The results of a phase II trial of cetuximab plus capecitabine in elderly patients with advanced colorectal cancer are presented. The response rate was 31.8% overall and 48.3% in patients with wild-type KRAS tumors.
Keywords: Capecitabine, Cetuximab, Colorectal neoplasms, Aged patients
Abstract
Single-agent cetuximab is safe and active in elderly patients with advanced colorectal cancer (CRC). A cetuximab–capecitabine combination has not previously been tested in elderly patients with advanced CRC.
Material and Methods.
Sixty-six patients with advanced CRC were treated with cetuximab as a 400 mg/m2 i.v. infusion followed by 250 mg/m2 i.v. weekly plus capecitabine at a dose of 1,250 mg/m2 every 12 hours. After the inclusion of 27 patients, the protocol was amended for safety reasons, reducing the dose of capecitabine to 1,000 mg/m2 every 12 hours. Thirty-nine additional patients were treated with the reduced dose of capecitabine.
Results.
The overall response rate was 31.8%. KRAS status was determined in 58 patients (88%). Fourteen of 29 patients with wild-type KRAS tumors responded (48.3%; 95% confidence interval [CI], 29.4%–67.5%), compared with six of 29 patients with mutant KRAS tumors (20.7%; 95% CI, 8.0%–39.7%). The median progression-free survival (PFS) interval was 7.1 months. The median PFS interval for patients whose tumors were wild-type KRAS was significantly longer than for those with mutant KRAS tumors (8.4 months versus 6.0 months; p = .024). The high incidence of severe paronychia (29.6%) declined (7.7%) after capecitabine dose adjustment.
Conclusions.
Cetuximab plus capecitabine at a dose of 1,000 mg/m2 every 12 hours may be an alternative to more aggressive regimens in elderly patients with advanced wild-type KRAS CRC.
Introduction
Colorectal cancer (CRC) is one of the most common malignancies in the elderly population. In the European Union, 40% of patients diagnosed with CRC are aged >74 years, and the incidence of CRC in elderly patients is expected to increase in the future [1]. Overall, elderly patients have been underrepresented in clinical trials [2, 3], and clinical decisions in routine practice are based on extrapolated data from nonelderly populations. The majority of previous trials specifically designed for elderly patients with advanced disease were phase II studies employing single-agent fluoropyrimidines or raltitrexed. The overall response rate (RR) in these studies was in the range of 13%–29% [4–6]. Single-agent capecitabine has been shown to be effective as first-line treatment for metastatic CRC, demonstrating a superior RR and at least equivalent time to progression and survival time to those seen with 5-fluorouracil (5-FU) plus leucovorin in controlled phase III trials [7]. Furthermore, its activity in elderly metastatic CRC patients, together with a good safety profile and its convenience as an oral drug, make it, in many cases, a treatment of choice for this patient population [8].
Cetuximab is a chimeric immunoglobulin G1 monoclonal antibody that targets the extracellular domain of the epidermal growth factor receptor (EGFR). EGFR is commonly expressed in many human tumors, including CRC, and is involved in signaling pathways affecting cell proliferation, differentiation, and angiogenesis [9]. Single-agent cetuximab has been shown to be safe and effective as first-line treatment for elderly patients with advanced CRC (RR, 14.6%) by our own group [10]. In 2006, the Spanish Cooperative Group for Gastrointestinal Tumor Therapy (TTD) decided to carry out a phase II trial of the combination of cetuximab plus capecitabine in order to increase the RR without increasing potential life-threatening toxicities such as neutropenia or diarrhea associated with more aggressive combination therapies. At the time this phase II trial was designed, cetuximab had previously been combined with chemotherapy (irinotecan) in refractory metastatic patients without a greater severity and incidence of expected chemotherapy adverse events [11]. Recently, it has become clear that cetuximab does not benefit patients with mutant KRAS tumors. Based on these data, a further subgroup analysis according to KRAS status was done and is also presented here.
Patients and Methods
Local ethics committee approval was obtained before enrollment of any patients into the study, which was performed in accordance with the Declaration of Helsinki and its subsequent amendments as well as Good Clinical Practice Guidelines. Signed informed consent was obtained from all patients before study entry.
Patient Selection
Inclusion criteria included: histologically confirmed metastatic nonresectable colorectal adenocarcinoma; age ≥70 years; the absence of previous treatment for advanced disease; at least one measurable lesion by World Health Organization (WHO) criteria; a Karnofsky performance status score of 80%–100%; an interval >12 months after the end of adjuvant chemotherapy; a life expectancy >3 months; adequate bone marrow, renal, and hepatic functions (neutrophils ≥1.5 × 109/L, hemoglobin ≥9 g/dL, platelets ≥100 × 109/L, total bilirubin <1.5× upper limit of normal [ULN], aspartate aminotransferase and alanine aminotransferase ≤2.5× ULN or 5× ULN in cases of liver metastases), and a serum creatinine clearance (CrCl) >30 mL/minute by the Cockroft–Gault method.
Exclusion criteria were: brain or leptomeningeal metastases; other malignancy except basal cell skin carcinoma or in situ carcinoma of the cervix; malabsorption; inflammatory intestinal disease; previous administration of anti-EGFR therapy; serious concomitant disease such as unstable heart disease, acute myocardial infarction in the last 12 months, uncontrolled active infection, or severe neurologic or psychiatric disorders; and the presence of one or more criteria for “frailty”—(a) dependent for activities of daily living according to the Katz scale; (b) the presence of three or more of the following comorbid conditions: congestive heart failure, other chronic heart disease, chronic obstructive pulmonary disease, cerebrovascular disease, peripheral neuropathy, chronic renal failure, hypertension, diabetes, systemic vasculitis, and severe arthritis; and (c) the presence of geriatric syndromes such as dementia, delirium in stressful situations, fecal or urinary incontinence in the absence of infection, the use of diuretics or laxatives or benign prostatic hyperplasia, severe depression, frequent falls, spontaneous bone fractures, and neglect.
Treatment Schedule
Cetuximab was administered i.v. at an initial dose of 400 mg/m2 body surface area as a 2-h i.v. infusion followed by weekly i.v. doses of 250 mg/m2. Premedication with an i.v. antihistamine was mandatory. Capecitabine was taken orally, 2 weeks on and 1 week off, at a dose of 1,250 mg/m2 every 12 hours for patients with a CrCl >50 mL/minute or 950 mg/m2 every 12 hours in cases of a CrCl of 30–50 mL/minute. During the trial, the protocol was amended for safety reasons, reducing the dose of capecitabine to 1,000 mg/m2 every 12 hours in patients with a CrCl >50 mL/minute and 750 mg/m2 every 12 hours in cases with a CrCl of 30–50 mL/minute. Treatment was maintained until disease progression, unacceptable toxicity, or withdrawal of patient consent. The cetuximab dose was reduced to 200 mg/m2 in cases of a second episode of grade 3 cutaneous toxicity and to 150 mg/m2 if repeated. Cetuximab was delayed until the patient recovered to grade 0–1 toxicity. Capecitabine was reduced by 25% in cases of a grade 3 or second episode of grade 2 nonhematologic toxicity and by 50% if a grade 4 or a second episode of grade 3 nonhematologic toxicity occurred. Capecitabine was also reduced by 25% in cases of grade 2 hand-foot syndrome and by 50% after a second episode of the same toxicity.
Study Evaluations
In the 14 days before the first infusion, patients underwent a clinical history and physical examination, assessment using the Independent Daily Activities Katz Scale, comorbidity evaluation, blood count measurements, liver and renal function tests, carcinoembryonic antigen (CEA) level measurement, and evaluation of their prothrombin time and electrolytes. An abdominal computed tomography (CT) scan together with a chest x-ray or chest CT scan were performed within 28 days before treatment commencement. During the treatment period, blood counts and biochemical parameters were determined every 3 weeks, and CEA levels were measured every 6 weeks for 6 months and every 3 months thereafter.
Evaluation of Efficacy and Toxicity
Tumor response was evaluated by the investigators using the radiologic WHO criteria at 6-week intervals for 6 months and every 3 months thereafter, until the disease progressed or the patient died. No independent radiological review committee was established. Patients were considered assessable for efficacy if they had received ≥6 weeks of treatment. All patients were included in the intent-to-treat analysis. Progression-free survival (PFS) and overall survival (OS) times were calculated from the date of inclusion into the study until documentation of progression or death, respectively. Toxicity was evaluated weekly and graded according to the National Cancer Institute Common Toxicity Criteria, version 2.0.
Statistical Analysis
The primary endpoint was the overall RR (ORR). Secondary endpoints were the safety profile and PFS and OS times. Survival was estimated using the Kaplan–Meier method. Sample size was calculated using Simon′s optimal two-staged design to detect a minimum expected RR of 25%. Patients were accrued in two stages. If no objective responses were observed in the first 11 patients, a RR ≥25% could be excluded at a level of significance of 95% and accrual would stop. If there was at least one complete or partial response, then a total of 40 patients would be included to validate the RR more accurately. The protocol was amended after 27 patients were included, so a further 39 patients were recruited to maintain statistical validity. Because KRAS mutations were demonstrated to be a predictive factor for response and survival at the end of our recruitment period, an analysis of the primary and secondary endpoints according to KRAS status was done as an unplanned post hoc analysis.
Results
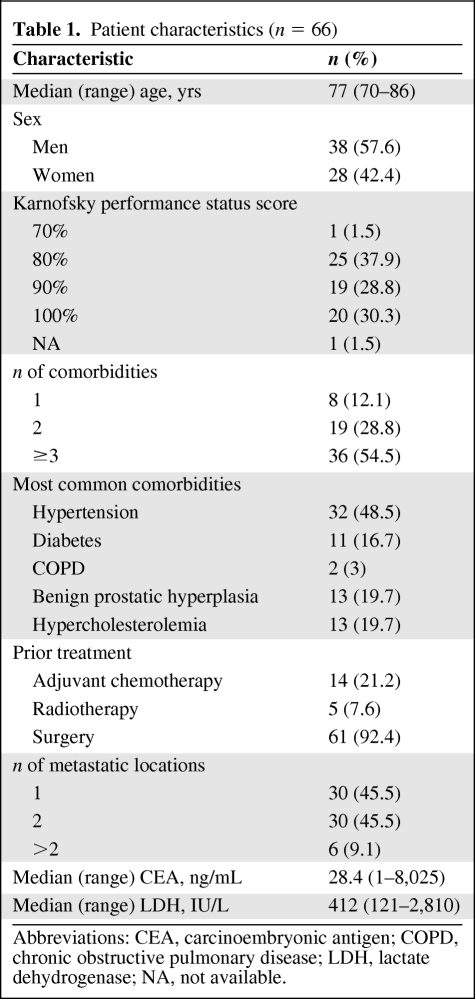
Table 1 summarizes the main characteristics of the 66 patients (27 in cohort 1 and 39 in cohort 2) recruited into the study from August 2006 to July 2007. The median age was 77 years. Slightly more than one third had a Karnofsky performance status score of 80%. Fifty-four percent of patients suffered from three or more comorbid conditions, although these did not affect the exclusion criteria. Almost half of the patients had metastatic disease in only one organ.
Table 1.
Patient characteristics (n = 66)
Abbreviations: CEA, carcinoembryonic antigen; COPD, chronic obstructive pulmonary disease; LDH, lactate dehydrogenase; NA, not available.
Treatment Compliance
The median number of cetuximab infusions was 17.5 (range, 1–82). The dose of cetuximab had to be reduced in 18 patients (27.3%). The relative median dose intensity of cetuximab was 92%. Thirty-eight patients (57.6%) received >90% of the planned dose of cetuximab. The median number of cycles of capecitabine was six (range, 1–27). The dose of capecitabine had to be reduced in 18 patients (66.6%) in cohort 1 and 12 patients (30.7%) in cohort 2. The relative median dose intensities of capecitabine in cohort 1 and cohort 2 were 80% and 94%, respectively.
Toxicity Profile
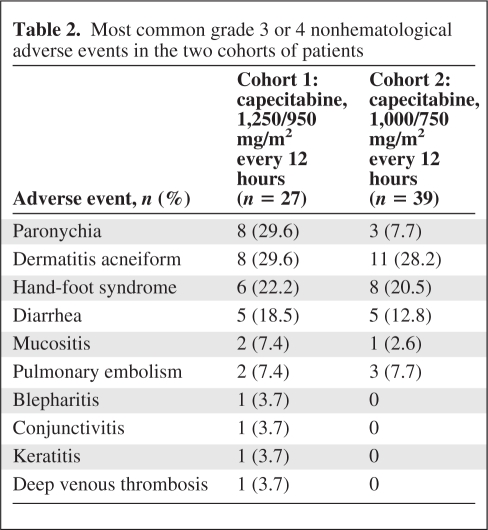
Table 2 shows the principal adverse events related to the study treatment by cohort. Severe hematological toxicity was rare, with only one case of grade 4 neutropenia reported. The incidence of severe paronychia in the first cohort of patients (29.6%) was considered unacceptable and prompted us to reduce the dose of capecitabine, which resulted in a clear decline to 7.7% in the next 39 patients recruited into the study. Grade 3 acneiform rash was reported in 29.6% of the first cohort of patients, and this was essentially unchanged after capecitabine dose adjustment (28.2%). Severe eye disorders also disappeared after dose adjustment. Diarrhea and hand-foot syndrome were slightly less frequent in cohort 2. In terms of cardiac and vascular disorders, one patient suffered from an acute myocardial infarction, five patients presented with pulmonary embolism, and one patient had deep venous thrombosis. Other typical toxicities related to capecitabine or cetuximab, such as dry skin, nausea, asthenia, anorexia, and hypomagnesemia, were mild to moderate.
Table 2.
Most common grade 3 or 4 nonhematological adverse events in the two cohorts of patients
Efficacy
All patients (from both cohorts) were included in the efficacy assessment in an intent-to-treat analysis. Two patients achieved a complete response and 19 patients had a partial response for an ORR of 31.8% (95% confidence interval [CI], 20.9%–44.4%). Thirty-five patients (53%) remained stable after treatment. A total disease control rate of 84.8% (95% CI, 73.9%–92.5%) was achieved. The median duration of response was 7.4 months. The RRs by treatment cohort were similar (33% in cohort 1 and 30.8% in cohort 2). Thirteen of 37 patients (35.1%) who developed grade 2 or 3 acne-like rash responded to treatment, in contrast to eight of 29 patients (27.6%) who did not experience cutaneous toxicity of grade >1. This difference, however, was not statistically significant (p = .129).
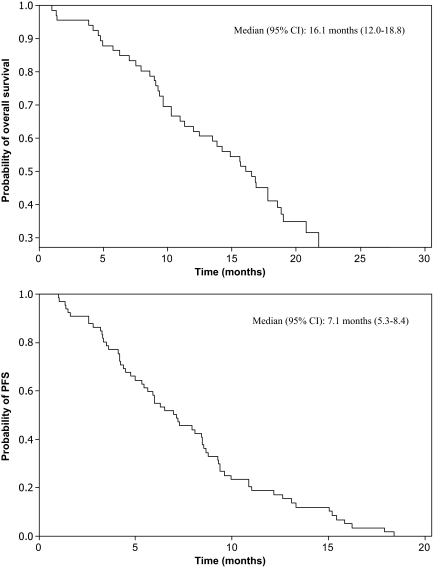
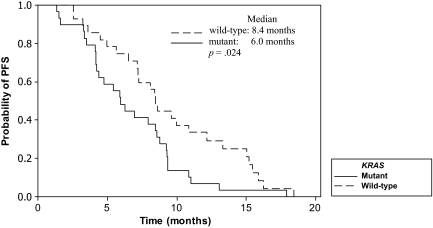
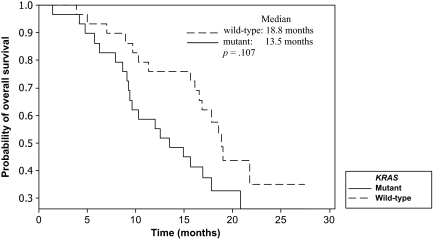
KRAS status was determined in 58 patients (88%). Eight patients did not consent for KRAS mutation analysis. The incidences of both wild-type and mutant KRAS tumors were 50%. Fourteen of 29 patients with wild-type KRAS tumors responded (48.3%; 95% CI, 29.4%–67.5%), compared with six of 29 patients with mutant KRAS tumors (20.7%; 95% CI, 8.0%–39.7%; p = .027). The median PFS and OS times for the entire group were 7.1 months (95% CI, 5.3–8.4 months) and 16.1 months (95% CI, 12.0–18.8 months), respectively (Fig. 1). A tendency to a longer median PFS interval for patients who experienced grade 2–3 acneiform rash, compared with those with grade 0–1 rash, was observed (7.9 months versus 5.0 months; p = .052). The median PFS duration for patients whose tumors were wild-type KRAS was significantly longer than that of those with mutant KRAS tumors (8.4 months versus 6.0 months; p = .024) (Fig. 2). A tendency for a longer OS duration was also observed for patients with wild-type KRAS tumors (18.8 months versus 13.5 months; p = .107) (Fig. 3).
Figure 1.
Overall survival and progression-free survival times for the whole population by the Kaplan–Meier method.
Abbreviations: CI, confidence interval; PFS, progression-free survival.
Figure 2.
Progression-free survival (PFS) time according to KRAS status by the Kaplan–Meier method.
Figure 3.
Overall survival time according to KRAS status by the Kaplan–Meier method.
Poststudy Treatment
Fifty-eight percent of patients received a second-line chemotherapeutic regimen consisting of an oxaliplatin- or irinotecan-based combination, and 26% received a third-line regimen. Three patients were submitted for surgical rescue for hepatic metastasis. A complete R0 resection was performed in only one patient.
Discussion
Systemic treatment for elderly patients with advanced CRC remains a challenge for medical oncologists. Since the mid-1990s, oncologists and geriatricians have tried to integrate the comprehensive geriatric assessment (CGA), a multidisciplinary evaluation of multiple problems of older persons, as a tool for individualized clinical decisions. Nevertheless, the best form of geriatric assessment for cancer patients remains to be defined. From a practical perspective, the CGA allows us to recognize three stages of aging: fit patients (functionally independent and without comorbidities), who are candidates for standard cancer treatment; patients who are frail (dependence in one or more activities of daily living, three or more comorbid conditions that may compromise survival, or one or more geriatric syndromes), who are candidates only for palliative treatment; and an intermediate group for whom pharmacological approaches such as dose reduction or any type of support should be considered [12]. Recent studies including combination therapy in fit elderly patients reported ORRs in the range of 35%–36% [13, 14], which are consistent with pooled and subgroup analyses from elderly patients included in phase III trials, and suggest a benefit similar to that of younger patients treated with combination therapy [15–18]. Nevertheless, only a few patients aged ≥70 years can be considered fit after a CGA. Although we excluded frail patients, 95% of the patients selected in our trial had at least one comorbid condition and 54% of them had three or more comorbid conditions, although some of these comorbidities did not affect major organs and they were not considered criteria for exclusion. Aparicio et al. [19] recently showed that half of elderly patients routinely receive substandard treatment for colon cancer, including those with metastatic disease. The lack of data on elderly CRC patients from phase III clinical trials confirming the benefit of combination therapy over single-agent therapy in terms of the PFS and OS times together with the frequent presence of comorbid conditions justifies the use of single-agent fluoropyrimidines in most elderly patients, especially capecitabine because of its convenience as an oral drug. Cetuximab is active as single-agent therapy for patients with advanced CRC [10], and because of its toxicity profile, it might be a good candidate to combine with capecitabine in elderly patients. This was the rationale for our phase II trial that was planned in 2006. On the whole, the observed ORR of 31.8% is higher than the reported RR with capecitabine alone, both in elderly and nonselected patients [7, 8] (24% and 25%, respectively). Furthermore, after our patient accrual was completed, KRAS mutation status was shown to be crucial for selecting candidates for cetuximab therapy. Only patients with wild-type KRAS tumors benefit from the addition of cetuximab [20, 21]. In our trial, the ORR in the wild-type KRAS subgroup of patients was more than double that in the mutant KRAS subgroup (48% versus 20.7%, respectively). The ORR in the wild-type KRAS group is in the range of those obtained with oxaliplatin- or irinotecan-based combination chemotherapy in the elderly patient population [13, 14, 22, 23]. Furthermore, the PFS and OS times achieved with cetuximab plus capecitabine in the subgroup of patients with wild-type KRAS tumors (8.4 months and 18.8 months, respectively) are also in the range of those expected with oxaliplatin- and irinotecan-based combinations. Nevertheless, comparative results with historical controls, especially among small phase II trials, should be viewed with caution.
The outcomes of the recent three-arm randomised controlled trial comparing either COntinuous chemotherapy plus cetuximab or INtermittent chemotherapy with standard continuous palliative combination chemotherapy (COIN) phase III trial have thrown doubt on the benefit of the addition of cetuximab to oxaliplatin-based combinations in patients with wild-type KRAS tumors [24]. The addition of cetuximab did not produce a longer PFS or OS time in wild-type KRAS patients. From a prespecified exploratory analysis, the authors observed a potential interaction between the choice of chemotherapy (5-FU or capecitabine) and the effect of adding cetuximab. Only patients receiving oxaliplatin plus 5-FU benefited from cetuximab, whereas patients receiving oxaliplatin plus capecitabine did not. The combined effect of capecitabine and cetuximab on skin and nail toxicity, which can lead to a reduced dose of capecitabine or even both drugs, might explain a negative interaction between these drugs. In fact, in our trial, patients included in cohort 1 experienced an unacceptably high rate of nail toxicity, leading to a reduction of the capecitabine dose from 1,250 mg/m2 every 12 hours to 1,000 mg/m2 every 12 hours. Nevertheless, the activity in terms of the RR was equivalent in the two cohorts of patients, probably related to the higher relative median dose intensity of capecitabine reached after the dose adjustment.
In the second cohort of patients, toxicity was mild to moderate. Acneiform rash was the most common toxicity and was grade 3 or 4 in approximately one quarter of patients. In our own experience, only 12% of elderly patients treated with first-line cetuximab alone experience grade 3 or 4 acneiform rash [10]. Additionally, the percentages of severe diarrhea and hand–food syndrome, typically associated with capecitabine, were double or more than those previously reported with capecitabine alone at a dose of 1,250 mg/m2 every 12 hours in a similar patient population [8]. Therefore, an interaction between cetuximab and capecitabine that increases the incidence of the typical side effects of both drugs as single agents cannot be ruled out. Nevertheless, in comparison with more aggressive regimens such as oxaliplatin or irinotecan combinations in patients aged ≥70 years [13, 14, 17, 18], capecitabine plus cetuximab seems to be less toxic in terms of diarrhea, asthenia, and especially myelosuppression.
To summarize, cetuximab plus capecitabine at a dose of 1,000 mg/m2 every 12 hours (or 750 mg/m2 in cases of moderate renal insufficiency) may be an alternative to more aggressive regimens in elderly patients with comorbidities and advanced wild-type KRAS CRC. Nevertheless, some statements should be addressed in the treatment of elderly patients with metastatic CRC. Is it necessary to use cetuximab upfront? Sequential drug administration of chemotherapeutic agents has been shown to be an effective approach for subgroups of patients with no aggressive disease, in terms of PFS and OS outcomes [25, 26]. Cetuximab prolongs survival in chemotherapy-refractory patients [27], and then sequential administration of cetuximab after resistance to chemotherapy may be as useful as combination cetuximab–chemotherapy upfront, avoiding additive adverse events, especially in the skin.
Acknowledgments
The authors thank the patients and the medical and nursing staff of all the participating institutions.
Supported by the TTD, Madrid, Spain. Financial support for this research was provided by Merck KGaA. Merck KGaA has reviewed the publication and the views and opinions described in the publication do not necessarily reflect those of Merck KGaA.
This work was presented previously as an oral presentation at the European Cancer Organisation (ECCO) 15/European Society for Medical Oncology (ESMO) 34 meeting in Berlin, Germany, September 20–24, 2009, and at the 2008 Annual Meeting of the American Society of Clinical Oncology, May 30 to June 3, 2008 in Chicago, Illinois (abstract 15027).
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Javier Sastre, Eduardo Díaz-Rubio
Provision of study material or patients: Javier Sastre, Cristina Grávalos, Fernando Rivera, Bartomeu Massuti, Manuel Valladares-Ayerbes, Eugenio Marcuello, José L. Manzano, Manuel Benavides, Manuel Hidalgo, Eduardo Díaz-Rubio, Enrique Aranda
Collection and/or assembly of data: Javier Sastre, Cristina Grávalos, Fernando Rivera, Bartomeu Massuti, Manuel Valladares-Ayerbes, Eugenio Marcuello, José L. Manzano, Manuel Benavides, Manuel Hidalgo, Eduardo Díaz-Rubio, Enrique Aranda
Data analysis and interpretation: Javier Sastre, Eduardo Díaz-Rubio
Manuscript writing: Javier Sastre, Cristina Grávalos, Fernando Rivera, Bartomeu Massuti, Manuel Valladares-Ayerbes, Eugenio Marcuello, José L. Manzano, Manuel Benavides, Manuel Hidalgo, Eduardo Díaz-Rubio, Enrique Aranda
Final approval of manuscript: Javier Sastre, Cristina Grávalos, Fernando Rivera, Bartomeu Massuti, Manuel Valladares-Ayerbes, Eugenio Marcuello, José L. Manzano, Manuel Benavides, Manuel Hidalgo, Eduardo Díaz-Rubio, Enrique Aranda
References
- 1.Gatta G, Faivre J, Capocaccia R, et al. The EUROCARE Working Group. Survival of colorectal cancer patients in Europe during period 1978–1989. Eur J Cancer. 1998;34:2176–2183. doi: 10.1016/s0959-8049(98)00327-x. [DOI] [PubMed] [Google Scholar]
- 2.Fentiman IS. Are the elderly receiving appropriate treatment for cancer? Ann Oncol. 1996;7:657–658. doi: 10.1093/oxfordjournals.annonc.a010712. [DOI] [PubMed] [Google Scholar]
- 3.Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 4.Feliu J, Gonzàlez Barón M, Espinosa E, et al. Uracil and tegafur modulated with leucovorin: An effective regimen with low toxicity for the treatment of colorectal carcinoma in the elderly. Oncopaz Cooperative Group. Cancer. 1997;79:1884–1889. doi: 10.1002/(sici)1097-0142(19970515)79:10<1884::aid-cncr7>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 5.Feliu J, Mel JR, Camps C, et al. Raltitrexed in the treatment of elderly patients with advanced colorectal cancer: An active and low toxicity regimen. Eur J Cancer. 2002;38:1204–1211. doi: 10.1016/s0959-8049(02)00005-9. [DOI] [PubMed] [Google Scholar]
- 6.Abad A, Aranda E, Navarro M, et al. Two consecutive studies using oral UFT-based chemotherapy regimens in elderly patients with advanced colorectal cancer. Rev Oncologia. 2000;2:154–158. [Google Scholar]
- 7.Van Cutsem E, Hoff PM, Harper P, et al. Oral capecitabine vs intravenous 5-fluorouracil and leucovorin: Integrated efficacy data and novel analyses from two large, randomised, phase III trials. Br J Cancer. 2004;90:1190–1197. doi: 10.1038/sj.bjc.6601676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feliu J, Escudero P, Llosa F, et al. Capecitabine as first-line treatment for patients older than 70 years with metastatic colorectal cancer: An Oncopaz Cooperative Group study. J Clin Oncol. 2005;23:3104–3111. doi: 10.1200/JCO.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein NI, Prewett M, Zulys K, et al. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res. 1995;1:1311–1318. [PubMed] [Google Scholar]
- 10.Sastre J, Aranda A, Gràvalos C, et al. First-line single-agent cetuximab in elderly patients with metastatic colorectal cancer. A phase II clinical and molecular study of the Spanish group for digestive tumor therapy (TTD) Crit Rev Oncol Hematol. 2011;77:78–84. doi: 10.1016/j.critrevonc.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 12.Balducci L, Extermann M. Management of cancer in the older person: A practical approach. The Oncologist. 2000;5:224–237. doi: 10.1634/theoncologist.5-3-224. [DOI] [PubMed] [Google Scholar]
- 13.Sastre J, Marcuello E, Masutti B, et al. Irinotecan in combination with fluorouracil in a 48-hour continuous infusion as first-line chemotherapy for elderly patients with metastatic colorectal cancer: A Spanish Cooperative Group for the Treatment of Digestive Tumors Study. J Clin Oncol. 2005;23:3545–3551. doi: 10.1200/JCO.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Feliu J, Salud A, Escudero P, et al. XELOX (capecitabine plus oxaliplatin) as first-line treatment for elderly patients over 70 years of age with advanced colorectal cancer. Br J Cancer. 2006;94:969–975. doi: 10.1038/sj.bjc.6603047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitry E, Douillard JY, Van Cutsem E, et al. Predictive factors of survival in patients with advanced colorectal cancer: An individual data analysis of 602 patients included in irinotecan phase III trials. Ann Oncol. 2004;15:1013–1017. doi: 10.1093/annonc/mdh267. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg RM, Tabah-Fisch I, Bleiberg H, et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol. 2006;24:4085–4091. doi: 10.1200/JCO.2006.06.9039. [DOI] [PubMed] [Google Scholar]
- 17.Sastre J, Aranda E, Massuti B, et al. Elderly patients with advanced colorectal cancer derive similar benefit without excessive toxicity after first-line chemotherapy with oxaliplatin-based combinations: Comparative outcomes from the 03-TTD-01 phase III study. Crit Rev Oncol Hemotol. 2009;70:134–144. doi: 10.1016/j.critrevonc.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Jackson NA, Barrueco J, Soufi-Mahjoubi R, et al. Comparing safety and efficacy of first-line irinotecan/fluoropyrimidine combinations in elderly versus nonelderly patients with metastatic colorectal cancer: Findings from the bolus, infusional, or capecitabine with camptostar-celecoxib study. Cancer. 2009;115:2617–2629. doi: 10.1002/cncr.24305. [DOI] [PubMed] [Google Scholar]
- 19.Aparicio T, Navazesh A, Boutron I, et al. Half of elderly patients routinely treated for colorectal cancer receive a sub-standard treatment. Crit Rev Oncol Hematol. 2009;71:249–257. doi: 10.1016/j.critrevonc.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Van Cutsem E, Köhne CH, Lang I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011–2019. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 21.Bokemeyer C, Bondarenko I, Hartmann JT, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: The OPUS study. Ann Oncol. 2011;22:1535–1546. doi: 10.1093/annonc/mdq632. [DOI] [PubMed] [Google Scholar]
- 22.Souglakos J, Pallis A, Kakolyris S, et al. Combination of irinotecan (CPT-11) plus 5-fluorouracil and leucovorin (FOLFIRI regimen) as first line treatment for elderly patients with metastatic colorectal cancer: A phase II trial. Oncology. 2005;69:384–390. doi: 10.1159/000089992. [DOI] [PubMed] [Google Scholar]
- 23.Comella P, Natale D, Farris A, et al. Capecitabine plus oxaliplatin for the first-line treatment of elderly patients with metastatic colorectal carcinoma: Final results of the Southern Italy Cooperative Oncology Group Trial 0108. Cancer. 2005;104:282–289. doi: 10.1002/cncr.21167. [DOI] [PubMed] [Google Scholar]
- 24.Maughan TS, Adams R, Smith CG, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: Results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377:2103–2114. doi: 10.1016/S0140-6736(11)60613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koopman M, Antonini NF, Douma J, et al. Sequential versus combination chemotherapy with capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer (CAIRO): A phase III randomised controlled trial. Lancet. 2007;370:135–142. doi: 10.1016/S0140-6736(07)61086-1. [DOI] [PubMed] [Google Scholar]
- 26.Seymour MT, Maughan TS, Lederman JA, et al. Different strategies of sequential and combination chemotherapy for patients with poor prognosis advanced colorectal cancer (MRC FOCUS): A randomised controlled trial. Lancet. 2007;370:143–152. doi: 10.1016/S0140-6736(07)61087-3. [DOI] [PubMed] [Google Scholar]
- 27.Jonker DJ, O'Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–2048. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]