The underlying molecular basis, current clinical evidence, and ongoing trials supporting the use of targeted agents in the treatment of patients with advanced gastric cancer are summarized. Future perspectives are explored, including predictive biomarkers and novel signaling pathways.
Keywords: Advanced gastric cancer, Angiogenic pathway, Biomarker, Epidermal growth factor Pathway, Targeted therapy
Learning Objectives
After completing this course, the reader will be able to:
Identify the subset of advanced gastric cancer patients who might benefit from approved anti-HER2 therapy.
Explain the cellular signaling pathways and the biological rationale of novel targeted agents in the management of advanced gastric cancer.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Background.
Gastric cancer is one of the leading causes of cancer death. With greater understanding of the molecular basis of carcinogenesis, targeted agents have led to a modest improvement in the outcome of advanced gastric cancer (AGC) patients.
Methods and Results.
We conducted an overview of the published evidence regarding the use of targeted therapy in AGC patients. Thus far, the human epidermal growth factor receptor (HER) pathway, angiogenic pathway, and phosphatidylinositol-3-kinase (PI3K)–Akt–mammalian target of rapamycin pathway have emerged as potential avenues for targeted therapy in AGC patients. The promising efficacy results of the Trastuzumab for Gastric Cancer trial led to the approved use of trastuzumab-based therapy as first-line treatment for patients with HER-2+ AGC. On the other hand, the Avastin® in Gastric Cancer trial evaluating bevacizumab in combination with chemotherapy did not meet its primary endpoint of a longer overall survival duration despite a significantly higher response rate and longer progression-free survival time in patients in the bevacizumab arm. Phase III data are awaited for other targeted agents, including cetuximab, panitumumab, lapatinib, and everolimus.
Conclusion.
Recent progress in targeted therapy development for AGC has been modest. Further improvement in the outcome of AGC patients will depend on the identification of biomarkers in different patient populations to facilitate the understanding of gastric carcinogenesis, combining different targeted agents with chemotherapy, and unraveling new molecular targets for therapeutic intervention.
Introduction
Gastric cancer is one of the leading causes of cancer death [1]; although its global incidence is declining, it remains highly prevalent in Asian countries [2]. Conventional treatment modalities, including surgery, radiotherapy, and chemotherapy, play a role mainly in patients with early disease, for whom adjuvant chemotherapy and chemoirradiation have led to 20%–35% longer overall survival (OS) times [3, 4]. These modalities, however, have modest efficacy in treating patients with advanced gastric cancer (AGC), conferring a median survival time in the range of 6–11 months, with considerable treatment-related toxicities [5]. Despite the better response rates (RRs) and tolerability with various new-generation combination regimens using capecitabine, oxaliplatin, S1, docetaxel, and irinotecan [6–9], the OS outcome of AGC patients remains dismal.
With the greater understanding of the biology and underlying molecular basis of carcinogenesis, several targeted agents have led to better outcomes for advanced lung, colon, breast, and kidney cancer patients, leading to their approval and widespread use for these entities. In this respect, the development of targeted agents for AGC is apparently making rather slow progress. Unlike other solid tumors, which are predominantly addicted to a particular signaling pathway, such as human epidermal growth factor receptor (HER)-2+ breast cancer, the molecular and genetic pathogenesis of gastric cancer may be more complex [10, 11]. Many pathways may play key roles in gastric tumor carcinogenesis whereas the predominant driving pathway is difficult to delineate.
Nevertheless, the release of the promising efficacy results of the Trastuzumab for Gastric Cancer (ToGA) trial [12, 13] marked the beginning of a new era. This pivotal trial led to the approval of trastuzumab as the first targeted agent in the first-line treatment of patients with HER-2+ AGC. In addition, other signaling pathways have also emerged as potential avenues for future therapeutic interventions.
In this review, we outline the underlying molecular basis and summarize the current clinical evidence and ongoing trials supporting the use of targeted agents in the treatment of patients with AGC. We also explore future perspectives, including predictive biomarkers and novel signaling pathways, that may potentially be exploited as strategic targets of treatment.
The HER Family
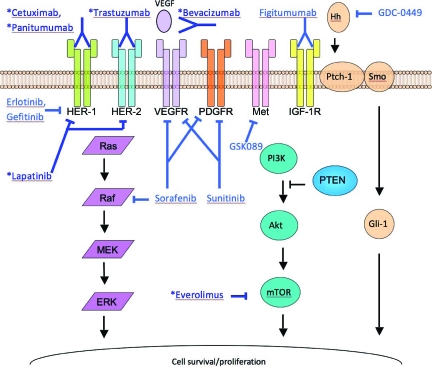
The HER family consists of four members: HER-1 (epidermal growth factor receptor [EGFR]), HER-2, HER-3, and HER-4. HER-1, HER-3, and HER-4 are all activated by ligand binding, whereas HER-2 does not require a ligand for activation. Activation of these receptors leads to homo- or heterodimerization that in turn initiates phosphorylation cascades and subsequent activation of the phosphatidylinositol-3-kinase (PI3K)–Akt–mammalian target of rapamycin (mTOR) and Ras–Raf–mitogen-activated protein kinase/extracellular signal–related kinase (ERK) kinase (MEK)–ERK pathways, which are important in cancer cell proliferation and survival [14, 15] (Fig. 1).
Figure 1.
Schematic diagram of key signaling pathways in gastric cancer cells, and mechanisms and sites of action of various targeted agents that may play a role in advanced gastric cancer treatment. Agents that have been or are currently under phase III testing are marked with an asterisk.
Abbreviations: ERK, extracellular signal–related kinase; HER, human epidermal growth factor receptor; Hh, hedgehog; IGF-1R, insulin-like growth factor 1 receptor; MEK, mitogen-activated protein kinase/ERK kinase; mTOR, mammalian target of rapamycin; PDGF, platelet-derived growth factor; PDGFR, PDGF receptor; PI3K, phosphatidylinositol 3-kinase; Ptch-1, Patched 1; PTEN, phosphatase and tensin homologue deleted on chromosome ten; Smo, Smoothened; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor.
Targeting HER-2
HER-2 overexpression is observed in 10%–38% of gastric cancer tumor samples [16–20], with a higher prevalence in intestinal-type and gastroesophageal junction (GEJ) tumors than in diffuse-type and gastric tumors [17–19]. The prognostic value of HER-2 overexpression in gastric cancer remains controversial; it is generally associated with a poorer outcome [21, 22] [23–25], although contradictory evidence exists [26, 27]. Notably, in the ToGA trial [13], HER-2+ patients had a superior outcome in the control arm consisting of chemotherapy without trastuzumab, a humanized recombinant monoclonal antibody that selectively binds to the extracellular domain of HER-2, thereby blocking its downstream signaling (Fig. 1). Although this suggests against a negative prognostic role for HER-2, it may be confounded by factors such as second-line therapy or a better intrinsic prognosis associated with the intestinal subtype. On the other hand, HER-2 overexpression has been shown to predict response to trastuzumab [28].
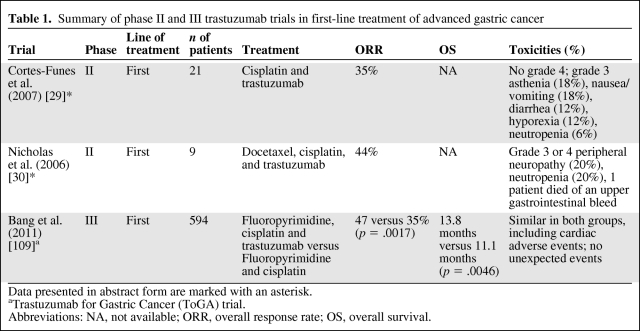
Table 1 summarizes the results of first-line trastuzumab-based trials in AGC patients. Trastuzumab in combination with chemotherapy achieved an overall response rate (ORR) of 35%–44% [29, 30]. The ToGA trial is a large, phase III, randomized controlled multicenter trial [12, 13] wherein tumor samples from >3,800 patients with locally advanced, recurrent, or metastatic gastric or gastroesophageal adenocarcinoma were centrally tested for HER-2 overexpression. Subsequently, 594 (22%) patients with HER-2+ disease, defined by an immunohistochemical (IHC) staining score of 3+ or positive fluorescence in situ hybridization (FISH) result, were randomized to receive chemotherapy with cisplatin and 5-fluorouracil (5-FU) or capecitabine for six cycles with or without trastuzumab until progression. The addition of trastuzumab to chemotherapy led to a significantly higher ORR, 47% versus 35% (p = .0017), significantly longer progression-free survival (PFS) interval, 6.7 months versus 5.5 months (p = .0002), and significantly longer OS duration, 13.8 months versus 11.1 months (p = .0046). The greatest benefit was seen in patients with higher levels of HER-2 expression, with either an IHC score of 3+ or 2+ plus FISH positivity; the OS time of those patients reached 16 months. The trastuzumab-containing regimen was generally well tolerated. Moreover, the addition of trastuzumab did not affect quality of life. To date, trastuzumab is the first and only targeted agent for gastric cancer approved by both the U.S. [31] and European [32] authorities. It is indicated in combination with cisplatin and capecitabine or 5-FU in the first-line treatment of HER-2–overexpressing AGC; strong HER-2 expression, with an IHC score of 3+ or 2+ plus FISH positivity, is required by the European guidelines. Despite these exciting results, it is worthwhile to note that only a relatively small proportion of AGC patients have HER-2+ disease after all.
Table 1.
Summary of phase II and III trastuzumab trials in first-line treatment of advanced gastric cancer
Data presented in abstract form are marked with an asterisk.
aTrastuzumab for Gastric Cancer (ToGA) trial.
Abbreviations: NA, not available; ORR, overall response rate; OS, overall survival.
In the second-line setting, after progression on platinum- or 5-FU–based chemotherapy, a trial studied single-agent trastuzumab in AGC patients, but it was limited by poor accrual [33].
Targeting EGFR
EGFR overexpression, observed in 27%–44% of gastric cancer cases, is generally reported to be a poor prognostic factor [34–36], despite contradictory evidence [37].
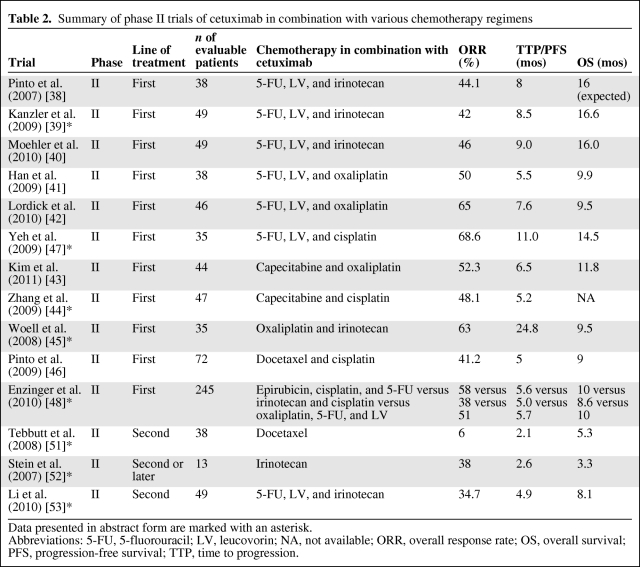
Cetuximab is a recombinant human–mouse chimeric monoclonal antibody against EGFR. In first-line phase II trials (Table 2), cetuximab was evaluated in combination with various chemotherapy regimens [38–48]. The most common side effects observed were neutropenia, diarrhea, and rash. The ORR was in the range of 40%–60%, the time to progression (TTP) was 5.5–8 months, and the OS time was 9.5–16 months. In particular, Enzinger et al. [48] reported on a recent three-arm randomized phase II study comparing cetuximab plus epirubicin, cisplatin, and 5-FU with irinotecan plus cisplatin) and with 5-FU, leucovorin and oxaliplatin. The trial was not designed to test the efficacy of cetuximab, but none of the treatment arms showed a better survival outcome than in historical controls. More recently, the preliminary results of a randomized, phase II study showed no clinically significant benefit when cetuximab was added to docetaxel plus oxaliplatin [49]. A randomized phase III trial, Erbitux® in Combination With Xeloda® and Cisplatin in Advanced Esophago-gastric Cancer [50], is ongoing to evaluate capecitabine and cisplatin with or without cetuximab as first-line treatment. In the pretreated setting, data are conflicting in the literature [51–53] (Table 2). Mature data from large-scale, randomized trials are needed to support the incorporation of cetuximab into the management of AGC patients.
Table 2.
Summary of phase II trials of cetuximab in combination with various chemotherapy regimens
Data presented in abstract form are marked with an asterisk.
Abbreviations: 5-FU, 5-fluorouracil; LV, leucovorin; NA, not available; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; TTP, time to progression.
In contrast to cetuximab, panitumumab is a fully humanized monoclonal antibody targeting EGFR. It showed activity in patients with advanced colorectal cancer after failure on 5-FU, irinotecan, and oxaliplatin [54]. Nonetheless, there is very limited experience with this agent in AGC patients. Recently, the Randomized ECF for Advanced and Locally Advanced Esophagogastric Cancer 3 trial [55] was started to explore the role of panitumumab in combination with epirubicin, oxaliplatin, and capecitabine (EOC). The ORR of patients treated with the chemotherapy triplet plus panitumumab was 52% in the phase II section of the study [56]; phase III results are awaited. On the other hand, other EGFR monoclonal antibodies, namely matuzumab and nimotuzumab, achieved even a shorter PFS time in combination with chemotherapy than with chemotherapy alone, in randomized phase II studies [57, 58].
The EGFR tyrosine kinase inhibitors (TKIs) gefitinib and erlotinib were evaluated in phase II trials but produced disappointing results as monotherapy for AGC. Response occurred in GEJ but not gastric cancer patients in a phase II first-line trial [59]. Other studies demonstrated minimal efficacy, mainly in pretreated patients [60–62]. On the other hand, a recent phase II trial showed an ORR >50% with the combination of 5-FU, oxaliplatin, and erlotinib in patients with esophageal or GEJ cancer [63].
Although a randomized trial is needed to clarify the role of EGFR TKIs in combination with chemotherapy, and phase III data on EGFR antibodies are awaited, biomarkers predictive of response may still be of research interest. EGFR mutation, high EGFR copy number, KRAS mutation status, and the development of a skin rash have all been suggested to predict response to EGFR inhibitors, but study results are conflicting. For example, EGFR overexpression evaluated using IHC with low serum EGF and transforming growth factor α levels was associated with response to cetuximab [41], although another study showed no such correlation [42]. Moreover, the RR was significantly higher (76.5% versus 40.0%) and the TTP was longer (6.8 months versus 3.0 months) in patients with grade 2–3 skin rash than in those with a less severe rash [44]; although this phenomenon is also observed in colon and lung cancer, further validation is needed in AGC patients.
Dual Targeting of HER-2 and EGFR
Lapatinib is a dual TKI inhibiting both HER-2 and EGFR. In a phase II trial of 47 patients with metastatic gastric cancer, lapatinib achieved an ORR of 7% and a 20% rate of disease stabilization [64]. The median TTP was only 2 months. One patient had grade 4 cardiac toxicity, two patients had grade 4 fatigue, and one patient had grade 4 vomiting. In another cohort of 21 previously treated patients, lapatinib had limited single-agent activity, with only two patients having durable stable disease [65]. Notably, in those two trials, lapatinib was given to both HER-2+ and HER-2− patients; thus, these disappointing results were not unexpected.
Two phase III trials are ongoing: the Lapatinib Optimization Study in ErbB2 (HER-2) Positive Gastric Cancer (LoGIC) trial, investigating first-line treatment with capecitabine and oxaliplatin with or without lapatinib [66], and the Lapatinib (Tykerb) with paclitaxel (Taxol) in Asian ErbB2+ (HER2+) Gastric Cancer Study (TYTAN) trial, investigating second-line paclitaxel with or without lapatinib in Asian patients [67]. In sharp contrast to their precedent studies, the LoGIC and TYTAN trials only target AGC patients with HER-2+ disease. The results from these trials will be instrumental in guiding the future role of lapatinib in treating AGC patients.
Intracellular Signaling Pathways
The PI3K–Akt–mTOR Pathway
mTOR is a key protein kinase that regulates cell growth and proliferation, cellular metabolism, and angiogenesis. It is mainly activated by PI3K through Akt (Fig. 1). mTOR activity is positively regulated by many receptors, including members of the EGFR and vascular endothelial growth factor receptor (VEGFR) families and their ligands, whereas phosphatase and tensin homologue deleted on chromosome ten (PTEN) is an example of a negative regulator. It is involved in the initiation of ribosomal translation of mRNA into proteins for cell growth, cell cycle progression, and cell metabolism [68].
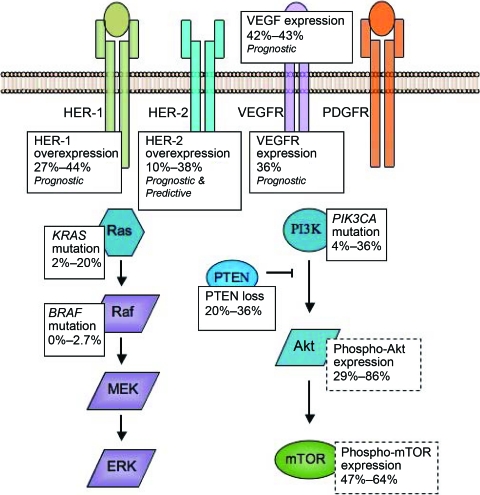
As illustrated in Figure 2, the PI3K–Akt–mTOR pathway is frequently activated in gastric cancer, as suggested by the prevalent expression of phospho-Akt (29%–86%) [69, 70] and phospho-mTOR (47%–64%) [69, 71, 72]. This can be mediated either by the overexpression of upstream receptors or by constitutively enhanced PI3K activity caused in turn by activating mutations of PIK3CA or PTEN loss. Whereas upstream receptors are overexpressed in only 20%–30% of gastric cancer cases, Akt is activated in >80% (Fig. 2). This observation suggests that the survival and proliferation of a significant proportion of gastric cancer cells are independent of the upstream receptors. In fact, PIK3CA activating mutation was reported in 4%–36% of gastric cancer cases [73, 74] and PTEN loss was reported in 20%–36% of cases [73, 75]. Specifically, for HER-2+ gastric cancer, recent preliminary evidence suggested that PTEN was lost in the majority of cases [76]. These findings may explain resistance to receptor blockade, and suggest rational targets for treatment.
Figure 2.
Summary of overexpression or mutation in the signaling pathways of gastric cancer cells. These studies are retrospective analyses of human gastric cancer cell lines and primary tumors. In general, overexpression was detected by immunohistochemistry, and mutations were detected by direct sequencing. Results specific for advanced gastric cancer, as opposed to early gastric cancer or cell lines, are summarized here if specified in the studies; otherwise, general results are quoted. HER-2 overexpression is established to be both predictive and prognostic. HER-1, VEGFR, and VEGF expression are generally regarded as prognostic, but the role of other gene mutations or protein overexpression is not well-defined.
Abbreviations: ERK, extracellular signal–related kinase; HER, human epidermal growth factor receptor; MEK, mitogen-activated protein kinase/ERK kinase; mTOR, mammalian target of rapamycin; PDGFR, platelet-derived growth factor receptor; PI3K, phosphatidylinositol 3-kinase; PTEN, phosphatase and tensin homologue deleted on chromosome ten; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor.
Inhibitors of the PI3K–Akt–mTOR pathway have been developed at multiple levels, such as PI3K–Akt inhibitors and mTOR inhibitors. In particular, everolimus is an mTOR inhibitor. In a multicenter phase II trial using everolimus as salvage therapy for pretreated AGC patients, the disease control rate was 55%, although no objective response was noted [77]. The median PFS and OS times were 2.7 months and 10.1 months, respectively. Subgroup analysis did not reveal a difference in PFS stratified by number of lines of chemotherapy. Putting these data into perspective, everolimus achieved similar, if not better, PFS and OS results to those seen in second-line chemotherapy trials [78–81]. Everolimus was generally well tolerated. Stomatitis (73.6%), anorexia (52.8%), and fatigue (50.9%) were the commonly encountered adverse events. Based on these provocative results, a phase III randomized trial is ongoing to compare everolimus with placebo plus best supportive care in patients with progressive disease after one or two prior lines of chemotherapy [82]. Given its second-line activity, its oral form of administration, and its good tolerability, the authors suggested that everolimus could also possibly be evaluated as maintenance therapy after induction of response by first-line systemic treatment for AGC.
The Ras–Raf–MEK–ERK Pathway
The Ras–Raf–MEK–ERK pathway is another key signaling pathway downstream of HER. For colon cancer, most evidence suggests that KRAS mutations are negative predictors of response to cetuximab and panitumumab [83, 84], but the predictive ability of BRAF mutations remains controversial. For gastric cancer, KRAS mutation was observed in 2%–20% of cases [85–90] and BRAF mutations was observed in 0%–2.7% of cases [85, 87]. The predictive ability of KRAS and BRAF has not been extensively studied, but small reports did not demonstrate such characteristics [42, 91].
Sorafenib is a multitargeted inhibitor of Raf and other pathways, in addition to its antiangiogenic properties. It was combined with docetaxel and cisplatin in a single-arm Eastern Cooperative Oncology Group phase II study [92]. Efficacy data were encouraging, with ORR of 41% in 44 evaluable chemotherapy-naive patients. The median PFS and OS times were 5.8 months and 13.6 months, respectively. Nevertheless, grade 3 neutropenia was common, and two patients possibly died as a result of treatment-related toxicities. In pretreated patients, single-agent sorafenib was studied in a phase II trial—preliminary analysis of 16 evaluable patients included one durable complete response and another case of protracted stable disease of >19 months [93]. More mature phase II–III data are needed to validate these results and, more importantly, to evaluate the long-term safety of the compound.
Tumor Angiogenesis
Misregulated angiogenesis is a key step in tumor growth and metastasis [94]. The VEGF family of proteins, as ligands, are important promoters of endothelial cell proliferation and new vessel formation by interacting with VEGFRs [95]. There are four VEGF members (VEGF-A through VEGF-D) and three VEGFRs (VEGFR-1 through VEGFR-3).
In gastric cancer, expression of VEGF and VEGFR was reported in ∼40% [35, 96] and 36% [97] of cases, respectively. In particular, the expression of VEGF was associated with tumor vascularity, hematogenous metastases, and poor prognosis [35, 98]. Certain VEGF polymorphisms also affected cancer risk [99] and prognosis [100]. The occurrence of hypertension during bevacizumab treatment and VEGF gene polymorphisms were suggested to be associated with clinical outcomes in metastatic breast cancer patients [101]. In the case of gastric cancer, however, the occurrence of hypertension has not been reported to predict benefit from bevacizumab [102].
Targeting VEGF
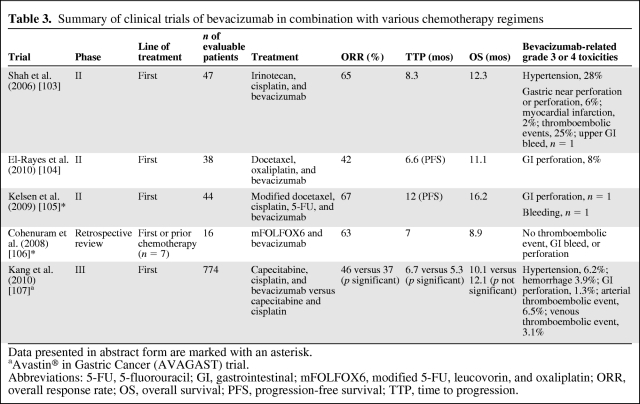
Bevacizumab is a monoclonal antibody that binds to VEGF-1, also commonly known as VEGF (Fig. 1). Several phase II trials studied bevacizumab in addition to chemotherapy [103–106] (Table 3). An ORR of 42%–67% was achieved; when available, the median TTP was 6.6–12 months and the OS time was 8.9–16.2 months. Nevertheless, grade 3–4 thromboembolic events occurred in up to 25% of patients and gastric perforation occurred in ∼8% of patients enrolled in phase II studies. Moreover, significant upper gastrointestinal bleeding was also noted in a minority of patients with unresected primary tumor.
Table 3.
Summary of clinical trials of bevacizumab in combination with various chemotherapy regimens
Data presented in abstract form are marked with an asterisk.
aAvastin® in Gastric Cancer (AVAGAST) trial.
Abbreviations: 5-FU, 5-fluorouracil; GI, gastrointestinal; mFOLFOX6, modified 5-FU, leucovorin, and oxaliplatin; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; TTP, time to progression.
In the phase III Avastin® in Gastric Cancer (AVAGAST) trial, 774 patients were randomized to receive capecitabine and cisplatin with or without bevacizumab [107, 108]. Cisplatin was given for six cycles; capecitabine and bevacizumab or placebo were given until progression or unmanageable toxicity. Although the study did not meet its primary endpoint of OS and was thus a negative trial for this endpoint, the ORR was significantly better in the bevacizumab arm (46% versus 37%; p = .0315) and the PFS interval was significantly longer, 6.7 months versus 5.3 months (hazard ratio, 0.8; p = .0037) [107, 108]. Interestingly, differences across geographical regions were reported. Survival was longer in patients in pan-America with the addition of bevacizumab, but not in Asians or Europeans despite the better prognosis of the latter. Differences in patient selection, clinical practice, population genetics, and second-line chemotherapy may explain the results, and biomarker studies are under way. The most commonly encountered grade 3–5 adverse events in both arms were neutropenia, anemia, and decreased appetite, the incidences of which were similar with or without bevacizumab. No new bevacizumab-related safety concerns were reported. Suffice it to say, it seems inappropriate to incorporate bevacizumab in the treatment algorithm of AGC patients now.
Targeting VEGFR and PDGFR
Multitargeted TKIs (MTIs), such as sorafenib and sunitinib, take another approach to suppress angiogenesis, by targeting VEGFR and other signaling pathways simultaneously. To date, most of these agents are in phase I/II of clinical development for treating AGC patients. Sunitinib inhibits PDGFR, Kit, REarranged during Transfection, and Flt-3 together with VEGFR. In a phase II study of single-agent sunitinib as second-line treatment for AGC patients treated with one prior chemotherapy regimen, 2.6% of the enrolled patients had a partial response and 25 patients (32.1%) had stable disease. The median PFS and OS times were 2.3 months and 6.8 months, respectively. Notably, grade ≥3 thrombocytopenia and neutropenia were reported in 34.6% and 29.4% of patients, respectively [109]. Another phase II study of pretreated AGC patients reported disease stabilization in five of 14 patients [110]. These data suggest that single-agent sunitinib has limited activity as salvage therapy for chemotherapy-refractory AGC patients. Moreover, it is rather unlikely that positive results will be yielded when sunitinib is tested in the first-line setting or in combination with chemotherapy because of the complete failure of sunitinib to change the survival outcome when combined with chemotherapy for other solid tumors, such as lung and colorectal cancers. Sunitinib is indeed very unlikely to be further developed in the AGC patient population.
Other MTIs are in early-phase clinical investigation. Telatinib in combination with capecitabine and cisplatin resulted in a preliminary ORR of 67% in a phase II first-line study [111]. Axitinib in combination with capecitabine and cisplatin is currently in phase I of clinical development [112]. In the third-line setting, single-agent apatinib produced an ORR on the order of 10% in another phase II trial [113].
Future Perspectives
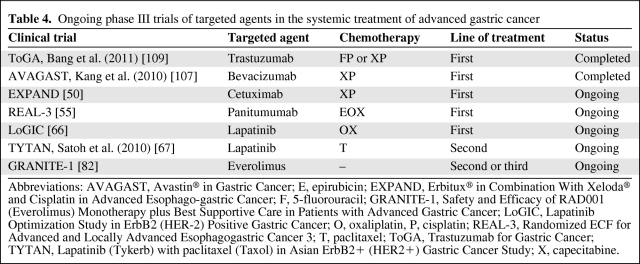
The ToGA and AVAGAST trials have marked the beginning of a new era in AGC treatment. A number of other phase III clinical trials are ongoing (Table 4). Nevertheless, our current understanding of the biology of gastric cancer is very preliminary. It is possible that many key molecular pathways play equally pivotal roles in AGC; the predominant pathway that the tumor is addicted to, if any, has yet to be conclusively defined. Moreover, only a limited proportion of patients with known activated molecular targets can benefit from the currently available therapeutics, and the achieved clinical benefit is only modest. Further improvement in the outcome of AGC patients will depend on the following.
Table 4.
Ongoing phase III trials of targeted agents in the systemic treatment of advanced gastric cancer
Abbreviations: AVAGAST, Avastin® in Gastric Cancer; E, epirubicin; EXPAND, Erbitux® in Combination With Xeloda® and Cisplatin in Advanced Esophago-gastric Cancer; F, 5-fluorouracil; GRANITE-1, Safety and Efficacy of RAD001 (Everolimus) Monotherapy plus Best Supportive Care in Patients with Advanced Gastric Cancer; LoGIC, Lapatinib Optimization Study in ErbB2 (HER-2) Positive Gastric Cancer; O, oxaliplatin, P, cisplatin; REAL-3, Randomized ECF for Advanced and Locally Advanced Esophagogastric Cancer 3; T, paclitaxel; ToGA, Trastuzumab for Gastric Cancer; TYTAN, Lapatinib (Tykerb) with paclitaxel (Taxol) in Asian ErbB2+ (HER2+) Gastric Cancer Study; X, capecitabine.
Patient Selection for Targeted Therapy Trials
Gastric carcinoma is a heterogeneous disease that results from a complex interaction among bacterial, environmental, host-genetic, and molecular mechanisms. The overexpression and amplification of many molecular targets for treatment vary with different histological, anatomical, epidemiological, and molecular AGC subtypes [114, 115]. For example, it is overall suggested that gastric cancer can be classified into three main subtypes, with distinct epidemiology and possibly genetic profiles, namely, distal intestinal-type tumors, distal diffuse-type tumors, and GEJ tumors. More HER-2+ disease is observed in intestinal-type and GEJ tumors than in diffuse-type and gastric tumors [17, 18]. Moreover, the prevalence of PIK3CA mutations is ∼23% in western gastric cancer populations but is rarely seen in Asian populations [74]. This, together with the population-based difference in the benefit of targeted therapy suggested by the AVAGAST trial [107], underscores the importance of conducting clinical trials stratified by clinical gastric cancer subtypes and by ethnic subgroups because of potential differences in tumor biology and pharmacogenomics.
Development of Biomarkers
In the era of personalized medicine, given the financial implications and potential toxicities associated with targeted therapy, identification of predictive biomarkers is crucial to enable the effective use of targeted therapy in AGC patients. Prospective biomarker-driven clinical trials dedicated to specific patient populations enriched with rational molecular targets would potentially enhance the efficacy results, and also allow evaluation of targeted agents as monotherapy to provide insight on gastric cancer biology and the prevalence and mechanisms of primary and secondary resistance.
Various markers, including EGFR and VEGF overexpression, skin rash, and hypertension, have not been validated to be predictive in AGC patients, and HER-2 overexpression and HER-2 amplification remain the only predictive biomarkers. It is imperative to refine and standardize techniques for HER-2 determination. Current IHC testing is associated with significant false-positive and false-negative results [116]. More accurate techniques, such as FISH, should be adopted and samples should be analyzed in a centralized laboratory. Notably, unlike breast cancer, the occurrence of basolateral membrane staining of glandular cells (resulting in incompletely stained membranes) and tumor heterogeneity in gastric cancer lead to discrepancies between IHC and FISH results. Modifications to the HercepTest™ (Dako, Glostrup, Denmark) score for gastric cancer [117] should be widely promoted. Further to the qualitative detection of HER-2 overexpression, the level of HER-2 may be associated with the magnitude of benefit [13].
Combination Strategies
The combination of targeted agents, the addition of chemotherapy to targeted agents, and the development of multitargeted agents may overcome resistance and improve clinical efficacy.
Primary and secondary resistance to targeted agents remain poorly defined problems. The addition of trastuzumab to chemotherapy results in only a 12% higher RR and 10% greater clinical benefit rate (complete responses plus partial responses) [13]. Although the prevalence of primary trastuzumab resistance in AGC cannot be clearly determined without first-line monotherapy trastuzumab trials, it can be inferred that this occurs in the majority of HER-2–overexpressing AGC patients, and all initially sensitive patients eventually become trastuzumab refractory. As discussed earlier, constitutive activation of the PI3K pathway through PIK3CA mutation or PTEN loss may play a role in resistance to receptor monoclonal antibodies, and they may represent rational targets irrespective of the upstream receptor expression status.
Resistance to targeted therapy may also be contributed to partly by intratumoral heterogeneity [118]. In initially sensitive disease, selection of a nonsensitive clone with continued targeted therapy may give rise to acquired resistance. Although intratumoral heterogeneity is better described for colon, breast, ovarian, and cervical cancers [119–122], it has also been demonstrated in gastric cancer [26, 123–125].
Multilevel blockade is therefore a promising strategy to tackle intratumor heterogeneity. For example, a preclinical study suggested that the combination of trastuzumab and lapatinib was synergistic in inhibiting cell growth in HER-2–amplified human upper gastrointestinal cell lines [126]; the combination lower rates of Akt and ERK activation, G0–G1 cell cycle arrest, and greater rates of apoptosis. These provocative data may provide a strong rationale for testing this interesting combination in early-phase clinical trials. Similarly, the combination of targeted therapy with chemotherapy was also shown to have a synergistic effect in gastric cancer cell lines [127]. More importantly, new-generation MTIs can target multiple molecular defects concurrently. The promising clinical activity of the pan-HER inhibitor neratinib in metastatic breast cancer patients serves as an example: neratinib resulted in an ORR of 56% [128], compared with the 23%–35% rates achieved by trastuzumab [129, 130] in HER-2–overexpressing disease, although definitive conclusions cannot be drawn from crosstrial comparisons.
Novel Molecular Targets
A few signaling pathways have attracted a lot of enthusiasm. First, the ubiquitin–proteosome pathway is involved in cell cycle control, through normal degradation of cellular proteins; disruption of this pathway contributes to tumor growth. Bortezomib, a proteosome inhibitor, was shown to induce apoptosis and suppress tumor growth in gastric cancer cell lines [131]. In a preliminary phase II study, bortezomib plus irinotecan led to an RR of 44% in chemotherapy-naive patients and an RR of 9% in pretreated patients when used as monotherapy [132]. Nevertheless, recent evidence showed disappointing results. Single-agent bortezomib did not achieve any objective response in 15 evaluable patients with advanced gastric or GEJ adenocarcinoma with up to one line of prior therapy [133]. Another trial evaluating bortezomib, paclitaxel, and carboplatin as first-line treatment for metastatic esophageal, gastric, and GEJ cancers showed a disappointing ORR of 23%, leading to premature termination of the study [134]. Thus, the initial enthusiasm in using bortezomib for AGC cannot be confirmed.
Second, the overexpression/activation of c-Met, a receptor for hepatocyte growth factor, leads to proliferation and antiapoptotic signals [135]. It was found to be activated both in vitro in human gastric cancer cell lines [136] and in vivo in human gastric cancer tissue [137], and this may result from the infection of gastric cells by Helicobacter pylori (HP) [138]. Amplification of MET predicts response to Met inhibition in vitro [139]. Interim results of a phase II study of GSK1363089 (GSK089, formerly XL880), a c-Met TKI, showed minimal activity in a cohort of metastatic gastric cancer patients unselected for c-Met but was well tolerated, with toxicities including liver function abnormalities, fatigue, and venous thromboembolism [140]. In another preliminary report, two of 10 gastroesophageal cancer patients with MET amplification had tumor shrinkage with the Met inhibitor crizotinib [141]. Notably, variable responses were noted in different Met-overexpressing esophageal cancer cell lines [142], suggesting that factors other than Met overexpression may play a role in predicting response. For example, a recent report showed that HER activation induced resistance to Met inhibition in Met-addicted gastric cancer cells in vitro and in vivo [143, 144]; this reiterates the importance of the development of predictive biomarkers.
The Hedgehog (Hh) pathway further complicates the complex signaling in gastric cancer cells [145]. The Hh protein family includes Sonic (Shh), Indian (Ihh), and Desert (Dhh) Hedgehogs. In gastric cancer, the aberrant activation of Shh, through binding Patched 1 receptor and subsequent disinhibition of Smoothened in turn activates the transcription factor Gli-1. Cyclopamine, an Hh inhibitor, induced gastric cancer cell apoptosis in vitro [145]. Clinical use of Hh inhibitors is currently only in early phases of development [146].
Inhibition of other biological pathways in AGC is in preclinical or early clinical evaluation. The expression of insulin-like growth factor 1 receptor (IGF-1R) is correlated with poor outcome in AGC patients [147], and the IGF-1R antibody figitumumab in combination with docetaxel was well tolerated in a phase I trial of patients with advanced solid tumors [148]. Fibroblast growth factor receptor (FGFR) mutations are associated with the development of gastric cancer [149], and FGFR inhibitors may play a role in AGC treatment [150]. Heat shock protein 90 (HSP90) regulates oncogenic protein stability, and an HSP90 inhibitor was shown to inhibit gastric cancer cell growth in vitro and in xenografts [151]. Histone deacetylase (HDAC) has an important role in cell cycle regulation; its expression was associated with tumor aggressiveness in gastric cancer [152]. A phase I trial demonstrated tolerability of the combination of the HDAC inhibitor vorinostat, irinotecan, and 5-FU in patients with upper gastrointestinal cancer [153]. Moreover, the expression of interleukin (IL)-6 is higher in HP-induced gastritis, and it has been implicated in carcinogenesis via activation of the Janus kinase–signal transducer and activator of transcription (JAK–STAT) pathway [154]. Both IL-6–neutralizing antibody and AZD 1480, a selective JAK-2 inhibitor with potent activity in blocking STAT-3 signaling, are in the early phase of clinical testing.
Conclusion
In conclusion, recent progress in targeted therapy development for AGC has been modest. The ToGA trial was practice changing for patients with HER-2–overexpressing AGC, for which trastuzumab is approved in combination with chemotherapy in the first-line setting, whereas other agents require vetting in well-designed phase III trials. There is still an unmet need for researchers to unravel the molecular carcinogenic mechanisms underlying AGC, to rationally design targeted therapy or combinations of such, and to develop predictive biomarkers to aid patient selection.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Thomas Yau, Hilda Wong
Collection and/or assembly of data: Thomas Yau, Hilda Wong
Data analysis and interpretation: Thomas Yau, Hilda Wong
Manuscript writing: Thomas Yau, Hilda Wong
Final approval of manuscript: Thomas Yau, Hilda Wong
References
- 1.Siegel R, Ward E, Jemal A. Cancer statistics, 2011. CA Cancer J Clin. 2011;41:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: Defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 3.Paoletti X, Oba K, Burzykowski T, et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: A meta-analysis. JAMA. 2010;303:1729–1737. doi: 10.1001/jama.2010.534. [DOI] [PubMed] [Google Scholar]
- 4.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 5.Wagner AD, Grothe W, Behl S, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2005;(2):CD004064. doi: 10.1002/14651858.CD004064.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 7.Tanabe K, Suzuki T, Tokumoto N, et al. Combination therapy with docetaxel and S-1 as a first-line treatment in patients with advanced or recurrent gastric cancer: A retrospective analysis. World J Surg Oncol. 2010;8:40. doi: 10.1186/1477-7819-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 Study Group. J Clin Oncol. 2006;24:4991–4997. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 9.Dank M, Zaluski J, Barone C, et al. Randomized phase III study comparing irinotecan combined with 5-fluorouracil and folinic acid to cisplatin combined with 5-fluorouracil in chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction. Ann Oncol. 2008;19:1450–1457. doi: 10.1093/annonc/mdn166. [DOI] [PubMed] [Google Scholar]
- 10.Wu K, Nie Y, Guo C, et al. Molecular basis of therapeutic approaches to gastric cancer. J Gastroenterol Hepatol. 2009;24:37–41. doi: 10.1111/j.1440-1746.2008.05753.x. [DOI] [PubMed] [Google Scholar]
- 11.Yin M, Hu Z, Tan D, et al. Molecular epidemiology of genetic susceptibility to gastric cancer: Focus on single nucleotide polymorphisms in gastric carcinogenesis. Am J Transl Res. 2009;1:44–54. [PMC free article] [PubMed] [Google Scholar]
- 12.Van Cutsem E, Kang Y, Chung H, et al. Efficacy results from the ToGA trial: A phase III study of trastuzumab added to standard chemotherapy in first-line human epidermal growth factor receptor 2-positive advanced gastric cancer. J Clin Oncol. 2009;27(15 suppl):LBA4509. [Google Scholar]
- 13.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 14.Dhanasekaran DN, Johnson GL. MAPKs: Function, regulation, role in cancer and therapeutic targeting. Oncogene. 2007;26:3097–3099. doi: 10.1038/sj.onc.1210395. [DOI] [PubMed] [Google Scholar]
- 15.Schlessinger J. Common and distinct elements in cellular signaling via EGF and FGF receptors. Science. 2004;306:1506–1507. doi: 10.1126/science.1105396. [DOI] [PubMed] [Google Scholar]
- 16.Yano T, Doi T, Ohtsu A, et al. Comparison of HER2 gene amplification assessed by fluorescence in situ hybridization and HER2 protein expression assessed by immunohistochemistry in gastric cancer. Oncol Rep. 2006;15:65–71. [PubMed] [Google Scholar]
- 17.Gravalos C, Màrquez A, García-Carbonero R, et al. Correlation between Her2/neu overexpression/amplification and clinicopathological parameters in advanced gastric cancer patients: A prospective study 2007 [abstract 189]. Presented at the 2007 American Society of Clinical Oncology Gastrointestinal Cancers Symposium; January 19–21, 2007; Orlando, FL. [Google Scholar]
- 18.Lordick F, Bang YJ, Kang YK, et al. HER2-positive advanced gastric cancer: Similar HER2-positivity levels to breast cancer [abstract 3541] Eur J Cancer. 2007;5:271. [Google Scholar]
- 19.Koeppen HK, Wright BD, Burt AD, et al. Overexpression of HER2/neu in solid tumours: An immunohistochemical survey. Histopathology. 2001;38:96–104. doi: 10.1046/j.1365-2559.2001.01084.x. [DOI] [PubMed] [Google Scholar]
- 20.Jaehne J, Urmacher C, Thaler HT, et al. Expression of Her2/neu oncogene product p185 in correlation to clinicopathological and prognostic factors of gastric carcinoma. J Cancer Res Clin Oncol. 1992;118:474–479. doi: 10.1007/BF01629433. [DOI] [PubMed] [Google Scholar]
- 21.Im SA, Lee KE, Nam E, et al. Potential prognostic significance of p185(HER2) overexpression with loss of PTEN expression in gastric carcinomas. Tumori. 2005;91:513–521. doi: 10.1177/030089160509100612. [DOI] [PubMed] [Google Scholar]
- 22.Uchino S, Tsuda H, Maruyama K, et al. Overexpression of c-erbB-2 protein in gastric cancer. Its correlation with long-term survival of patients. Cancer. 1993;72:3179–3184. doi: 10.1002/1097-0142(19931201)72:11<3179::aid-cncr2820721108>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 23.Nakajima M, Sawada H, Yamada Y, et al. The prognostic significance of amplification and overexpression of c-met and c-erb B-2 in human gastric carcinomas. Cancer. 1999;85:1894–1902. doi: 10.1002/(sici)1097-0142(19990501)85:9<1894::aid-cncr3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 24.Begnami MD, Fukuda E, Fregnani JH, et al. Prognostic implications of altered human epidermal growth factor receptors (HERs) in gastric carcinomas: HER2 and HER3 are predictors of poor outcome. J Clin Oncol. 2011;29:3030–3036. doi: 10.1200/JCO.2010.33.6313. [DOI] [PubMed] [Google Scholar]
- 25.Kim KC, Koh YW, Chang HM, et al. Evaluation of HER2 protein expression in gastric carcinomas: Comparative analysis of 1,414 cases of whole-tissue sections and 595 cases of tissue microarrays. Ann Surg Oncol. 2011;18:2833–2840. doi: 10.1245/s10434-011-1695-2. [DOI] [PubMed] [Google Scholar]
- 26.Grabsch H, Sivakumar S, Gray S, et al. HER2 expression in gastric cancer: Rare, heterogeneous and of no prognostic value—conclusions from 924 cases of two independent series. Cell Oncol. 2010;32:57–65. doi: 10.3233/CLO-2009-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chua TC, Merrett ND. Clinicopathologic factors associated with HER2-positive gastric cancer and its impact on survival outcomes—a systematic review. Int J Cancer. 2011 Jul 21; doi: 10.1002/ijc.26292. [Epub ahead of print]. doi: 10.1002/ijc.2692. [DOI] [PubMed] [Google Scholar]
- 28.Moelans CB, van Diest PJ, Milne AN, et al. Her-2/neu testing and therapy in gastroesophageal adenocarcinoma. Patholog Res Int. 2010;2011:674182. doi: 10.4061/2011/674182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cortés-Funes H, Rivera F, Alés I, et al. Phase II of trastuzumab and cisplatin in patients with advanced gastric cancer with HER2/neu overexpression/amplification. J Clin Oncol. 2007;15(18 suppl):4613. [Google Scholar]
- 30.Nicholas G, Cripps C, Au HP, et al. Early results of a trial of trastuzumab, cisplatin and docetaxel for the treatment of metastatic gastric cancer overexpressing HER-2 [abstract 1105] ESMO. 2006;17:316. [Google Scholar]
- 31.U.S. Food and Drug Administration. Herceptin® (trastuzumab) [prescribing information] [accessed October 26, 2011]. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/103792s5250lbl.pdf.
- 32.European Medicines Agency. Herceptin® [summary of product characteristics] [accessed October 26, 2011]. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000278/WC500074922.pdf.
- 33.Rech J, Arnold D, Folprecht G, et al. A pilot study of trastuzumab monotherapy in patients who progressed while on chemotherapy for metastatic or locally advanced HER-2 positive gastric cancer [abstract 1096] ESMO. 2006;17:314. [Google Scholar]
- 34.Kim MA, Lee HS, Lee HE, et al. EGFR in gastric carcinomas: Prognostic significance of protein overexpression and high gene copy number. Histopathology. 2008;52:738–746. doi: 10.1111/j.1365-2559.2008.03021.x. [DOI] [PubMed] [Google Scholar]
- 35.Lieto E, Ferraraccio F, Orditura M, et al. Expression of vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) is an independent prognostic indicator of worse outcome in gastric cancer patients. Ann Surg Oncol. 2008;15:69–79. doi: 10.1245/s10434-007-9596-0. [DOI] [PubMed] [Google Scholar]
- 36.Gamboa-Dominguez A, Dominguez-Fonseca C, Quintanilla-Martinez L, et al. Epidermal growth factor receptor expression correlates with poor survival in gastric adenocarcinoma from Mexican patients: A multivariate analysis using a standardized immunohistochemical detection system. Mod Pathol. 2004;17:579–587. doi: 10.1038/modpathol.3800085. [DOI] [PubMed] [Google Scholar]
- 37.Matsubara J, Nishina T, Yamada Y, et al. Impacts of excision repair cross-complementing gene 1 (ERCC1), dihydropyrimidine dehydrogenase, and epidermal growth factor receptor on the outcomes of patients with advanced gastric cancer. Br J Cancer. 2008;98:832–839. doi: 10.1038/sj.bjc.6604211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pinto C, Di Fabio F, Siena S, et al. Phase II study of cetuximab in combination with FOLFIRI in patients with untreated advanced gastric or gastroesophageal junction adenocarcinoma (FOLCETUX study) Ann Oncol. 2007;18:510–517. doi: 10.1093/annonc/mdl459. [DOI] [PubMed] [Google Scholar]
- 39.Kanzler S, Trarbach T, Seufferlein T, et al. German Arbeitsgemeinschaft Internistische O. Cetuximab with irinotecan/folinic acid/5-FU as first-line treatment in advanced gastric cancer: A nonrandomized multicenter AIO phase II study. J Clin Oncol. 2009;27(15 suppl):4534. [Google Scholar]
- 40.Moehler M, Mueller A, Trarbach T, et al. Cetuximab with irinotecan, folinic acid and 5-fluorouracil as first-line treatment in advanced gastroesophageal cancer: A prospective multi-center biomarker-oriented phase II study. Ann Oncol. 2011;22:1358–1366. doi: 10.1093/annonc/mdq591. [DOI] [PubMed] [Google Scholar]
- 41.Han SW, Oh DY, Im SA, et al. Phase II study and biomarker analysis of cetuximab combined with modified FOLFOX6 in advanced gastric cancer. Br J Cancer. 2009;100:298–304. doi: 10.1038/sj.bjc.6604861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lordick F, Luber B, Lorenzen S, et al. Cetuximab plus oxaliplatin/leucovorin/5-fluorouracil in first-line metastatic gastric cancer: A phase II study of the Arbeitsgemeinschaft Internistische Onkologie (AIO) Br J Cancer. 2010;102:500–505. doi: 10.1038/sj.bjc.6605521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim C, Lee JL, Ryu MH, et al. A prospective phase II study of cetuximab in combination with XELOX (capecitabine and oxaliplatin) in patients with metastatic and/or recurrent advanced gastric cancer. Invest New Drugs. 2011;29:366–373. doi: 10.1007/s10637-009-9363-0. [DOI] [PubMed] [Google Scholar]
- 44.Zhang X, Xu J, Shen L, et al. A phase II study of cetuximab with cisplatin and capecitabine as first-line treatment in advanced gastric cancer [abstract LBA39]. Presented at the 2009 American Society of Clinical Oncology Gastrointestinal Cancers Symposium; January 15–17, 2009; San Francisco, CA. [Google Scholar]
- 45.Woell E, Greil R, Eisterer W, et al. Oxaliplatin, irinotecan, and cetuximab in advanced gastric cancer. First efficacy results of a multicenter phase II trial (AGMT Gastric-2) [abstract 15587)] Proc Am Soc Clin Oncol. 2008;26:662s. [Google Scholar]
- 46.Pinto C, Di Fabio F, Barone C, et al. Phase II study of cetuximab in combination with cisplatin and docetaxel in patients with untreated advanced gastric or gastro-oesophageal junction adenocarcinoma (DOCETUX study) Br J Cancer. 2009;101:1261–1268. doi: 10.1038/sj.bjc.6605319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeh K, Hsu C, Hsu C, et al. Phase II study of cetuximab plus weekly cisplatin and 24-hour infusion of high-dose 5-fluorouracil and leucovorin for the first-line treatment of advanced gastric cancer. J Clin Oncol. 2009;27(15 suppl):4567. [Google Scholar]
- 48.Enzinger PC, Burtness B, Hollis D, et al. CALGB 80403/ECOG 1206: A randomized phase II study of three standard chemotherapy regimens (ECF, IC, FOLFOX) plus cetuximab in metastatic esophageal and GE junction cancer. J Clin Oncol. 2010;28(15 suppl):4006. doi: 10.1200/JCO.2015.65.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richards DA, Kocs DM, Spira AI, et al. Results of docetaxel plus oxaliplatin (DOCOX) with or without cetuximab in patients with metastatic gastric and/or gastroesophageal junction adenocarcinoma: Results of a randomized phase II study. J Clin Oncol. 2011;29(15 suppl):4015. doi: 10.1016/j.ejca.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 50.ClinicalTrials.gov. Erbitux in Combination With Xeloda and Cisplatin in Advanced Esophago-gastric Cancer (EXPAND) [accessed January 21, 2012]. Available at http://www.clinicaltrials.gov/ct/show/NCT00678535.
- 51.Tebbutt N, Sourjina T, Strickland AH, et al. ATTAX2: Docetaxel plus cetuximab as second-line treatment for docetaxel refractory oesophago-gastric cancer—final results of a multicentre phase II trial by the AGITG [abstract 15554] Proc Am Soc Clin Oncol. 2008;26:659s. [Google Scholar]
- 52.Stein A, Al-Batran SE, Arnold D, et al. Cetuximab with irinotecan as salvage therapy in heavily pretreated patients with metastatic gastric cancer. Presented at the 2007 American Society of Clinical Oncology Gastrointestinal Cancers Symposium; January 19–21, 2007; Orlando, FL. [Google Scholar]
- 53.Li J, Liu X, Wang BY, et al. Phase II study of cetuximab in combination with modified FOLFIRI in patients with advanced gastric cancer who failed first-line chemotherapy (EFFI study) J Clin Oncol. 2010;28(15 suppl):4107. [Google Scholar]
- 54.Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–1664. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 55.ClinicalTrials.gov. REAL 3 Version 2.0: Trial of the Efficacy of Epirubicin, Oxaliplatin and Capecitabine (EOX) With or Without Panitumumab in Previously Untreated Advanced Oesophago-Gastric Cancer. [accessed January 21, 2012]. Available at http://www.clinicaltrials.gov/ct/show/NCT00824785.
- 56.Chau I, Okines AFC, Gonzalez de Castro D, et al. REAL3: A multicenter randomized phase II/III trial of epirubicin, oxaliplatin, and capecitabine (EOC) versus modified (m) EOC plus panitumumab (P) in advanced oesophagogastric (OG) cancer—response rate (RR), toxicity, and molecular analysis from phase II. J Clin Oncol. 2011;29(15 suppl):4131. [Google Scholar]
- 57.Rao S, Starling N, Cunningham D, et al. Matuzumab plus epirubicin, cisplatin and capecitabine (ECX) compared with epirubicin, cisplatin and capecitabine alone as first-line treatment in patients with advanced oesophago-gastric cancer: A randomised, multicentre open-label phase II study. Ann Oncol. 2010;21:2213–2219. doi: 10.1093/annonc/mdq247. [DOI] [PubMed] [Google Scholar]
- 58.Kim YH, Sasaki Y, Lee KH, et al. Randomized phase II study of nimotuzumab, an anti-EGFR antibody, plus irinotecan in patients with 5-fluorouracil-based regimen-refractory advanced or recurrent gastric cancer in Korea and Japan: Preliminary results. J Clin Oncol. 2011;29(suppl 4):87. [Google Scholar]
- 59.Dragovich T, McCoy S, Fenoglio-Preiser CM, et al. Phase II trial of erlotinib in gastroesophageal junction and gastric adenocarcinomas: SWOG 0127. J Clin Oncol. 2006;24:4922–4927. doi: 10.1200/JCO.2006.07.1316. [DOI] [PubMed] [Google Scholar]
- 60.Rojo F, Tabernero J, Albanell J, et al. Pharmacodynamic studies of gefitinib in tumor biopsy specimens from patients with advanced gastric carcinoma. J Clin Oncol. 2006;24:4309–4316. doi: 10.1200/JCO.2005.04.2424. [DOI] [PubMed] [Google Scholar]
- 61.Doi T, Koizumi S, Siena S. Efficacy, tolerability and pharmacokinetics of gefitinib (ZD1839) in pretreated patients with metastatic gastric cancer. Proc Am Soc Clin Oncol. 2003;22:1036. al. e. [Google Scholar]
- 62.Adelstein DJ, Rybicki L, Carrol MA, et al. Phase II trial of gefitinib for recurrent or metastatic esophageal or gastroesophageal junction (GEJ) cancer. Proc Am Soc Clin Oncol. 2005;23:A4054. [Google Scholar]
- 63.Wainberg ZA, Lin LS, DiCarlo B, et al. Phase II trial of modified FOLFOX6 and erlotinib in patients with metastatic or advanced adenocarcinoma of the oesophagus and gastro-oesophageal junction. Br J Cancer. 2011;105:760–765. doi: 10.1038/bjc.2011.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iqbal S, Goldman H, Lenz HJ, et al. S0413: A phase II SWOG study of GW572016 (lapatinib) as first line therapy in patients with advanced or metastatic gastric cancer. J Clin Oncol. 2007;25:4621. doi: 10.1093/annonc/mdr021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hecht JR, Urba SG, Koehler M, et al. Lapatinib monotherapy in recurrent upper gastrointestinal malignancy: Phase II efficacy and biomarker analyses [abstract 43]. Presented at the 2008 American Society of Clinical Oncology Gastrointestinal Cancers Symposium; January 25–27, 2008; Orlando, FL. [Google Scholar]
- 66.ClinicalTrials.gov. LOGiC - Lapatinib Optimization Study in ErbB2 (HER2) Positive Gastric Cancer: A Phase III Global, Blinded Study Designed to Evaluate Clinical Endpoints and Safety of Chemotherapy Plus Lapatinib. [accessed January 21, 2012]. Available at http://www.clinicaltrials.gov/ct/show/NCT00680901.
- 67.Satoh T, Bang Y, Wang J, et al. Interim safety analysis from TYTAN: A phase III Asian study of lapatinib in combination with paclitaxel as second-line therapy in gastric cancer. J Clin Oncol. 2010;28(15 suppl):4057. [Google Scholar]
- 68.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 69.Murayama T, Inokuchi M, Takagi Y, et al. Relation between outcomes and localisation of p-mTOR expression in gastric cancer. Br J Cancer. 2009;100:782–788. doi: 10.1038/sj.bjc.6604915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bellacosa A, Kumar CC, Di Cristofano A, et al. Activation of AKT kinases in cancer: Implications for therapeutic targeting. Adv Cancer Res. 2005;94:29–86. doi: 10.1016/S0065-230X(05)94002-5. [DOI] [PubMed] [Google Scholar]
- 71.Lang SA, Gaumann A, Koehl GE, et al. Mammalian target of rapamycin is activated in human gastric cancer and serves as a target for therapy in an experimental model. Int J Cancer. 2007;120:1803–1810. doi: 10.1002/ijc.22442. [DOI] [PubMed] [Google Scholar]
- 72.Yu G, Wang J, Chen Y, et al. Overexpression of phosphorylated mammalian target of rapamycin predicts lymph node metastasis and prognosis of Chinese patients with gastric cancer. Clin Cancer Res. 2009;15:1821–1829. doi: 10.1158/1078-0432.CCR-08-2138. [DOI] [PubMed] [Google Scholar]
- 73.Byun DS, Cho K, Ryu BK, et al. Frequent monoallelic deletion of PTEN and its reciprocal association with PIK3CA amplification in gastric carcinoma. Int J Cancer. 2003;104:318–327. doi: 10.1002/ijc.10962. [DOI] [PubMed] [Google Scholar]
- 74.Li VS, Wong CW, Chan TL, et al. Mutations of PIK3CA in gastric adenocarcinoma. BMC Cancer. 2005;5:29. doi: 10.1186/1471-2407-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kang YH, Lee HS, Kim WH. Promoter methylation and silencing of PTEN in gastric carcinoma. Lab Invest. 2002;82:285–291. doi: 10.1038/labinvest.3780422. [DOI] [PubMed] [Google Scholar]
- 76.Linos K, Sheehan CE, Ross JS. Correlation of HER2 and PTEN status with clinical outcome in esophageal (E), gastric (G), and gastroesophageal junction (GEJ) adenocarcinomas (ACs) J Clin Oncol. 2011;29(15 suppl):4066. [Google Scholar]
- 77.Doi T, Muro K, Boku N, et al. Multicenter phase II study of everolimus in patients with previously treated metastatic gastric cancer. J Clin Oncol. 2010;28:1904–1910. doi: 10.1200/JCO.2009.26.2923. [DOI] [PubMed] [Google Scholar]
- 78.Jo JC, Lee JL, Ryu MH, et al. Docetaxel monotherapy as a second-line treatment after failure of fluoropyrimidine and platinum in advanced gastric cancer: Experience of 154 patients with prognostic factor analysis. Jpn J Clin Oncol. 2007;37:936–941. doi: 10.1093/jjco/hym123. [DOI] [PubMed] [Google Scholar]
- 79.Lee KW, Kim JH, Yun T, et al. Phase II study of low-dose paclitaxel and cisplatin as a second-line therapy after 5-fluorouracil/platinum chemotherapy in gastric cancer. J Korean Med Sci. 2007;22(suppl):S115–S121. doi: 10.3346/jkms.2007.22.S.S115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Park SH, Kim YS, Hong J, et al. Mitomycin C plus S-1 as second-line therapy in patients with advanced gastric cancer: A noncomparative phase II study. Anticancer Drugs. 2008;19:303–307. doi: 10.1097/cad.0b013e3282f46ad8. [DOI] [PubMed] [Google Scholar]
- 81.Takiuchi H, Goto M, Imamura H, et al. Multi-center phase II study for combination therapy with paclitaxel/doxifluridine to treat advanced/recurrent gastric cancer showing resistance to S-1 (OGSG 0302) Jpn J Clin Oncol. 2008;38:176–181. doi: 10.1093/jjco/hyn003. [DOI] [PubMed] [Google Scholar]
- 82.ClinicalTrials.gov. Safety and Efficacy of RAD001 (Everolimus) Monotherapy Plus Best Supportive Care in Patients With Advanced Gastric Cancer (AGC) (GRANITE-1) [accessed January 21, 2012]. Available at http://www.clinicaltrials.gov/ct/show/NCT00879333.
- 83.Normanno N, Tejpar S, Morgillo F, et al. Implications for KRAS status and EGFR-targeted therapies in metastatic CRC. Nat Rev Clin Oncol. 2009;6:519–527. doi: 10.1038/nrclinonc.2009.111. [DOI] [PubMed] [Google Scholar]
- 84.Bardelli A, Siena S. Molecular mechanisms of resistance to cetuximab and panitumumab in colorectal cancer. J Clin Oncol. 2010;28:1254–1261. doi: 10.1200/JCO.2009.24.6116. [DOI] [PubMed] [Google Scholar]
- 85.Lee SH, Lee JW, Soung YH, et al. BRAF and KRAS mutations in stomach cancer. Oncogene. 2003;22:6942–6945. doi: 10.1038/sj.onc.1206749. [DOI] [PubMed] [Google Scholar]
- 86.Hiyama T, Haruma K, Kitadai Y, et al. K-ras mutation in Helicobacter pylori-associated chronic gastritis in patients with and without gastric cancer. Int J Cancer. 2002;97:562–566. doi: 10.1002/ijc.1644. [DOI] [PubMed] [Google Scholar]
- 87.Kim IJ, Park JH, Kang HC, et al. Mutational analysis of BRAF and K-ras in gastric cancers: Absence of BRAF mutations in gastric cancers. Hum Genet. 2003;114:118–120. doi: 10.1007/s00439-003-1027-0. [DOI] [PubMed] [Google Scholar]
- 88.Lee KH, Lee JS, Suh C, et al. Clinicopathologic significance of the K-ras gene codon 12 point mutation in stomach cancer. An analysis of 140 cases. Cancer. 1995;75:2794–2801. doi: 10.1002/1097-0142(19950615)75:12<2794::aid-cncr2820751203>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 89.Nanus DM, Kelsen DP, Mentle IR, et al. Infrequent point mutations of ras oncogenes in gastric cancers. Gastroenterology. 1990;98:955–960. doi: 10.1016/0016-5085(90)90019-w. [DOI] [PubMed] [Google Scholar]
- 90.Hongyo T, Buzard GS, Palli D, et al. Mutations of the K-ras and p53 genes in gastric adenocarcinomas from a high-incidence region around Florence, Italy. Cancer Res. 1995;55:2665–2672. [PubMed] [Google Scholar]
- 91.Park SR, Kook MC, Choi IJ, et al. Predictive factors for the efficacy of cetuximab plus chemotherapy as salvage therapy in metastatic gastric cancer patients. Cancer Chemother Pharmacol. 2010;65:579–587. doi: 10.1007/s00280-009-1067-9. [DOI] [PubMed] [Google Scholar]
- 92.Sun W, Powell M, O'Dwyer PJ, et al. Phase II study of sorafenib in combination with docetaxel and cisplatin in the treatment of metastatic or advanced gastric and gastroesophageal junction adenocarcinoma: ECOG 5203. J Clin Oncol. 2010;28:2947–2951. doi: 10.1200/JCO.2009.27.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ilson D, Janjigian YY, Shah MA, et al. Phase II trial of sorafenib in esophageal (E) and gastroesophageal junction (GEJ) cancer: Response and protracted stable disease observed in adenocarcinoma. J Clin Oncol. 2011;29(15 suppl):4100. [Google Scholar]
- 94.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 95.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 96.Maeda K, Chung YS, Ogawa Y, et al. Prognostic value of vascular endothelial growth factor expression in gastric carcinoma. Cancer. 1996;77:858–863. doi: 10.1002/(sici)1097-0142(19960301)77:5<858::aid-cncr8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 97.Ni XF, Wu CP, Jiang JT. Serum VEGFR-3 and survival of advanced gastric cancer patients treated with FOLFOX. World J Gastroenterol. 2010;16:2163–2169. doi: 10.3748/wjg.v16.i17.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tanigawa N, Amaya H, Matsumura M, et al. Extent of tumor vascularization correlates with prognosis and hematogenous metastasis in gastric carcinomas. Cancer Res. 1996;56:2671–2676. [PubMed] [Google Scholar]
- 99.Guan X, Zhao H, Niu J, et al. The VEGF -634G>C promoter polymorphism is associated with risk of gastric cancer. BMC Gastroenterol. 2009;9:77. doi: 10.1186/1471-230X-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim JG, Sohn SK, Chae YS, et al. Vascular endothelial growth factor gene polymorphisms associated with prognosis for patients with gastric cancer. Ann Oncol. 2007;18:1030–1036. doi: 10.1093/annonc/mdm085. [DOI] [PubMed] [Google Scholar]
- 101.Schneider BP, Wang M, Radovich M, et al. Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol. 2008;26:4672–4678. doi: 10.1200/JCO.2008.16.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Okines AF, Reynolds AR, Cunningham D. Targeting angiogenesis in esophagogastric adenocarcinoma. The Oncologist. 2011;16:844–858. doi: 10.1634/theoncologist.2010-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shah MA, Ramanathan RK, Ilson DH, et al. Multicenter phase II study of irinotecan, cisplatin, and bevacizumab in patients with metastatic gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol. 2006;24:5201–5206. doi: 10.1200/JCO.2006.08.0887. [DOI] [PubMed] [Google Scholar]
- 104.El-Rayes BF, Zalupski M, Bekai-Saab T, et al. A phase II study of bevacizumab, oxaliplatin, and docetaxel in locally advanced and metastatic gastric and gastroesophageal junction cancers. Ann Oncol. 2010;21:1999–2004. doi: 10.1093/annonc/mdq065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kelsen D, Jhawer M, Ilson D, et al. Analysis of survival with modified docetaxel, cisplatin, fluorouracil, and bevacizumab in patients with metastatic gastroesophageal adenocarcinoma: Results of a phase II clinical trial. J Clin Oncol. 2009;27:4512. doi: 10.1200/JCO.2010.32.0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cohenuram MK, Lacy J. FOLFOX6 and bevacizumab (FOLFOX6/B) for metastatic esophageal (E), gastroesophageal (GE), and gastric (G) adenocarcinoma: A single institution's initial clinical experience [abstract 74]. Presented at the 2008 American Society of Clinical Oncology Gastrointestinal Cancers Symposium; January 25–27, 2008; Orlando, FL. [Google Scholar]
- 107.Kang Y, Ohtsu A, Van Cutsem E, et al. AVAGAST: A randomized, double-blind, placebo-controlled, phase III study of first-line capecitabine and cisplatin plus bevacizumab or placebo in patients with advanced gastric cancer (AGC) J Clin Oncol. 2010;28(18 suppl):LBA4007. [Google Scholar]
- 108.Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: A randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29:3968–3976. doi: 10.1200/JCO.2011.36.2236. [DOI] [PubMed] [Google Scholar]
- 109.Bang YJ, Kang YK, Kang WK, et al. Phase II study of sunitinib as second-line treatment for advanced gastric cancer. Invest New Drugs. 2011;29:1449–1458. doi: 10.1007/s10637-010-9438-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Moehler M, Hartmann JT, Lordick F, et al. Sunitinib in patients with chemo-refractory metastatic gastric cancer: Preliminary results of an open-label, prospective nonrandomized multicenter AIO phase II trial [abstract 61]. Presented at the 2009 American Society of Clinical Oncology Gastrointestinal Cancers Symposium; January 15–17, 2009; San Francisco, CA. [Google Scholar]
- 111.Alsina M, Ko AH, Garcia De Paredes M, et al. Clinical and pharmacodynamic (PD) results of TEL0805 trial: A phase II study of telatinib (TEL) in combination with capecitabine (X) and cisplatin (P) as first-line treatment in patients (pts) with advanced gastric or gastroesophageal junction (GEJ) cancer. J Clin Oncol. 2011;29(15 suppl):4122. [Google Scholar]
- 112.ClinicalTrials.gov. Study of Axitinib in Combination With Cisplatin and Capecitabine in Patients With Advanced Gastric Cancer. [accessed January 21, 2012]. Available at http://clinicaltrials.gov/ct2/show/NCT00842244.
- 113.Li J, Qin S, Xu J, et al. A randomized, double-blind, multicenter, phase II, three-arm, placebo-control study of apatinib as third-line treatment in patients with metastatic gastric carcinoma. J Clin Oncol. 2011;29(15 suppl):4019. [Google Scholar]
- 114.Shah MA, Khanin R, Tang L, et al. Molecular classification of gastric cancer: A new paradigm. Clin Cancer Res. 2011;17:2693–2701. doi: 10.1158/1078-0432.CCR-10-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yamashita K, Sakuramoto S, Watanabe M. Genomic and epigenetic profiles of gastric cancer: Potential diagnostic and therapeutic applications. Surg Today. 2011;41:24–38. doi: 10.1007/s00595-010-4370-5. [DOI] [PubMed] [Google Scholar]
- 116.Sauter G, Lee J, Bartlett JM, et al. Guidelines for human epidermal growth factor receptor 2 testing: Biologic and methodologic considerations. J Clin Oncol. 2009;27:1323–1333. doi: 10.1200/JCO.2007.14.8197. [DOI] [PubMed] [Google Scholar]
- 117.Hofmann M, Stoss O, Shi D, et al. HER2 status evaluation for gastric cancer: A consensus study [abstract 24]. Presented at the 2006 American Society of Clinical Oncology Gastrointestinal Cancers Symposium; Jauary, 26–28, 2006; San Francisco. [Google Scholar]
- 118.Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 119.Richman SD, Chambers P, Seymour MT, et al. Intra-tumoral heterogeneity of KRAS and BRAF mutation status in patients with advanced colorectal cancer (aCRC) and cost-effectiveness of multiple sample testing. Anal Cell Pathol (Amst) 2011;34:61–66. doi: 10.3233/ACP-2011-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Choi YP, Shim HS, Gao MQ, et al. Molecular portraits of intratumoral heterogeneity in human ovarian cancer. Cancer Lett. 2011;307:62–71. doi: 10.1016/j.canlet.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 121.Nassar A, Radhakrishnan A, Cabrero IA, et al. Intratumoral heterogeneity of immunohistochemical marker expression in breast carcinoma: A tissue microarray-based study. Appl Immunohistochem Mol Morphol. 2010;18:433–441. doi: 10.1097/PAI.0b013e3181dddb20. [DOI] [PubMed] [Google Scholar]
- 122.Guo Z, Wu F, Asplund A, et al. Analysis of intratumoral heterogeneity of chromosome 3p deletions and genetic evidence of polyclonal origin of cervical squamous carcinoma. Mod Pathol. 2001;14:54–61. doi: 10.1038/modpathol.3880256. [DOI] [PubMed] [Google Scholar]
- 123.Yoshimura A, Sugihara H, Ling ZQ, et al. How wild-type TP53 is inactivated in undifferentiated-type gastric carcinomas: Analyses of intratumoral heterogeneity in deletion and mutation of TP53. Pathobiology. 2006;73:40–49. doi: 10.1159/000093090. [DOI] [PubMed] [Google Scholar]
- 124.Furuya T, Uchiyama T, Murakami T, et al. Relationship between chromosomal instability and intratumoral regional DNA ploidy heterogeneity in primary gastric cancers. Clin Cancer Res. 2000;6:2815–2820. [PubMed] [Google Scholar]
- 125.Anzai H, Kitadai Y, Bucana CD, et al. Intratumoral heterogeneity and inverse correlation between expression of E-cadherin and collagenase type IV in human gastric carcinomas. Differentiation. 1996;60:119–127. doi: 10.1046/j.1432-0436.1996.6020119.x. [DOI] [PubMed] [Google Scholar]
- 126.Wainberg ZA, Anghel A, Desai AJ, et al. Lapatinib, a dual EGFR and HER2 kinase inhibitor, selectively inhibits HER2-amplified human gastric cancer cells and is synergistic with trastuzumab in vitro and in vivo. Clin Cancer Res. 2010;16:1509–1519. doi: 10.1158/1078-0432.CCR-09-1112. [DOI] [PubMed] [Google Scholar]
- 127.Tanizaki J, Okamoto I, Takezawa K, et al. Synergistic antitumor effect of S-1 and HER2-targeting agents in gastric cancer with HER2 amplification. Mol Cancer Ther. 2010;9:1198–1207. doi: 10.1158/1535-7163.MCT-10-0045. [DOI] [PubMed] [Google Scholar]
- 128.Burstein HJ, Sun Y, Dirix LY, et al. Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J Clin Oncol. 2010;28:1301–1307. doi: 10.1200/JCO.2009.25.8707. [DOI] [PubMed] [Google Scholar]
- 129.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 130.Burstein HJ, Kuter I, Campos SM, et al. Clinical activity of trastuzumab and vinorelbine in women with HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2001;19:2722–2730. doi: 10.1200/JCO.2001.19.10.2722. [DOI] [PubMed] [Google Scholar]
- 131.Bae SH, Ryoo HM, Kim MK, et al. Effects of the proteasome inhibitor bortezomib alone and in combination with chemotherapeutic agents in gastric cancer cell lines. Oncol Rep. 2008;19:1027–1032. [PubMed] [Google Scholar]
- 132.Ocean AJ, Schnoll-Sussman F, Keresztes R, et al. Phase II study of PS-341 (bortezomib) with or without irinotecan in patients with advanced gastric adenocarcinomas. J Clin Oncol. 2006;24(18 suppl):4040. [Google Scholar]
- 133.Shah MA, Power DG, Kindler HL, et al. A multicenter, phase II study of bortezomib (PS-341) in patients with unresectable or metastatic gastric and gastroesophageal junction adenocarcinoma. Invest New Drugs. 2011;29:1475–1481. doi: 10.1007/s10637-010-9474-7. [DOI] [PubMed] [Google Scholar]
- 134.Jatoi A, Dakhil SR, Foster NR, et al. Bortezomib, paclitaxel, and carboplatin as a first-line regimen for patients with metastatic esophageal, gastric, and gastroesophageal cancer: Phase II results from the North Central Cancer Treatment Group (N044B) J Thorac Oncol. 2008;3:516–520. doi: 10.1097/JTO.0b013e31816de276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Migliore C, Giordano S. Molecular cancer therapy: Can our expectation be MET? Eur J Cancer. 2008;44:641–651. doi: 10.1016/j.ejca.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 136.Ponzetto C, Giordano S, Peverali F, et al. c-met is amplified but not mutated in a cell line with an activated met tyrosine kinase. Oncogene. 1991;6:553–559. [PubMed] [Google Scholar]
- 137.Inoue T, Kataoka H, Goto K, et al. Activation of c-Met (hepatocyte growth factor receptor) in human gastric cancer tissue. Cancer Sci. 2004;95:803–808. doi: 10.1111/j.1349-7006.2004.tb02185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Oliveira MJ, Costa AC, Costa AM, et al. Helicobacter pylori induces gastric epithelial cell invasion in a c-Met and type IV secretion system-dependent manner. J Biol Chem. 2006;281:34888–34896. doi: 10.1074/jbc.M607067200. [DOI] [PubMed] [Google Scholar]
- 139.Smolen GA, Sordella R, Muir B, et al. Amplification of MET may identify a subset of cancers with extreme sensitivity to the selective tyrosine kinase inhibitor PHA-665752. Proc Natl Acad Sci U S A. 2006;103:2316–2321. doi: 10.1073/pnas.0508776103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jhawer MP, Kindler HL, Wainberg ZA, et al. Assessment of two dosing schedules of GSK1363089 (GSK089), a dual MET/VEGFR2 inhibitor, in metastatic gastric cancer (GC): Interim results of a multicenter phase II study. J Clin Oncol. 2009;27(15 suppl):4502. [Google Scholar]
- 141.Lennerz JK, Kwak EL, Michael M, et al. Identification of a small and lethal subgroup of esophagogastric adenocarcinoma with evidence of responsiveness to crizotinib by MET amplification. J Clin Oncol. 2011;29(15 suppl):4130. doi: 10.1200/JCO.2011.35.4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Watson GA, Zhang X, Stang MT, et al. Inhibition of c-Met as a therapeutic strategy for esophageal adenocarcinoma. Neoplasia. 2006;8:949–955. doi: 10.1593/neo.06499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Corso S, Ghiso E, Cepero V, et al. Activation of HER family members in gastric carcinoma cells mediates resistance to MET inhibition. Mol Cancer. 2010;9:121. doi: 10.1186/1476-4598-9-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bachleitner-Hofmann T, Sun MY, Chen CT, et al. HER kinase activation confers resistance to MET tyrosine kinase inhibition in MET oncogene-addicted gastric cancer cells. Mol Cancer Ther. 2008;7:3499–3508. doi: 10.1158/1535-7163.MCT-08-0374. [DOI] [PubMed] [Google Scholar]
- 145.Han ME, Lee YS, Baek SY, et al. Hedgehog signaling regulates the survival of gastric cancer cells by regulating the expression of Bcl-2. Int J Mol Sci. 2009;10:3033–3043. doi: 10.3390/ijms10073033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.ClinicalTrials.gov. Combination Chemotherapy With or Without GDC-0449 in Treating Patients With Advanced Stomach Cancer or Gastroesophageal Junction Cancer. [accessed January 21, 2012]. Available at http://www.clinicaltrials.gov/ct/show/NCT00982592.
- 147.Matsubara J, Yamada Y, Nakajima TE, et al. Clinical significance of insulin-like growth factor type 1 receptor and epidermal growth factor receptor in patients with advanced gastric cancer. Oncology. 2008;74:76–83. doi: 10.1159/000139127. [DOI] [PubMed] [Google Scholar]
- 148.Molife LR, Fong PC, Paccagnella L, et al. The insulin-like growth factor-I receptor inhibitor figitumumab (CP-751,871) in combination with docetaxel in patients with advanced solid tumours: Results of a phase Ib dose-escalation, open-label study. Br J Cancer. 2010;103:332–339. doi: 10.1038/sj.bjc.6605767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jang JH, Shin KH, Park JG. Mutations in fibroblast growth factor receptor 2 and fibroblast growth factor receptor 3 genes associated with human gastric and colorectal cancers. Cancer Res. 2001;61:3541–3543. [PubMed] [Google Scholar]
- 150.Squires M, Ward G, Saxty G, et al. Potent, selective inhibitors of fibroblast growth factor receptor define fibroblast growth factor dependence in preclinical cancer models. Mol Cancer Ther. 2011;10:1542–1552. doi: 10.1158/1535-7163.MCT-11-0426. [DOI] [PubMed] [Google Scholar]
- 151.Lang SA, Klein D, Moser C, et al. Inhibition of heat shock protein 90 impairs epidermal growth factor-mediated signaling in gastric cancer cells and reduces tumor growth and vascularization in vivo. Mol Cancer Ther. 2007;6:1123–1132. doi: 10.1158/1535-7163.MCT-06-0628. [DOI] [PubMed] [Google Scholar]
- 152.Weichert W, Roske A, Gekeler V, et al. Association of patterns of class I histone deacetylase expression with patient prognosis in gastric cancer: A retrospective analysis. Lancet Oncol. 2008;9:139–148. doi: 10.1016/S1470-2045(08)70004-4. [DOI] [PubMed] [Google Scholar]
- 153.Fetterly GJ, Brady WE, LeVea CM, et al. A phase I pharmacokinetic (PK) study of vorinostat (V) in combination with irinotecan (I), 5-fluorouracil (5FU), and leucovorin (FOLFIRI) in advanced upper gastrointestinal cancers (AGC) J Clin Oncol. 2009;27(15 suppl):el5540. [Google Scholar]
- 154.Chiba T, Marusawa H, Seno H, et al. Mechanism for gastric cancer development by Helicobacter pylori infection. J Gastroenterol Hepatol. 2008;23:1175–1181. doi: 10.1111/j.1440-1746.2008.05472.x. [DOI] [PubMed] [Google Scholar]