likewise, noncompletion of chemotherapy has been associated with worse outcomes [4]. The causes of nonadherence to chemotherapy are unknown because few studies have addressed this issue outside the clinical trials setting.
For several years, anthracyclines have been the backbone of chemotherapy combinations used in the adjuvant and neoadjuvant settings. A minimum of four cycles of an anthracycline-based regimen is considered a standard chemotherapy regimen [5]. During the time period of this study, in the U.S., the most widely used anthracycline-based regimens were: doxorubicin (A) with cyclophosphamide (C) for four cycles, fluorouracil (F) with AC for six cycles, F with epirubicin at 100 mg/m2 and C for six cycles, and other regimens that use these same chemotherapy agents with different dosing schedules and/or in combination with taxanes such as paclitaxel and docetaxel [3, 6].
We used the Surveillance, Epidemiology, and End Results (SEER)–Medicare linked database [7] to describe the extent of nonadherence to anthracycline-based chemotherapy regimens in older patients with early breast cancer and to explore factors associated with nonadherence.
Materials and Methods
Data Source
We used data provided from the SEER–Medicare linked database. SEER is a national population-based tumor registry of incident cancer cases. SEER subjects who are eligible for Medicare services have been linked to their Medicare records. The SEER–Medicare database links the SEER data with Medicare records for the U.S. population aged ≥65 years, which results in a unique, large, population-based cancer registry data for elder men and women.
Study Population
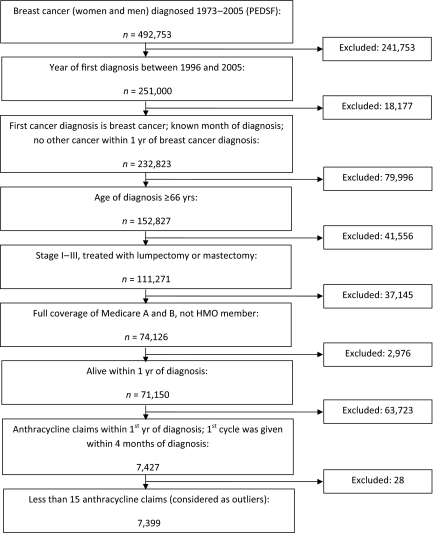
We initially identified 492,753 breast cancer patients (men and women) with a date of diagnosis in 1973–2005 recorded in the Patient Entitlement and Diagnosis Summary File (Fig. 1). We selected those with a first breast cancer diagnosed in 1996–2005 (removed 241,753). We excluded those who had a previous cancer before their diagnosis of breast cancer (n = 6,596) and also individuals who had a second cancer within 1 year of the diagnosis of breast cancer (n = 10,666). We also excluded those with an unknown month of diagnosis (n = 915). We included patients with an age at diagnosis ≥66 years (removed 79,996), with stage I–III disease (removed 38,626), and who were treated with definitive surgery (removed 2,930). To ensure complete Medicare claims, we selected individuals who had full coverage of both Medicare Part A and Part B during the 12 months before and 12 months after diagnosis and had not been health maintenance organization member during this time (removed 37,145). We also excluded patients who died within 1 year of diagnosis (n = 2,853) because they may have not survived long enough to receive a complete course of chemotherapy. We also removed 123 patients with a non–breast cancer histology code.
Figure 1.
Cohort pooling procedure.
Abbreviations: HMO, health maintenance organization; PEDSF, Patient Entitlement and Diagnosis Summary File.
We had narrowed the study population down to 71,150 breast cancer patients. We then retrieved individuals' anthracycline claims data from the Medicare databases, including the Durable Medical Equipment, National Claims History Physician/Supplier, and Outpatient files, which covered claims for 1995–2007. Anthracycline claims were identified using level II Healthcare Common Procedure Coding System codes J9000, J9001, J9010 (only from claims years 1995–1997), J9178, and J9180 [8]. We only included anthracycline claims made within the first year after diagnosis, limited to those patients who received their first cycle of anthracycline within 4 months of diagnosis, and we also excluded denied claims. We identified 31,618 anthracycline Medicare claims that were made by 7,427 breast cancer patients. We finally excluded 28 individuals who had ≥15 anthracycline claims within the first year after diagnosis because they were considered outliers.
To evaluate comorbid conditions as a possible risk factor for anthracycline nonadherence, we calculated a comorbidity score using the Klabunde adaptation of the Charlson comorbidity index, which is a summary of weighted comorbidities calculated using diagnostic and procedural codes available in the Medicare inpatient and outpatient claims datasets. This comorbidity index has been demonstrated to predict mortality and is widely used in research that uses claims datasets [9–11]. We used the SAS (SAS Institute Inc., Cary, NC) macro provided by the National Cancer Institute. For those who did not have a comorbidity disease claim, we assigned a comorbidity score of zero.
We considered the number of hospitalization admissions between each patient's first anthracycline claim and within 1 year of diagnosis as another possible factor associated with nonadherence. Hospitalization claims falling within this time frame were retrieved from the Medicare Provider Analysis and Review file, which contains inpatient hospital information, including dates of admission and discharge and diagnosis and procedural codes. Hospitalization claims that occurred 60 days after each patient's last chemotherapy date were excluded because we thought they were unlikely to be a result of anthracycline side effects. The accumulated number of days that each patient remained hospitalized was also calculated.
Statistical Analysis
Patients' demographic, tumor, and treatment information was depicted using descriptive analysis. The distributions of anthracycline cycles were calculated and illustrated in a column graph. A flowchart-type diagram was used to describe the cohort pooling procedures. Patients who only finished one to three anthracycline cycles were considered as nonadherent cases, whereas those who finished 4 or more anthracycline cycles were considered as adherent cases. A χ2 analysis was conducted to compare the frequencies of demographic, tumor, and treatment variables between nonadherent and adherent cases.
To identify risk factors associated with nonadherence to anthracycline-based chemotherapy, we conducted both univariate and multivariate logistic regressions. Odds ratios (ORs) and 95% confident intervals (CIs) were calculated. The multivariate model was adjusted for risk factors including age at diagnosis (66–70 years, 71–75 years, >75 years), race (white, black, other), marital status, educational level, poverty level, SEER region, year of diagnosis (before 2000, after 2000), lymph node involvement (negative, positive, unknown), tumor size (<2.0 cm, 2.0–4.9 cm, ≥5.0 cm, unknown), tumor grade (1, 2, 3, unknown), progesterone receptor (PR) and estrogen receptor (ER) status (negative, positive, unknown), surgery (lumpectomy, mastectomy), Charlson comorbidity index score (0, 1, 2+), radiation therapy (yes, no, unknown), and number of hospitalizations (0, 1, 2+).
The values of census tract variables for education and poverty were categorized in quartiles calculated in increasing order. The quartiles for percentage of persons aged ≥25 years with <12 years of education were: 0%–8.25% (representing a high educational level), 8.25%–14.25% (medium), 14.25%–23.45% (lower), and >23.45% (lowest). The quartiles for percentage of residents living below the poverty level were: 0%–4.06% (representing the lowest poverty level), 4.06%–7.38% (lower level), 7.38%–13.81% (medium level), and >13.81% (high level). Census data from the 2000 files were supplemented with 1990 files if missing or unknown information was found.
Besides the variables cited above, a univariate logistic regression model also examined the association between the number of accumulated hospitalization days and the odds of anthracycline nonadherence. This variable was stratified as 0 days, 1–7 days, and ≥8 days, and was not included in the multivariate model because of its collinearity with the number of hospitalizations. Tumor stage was not included in the logistic regression analysis because it provided redundant information with lymph node status and tumor size.
SAS system version 9.2 (SAS Institute, Inc., Cary, NC) was used to perform all the data management and statistical analyses.
Results
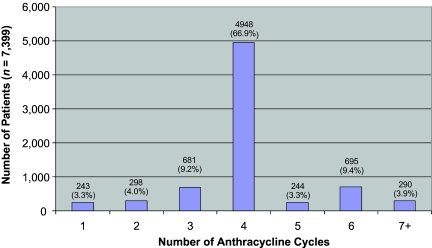
After applying the cohort pooling procedures described above, the final cohort population sample consisted of 7,399 breast cancer patients who received anthracycline-based chemotherapy. The distribution of the number of claims for these patients is shown in Figure 2. In total, 6,177 patients (83%) had four or more anthracycline claims and were, therefore, considered to be adherent. Among the 1,222 patients who had fewer than four claims, more than half (n = 681) had three claims, 20% (n = 243) had only one claim, and 24% (n = 298) had two claims. Among the adherent patients, ∼80% (n = 4,948) had exactly four claims, 4% (n = 244) had five claims, 11% (n = 695) had six claims, and the rest (5%) had seven or more claims.
Figure 2.
Distribution of anthracycline cycles.
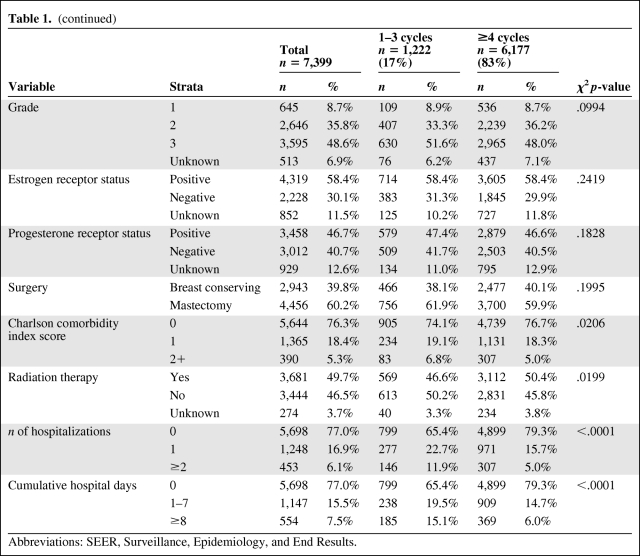
Table 1 presents rates of nonadherence stratified by patient characteristics and other factors. The study sample only included 75 male breast cancer patients, of whom nine (12%) were nonadherent. The majority of patients were white (84%), married (55%), and with an age at diagnosis of 66–70 years (53%). Regarding the tumor characteristics, 15% were stage I, 64% were stage II, and the remaining were stage III. Almost half the tumors (49%) were histologic grade 3, 67% of patients had positive lymph nodes, and 60% of tumors were either ER+ or PR+. Around 60% of the patients had a mastectomy performed and almost half received radiation therapy.
Table 1.
Patient characteristics
Table 1a.
(continued)
Abbreviations: SEER, Surveillance, Epidemiology, and End Results.
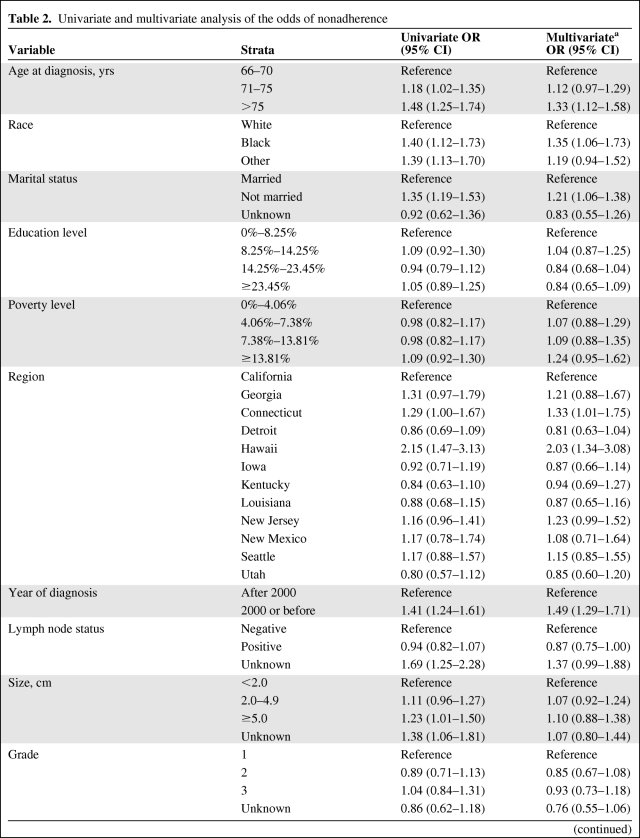
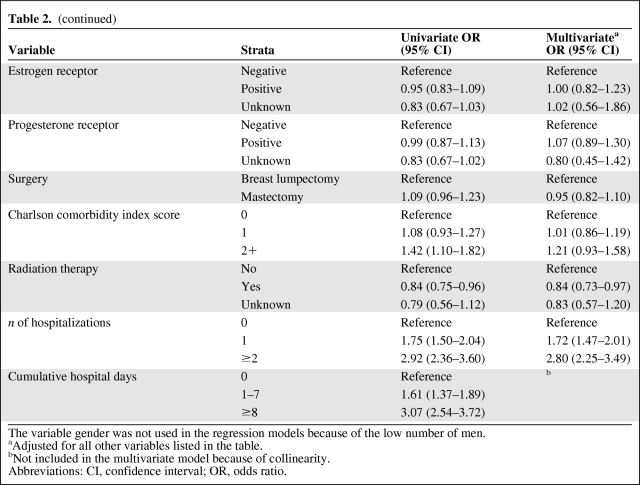
The univariate analysis (Table 2) showed that the following factors were associated with nonadherence: older age, black and “other” race, unmarried status, Charlson comorbidity index score ≥2, registration in the SEER geographic regions of Connecticut and Hawaii, diagnosis in the year 2000 or earlier, unknown lymph node status, tumor size ≥5.0 cm, greater number of hospitalizations, and greater number of days hospitalized. Interestingly, neither the educational level nor the poverty level was associated with being nonadherent. Certain tumor characteristics, such as grade and receptor status, were also not associated.
Table 2.
Univariate and multivariate analysis of the odds of nonadherence
Table 2a.
(continued)
The variable gender was not used in the regression models because of the low number of men.
aAdjusted for all other variables listed in the table.
bNot included in the multivariate model because of collinearity.
Abbreviations: CI, confidence interval; OR, odds ratio.
The results of the multivariate logistic regression model are presented in Table 2. Age >75 years (33% higher odds), black race (35% higher odds), and an unmarried status (21% higher odds) were statistically significantly associated with nonadherence. Interestingly, the SEER regions of Connecticut and Hawaii (with a twofold greater odds) were independently associated with nonadherence. Those with a diagnosis in the year 2000 or earlier had a 49% higher risk for nonadherence. None of the tumor characteristics were associated with nonadherence, after adjusting for all other variables included in the model. Similarly, the Charlson comorbidity index score did not maintain the strength of the association in the multivariate model. However, the number of hospitalizations was strongly associated with nonadherence and there was also a dose effect, whereby those who had one hospitalization had an OR of 1.72 (95% CI, 2.26–3.50) and those who had two or more hospitalizations had an OR of 2.81 (95% CI, 2.25–3.49).
Discussion
This population-based study shows that greater age at diagnosis, black race, unmarried status, diagnosis before the year 2001, and higher number of hospitalizations were all factors independently associated with nonadherence with an anthracycline-based chemotherapy regimen in older patients with early breast cancer. To our knowledge, this is the first population-based study that specifically addressed nonadherence with anthracyclines in older patients with early breast cancer.
In a previous population-based study of older women with early breast cancer, factors associated with noncompletion of adjuvant radiation therapy were: having a mastectomy, hospitalization during treatment, earlier year of diagnosis, and black race [12]. Interestingly, both studies had analogous results regarding factors associated with nonadherence, such as race, year of diagnosis, and hospitalization, and also regarding factors not associated with nonadherence, such as comorbidities and tumor characteristics. In contrast to the present study, age was not associated with adherence to radiotherapy.
Previous studies have shown that older women are less likely to receive standard-of-care adjuvant chemotherapy. A small single-center experience in Italy showed that poor compliance with adjuvant cyclophosphamide, methotrexate, and fluorouracil was more frequent among older patients [13]. DeMichele et al. [14] reported that the odds of receiving chemotherapy was 22% lower for each year of increasing age. The use of chemotherapy declines significantly with age across all stages in women with breast cancer [15]. A previous study from our institution found that women aged 65–74 years were 76% less likely and those aged >75 years were 97% less likely to receive chemotherapy, compared with women aged 55–64 years [16]. In the present study, all patients received at least one cycle of an anthracycline, so we assume that the recommendation to provide this chemotherapy regimen was initially given by the patient's medical oncologist, and also that the patient would have consented.
African-American race has repeatedly been shown to be an independent risk factor for worse survival from breast cancer than with white race [17–19]. This disparity has persisted even after controlling for known prognostic factors, such as stage at diagnosis and unfavorable biologic factors [18]. Using a single-institution tumor registry, Hershman et al. [4] demonstrated that premature termination of chemotherapy in early-stage breast cancer patients was associated with both black race and poorer survival. Our study shows, on a population-based level, that black race is a risk factor independently associated with nonadherence with an anthracycline-based chemotherapy regimen, which could contribute to survival disparities.
The explanation for a survival advantage among married persons with cancer, compared with the unmarried, is not well understood. Suggested reasons are a higher economic status, healthier lifestyle behaviors, and more social support networks. Using a SEER–Medicare database, Osborne et al. [20] demonstrated that unmarried women with early-stage breast cancer were less likely to receive definitive surgical intervention and also had a higher risk for death from breast cancer, after controlling for known prognostic factors. Silliman et al. [21] described that older unmarried women were more concerned than married women about postsurgical-related problems and possible out-of-pocket costs of their care, which could lead them to choose a less intense regimen. Our study adds to the body of research knowledge showing that unmarried individuals with breast cancer are less likely to complete an anthracycline-based chemotherapy regimen than married patients.
Anthracycline-based chemotherapy is associated with higher rates of chemotherapy-related toxicity in older patients [15]. It seems intuitive that the number of hospitalizations and the number of cumulative hospitalization days after initiating chemotherapy with an anthracycline-based regimen are associated with nonadherence, considering the well-known toxicities, such febrile neutropenia, infections, thrombocytopenia, mucositis, dehydration resulting from emesis, and cardiac toxicity. In addition, patients who develop an intervening severe medical illness might not be appropriate candidates for further chemotherapy.
An independent factor found to be associated with nonadherence is being diagnosed with breast cancer before the year 2001, compared with a diagnosis after 2001. Better adherence at later dates may relate to progress made in the symptom control of chemotherapy-induced side effects and possible greater awareness of the importance of completion of chemotherapy regimens to breast cancer outcomes.
Other studies are evaluating interventions that could help improve completion rates of chemotherapy. The Improving Patient Access and Adherence to Cancer Treatment randomized controlled trial in low-income Hispanic women suggested that active telephone patient navigation or written resource informational materials could improve adherence to chemotherapy in breast cancer patients [22].
Using population-based claims databases has its inherent limitations. One of them is that we cannot determine the actual chemotherapy regimen used by each individual patient. We reviewed chemotherapy claims made within 3 months after the last anthracycline claim in nonadherent patients and found that 38% had at least one claim for a taxane. It is likely that some of these patients were switched to a nonanthracycline regimen or completed their chemotherapy schedule with a taxane. However, because of issues with the timing of the billing, it is impossible to determine which patients actually received further chemotherapy.
Another limitation is that we cannot determine the exact reason for nonadherence for each patient. Other reasons could be the patient's oncologist recommending less than four cycles of chemotherapy, the patient's preference to stop treatment, discontinuation resulting from chemotherapy-induced toxicities, or the presence of a severe illness that impeded continuation of the chemotherapy plan. Given that individual chemotherapy agents are not discernable using Medicare claims when patients receive chemotherapy in the inpatient setting [23], we can also speculate that some of the nonadherent cases could have received chemotherapy while hospitalized. Medicare billing errors and inaccuracies capturing billing information can also occur, despite the strong incentives to bill correctly [23]. Although all the patients included in this study had medical insurance coverage provided by Medicare, it is possible that out-of-pocket obligations, such as copays, could have influenced a possible decision to not continue the planned chemotherapy schedule. Our findings also only apply to older U.S. breast cancer patients and may not be representative of a younger population.
The reasons for nonadherence to chemotherapy schedules could be different in developing countries, such as the experience described by Adisa et al. [24] in Nigeria, where the most important reason to not complete the scheduled cycles was the patient's inability to afford the cost of the treatment, whereas other causes were the unavailability of the drugs, hospital staff strikes, lack of transportation to the treatment center, and opting out of the treatment plan to attend spiritual healing.
Summary
Our study shows, on a population-based level, that ∼83% of patients diagnosed at age ≥66 years with stage I–III breast cancer who initiate an anthracycline-based chemotherapy regimen complete the scheduled cycles. We also identified a subset of patients most likely to not adhere to the planned anthracycline-based regimen. Further research is needed to determine reasons for noncompletion of chemotherapy and to develop interventions to optimize chemotherapy treatment in older patients.
Acknowledgments
This work was supported by the National Institutes of Health (K07CA109064) and the Cancer Prevention and Research Institute of Texas (RP101207).
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Sharon H. Giordano, Carlos H. Barcenas, Hui Zhao, Gabriel N. Hortobagyi
Provision of study material or patients: Sharon H. Giordano
Collection and/or assembly of data: Sharon H. Giordano, Ning Zhang, Zhigang Duan
Data analysis and interpretation: Sharon H. Giordano, Carlos H. Barcenas, Ning Zhang, Hui Zhao, Zhigang Duan, Thomas A. Buchholz, Gabriel N. Hortobagyi
Manuscript writing: Sharon H. Giordano, Carlos H. Barcenas, Ning Zhang, Hui Zhao, Zhigang Duan, Thomas A. Buchholz, Gabriel N. Hortobagyi
Final approval of manuscript: Sharon H. Giordano, Carlos H. Barcenas, Ning Zhang, Hui Zhao, Zhigang Duan, Thomas A. Buchholz, Gabriel N. Hortobagyi
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Rigden C, Chavez-MacGregor M. Breast cancer. In: Cashen A, Wildes T, editors. The Washington Manual Hematology and Oncology Subspecialty Consult. Second Edition. Philadelphia, PA: Wolters Kluwer, Lippincott Williams & Wilkins; 2008. pp. 189–203. [Google Scholar]
- 3.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 4.Hershman D, McBride R, Jacobson JS, et al. Racial disparities in treatment and survival among women with early-stage breast cancer. J Clin Oncol. 2005;23:6639–6646. doi: 10.1200/JCO.2005.12.633. [DOI] [PubMed] [Google Scholar]
- 5.Jones SE, Savin MA, Holmes FA, et al. Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol. 2006;24:5381–5387. doi: 10.1200/JCO.2006.06.5391. [DOI] [PubMed] [Google Scholar]
- 6.Harlan LC, Abrams J, Warren JL, et al. Adjuvant therapy for breast cancer: Practice patterns of community physicians. J Clin Oncol. 2002;20:1809–1817. doi: 10.1200/JCO.2002.07.052. [DOI] [PubMed] [Google Scholar]
- 7.Potosky AL, Riley GF, Lubitz JD, et al. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31:732–748. [PubMed] [Google Scholar]
- 8.Warren JL, Harlan LC, Fahey A, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40(8 suppl):IV-55–IV-61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- 9.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 10.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: Differing perspectives. J Clin Epidemiol. 1993;46:1075–1079. doi: 10.1016/0895-4356(93)90103-8. discussion 1081–1090. [DOI] [PubMed] [Google Scholar]
- 11.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 12.Srokowski TP, Fang S, Duan Z, et al. Completion of adjuvant radiation therapy among women with breast cancer. Cancer. 2008;113:22–29. doi: 10.1002/cncr.23513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Maio E, Gravina A, Pacilio C, et al. Compliance and toxicity of adjuvant CMF in elderly breast cancer patients: A single-center experience. BMC Cancer. 2005;5:30. doi: 10.1186/1471-2407-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeMichele A, Putt M, Zhang Y, et al. Older age predicts a decline in adjuvant chemotherapy recommendations for patients with breast carcinoma: Evidence from a tertiary care cohort of chemotherapy-eligible patients. Cancer. 2003;97:2150–2159. doi: 10.1002/cncr.11338. [DOI] [PubMed] [Google Scholar]
- 15.Du X, Goodwin JS. Patterns of use of chemotherapy for breast cancer in older women: Findings from Medicare claims data. J Clin Oncol. 2001;19:1455–1461. doi: 10.1200/JCO.2001.19.5.1455. [DOI] [PubMed] [Google Scholar]
- 16.Giordano SH, Hortobagyi GN, Kau SW, et al. Breast cancer treatment guidelines in older women. J Clin Oncol. 2005;23:783–791. doi: 10.1200/JCO.2005.04.175. [DOI] [PubMed] [Google Scholar]
- 17.Menashe I, Anderson WF, Jatoi I, et al. Underlying causes of the black-white racial disparity in breast cancer mortality: A population-based analysis. J Natl Cancer Inst. 2009;101:993–1000. doi: 10.1093/jnci/djp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albain KS, Unger JM, Crowley JJ, et al. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst. 2009;101:984–992. doi: 10.1093/jnci/djp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barcenas CH, Wells J, Chong D, et al. Race as an independent risk factor for breast cancer survival: Breast cancer outcomes from the Medical College of Georgia tumor registry. Clin Breast Cancer. 2010;10:59–63. doi: 10.3816/CBC.2010.n.008. [DOI] [PubMed] [Google Scholar]
- 20.Osborne C, Ostir GV, Du X, et al. The influence of marital status on the stage at diagnosis, treatment, and survival of older women with breast cancer. Breast Cancer Res Treat. 2005;93:41–47. doi: 10.1007/s10549-005-3702-4. [DOI] [PubMed] [Google Scholar]
- 21.Silliman RA, Troyan SL, Guadagnoli E, et al. The impact of age, marital status, and physician-patient interactions on the care of older women with breast carcinoma. Cancer. 1997;80:1326–1334. [PubMed] [Google Scholar]
- 22.Ell K, Vourlekis B, Xie B, et al. Cancer treatment adherence among low-income women with breast or gynecologic cancer: A randomized controlled trial of patient navigation. Cancer. 2009;115:4606–4615. doi: 10.1002/cncr.24500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamont EB, Herndon JE, 2nd, Weeks JC, et al. Criterion validity of Medicare chemotherapy claims in Cancer and Leukemia Group B breast and lung cancer trial participants. J Natl Cancer Inst. 2005;97:1080–1083. doi: 10.1093/jnci/dji189. [DOI] [PubMed] [Google Scholar]
- 24.Adisa AO, Gukas ID, Lawal OO, et al. Breast cancer in Nigeria: Is non-adherence to chemotherapy schedules a major factor in the reported poor treatment outcome? Breast J. 2010;16:206–207. doi: 10.1111/j.1524-4741.2009.00883.x. [DOI] [PubMed] [Google Scholar]