Carcinoids and pancreatic neuroendocrine tumors are becoming increasingly common, with the majority of patients presenting with either lymph node involvement or metastatic disease. An improved understanding of the molecular mechanisms involved in these tumors has implicated several pathways that have led to new therapeutic approaches. Phase III studies indicate that pharmacologic inhibition of the vascular endothelial growth factor pathway with sunitinib, and of the mammalian target of rapamycin pathway with everolimus, appears to have altered the natural history of these diseases.
Keywords: Carcinoid, Neuroendocrine tumor, Gastroenteropancreatic neuroendocrine tumor, Islet cell tumor, Pancreatic neuroendocrine tumor
Learning Objectives
After completing this course, the reader will be able to:
Describe the underlying biology of neuroendocrine tumors including pancreatic neuroendocrine tumors (PNETs) and carcinoids and the importance of these biologic features in the evolution of new drugs for these diseases.
Cite the historical data regarding the use of cytotoxic agents in the treatment of pancreatic neuroendocrine tumors and carcinoids.
Explain the significance of recent clinical trials utilizing biologic agents, in particular octreotide, the small molecule tyrosine kinase inhibitor, sunitinib and the mammalian target of rapamycin (mTOR) inhibitor, everolimus, and how these medications have altered the natural history of both pancreatic neuroendocrine tumors and carcinoids.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Carcinoids and pancreatic neuroendocrine tumors are becoming increasingly common, with the majority of patients presenting with either lymph node involvement or metastatic disease. An improved understanding of the molecular mechanisms involved in these tumors has implicated several pathways that have led to new therapeutic approaches. In this manuscript, we describe the biology of neuroendocrine tumors and approaches to systemic therapy. We review early data regarding the use of cytotoxics and several recent studies employing more targeted approaches that promise to change the standard of care. Specifically, phase III studies indicate that pharmacologic inhibition of the vascular endothelial growth factor pathway with sunitinib, and of the mammalian target of rapamycin pathway with everolimus, appears to have altered the natural history of these diseases. These successes set the stage for further advances in the management of patients with neuroendocrine tumors.
Introduction
Historically, neuroendocrine tumors (both carcinoids and pancreatic neuroendocrine tumors) have been thought to arise from neuroendocrine cells located throughout the body that are capable of undergoing neoplastic transformation. More recent data suggests that there may be a role for cancer stem cells in the pathogenesis of these tumors [1]. Neuroendocrine tumors (NETs) are characterized as functional or nonfunctional depending on whether they produce hormones, which in turn may result in specific symptoms. The majority of NETs are gastrointestinal in origin, arising in the foregut, midgut, or hindgut. The Surveillance, Epidemiology, and End Results (SEER) database approximates that 1%–4% of NETs arise within the pancreas, although case ascertainment may underestimate true incidence. An additional 75%–86% represent carcinoids from other gastrointestinal sites. Whereas most NETs follow a relatively indolent course, a small percentage (9.1%) are aggressive high-grade neoplasms with poor differentiation [2]. Here we review the medical management of well differentiated and moderately differentiated metastatic carcinoids and pancreatic neuroendocrine tumors (PNETs) and describe the impact of new targeted therapies on the natural history of these diseases.
Epidemiology, Clinical Presentation, and Staging
Although considered rare malignancies, the reported incidence of carcinoids and PNETs is increasing, due in part to improved classification systems and increased use of endoscopy [3]. A recent evaluation of the SEER database noted a significant rise in the age-adjusted annual incidence of both carcinoids and PNETs since 1973 with a two- to sixfold increase in the number of cases. Gastrointestinal carcinoids have increased from approximately 0.61/100,000 to 3.58/100,000 in 2004, whereas PNETs have risen from 0.17/100,000 to 0.32/100,000 [3]. Median age at the time of diagnosis is 60 years with most patients (60%–80%) presenting with metastatic disease [3, 4]. For patients presenting with localized disease, survival is reported from 9.3–18.6 years. However, patients with metastatic disease have an inferior prognosis with a median survival of only 39 months [3]. Of the 43,000 pancreatic neoplasms diagnosed annually [5], PNETs represent only 1%–10% while the remainder are ductal adenocarcinomas [6, 7].
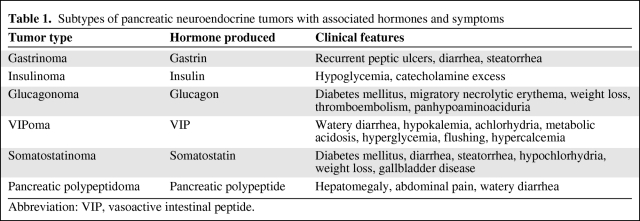
At the time of presentation, patients may have symptoms related to tumor bulk or, in 20%–50% of cases, hormone production [6, 8, 9]. The likelihood of presenting with hormone-related symptoms depends on the site of tumor origin, with PNETs and midgut carcinoids (jejunum, ileum, appendix, and proximal colon) being more likely to present with symptoms than carcinoids arising from the foregut (lungs, thymus, stomach, and duodenum) or hindgut (distal colon and rectum). PNETs specifically involve pancreatic islet cells that produce various hormones and result in symptoms depending on the underlying cellular subtype involved (Table 1) [9]. In contrast, midgut carcinoids produce symptoms by virtue of having high levels of serotonin production. This may result in carcinoid syndrome, characterized by diarrhea, flushing, and, particularly in patients with liver involvement, cardiac carcinoid with fibrotic endocardial plaque formation, tricuspid insufficiency, and ultimately pulmonary hypertension [10].
Table 1.
Subtypes of pancreatic neuroendocrine tumors with associated hormones and symptoms
Abbreviation: VIP, vasoactive intestinal peptide.
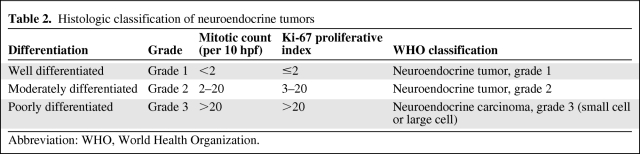
The TNM staging system as outlined by the American Joint Committee on Cancer (AJCC) is recommended for both carcinoids and PNETs [11, 12], with staging based on the site of origin. Tumor histology ranges from well differentiated with a relatively indolent course to poorly differentiated with a very aggressive course similar to that of small cell lung cancer. Although not officially part of any standard staging system, histologic features including degree of differentiation, mitotic count, and Ki-67 level (Table 2) have prognostic significance and can guide therapy [4, 8, 12].
Table 2.
Histologic classification of neuroendocrine tumors
Abbreviation: WHO, World Health Organization.
Neuroendocrine Tumor Biology
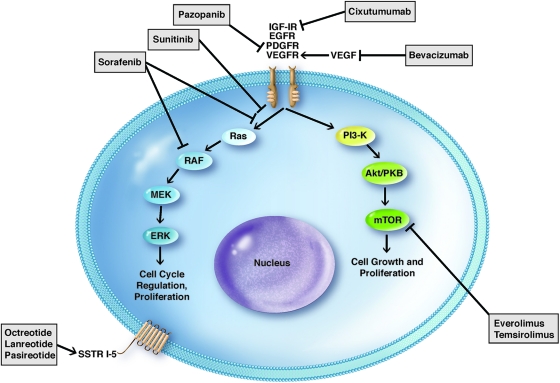
Several molecular mechanisms have been identified in the pathogenesis and behavior of neuroendocrine tumors (Fig. 1). This renders the treatment strategy for these tumors complex but also provides a basis for targeted approaches. In 80%–100% of PNETs and carcinoids, somatostatin receptors (SSTRs) are expressed of which there are five types (SSTR1–SSTR5). SSTR2 is the most common [13], but all five receptor types may be expressed with well differentiated tumors expressing higher levels and a wider variety of receptors than their poorly differentiated counterparts [14]. These are G protein coupled receptors that, upon binding somatostatin, are internalized as a receptor-ligand complex where they exert direct and indirect downstream effects. Direct effects on proliferation occur via inhibition of several pathways that differ depending on the receptor subtype, whereas indirect effects occur via suppression of growth factors such as insulin-like growth factor 1 (IGF-1) [13]. Binding of somatostatin (and thus its analogues) to somatostatin receptors also results in decreased hormone production by NETs, making it an attractive therapy for control of hormone-mediated symptoms.
Figure 1.
Molecular pathways implicated in neuroendocrine tumors and targets of action for therapeutic agents. Arrows represent activation whereas lines with a perpendicular block represent inhibition.
Abbreviations: EGFR, endothelial growth factor receptor; IGF-1R, insulin-like growth factor 1 receptor; mTOR, mammalian target of rapamycin; PDGFR, platelet-derived growth factor receptor; SSTR, somatostatin receptor; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Vascular endothelial growth factor (VEGF) is known to influence angiogenesis in PNETs and carcinoids [15, 16]. Additionally, several other growth factors and receptors including platelet-derived growth factor, platelet-derived growth factor receptors alpha and beta, stem-cell factor receptor (c-kit), VEGFR-2, VEGFR-3, insulin-like growth factor-1, insulin-like growth factor receptor, basic fibroblast growth factor, transforming growth factors alpha and beta, epidermal growth factor receptor, and stem-cell factor receptor have also been implicated in the cell signaling processes of NETs [15, 16]. Many of these pathways can be disrupted by small-molecule tyrosine kinase inhibitors and vascular endothelial growth factor inhibitors, which have led to clinical trials to evaluate the role of these agents in the treatment of NETs.
Mammalian target of rapamycin (mTOR) is an intracellular protein kinase involved in cell signaling and metabolism, which acts by mediating cell signaling via growth factor pathways [17–19]. One such growth factor is IGF-1, which has been shown to activate mTOR, resulting in cellular proliferation. Inhibition of mTOR has been shown to suppress neuroendocrine tumor growth [19, 20] and has recently proven to be a promising target for drug therapy.
Multiple inherited syndromes associated with NETs exist including multiple endocrine neoplasia types 1 and 2, von Hippel–Lindau disease, neurofibromatosis 1, and tuberous sclerosis, although most NETs are sporadic [12]. Among PNETs, 10%–15% are part of an inherited disorder [21], whereas the hereditary aspect of carcinoid tumors is less well characterized but believed to be less common. Among PNETs, the finding that somatic mutations in the MEN1, DAXX/ATRX, and mTOR pathway genes are most common (44%, 43%, and 14%, respectively) was recently described, with tumors containing a MEN1 or DAXX/ATRX mutation conferring a better prognosis [22]. Mechanisms including point mutations, deletions, DNA methylation, chromosomal loss, and chromosomal gains have been identified [23].
Treatment of NETs
The treatment approach for patients with NETs varies according to stage of disease. For localized disease, surgical resection is the standard of care. For patients with metastatic disease, goals of therapy include control of tumor growth and alleviation of hormone-mediated symptoms. Surgery may be appropriate in this setting for palliative debulking to decrease tumor burden or help control hormone production. Until recently, standard therapies for treatment of metastatic NETs included somatostatin analogues such as octreotide for hormonal control, hepatic-directed therapy for regional control, cytotoxic agents (including streptozocin, 5-fluorouracil, doxorubicin, capecitabine, dacarbazine, and temozolomide), or participation in a clinical trial. Over the last decade, development of targeted systemic therapies has resulted in significant impact as we understand more about the underlying biology of these tumors. Recently, two agents inhibiting relevant molecular targets have been approved by the U.S. Food and Drug Administration (FDA) for the treatment of well differentiated PNETs with promising data also emerging for carcinoids. These include the mTOR inhibitor, everolimus, and the VEGF receptor tyrosine kinase inhibitor (TKI), sunitinib. Despite having low response rates because they are cytostatic rather than cytotoxic, significantly longer progression-free survival (PFS) has been reported as compared to placebo, leading to a new standard for treatment of PNETs [16, 19].
Advancements in hepatic-directed therapies have also taken place over the last decade. Although mainly small phase I and phase II studies, multiple treatment modalities including bland hepatic artery embolization, chemoembolization, radiofrequency ablation, and administration of 90-Yttrium labeled microspheres are showing promising results, particularly in PNETs [24–30]. Although the specifics of these treatment modalities are beyond the scope of this review, these approaches should be considered as part of the management of localized liver metastases.
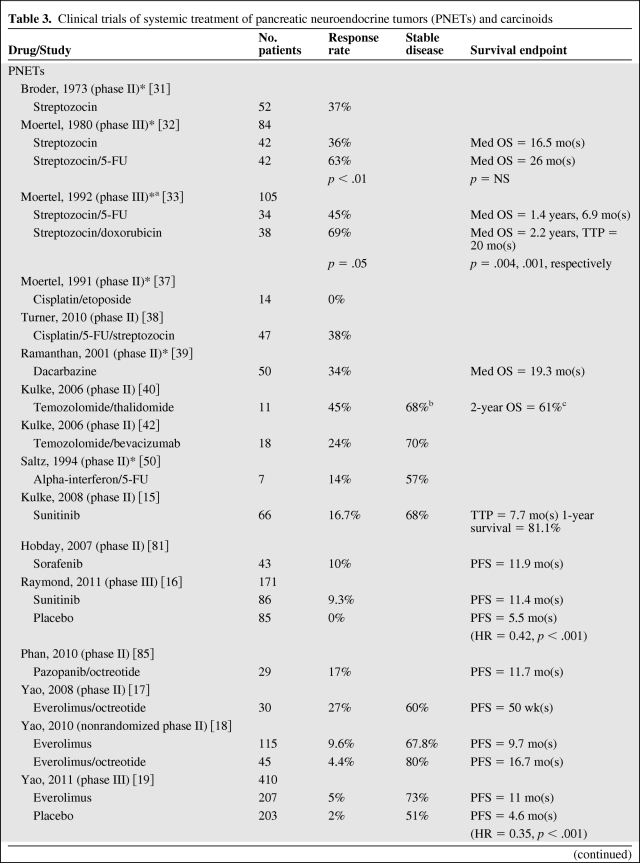
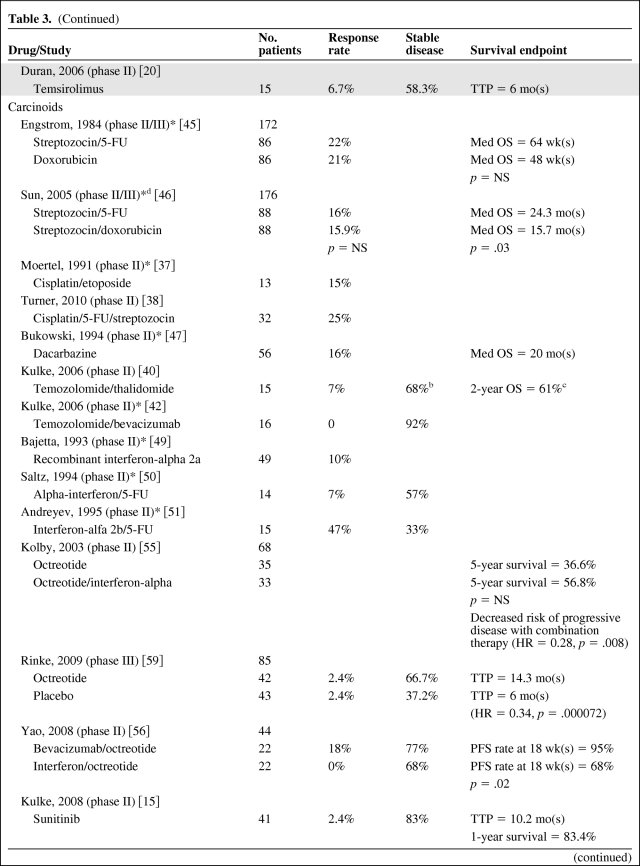
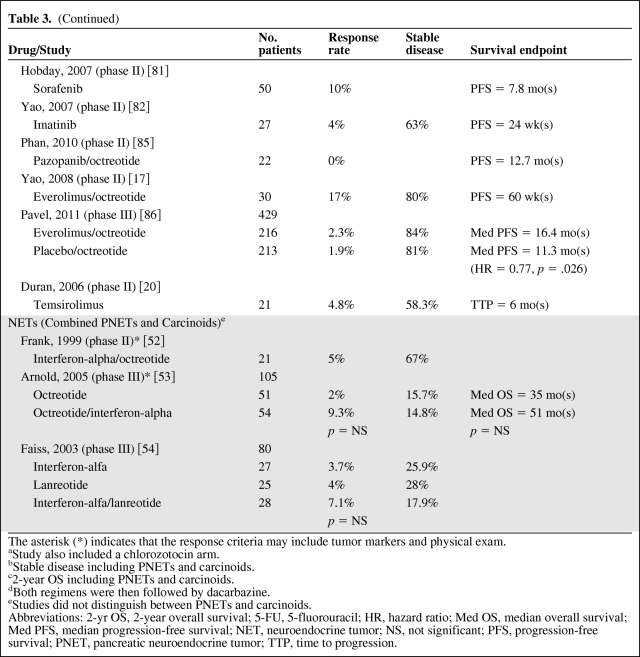
In evaluating results of treatment clinical trials for NETs, it is important to note that most trials conducted have been noncomparative phase II studies. Given the prolonged and variable natural history of NETs, nonrandomized studies must be interpreted with caution. Additionally, recent studies assessing targeted, cytostatic therapies have highlighted that a significant progression-free survival can be seen in the absence of a radiographic response, suggesting that response rate may be a suboptimal primary end point. This is not altogether surprising given that the indolent nature of these tumors often leads to stable disease both with and without treatment. Thus, small, single-arm, early studies with response end points are difficult to compare with more recent clinical trials. Table 3 summarizes results from selected trials discussed below.
Table 3.
Clinical trials of systemic treatment of pancreatic neuroendocrine tumors (PNETs) and carcinoids
Table 3a.
(Continued)
Table 3b.
(Continued)
The asterisk (*) indicates that the response criteria may include tumor markers and physical exam.
aStudy also included a chlorozotocin arm.
bStable disease including PNETs and carcinoids.
c2-year OS including PNETs and carcinoids.
dBoth regimens were then followed by dacarbazine.
eStudies did not distinguish between PNETs and carcinoids.
Abbreviations: 2-yr OS, 2-year overall survival; 5-FU, 5-fluorouracil; HR, hazard ratio; Med OS, median overall survival; Med PFS, median progression-free survival; NET, neuroendocrine tumor; NS, not significant; PFS, progression-free survival; PNET, pancreatic neuroendocrine tumor; TTP, time to progression.
Cytotoxic Agents
PNETs
In 1973, streptozocin was reported to have a 37% objective response rate and a 54% biochemical response rate in patients with metastatic PNETs [31]. This led to subsequent studies further investigating the role of streptozocin as well as doxorubicin and 5-fluorouracil in PNETs and carcinoids. A randomized phase III study of 84 patients with PNETs evaluated streptozocin versus streptozocin plus fluorouracil and reported an improved response rate of 63% with combination therapy as compared to 36% with streptozocin alone; however, a significant survival advantage was not observed (24 months versus 17 months) [32]. Combination therapy with streptozocin and doxorubicin versus streptozocin and fluorouracil versus chlorozotocin was compared in a randomized phase III trial of 102 PNET patients with significantly higher response rates and overall survival in the streptozocin plus doxorubicin group (69%, 2.2 years) as compared to the streptozocin plus fluorouracil group and chlorozotocin group (45%, 1.4 years and 30%, 1.5 years, respectively) [33]. It is important to note that all of these studies evaluated response using a combination of measurable tumor on physical exam, decrease in size of hepatomegaly, and improvement in endocrine parameters, which are not used as standard measurements in modern studies. Two retrospective studies assessed response to streptozocin plus doxorubicin using modern radiographic response criteria. A total of 32 patients were evaluated between the two studies, and a radiographic response rate of only 6% was seen [34, 35]. A retrospective study of 84 patients with metastatic PNETs who received streptozocin, fluorouracil, and doxorubicin evaluated efficacy and survival using the Response Evaluation Criteria in Solid Tumors (RECIST) criteria and showed a response rate of 39% and median overall survival of 37 months [36]. Unfortunately, no prospective studies have been conducted in the modern era using standard radiologic response criteria to determine outcome of these regimens in PNETs. Cisplatin shows activity in poorly differentiated NETs and thus has been assessed in PNETs and carcinoids. A phase II study of cisplatin and etoposide in 14 PNET patients showed disappointing results with a 0% response rate as assessed by change in tumor size on exam or imaging, hepatomegaly, or change in endocrine function [37]. A second phase II study of cisplatin, fluorouracil, and streptozocin in 47 PNET patients yielded a response rate of 38% as assessed by the RECIST criteria—however, this study also included poorly differentiated tumors, which are known to be much more chemosensitive than well differentiated or moderately differentiated tumors [38]. The poor chemotherapeutic response seen with well differentiated and moderately differentiated tumors further confirms the biologic differences observed as compared to their poorly differentiated counterparts.
Dacarbazine has shown promising results in patients with PNETs with a phase II study of 50 patients reporting a response rate of 34% [39]. Follow-up trials using temozolomide (an inhibitor of nucleoside incorporation and an oral alternative to dacarbazine) were then conducted in light of these positive results. When used with thalidomide (which is thought to have disease-stabilizing effects in metastatic NETs secondary to its antiangiogenic effect via inhibition of VEGF and basic fibroblast growth factor), a response rate of 45% was seen, but this regimen was associated with significant toxicity including neuropathy and lymphopenia and resulted in opportunistic infections in 10% of patients [40]. The relative contribution of each agent could not be evaluated in this study, but a retrospective evaluation of temozolomide in patients with advanced NETs showed a response rate of 14% [41]. The response rate in PNETs and carcinoids specifically, however, could not be assessed from this study because both were included. Combination studies including temozolomide/bevacizumab [42] and temozolomide/capecitabine [43] appear promising with response rates of 24% and 70%, respectively, although the latter was a retrospective study. The mechanism of sensitivity to temozolomide is unclear but may be related to a deficiency in the enzyme methylguanine methyltransferase (MGMT). Immunohistochemical analysis of MGMT in archived NETs revealed that 51% of PNETs and 0% of carcinoids were MGMT deficient [44]. Furthermore, of 21 temozolomide-treated patients with tissue available for immunohistochemical evaluation, 80% of MGMT-deficient tumors responded to therapy (all PNETs) while no tumors with normal MGMT expression responded [44]. This difference in MGMT expression further suggests the existence of underlying molecular differences between PNETs and carcinoids. To date, temozolomide has not been compared to any streptozocin-containing regimen, nor have the various temozolomide regimens been compared head-to-head. Further studies are needed to determine if temozolomide is superior to streptozocin-containing regimens.
Carcinoids
In general, cytotoxic agents have less activity against carcinoid tumors than against PNETs. After positive results were seen with streptozocin in PNETs, a phase II/III study was conducted in 210 carcinoid patients (172 randomized, 38 directly assigned) who were treated with streptozocin plus fluorouracil or single-agent doxorubicin. A response rate of 22% and 21% was observed in the two groups, respectively, with no significant difference in overall survival [45]. A phase II/III study randomizing patients to either streptozocin/fluorouracil or streptozocin/doxorubicin showed a response rate of only 16% in both groups, but there was an improved median overall survival in the streptozocin/fluorouracil group (24.3 months versus 15.7 months, p = .03) [46]. It is again important to note that these studies also evaluated response using methods not currently considered as standard in modern studies.
Cisplatin-containing regimens have also been evaluated in carcinoids. A phase II study of cisplatin/etoposide in 13 patients yielded a response rate of only 15% as assessed by change in tumor size on physical exam or imaging, hepatomegaly, or change in endocrine function [37]. A phase II study of cisplatin/fluorouracil/streptozocin in 32 patients yielded a response rate of 25% [38]. As of now, cisplatin is not considered as a standard therapy in the treatment of carcinoid tumors.
Dacarbazine and temozolomide show less activity against carcinoids than in PNETs. A phase II study of 56 patients with carcinoid treated with dacarbazine reported a response rate of 16% [47], whereas two smaller phase II studies of temozolomide/thalidomide (15 patients) and temozolomide/bevacizumab (16 patients) reported response rates of 7% and 0%, respectively [40, 42].
Interferon
Leukocyte interferon was first reported to be of benefit in treating carcinoid syndrome in 1983 [48]. Several subsequent phase II studies of recombinant interferon-alpha-2a, alone or in combination with 5-fluorouracil, reported symptomatic improvement and variable response rates in patients with NETs [49–51]. Combination studies of interferon-alpha plus a somatostatin analogue as compared to somatostatin analogue monotherapy have shown response rates of 5%–6% with one of three studies favoring an improved overall survival with combination therapy [52–54]. In each of these studies, carcinoids and PNETs were evaluated together. A small randomized study of octreotide with or without interferon-alpha in 68 patients with midgut carcinoids only showed a reduced risk of tumor progression with combination therapy but no difference in overall survival [55]. Recent clinical data regarding the combination of bevacizumab with depot octreotide [56] has led to an ongoing national, randomized phase III study of octreotide and interferon-alpha-2b versus octreotide and bevacizumab (SWOG 0518). Most recently, a small randomized phase III study of 64 patients receiving either 5-fluorouracil/streptozocin or recombinant interferon-alpha-2a showed no significant difference between the two treatment arms in terms of response rate or progression-free or overall survival; however, there was a trend for improved PFS in the interferon arm of 14.1 months versus 5.5 months in the chemotherapy arm [57]. A definitive role for interferon therapy in treating NETs, particularly carcinoids, is still under investigation.
Somatostatin Analogues
Somatostatin is a hormone originally identified as produced by the hypothalamus and inhibiting the release of growth hormone [13]. It has since been found to also be produced by the endocrine pancreas and the gastrointestinal tract [58]. The presence of somatostatin receptors on the surface of most NETs makes targeting these receptors a natural therapeutic approach. Although a targeted approach may suggest inhibiting somatostatin receptors, the natural activity of somatostatin is to inhibit the release of hormones and, in some cases, inhibit cellular proliferation. As such, somatostatin analogues were originally approved for controlling hormone production by functional NETs but recently were shown to have antitumor effects as well [59]. Natural somatostatin is rapidly degraded and therefore is of limited pharmaceutical potential [13]. Instead, several synthetic somatostatin analogues have been produced including octreotide, lanreotide, and pasireotide.
Octreotide is the most widely studied of the somatostatin analogues and acts primarily upon the SST2 receptor. Multiple studies show benefit in the treatment of hormone-mediated symptoms from functional PNETs and carcinoids [60–65], and the first early evidence of an antitumor effect was seen in 1993 when 50% of patients were noted to have disease stabilization with a possible survival advantage [66]. However, until recently there was substantial controversy regarding whether octreotide had benefit beyond symptom management. Rinke et al. recently published results of a phase III trial (the PROMID study) confirming the antitumor effect of octreotide in functional and nonfunctional well differentiated metastatic midgut NETs. Patients receiving octreotide long-acting repeatable (LAR) as compared to placebo had an 8.3 month improvement in time to progression (14.3 months versus 6 months), and a greater percentage of patients achieved disease stability (66.7% versus 37.2%) [59]. This study has established octreotide as a standard option for patients with midgut carcinoids, both functional and nonfunctional. The antitumor activity of somatostatin analogue treatment in PNETs has not been fully characterized; however, a nonrandomized phase II study of everolimus that was stratified for concurrent octreotide use suggested a potential prolongation of progression-free survival in patients receiving octreotide (16.7 months versus 9.7 months) [18].
Pasireotide (SOM 230) is a somatostatin analogue with affinity for four of five somatostatin receptors (SSTR1, SSTR2, SSTR3, and SSTR5) [13]. In a study assessing control of carcinoid-associated symptoms in patients refractory to octreotide, pasireotide provided symptomatic benefit in 25% [67]. A phase II study (the COOPERATE-2 study) using pasireotide in combination with everolimus in the treatment of PNETs is ongoing.
Therapy with radiolabeled somatostatin analogues for metastatic PNETs and carcinoids is under investigation. Although multiple small phase I and II studies have been conducted using a variety of radiolabels [68–78], the largest phase II study thus far included 1,109 patients with carcinoids, PNETs, rare neuroendocrine tumors, and neuroendocrine tumors of unknown primary who were treated with 2,472 cycles of [90Y-DOTA]-TOC (90yttrium-labeled tetraazacyclododecane tetraacetic acid modified Tyr-octreotide). A “morphologic” response (any radiologic improvement) was observed in 34.1%, a biochemical response (any improvement in tumor markers) was observed in 15.5%, and a clinical response (decrease in hormonal symptoms) was observed in 29.7% of patients, respectively. Response was associated with prolonged overall survival—44.7 months versus 18.3 months for morphologic (p < .001), 35.3 months versus 25.7 months for biochemical (p = .023), and 36.8 months versus 23.5 months (p < .001) for clinical response groups [79]. Although promising, given the variable natural history of NETs, the activity of [90Y-DOTA]-TOC requires further prospective evaluation using standard response criteria in randomized clinical trials of homogeneous patient populations.
TKIs
Sunitinib malate is a small molecule that inhibits multiple targets including platelet-derived growth factor receptor alpha (PDGFRα), PDGFRβ, VEGFR1, VEGFR2, VEGFR3, stem cell factor receptor (KIT), FML-like tyrosine kinase-3 (FLT3), colony stimulating factor receptor type I, and the glial cell-line derived neurotrophic factor receptor (RET). A phase II study showed an overall response rate of 16.7% in PNET patients and 2.4% in carcinoid patients [15], prompting a phase III randomized study in patients with PNETs. Here, 171 patients were randomized to receive 37.5 mg of sunitinib daily or placebo. Patients randomized to the sunitinib arm showed improved PFS compared with those in the placebo arm (11.4 months versus 5.5 months, hazard ratio [HR] 0.42, p < .001), and a response rate of 9.3% was observed [16]. Patients were allowed to receive a somatostatin analogue as well, but in a subgroup analysis this did not affect PFS. Patients with disease progression while receiving placebo were permitted to enter an open-label sunitinib extension protocol. The most commonly seen adverse events with sunitinib included diarrhea, nausea, vomiting, asthenia, and fatigue, with the most severe adverse events being neutropenia and hypertension. The use of sunitinib did not adversely affect quality of life. Of note, this study was terminated early by the data safety and monitoring committee due to a greater number of deaths and serious adverse events noted in the placebo group in addition to the favorable progression-free survival seen in the sunitinib group. As a result of early termination as well as multiple unplanned interim analyses, there is potential for overestimation of the benefit of sunitinib in prolonging PFS. Furthermore, with additional follow-up, a significant difference in overall survival has not been observed. Nevertheless, these promising findings led to FDA approval of sunitinib in May, 2011, for treatment of metastatic PNETs. Although the exact mechanism of action is still under investigation, it is thought that this is mediated by sunitinib's effect on PDGFRα, PDGFRβ, c-kit, VEGFR2, or VEGFR3, as these are all known to be expressed by PNETs [16].
Sorafenib is a small-molecule TKI that inhibits both intracellular and cell surface kinases (BRAF, CRAF, KIT, FLT-3, RET, VEGFR1, VEGFR2, VEGFR3, and PDGFRβ) [80]. In 50 patients with well differentiated carcinoids and 43 patients with PNETs, a 10% response rate was observed in each group [81]. Among PNETs, the response rates and PFS were similar to those seen with sunitinib; however, no phase III studies have compared sunitinib to sorafenib. Imatinib inhibits Abelson tyrosine kinase (ABL), platelet-derived growth factor receptor (PDGFR), and stem cell ligand receptor (c-kit), the latter two of which have been identified on NETs. In a small study of 27 patients with advanced carcinoid tumors treated with imatinib, the response rate (4%) and PFS (24 weeks) appear inferior [82] as compared to those for sunitinib or sorafenib. Cixutumumab is a fully human monoclonal antibody targeting insulin-like growth factor receptor 1. In a phase I study, 2 of 5 patients with carcinoid tumors showed tumor regression (one partial response and one minor response) [83]. A follow-up phase II study of cixutumumab in combination with octreotide in patients with carcinoids and PNETs is underway [84]. Pazopanib is a selective small-molecule TKI that inhibits the VEGFR-1, VEGFR-2, VEGFR-3, PDGF-α, PDGF-β, and c-kit tyrosine kinases. A phase II study of 29 patients with PNETs and 22 patients with carcinoids treated with a combination of pazopanib and octreotide LAR showed a response rate of 17% in the PNET group with a median PFS of 11.7 and 12.7 months in the PNET and carcinoid groups, respectively [85]. A combination study of pazopanib with temozolomide is currently underway.
Vascular Endothelial Growth Factor Inhibitors
Bevacizumab has been evaluated in unresectable and metastatic carcinoid tumors. A small phase II study randomizing 44 patients to octreotide plus bevacizumab versus octreotide plus interferon alfa-2b showed improved response rates and PFS in the bevacizumab arm as compared to interferon [56]. An ongoing large confirmatory cooperative group study (SWOG 0518) is comparing octreotide and bevacizumab to octreotide and interferon alfa-2b in 400 carcinoid patients with a primary outcome of PFS. Data assessing bevacizumab in patients with PNETs has not been reported, but a phase II study (CALGB 80701) of everolimus ± bevacizumab is currently ongoing.
Mammalian Target of Rapamycin Inhibitors
Recently, two phase II studies reported that the orally administered small-molecule mTOR inhibitor everolimus (RAD001) has promising anti-tumor activity in PNETs and carcinoids [17, 18]. Response rates were low in these studies, but progression-free survival was similar to values seen with the tyrosine kinase inhibitors sunitinib and sorafenib. A follow-up phase III randomized placebo-controlled trial of 410 patients with PNETs showed a response rate of only 5% but demonstrated a significantly improved PFS with everolimus as compared to placebo (11.0 months versus 4.6 months, HR 0.35, p < .001) [19], and in May 2011, the FDA approved everolimus for treatment of metastatic PNETs. In a nonrandomized phase II study, PFS in a patient cohort receiving octreotide in addition to everolimus (16.7 months) was superior to those treated with everolimus alone (9.7 months) [18].
Although not FDA approved for use in carcinoid tumors, the RADIANT-2 trial, a randomized, placebo-controlled, phase III study assessing everolimus plus octreotide versus placebo plus octreotide in patients with metastatic carcinoid tumors showed a PFS of 16.4 months versus 11.3 months in the two arms, respectively. Although the improvement did not reach statistical significance per central radiology review, local investigator assessment found improved PFS with combination therapy as compared to octreotide therapy alone [86]. Although these results are promising, further phase III studies will be needed to evaluate the role of everolimus both as a single agent and in combination with other therapies in the treatment of well differentiated, advanced carcinoid tumors.
A phase II study assessing temsirolimus, an intravenous mTOR inhibitor, in patients with advanced carcinoids and PNETs showed similar response rates to those seen with everolimus (4.8% in carcinoid patients, 6.7% in PNET patients) [20]. Median time to progression was estimated to be 6.0 months.
Recommendations
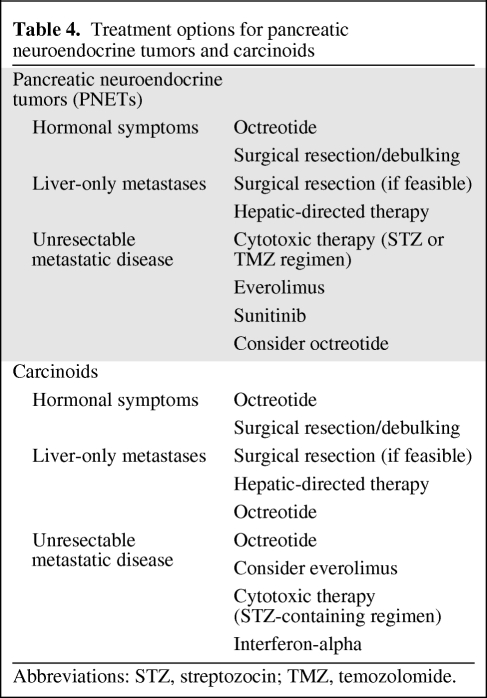
Treatment selection for metastatic PNETs and carcinoids is difficult given the paucity of comparative trials assessing various approaches in these diseases. On the basis of the data reviewed above, suggested treatment options are provided in Table 4. For patients with localized or fully resectable metastatic disease, surgical excision of both the primary tumor and metastatic foci is appropriate. Surgical resection should also be considered for debulking metastatic lesions, particularly for symptomatic carcinoids because there are limited effective systemic treatment options. In considering cytotoxic therapies, patients with PNETs should be considered for treatment with a streptozocin- or temozolomide-based regimen because response rates of up to 69% have been observed. Recent evidence from phase III randomized clinical trials also supports the use of everolimus and sunitinib as initial treatment of patients with metastatic PNETs. At this point, prospective comparative studies of cytotoxics and these pathway inhibitors have not been completed. Although not yet approved for use by the FDA in the treatment of carcinoids, we recommend consideration of everolimus in combination with octreotide for patients with carcinoid in light of the recently published RADIANT-2 trial. This is in stark contrast to cytotoxic efficacy in carcinoids, which is poor. In regard to somatostatin analogues, once metastatic disease is present, we favor initiation of octreotide in all midgut carcinoid patients and both carcinoid and PNET patients with hormone-mediated symptoms. Finally, hepatic-directed therapy may be considered an option for control of liver-only metastases, particularly those that are symptomatic. Unfortunately, there are no large or randomized studies evaluating the various hepatic-directed treatment modalities to guide the timing or selection of these interventions.
Table 4.
Treatment options for pancreatic neuroendocrine tumors and carcinoids
Abbreviations: STZ, streptozocin; TMZ, temozolomide.
Conclusions
Recent phase III studies of octreotide, everolimus, and sunitinib suggest that these targeted approaches can alter the natural history of neuroendocrine tumors. It is apparent that carcinoids and PNETs are indeed two separate diseases that must be approached differently. It has also become evident that utilizing response rate as a primary end point for treatment trials is suboptimal. These tumors are typically quite indolent, and even without treatment, stable disease may be observed in a significant proportion of cases. Indeed, a National Cancer Institute GI Steering Committee Clinical Trials Planning meeting report recommends that further trials in carcinoids and PNETs utilize progression-free survival as a primary end point [12].
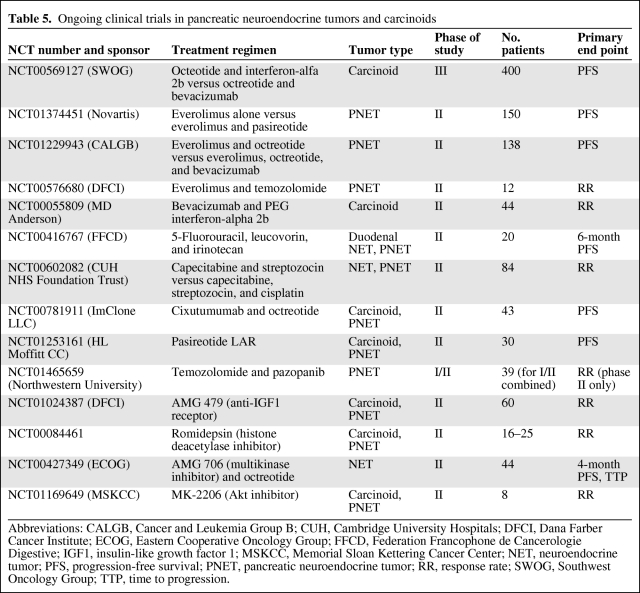
The discovery of multiple molecular mechanisms associated with PNETs and carcinoids, combined with clinical data to support drug efficacy against these targets, makes this a very exciting time in the investigation and clinical management of NETs. Clinical trials assessing various combinations of somatostatin analogues, mTOR inhibitors, tyrosine kinase inhibitors, and cytotoxic agents are ongoing (Table 5), and there is substantial promise that a multitargeted approach to therapy will translate into improved patient outcomes.
Table 5.
Ongoing clinical trials in pancreatic neuroendocrine tumors and carcinoids
Abbreviations: CALGB, Cancer and Leukemia Group B; CUH, Cambridge University Hospitals; DFCI, Dana Farber Cancer Institute; ECOG, Eastern Cooperative Oncology Group; FFCD, Federation Francophone de Cancerologie Digestive; IGF1, insulin-like growth factor 1; MSKCC, Memorial Sloan Kettering Cancer Center; NET, neuroendocrine tumor; PFS, progression-free survival; PNET, pancreatic neuroendocrine tumor; RR, response rate; SWOG, Southwest Oncology Group; TTP, time to progression.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Jennifer R. Eads, Neal J. Meropol
Collection and/or assembly of data: Jennifer R. Eads, Neal J. Meropol
Data analysis and interpretation: Jennifer R. Eads, Neal J. Meropol
Manuscript writing: Jennifer R. Eads, Neal J. Meropol
Final approval of manuscript: Jennifer R. Eads, Neal J. Meropol
References
- 1.Gaur P, Sceusi EL, Samuel S, et al. Identification of cancer stem cells in human gastrointestinal carcinoid and neuroendocrine tumors. Gastroenterology. 2011;141:1728–1737. doi: 10.1053/j.gastro.2011.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niederle MB, Hackl M, Kaserer K, et al. Gastroenteropancreatic neuroendocrine tumors: the current incidence and staging based on the WHO and European Neuroendocrine Tumour Society classification: an analysis based on prospectively collected parameters. Endocr Relat Cancer. 2010;17:909–918. doi: 10.1677/ERC-10-0152. [DOI] [PubMed] [Google Scholar]
- 3.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 4.Panzuto F, Boninsegna L, Fazio N, et al. Metastatic and locally advanced pancreatic endocrine carcinomas: analysis of factors associated with disease progression. J Clin Oncol. 2011;29:2372–2377. doi: 10.1200/JCO.2010.33.0688. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 6.Fischer L, Kleeff J, Esposito I, et al. Clinical outcome and long-term survival in 118 consecutive patients with neuroendocrine tumors of the pancreas. Br J Surg. 2008;95:627–635. doi: 10.1002/bjs.6051. [DOI] [PubMed] [Google Scholar]
- 7.Tan EH, Tan CH. Imaging of gastroenteropancreatic neuroendocrine tumors. World J Clin Oncol. 2011;2:28–43. doi: 10.5306/wjco.v2.i1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pape UF, Jann H, Müller-Nordhorn J, et al. Prognostic relevance of a novel TNM classification system for upper gastroenteropancreatic neuroendocrine tumors. Cancer. 2008;113:256–265. doi: 10.1002/cncr.23549. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology. Neuroendocrine Tumors Version 1.2011. 2011. [accessed June 15, 2011]. Available at http://www.nccn.org. [DOI] [PubMed]
- 10.Pinchot SN, Holen K, Sippel RS, et al. Carcinoid tumors. The Oncologist. 2008;13:1255–1269. doi: 10.1634/theoncologist.2008-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edge SB, Byrd DR, Compton CC, et al., editors. AJCC Cancer Staging Manual. Seventh edition. New York: Springer; 2009. pp. 1–646. [Google Scholar]
- 12.Kulke MH, Siu LL, Tepper JE, et al. Future directions in the treatment of neuroendocrine tumors: consensus report of the National Cancer Institute Neuroendocrine Tumor clinical trials planning meeting. J Clin Oncol. 2011;29:934–943. doi: 10.1200/JCO.2010.33.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oberg KE, Reubi JC, Kwekkeboom DJ, et al. Role of somatostatins in gastroenteropancreatic neuroendocrine tumor development and therapy. Gastroenterology. 2010;139:742–753. doi: 10.1053/j.gastro.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Reubi JC, Kvols LK, Waser B, et al. Detection of somatostatin receptors in surgical and percutaneous needle biopsy samples of carcinoids and islet cell carcinomas. Cancer Res. 1990;50:5969–5977. [PubMed] [Google Scholar]
- 15.Kulke MH, Lenz HJ, Meropol NJ, et al. Activity of sunitinib in patients with advanced neuroendocrine tumors. J Clin Oncol. 2008;26:3403–3410. doi: 10.1200/JCO.2007.15.9020. [DOI] [PubMed] [Google Scholar]
- 16.Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 17.Yao JC, Phan AT, Chang DZ, et al. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. J Clin Oncol. 2008;26:4311–4318. doi: 10.1200/JCO.2008.16.7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao JC, Lombard-Bohas C, Baudin E, et al. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: a phase II trial. J Clin Oncol. 2010;28:69–76. doi: 10.1200/JCO.2009.24.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duran I, Kortmansky J, Singh D, et al. A phase II clinical and pharmacodynamic study of temsirolimus in advanced neuroendocrine carcinomas. Br J Cancer. 2006;95:1148–1154. doi: 10.1038/sj.bjc.6603419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oberg K. Pancreatic endocrine tumors. Semin Oncol. 2010;37:594–618. doi: 10.1053/j.seminoncol.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Jiao Y, Shi C, Edil BH, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331:1199–1203. doi: 10.1126/science.1200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leotlela PD, Jauch A, Holtgreve-Graz H, et al. Genetics of neuroendocrine and carcinoid tumours. Endocr Relat Cancer. 2003;10:437–450. doi: 10.1677/erc.0.0100437. [DOI] [PubMed] [Google Scholar]
- 24.Gupta S, Yao JC, Ahrar K, et al. Hepatic artery embolization and chemoembolization for treatment of patients with metastatic carcinoid tumors: The M.D. Anderson experience. Cancer J. 2003;9:261–267. doi: 10.1097/00130404-200307000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Strosberg JR, Choi J, Cantor AB, et al. Selective hepatic artery embolization for treatment of patients with metastatic carcinoid and pancreatic endocrine tumors. Cancer Control. 2006;13:72–78. doi: 10.1177/107327480601300110. [DOI] [PubMed] [Google Scholar]
- 26.Gupta S, Johnson MM, Murthy R, et al. Hepatic arterial embolization and chemoembolization for the treatment of patients with metastatic neuroendocrine tumors: variables affecting response rates and survival. Cancer. 2005;104:1590–1602. doi: 10.1002/cncr.21389. [DOI] [PubMed] [Google Scholar]
- 27.Akyildiz HY, Mitchell J, Milas M, et al. Laparoscopic radiofrequency thermal ablation of neuroendocrine hepatic metastases: long-term follow-up. Surgery. 2010;148:1288–1293. doi: 10.1016/j.surg.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 28.Mazzaglia PJ, Berber E, Milas M, et al. Laparoscopic radiofrequency ablation of neuroendocrine liver metastases: a 10-year experience evaluating predictors of survival. Surgery. 2007;142:10–19. doi: 10.1016/j.surg.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 29.Kalinowski M, Dressler M, König A, et al. Selective internal radiotherapy with Yttrium-90 microspheres for hepatic metastatic neuroendocrine tumors: a prospective single center study. Digestion. 2009;79:137–142. doi: 10.1159/000209849. [DOI] [PubMed] [Google Scholar]
- 30.Rhee TK, Lewandowski RJ, Liu DM, et al. 90Y Radioembolization for metastatic neuroendocrine liver tumors: preliminary results from a multi-institutional experience. Ann Surg. 2008;247:1029–1035. doi: 10.1097/SLA.0b013e3181728a45. [DOI] [PubMed] [Google Scholar]
- 31.Broder LE, Carter SK. Pancreatic islet cell carcinoma. II. Results of therapy with streptozotocin in 52 patients. Ann Intern Med. 1973;79:108–118. doi: 10.7326/0003-4819-79-1-108. [DOI] [PubMed] [Google Scholar]
- 32.Moertel CG, Hanley JA, Johnson LA. Streptozocin alone compared with streptozocin plus fluorouracil in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1980;303:1189–1194. doi: 10.1056/NEJM198011203032101. [DOI] [PubMed] [Google Scholar]
- 33.Moertel CG, Lefkopoulo M, Lipsitz S, et al. Streptozocin-doxorubicin, streptozocin-fluorouracil, or chlorozotocin the treatment of advanced islet-cell carcinoma. N Engl J Med. 1992;326:519–523. doi: 10.1056/NEJM199202203260804. [DOI] [PubMed] [Google Scholar]
- 34.Cheng PN, Saltz LB. Failure to confirm major objective antitumor activity for streptozocin and doxorubicin in the treatment of patients with advanced islet cell carcinoma. Cancer. 1999;86:944–948. [PubMed] [Google Scholar]
- 35.McCollum AD, Kulke MH, Ryan DP, et al. Lack of efficacy of streptozocin and doxorubicin in patients with advanced pancreatic endocrine tumors. Am J Clin Oncol. 2004;27:485–488. doi: 10.1097/01.coc.0000135343.06038.eb. [DOI] [PubMed] [Google Scholar]
- 36.Kouvaraki MA, Ajani JA, Hoff P, et al. Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol. 2004;22:4762–4771. doi: 10.1200/JCO.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 37.Moertel CG, Kvols LK, O'Connell MJ, et al. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer. 1991;68:227–232. doi: 10.1002/1097-0142(19910715)68:2<227::aid-cncr2820680202>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 38.Turner NC, Strauss SJ, Sarker D, et al. Chemotherapy with 5-fluorouracil, cisplatin and streptozocin for neuroendocrine tumours. Br J Cancer. 2010;102:1106–1112. doi: 10.1038/sj.bjc.6605618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramanathan RK, Cnaan A, Hahn RG, et al. Phase II trial of dacarbazine (DTIC) in advanced pancreatic islet cell carcinoma: Study of the Eastern Cooperative Oncology Group E-6282. Ann Oncol. 2001;12:1139–1143. doi: 10.1023/a:1011632713360. [DOI] [PubMed] [Google Scholar]
- 40.Kulke MH, Stuart K, Enzinger PC, et al. Phase II study of temozolomide and thalidomide in patients with metastatic neuroendocrine tumors. J Clin Oncol. 2006;24:401–406. doi: 10.1200/JCO.2005.03.6046. [DOI] [PubMed] [Google Scholar]
- 41.Ekeblad S, Sundin A, Tiensuu Janson E, et al. Temozolomide as monotherapy is effective in treatment of advanced malignant neuroendocrine tumors. Clin Cancer Res. 2007;13:2986–2991. doi: 10.1158/1078-0432.CCR-06-2053. [DOI] [PubMed] [Google Scholar]
- 42.Kulke MH, Stuart K, Earle CC, et al. A phase II study of temozolomide and bevacizumab in patients with advanced neuroendocrine tumors. J Clin Oncol. 2006;24(18S):4044. [Google Scholar]
- 43.Strosberg JR, Fine RL, Choi J, et al. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer. 2011;117:268–275. doi: 10.1002/cncr.25425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kulke MH, Hornick JL, Frauenhoffer C, et al. O6-methylguanine DNA methyltransferase deficiency and response to temozolomide-based therapy in patients with neuroendocrine tumors. Clin Cancer Res. 2009;15:338–345. doi: 10.1158/1078-0432.CCR-08-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Engstrom PF, Lavin PT, Moertel CG, et al. Streptozocin plus fluorouracil versus doxorubicin therapy for metastatic carcinoid tumor. J Clin Oncol. 1984;2:1255–1259. doi: 10.1200/JCO.1984.2.11.1255. [DOI] [PubMed] [Google Scholar]
- 46.Sun W, Lipsitz S, Catalano P, et al. Phase II/III study of doxorubicin with fluorouracil compared with streptozocin with fluorouracil or dacarbazine in the treatment of advanced carcinoid tumors: Eastern Cooperative Oncology Group Study E1281. J Clin Oncol. 2005;23:4897–4904. doi: 10.1200/JCO.2005.03.616. [DOI] [PubMed] [Google Scholar]
- 47.Bukowski RM, Tangen CM, Peterson RF, et al. Phase II trial of dimethyltriazenoimidazole carboxamide in patients with metastatic carcinoid. A Southwest Oncology Group study. Cancer. 1994;73:1505–1508. doi: 10.1002/1097-0142(19940301)73:5<1505::aid-cncr2820730530>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 48.Oberg K, Funa K, Alm G. Effects of leukocyte interferon on clinical symptoms and hormone levels in patients with mid-gut carcinoid tumors and carcinoid syndrome. N Engl J Med. 1983;309:129–133. doi: 10.1056/NEJM198307213090301. [DOI] [PubMed] [Google Scholar]
- 49.Bajetta E, Zilembo N, Di Bartolomeo M, et al. Treatment of metastatic carcinoids and other neuroendocrine tumors with recombinant interferon-alpha-2a. A study by the Italian Trials in Medical Oncology Group. Cancer. 1993;72:3099–3105. doi: 10.1002/1097-0142(19931115)72:10<3099::aid-cncr2820721035>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 50.Saltz L, Kemeny N, Schwartz G, et al. A phase II trial of alpha-interferon and 5-fluorouracil in patients with advanced carcinoid and islet cell tumors. Cancer. 1994;74:958–961. doi: 10.1002/1097-0142(19940801)74:3<958::aid-cncr2820740326>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 51.Andreyev HJN, Scott-Mackie P, Cunningham D, et al. Phase II study of continuous infusion fluorouracil and interferon alfa-2b in the palliation of malignant neuroendocrine tumors. J Clin Oncol. 1995;13:1486–1492. doi: 10.1200/JCO.1995.13.6.1486. [DOI] [PubMed] [Google Scholar]
- 52.Frank M, Klose KJ, Wied M, et al. Combination therapy with octreotide and alpha-interferon: Effect on tumor growth in metastatic endocrine gastroenteropancreatic tumors. Am J Gastroenterol. 1999;94:1381–1387. doi: 10.1111/j.1572-0241.1999.01090.x. [DOI] [PubMed] [Google Scholar]
- 53.Arnold R, Rinke A, Klose KJ, et al. Octreotide versus octreotide plus interferon-alpha in endocrine gastroenteropancreatic tumors: a randomized trial. Clin Gastroenterol Hepatol. 2005;3:761–771. doi: 10.1016/s1542-3565(05)00481-7. [DOI] [PubMed] [Google Scholar]
- 54.Faiss S, Pape UF, Bohmig M, et al. Prospective, randomized, multicenter trial on the antiproliferative effect of lanreotide, interferon alfa, and their combination for therapy of metastatic neuroendocrine gastroenteropancreatic tumors—The International Lanreotide and Interferon Alfa Study Group. J Clin Oncol. 2003;21:2689–2696. doi: 10.1200/JCO.2003.12.142. [DOI] [PubMed] [Google Scholar]
- 55.Kölby L, Persson G, Franzen S, et al. Randomized clinical trial of the effect of interferon α on survival in patients with disseminated midgut carcinoid tumours. Br J Surg. 2003;90:687–693. doi: 10.1002/bjs.4149. [DOI] [PubMed] [Google Scholar]
- 56.Yao JC, Phan A, Hoff PM, et al. Targeting vascular endothelial growth factor in advanced carcinoid tumor: A random assignment phase II study of depot octreotide with bevacizumab and pegylated interferon alpha-2b. J Clin Oncol. 2008;26:1316–1323. doi: 10.1200/JCO.2007.13.6374. [DOI] [PubMed] [Google Scholar]
- 57.Dahan L, Bonnetain F, Rougier P, et al. Phase III trial of chemotherapy using 5-fluorouracil and streptozocin compared with interferon α for advanced carcinoid tumors: FNCLCC-FFCD 9710. Endocr Relat Cancer. 2009;16:1351–1361. doi: 10.1677/ERC-09-0104. [DOI] [PubMed] [Google Scholar]
- 58.Guillemin R. Hypothalamic hormones a.k.a. hypothalamic releasing factors. J Endocrinol. 2005;184:11–28. doi: 10.1677/joe.1.05883. [DOI] [PubMed] [Google Scholar]
- 59.Rinke A, Muller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID study group. J Clin Oncol. 2009;27:4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 60.Ch'ng JL, Anderson JV, Williams SJ, et al. Remission of symptoms during long term treatment of metastatic pancreatic endocrine tumors with long acting somatostatin analogue. Br Med J (Clin Res Ed) 1986;292:981–982. doi: 10.1136/bmj.292.6526.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maton PN, O'Dorisio TM, Howe BA, et al. Effect of a long-acting somatostatin analogue (SMS 201–995) in a patient with pancreatic cholera. N Engl J Med. 1985;312:17–21. doi: 10.1056/NEJM198501033120104. [DOI] [PubMed] [Google Scholar]
- 62.Boden G, Ryan IG, Eisenschmid BL, et al. Treatment of inoperable glucagonoma with the long acting somatostatin analog SMS 201–995. N Engl J Med. 1986;314:1686–1689. doi: 10.1056/NEJM198606263142606. [DOI] [PubMed] [Google Scholar]
- 63.Kvols LK, Buck M, Moertel CG, et al. Treatment of metastatic islet cell carcinoma with somatostatin analogue (SMS 201–995) Ann Intern Med. 1987;107:162–168. doi: 10.7326/0003-4819-107-2-162. [DOI] [PubMed] [Google Scholar]
- 64.Kvols LK, Moertel CG, O'Connell MJ, et al. Treatment of malignant carcinoid syndrome. Evaluation of a long acting somatostatin analogue. N Engl J Med. 1986;315:663–666. doi: 10.1056/NEJM198609113151102. [DOI] [PubMed] [Google Scholar]
- 65.Levi S, Ellis M, Leung E, et al. Long acting somatostatin in carcinoid syndrome [abstract] Gut. 1988;29:A714. [Google Scholar]
- 66.Saltz L, Trochanowski B, Buckley M, et al. Octreotide as an antineoplastic agent in the treatment of functional and nonfunctional neuroendocrine tumors. Cancer. 1993;72:244–248. doi: 10.1002/1097-0142(19930701)72:1<244::aid-cncr2820720143>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 67.Kvols L, Wiedenmann B, Oberg K, et al. Safety and efficacy of pasireotide (SOM230) in patients with metastatic carcinoid tumors refractory or resistant to octreotide LAR: Results of a phase II study. J Clin Oncol. 2006;24(18S):4082. [Google Scholar]
- 68.Frilling A, Weber F, Saner F, et al. Treatment with 90Y- and 177Lu-DOTATOC in patients with metastatic neuroendocrine tumors. Surgery. 2006;140:968–977. doi: 10.1016/j.surg.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 69.Waldherr C, Pless M, Maecke HR, et al. Tumor response and clinical benefit in neuroendocrine tumors after 7.4 GBq 90Y-DOTATOC. J Nucl Med. 2002;43:610–616. [PubMed] [Google Scholar]
- 70.Paganelli G, Bodei L, Handkiewicz Junak D, et al. 90Y-DOTA-D-Phe1-Tyr3-octretide in therapy of neuroendocrine malignancies. Biopolymers. 2002;66:393–398. doi: 10.1002/bip.10349. [DOI] [PubMed] [Google Scholar]
- 71.Bodei L, Cremonesi M, Zoboli S, et al. Receptor-mediated radionucide therapy with 90Y-DOTATOC in association with amino acid infusion: a phase I study. Eur J Nucl Med Mol Imaging. 2003;30:207–216. doi: 10.1007/s00259-002-1023-y. [DOI] [PubMed] [Google Scholar]
- 72.Kwekkeboom DJ, Bakker WH, Kam BL, et al. Treatment of patients with gastro-entero-pancreatic (GEP) tumours with the novel radiolabelled somatostatin analogue [177Lu-DOTA0,Tyr3]octreotate. Eur J Nucl Med Mol Imaging. 2003;30:417–422. doi: 10.1007/s00259-002-1050-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kwekkeboom DJ, de Herder WW, Kam BL, et al. Treatment with the radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124–2130. doi: 10.1200/JCO.2007.15.2553. [DOI] [PubMed] [Google Scholar]
- 74.De Jong M, Valkema R, Jamar R, et al. Somatostatin receptor-targeted radionuclide therapy of tumors: preclinical and clinical findings. Semin Nucl Med. 2002;32:133–140. doi: 10.1053/snuc.2002.31027. [DOI] [PubMed] [Google Scholar]
- 75.Baum R, Wehrmann C, Zachert C, et al. Long-term results of peptide receptor radionuclide therapy (PRRT): 5 year follow-up of 1,150 courses in 360 patients with progressive, somatostatin receptor positive neuroendocrine tumors in one clinical center. J Nucl Med. 2007;48(Suppl 2):37P. [Google Scholar]
- 76.Bushnell DL, Jr., O'Dorisio TM, O'Dorisio MS, et al. 90Y-edotreotide for metastatic carcinoid refractory to octreotide. J Clin Oncol. 2010;28:1652–1659. doi: 10.1200/JCO.2009.22.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Claringbold PG, Brayshaw PA, Price RA, et al. Phase II study of radiopeptide 177Lu-octreotate and capecitabine therapy of progressive disseminated neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2011;38:302–311. doi: 10.1007/s00259-010-1631-x. [DOI] [PubMed] [Google Scholar]
- 78.Kong G, Johnston V, Ramdave S, et al. High-administered activity In-111 octreotide therapy with concomitant radiosensitizing 5FU chemotherapy for treatment of neuroendocrine tumors: preliminary experience. Cancer Biother Radiopharm. 2009;24:527–533. doi: 10.1089/cbr.2009.0644. [DOI] [PubMed] [Google Scholar]
- 79.Imhof A, Brunner P, Marincek N, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol. 2011;29:2416–2423. doi: 10.1200/JCO.2010.33.7873. [DOI] [PubMed] [Google Scholar]
- 80.Bayer HealthCare Pharmaceuticals. Sorafenib prescribing information. 2011:1–20. [Google Scholar]
- 81.Hobday TJ, Rubin J, Holen K, et al. MC044h, a phase II trial of sorafenib in patients with metastatic neuroendocrine tumors (NET): A Phase II Consortium (P2C) study. J Clin Oncol; 2007 ASCO Annual Meeting Proceedings Part 1; 2007. p. 4504. [Google Scholar]
- 82.Yao JC, Zhang JX, Rashid A, et al. Clinical and in vitro studies of imatinib in advanced carcinoid tumors. Clin Cancer Res. 2007;13:234–240. doi: 10.1158/1078-0432.CCR-06-1618. [DOI] [PubMed] [Google Scholar]
- 83.Tolcher AW, Sarantopoulos J, Patnaik A, et al. Phase I, pharmacokinetic, and pharmacodynamic study of AMG 479, a fully human monoclonal antibody to insulin-like growth factor receptor 1. J Clin Oncol. 2009;27:5800–5807. doi: 10.1200/JCO.2009.23.6745. [DOI] [PubMed] [Google Scholar]
- 84.Anthony LB, Loehrer PJ, Leong S, et al. Phase II study of cixutumumab (IMC-A12) plus depot octreotide for patients with metastatic carcinoid or islet cell carcinoma. J Clin Oncol. 2010;28(15 suppl):TPS220. [Google Scholar]
- 85.Phan AT, Yao JC, Fogelman DR, et al. A prospective, multi-institutional phase II study of GW786034 (pazopanib) and depot octreotide (sandostatin LAR) in advanced low-grade neuroendocrine carcinoma (LGNEC) J Clin Oncol. 2010;28(15 suppl):4001. [Google Scholar]
- 86.Pavel ME, Hainsworth JD, Baudin E, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomized, placebo-controlled, phase 3 study. Lancet. 2011;378:2005–2012. doi: 10.1016/S0140-6736(11)61742-X. [DOI] [PubMed] [Google Scholar]