The frequency with which the distal forearm T score based on dual x-ray absorptiometry scan was the worst T score in patients with primary hyperparathyroidism was examined, and whether or not this T score alone could lead to higher rate of diagnosis of osteopenia or osteoporosis was evaluated.
Keywords: Primary hyperparathyroidism, Osteoporosis, Forearm, Dual x-ray absorptiometry
Abstract
Background.
Primary hyperparathyroidism (PHPT) leads to increased bone turnover, low bone mineral density, and increased fracture risk. These effects are, however, preferentially seen in the distal forearm, which is rich in cortical bone. This study aimed to determine how frequently the distal forearm T score was the worst T score and if this T score alone led to higher rate of diagnosis of osteopenia or osteoporosis.
Materials and Methods.
We retrospectively reviewed a prospective database of 300 patients undergoing parathyroidectomy at our institution between November 2000 and January 2009. The bone mineral density of the lumbar spine, total proximal femurs, and distal third of the nondominant radius was measured by dual x-ray absorptiometry. Data on bone density are reported as T scores.
Results.
The mean T scores were −1.30 ± 0.2 in the distal forearm, −1.0 ± 0.1 in the total proximal femurs, and −0.9 ± 0.1 in the spine. The distal forearm T score was the worst bone mineral density T score in 39% of patients. This T score alone led to an upstaging in diagnosis to osteopenia or osteoporosis in 9.4% of patients.
Conclusion.
In patients with PHPT, the worst T score is commonly found in the distal forearm. This T score can identify additional patients with a diagnosis of osteopenia or osteoporosis. Distal forearm bone mineral density should, therefore, be assessed in all patients who have a diagnosis of PHPT.
Introduction
Primary hyperparathyroidism (PHPT) is a common endocrine disorder with a population prevalence of 2.1% in healthy postmenopausal women [1]. It is caused by a single adenoma in ∼80% of cases and by parathyroid hyperplasia in 15% of cases. Parathyroid cancer is rare and accounts for 1%–2% of cases.
Chronic parathyroid hormone (PTH) excess occurs in PHPT patients and causes increased bone resorption through stimulation of osteoclast-activating factors such as interleukin-6 from osteoblasts [2]. The catabolic effects of PTH are also mediated by increased production of osteoclast differentiating factor (receptor activator of nuclear factor κB ligand [RANKL]) and decreased production of osteoprotegerin (OPG) by osteoblasts and stromal cells. This increased ratio of RANKL to OPG enhances osteoclast differentiation and activation [3].
The resultant high bone turnover in PHPT patients is associated with a reduction in bone mineral density. This reduction varies among skeletal sites, with both densitometry and histomorphometric analysis showing preferential involvement of cortical bone (forearm and hip) as opposed to cancellous bone (spine). Bone mineral density in the spine may be well preserved or even slightly increased [4]. After surgical cure of PHPT, the bone mineral density increases at all three sites; however, this occurs more so in the spine than in the hip or forearm [5]. Other long-term studies have found a permanent decrease in bone mineral density in the forearm even in patients who have surgery, suggesting that cortical bone loss in patients with PHPT is not easily reversible [6].
Decreased bone mineral density increases the risk for fracture. A high prevalence of fractures in patients with PHPT at the time of diagnosis has been reported. The sites at which the risk for fracture is greater are the forearm, spine, and femoral neck [7, 8]. Parathyroidectomy also influences fracture risk. One study showed that the risk for fracture decreased and returned to normal after parathyroidectomy. However, a small increase in the risk for fracture of the distal forearm emerged >10 years after surgery [9].
Because data show that parathyroidectomy leads to an increase in bone mineral density and a reduction in fracture risk, surgery is recommended for asymptomatic patients if the T score (standard deviation from the mean bone mineral density of the young adult population) at any of the three sites (forearm, hip, and spine) is <−2.5 [10].
Based on the preferential involvement of the forearm in PHPT, this study aimed to determine how frequently the bone mineral density T score was the worst at the distal forearm and if this T score alone led to a diagnosis of osteopenia or osteoporosis.
Materials and Methods
We retrospectively reviewed a prospective database of patients undergoing parathyroidectomy at our institution between November 2000 and January 2009. Nine hundred eighty-six patients had surgery for PHPT. This diagnosis was based on the finding of an elevated serum calcium concentration, an elevated or inappropriately normal PTH level, and an absence of other causes of hypercalcemia. The study cohort was identified by examining those patients who had a baseline dual x-ray absorptiometry (DXA) scan as part of their preoperative surgical assessment.
Clinical data collected included age, sex, fracture occurrence, and baseline laboratory values of calcium and PTH. The bone mineral density of the lumbar spine, total proximal femur, and distal third of the nondominant radius was measured using DXA (Lunar Radiation, Madison, WI). Data on bone density are reported as T scores. We also assessed if these patients were seen by an endocrinologist or another provider. Statistical analysis among these groups was performed using Student's t-test.
Results
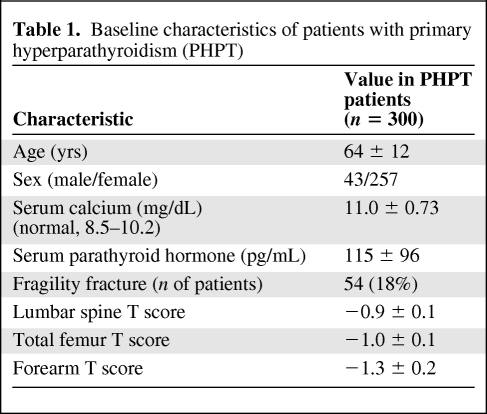
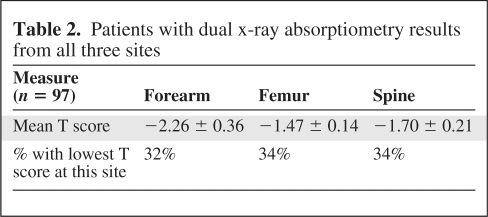
Three hundred patients had DXA scan results documented as part of their preoperative surgical assessment. There were 257 women and 43 men, with a mean age of 64 ± 12 years. The mean preoperative calcium concentration was 11.0 ± 0.73 mg/dL and the mean PTH level was 115 ± 96 pg/mL. The mean T scores were −1.30 ± 0.2 in the distal forearm, −1.0 ± 0.1 in the total proximal femurs, and −0.9 ± 0.1 in the spine (Table 1). Twenty-seven percent of patients had a normal bone mineral density, 36% had osteopenia, and 37% had osteoporosis according to World Health Organization (WHO) criteria. Eighteen percent of patients had a fragility fracture preoperatively and 39% of these occurred in patients whose lowest T score was in their forearm. This was defined as a fracture that occurs following relatively low trauma, such as a fall from standing height or less [11]. The distal forearm T score was the worst bone mineral density T score in 55 (39%) patients who had a forearm DXA scan performed in addition to the traditional spine and/or femur views. When we looked at only the 97 patients who had all three sites examined and interpretable results reported (i.e., they did not have degenerative disease, hardware, or scoliosis that made the results uninterpretable), the T score was worst in the spine in 34%, in the proximal femurs in 34%, and in the distal forearm in 32% of patients (Table 2). The forearm T score alone led to an upgrade in diagnosis from normal bone mass to osteopenia in 3% of patients and from osteopenia to osteoporosis in 6.4% of patients. Overall, the inclusion of forearm bone mineral density upstaged 9.4% of patients.
Table 1.
Baseline characteristics of patients with primary hyperparathyroidism (PHPT)
Table 2.
Patients with dual x-ray absorptiometry results from all three sites
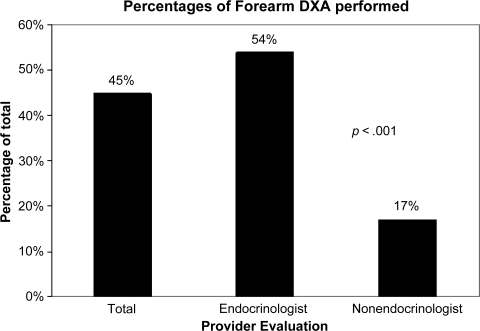
The distal forearm was assessed in only 45% of patients who had a preoperative DXA scan. Endocrinologists ordered a distal forearm assessment in 54% of their patients. However, this occurred in only 17% of patients seen by nonendocrinologists (Fig. 1).
Figure 1.
Percentage of patients with a forearm dual x-ray absorptiometry (DXA) scan performed.
Discussion
Seventy-three percent of patients in our series had osteopenia or osteoporosis according to the WHO criteria. These findings were similar to those from a study that showed that 79% of patients with primary hyperparathyroidism had osteopenia or osteoporosis [12]. The chronic hypersecretion of PTH that occurs with mild PHPT has been associated with anabolic effects on cancellous bone sites, such as the lumbar spine, but catabolic effects on cortical bone–rich sites, such as the distal one third of the radius. Changes in cortical bone consist of increased resorption of the endosteal envelope, increased cortical porosity, and thinning. In cancellous bone, a different response is seen. Although both bone resorption and bone formation are increased, resorption is decreased in the bone multicellular units (BMUs), so that the resorption bays are lower. Bone balance at individual BMUs is preserved or may even increase [13].
The classic pattern of reduced bone mineral density in PHPT patients is, therefore, maximal in the distal radius, which is predominantly cortical bone, minimal in the lumbar spine, which is enriched with cancellous bone, and intermediate in the hip region, which contains an equal content of both cortical and cancellous bone [14]. In one study, the mean Z score in patients with PHPT was −0.24 ± 0.20 in the lumbar spine, −0.76 ± 0.13 in the femoral neck, and −1.34 ± 0.15 in the distal radius [15]. Our study confirmed this, because the mean T score was worst in the distal forearm, at −1.3 ± 1.0.2, versus −1.0 ± 0.1 in the total proximal femurs and −0.9 ± 0.1 in the spine. In addition to this, the distal forearm T score was the worst bone mineral density T score in 39% of patients who had their forearm assessed and in 32% of those who had all three sites assessed and reported.
Because of the differing skeletal effects of PHPT, a 2002 workshop by the National Institutes of Health recommended that routine measurement of bone mineral density at three skeletal sites—the lumbar spine, hip, and forearm—be performed for assessment of PHPT [16]. Despite this, we found that the distal forearm was assessed in under half of our patients. Although endocrinologists ordered a forearm assessment more frequently than nonendocrinologists, this still occurred in just >50% of patients. This could result in the underestimation of the severity of skeletal effects of PHPT, because the forearm T score alone led to a change in diagnosis to osteopenia or osteoporosis in 9.4% of patients. At our institution, forearm DXA assessment exists as a separate order from an axial DXA scan, and this may be one of the reasons for its underuse.
Parathyroidectomy remains the only cure for PHPT and results in a significant gain in bone mineral density. The gain is maximal in the hip and the spine but only modest in the distal forearm, except in a group of patients with the lowest preoperative bone density, for whom the increase was also marked [15]. Surgery also significantly reduces the risk for osteoporotic fractures. One study showed that this reduction was maximal at the spine, followed by the distal one third of the radius [7]. Current guidelines recommend parathyroidectomy in patients with a T score <−2.5 at any of the three sites [10]. If forearm assessment is not performed, there may be a proportion of patients with osteoporosis based on the forearm T score alone who may not be appropriately referred for surgery.
Summary
Primary hyperparathyroidism leads to low bone mineral density that is predominantly seen in the distal forearm, which is rich in cortical bone. Our study confirmed this because the distal forearm T score was the worst T score in 39% of patients. Mean T scores were also the lowest at this site. Despite this involvement, preoperative forearm DXA assessments are underused. This underestimates the extent of skeletal involvement and the proportion of patients who meet the requirements for surgery based on bone density alone. Because a distal forearm DXA scan may not be part of the standard order, it is essential that providers caring for patients with PHPT recognize the importance of obtaining it. Forearm assessment should therefore be included if a preoperative DXA scan is performed in patients with PHPT.
Author Contributions
Conception/Design: Rebecca Sippel, Kelly Wood, Herbert Chen
Provision of study material or patients: Rebecca Sippel, Herbert Chen
Collection and/or assembly of data: Kelly Wood, Subarna Dhital
Data analysis and interpretation: Rebecca Sippel, Kelly Wood, Herbert Chen, Subarna Dhital
Manuscript writing: Rebecca Sippel, Kelly Wood, Subarna Dhital
Final approval of manuscript: Rebecca Sippel, Kelly Wood, Herbert Chen
References
- 1.Lundgren E, Rastad J, Thrufjell E, et al. Population-based screening for primary hyperparathyroidism with serum calcium and parathyroid hormone values in menopausal women. Surgery. 1997;121:287–294. doi: 10.1016/s0039-6060(97)90357-3. [DOI] [PubMed] [Google Scholar]
- 2.Grey A, Mitnick MA, Shapses S, et al. Circulating levels of interleukin-6 and tumor necrosis factor-α are elevated in primary hyperparathyroidism and correlate with markers of bone resorption—a clinical research center study. J Clin Endocrinol Metab. 1996;81:3450–3454. doi: 10.1210/jcem.81.10.8855783. [DOI] [PubMed] [Google Scholar]
- 3.Saleem TF, Horwith M, Stack BC., Jr Significance of primary hyperparathyroidism in the management of osteoporosis. Otolaryngol Clin North Am. 2004;37:751–761. viii–ix. doi: 10.1016/j.otc.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Silverberg SJ, Shane E, de la Cruz L, et al. Skeletal disease in primary hyperparathyroidism. J Bone Miner Res. 1989;4:283–291. doi: 10.1002/jbmr.5650040302. [DOI] [PubMed] [Google Scholar]
- 5.Rao DS, Phillips ER, Divine GW, et al. Randomized controlled clinical trial of surgery versus no surgery in patients with mild asymptomatic primary hyperparathyroidism. J Clin Endocrinol Metab. 2004;89:5415–5422. doi: 10.1210/jc.2004-0028. [DOI] [PubMed] [Google Scholar]
- 6.Silverberg SJ, Shane E, Jacobs TP, et al. A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med. 1999;341:1249–1255. doi: 10.1056/NEJM199910213411701. [DOI] [PubMed] [Google Scholar]
- 7.Khosla S, Melton LJ, 3rd, Wermers RA, et al. Primary hyperparathyroidism and the risk of fracture: A population-based study. J Bone Miner Res. 1999;14:1700–1707. doi: 10.1359/jbmr.1999.14.10.1700. [DOI] [PubMed] [Google Scholar]
- 8.Melton LJ, 3rd, Atkinson EJ, O'Fallon WM, et al. Risk of age-related fractures in patients with primary hyperparathyroidism. Arch Intern Med. 1992;152:2269–2273. [PubMed] [Google Scholar]
- 9.Vestergaard P, Mollerup CL, Frk̸jaer VG, et al. Cohort study of risk of fracture before and after surgery for primary hyperparathyroidism. BMJ. 2000;321:598–602. doi: 10.1136/bmj.321.7261.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bilezikian JP, Khan AA, Potts JT, Jr, et al. Guidelines for the management of asymptomatic primary hyperparathyroidism: Summary statement from the third international workshop. J Clin Endocrinol Metab. 2009;94:335–339. doi: 10.1210/jc.2008-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Center JR, Bliuc D, Nguyen TV, et al. Risk of subsequent fracture after low-trauma fracture in men and women. JAMA. 2007;297:387–394. doi: 10.1001/jama.297.4.387. [DOI] [PubMed] [Google Scholar]
- 12.Grant CS, Thompson G, Farley D, et al. Primary hyperparathyroidism surgical management since the introduction of minimally invasive parathyroidectomy: Mayo Clinic experience. Arch Surg. 2005;140:472–478. doi: 10.1001/archsurg.140.5.472. discussion 478–479. [DOI] [PubMed] [Google Scholar]
- 13.Eriksen EF. Primary hyperparathyroidism: Lessons from bone histomorphometry. J Bone Miner Res. 2002;17(suppl 2):N95–N97. [PubMed] [Google Scholar]
- 14.Miller PD, Bilezikian JP. Bone densitometry in asymptomatic primary hyperparathyroidism. J Bone Miner Res. 2002;17(suppl 2):N98–N102. [PubMed] [Google Scholar]
- 15.Silverberg SJ, Gartenberg F, Jacobs TP, et al. Longitudinal measurements of bone density and biochemical indices in untreated primary hyperparathyroidism. J Clin Endocrinol Metab. 1995;80:723–728. doi: 10.1210/jcem.80.3.7883823. [DOI] [PubMed] [Google Scholar]
- 16.Bilezikian JP, Potts JT, Jr, Fuleihan Gel-H, et al. Summary statement from a workshop on asymptomatic primary hyperparathyroidism: A perspective for the 21st century. J Clin Endocrinol Metab. 2002;87:5353–5361. doi: 10.1210/jc.2002-021370. [DOI] [PubMed] [Google Scholar]