The management of patients with relapsed or refractory Hodgkin's lymphoma is discussed and several newer agents showing clinical promise are discussed.
Abstract
Hodgkin's lymphoma (HL) is diagnosed in 20,000 men and women annually in North America and Europe. Despite treatment advancements for HL resulting in an overall survival rate of 80%, patients with advanced stage disease continue to have suboptimal outcomes, with relapse rates of 30%–40%. An additional 10%–15% of patients present with primary refractory disease. For patients who relapse after initial treatment, salvage chemotherapy followed by autologous stem cell transplant in those with chemotherapy-sensitive disease is the standard of care. Patients who relapse after second-line therapy have a median survival time in the range of 6–36 months, and the optimal management of these patients remains unclear. Unfortunately, there have been no new agents approved for relapsed HL treatment since the 1970s. Consequently, clinical decision making in this population is difficult. Recently however, several agents have emerged that have shown clinical promise in this poor-risk population. This review discusses the management of these patients and also discusses several newer agents showing clinical promise in the treatment of HL.
Introduction
Hodgkin's lymphoma (HL), a lymphoid malignancy characterized by the presence of large, dysplastic, multinucleated cells (Reed–Sternberg cells), is diagnosed in 20,000 men and women annually in North America and Europe [1]. In the early 20th century, radiotherapy was identified as an effective agent in treating HL patients. The subsequent discovery of mechlorethamine in the 1940s and the advent of combination chemotherapy and radiotherapy in the 1960s, initially with mechlorethamine, vincristine, procarbazine, and prednisone and subsequently with doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD), defined HL as a largely curable malignancy with an overall survival (OS) rate of 80% at 5 years [2, 3]. However, it can be argued that these advances were not necessarily a product of progress in understanding the underlying disease biology, but rather a result of progress in the fields of drug development and evidence-based medicine. Indeed, it was not until the late 1990s that questions surrounding the nature and lineage of Hodgkin Reed-Sternberg (HRS) cells were answered, when molecular genetic studies identified these cells to be of malignant B-cell origin.
As a result of this early success, ABVD continued to remain the standard of care in 2011. However, patients with advanced stage disease continue to have suboptimal outcomes, with 5-year freedom from progression rates of 47%–79% and relapse rates of 30%–40% [4, 5]. Moreover, an additional 10%–15% of patients fail to enter remission with frontline therapy (primary refractory disease). These shortcomings have persisted despite extraordinary initial success and illustrate the complex nature of the tumor biology and the difficult task of eliminating a multifaceted disease process [6, 7]. This review briefly discusses the challenges posed by recurrent or refractory HL and discusses recent breakthroughs in drug development.
Refractory or Relapsed HL
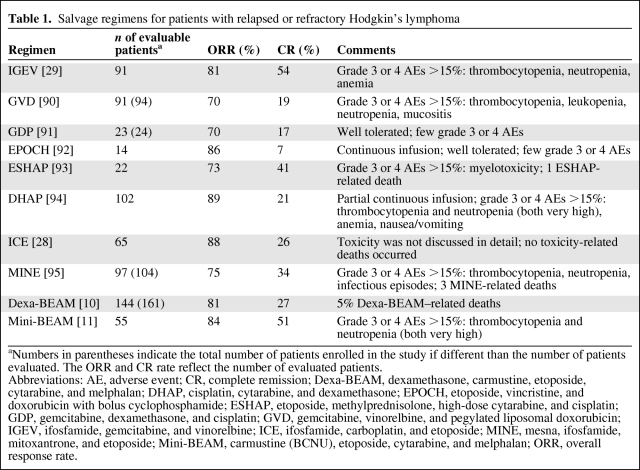
For patients who relapse with nonlocalized disease after initial treatment, salvage chemotherapy followed by autologous stem cell transplant (ASCT) in those with chemotherapy-sensitive disease is the standard of care and results in cure rates of 40%–50% [8]. Although there have been many phase II studies reporting results using salvage regimens for relapsed or refractory HL, there are no randomized trials and no consensus on the most effective second-line chemotherapy regimen (Table 1). These trials reported overlapping response and complete remission (CR) rates [9, 10–19]. Several studies identified the duration of remission after initial chemotherapy as a significant prognostic factor in obtaining a subsequent remission. Patients who have an initial remission >12 months have a 75% chance of achieving a durable second remission with salvage therapy and ASCT. In contrast, patients who have remissions lasting <12 months or who have primary progressive disease achieve a durable second remission only 40% and 20% of the time, respectively [20].
Table 1.
Salvage regimens for patients with relapsed or refractory Hodgkin's lymphoma
aNumbers in parentheses indicate the total number of patients enrolled in the study if different than the number of patients evaluated. The ORR and CR rate reflect the number of evaluated patients.
Abbreviations: AE, adverse event; CR, complete remission; Dexa-BEAM, dexamethasone, carmustine, etoposide, cytarabine, and melphalan; DHAP, cisplatin, cytarabine, and dexamethasone; EPOCH, etoposide, vincristine, and doxorubicin with bolus cyclophosphamide; ESHAP, etoposide, methylprednisolone, high-dose cytarabine, and cisplatin; GDP, gemcitabine, dexamethasone, and cisplatin; GVD, gemcitabine, vinorelbine, and pegylated liposomal doxorubicin; IGEV, ifosfamide, gemcitabine, and vinorelbine; ICE, ifosfamide, carboplatin, and etoposide; MINE, mesna, ifosfamide, mitoxantrone, and etoposide; Mini-BEAM, carmustine (BCNU), etoposide, cytarabine, and melphalan; ORR, overall response rate.
Patients who relapse after second-line therapy have a median survival time in the range of 6–36 months, and the optimal management of these patients remains unclear [8, 20, 21]. Options for these patients include palliative chemotherapy, radiotherapy, as well as supportive care or observation in selected cases. Because of the limited options for these patients, their relatively young age, and the potential for curative therapy, allogeneic SCT has intrinsic appeal. Studies evaluating allogeneic SCT, however, have yielded suboptimal results, with a relatively large treatment-related mortality rate. Myeloablative regimens have been evaluated by multiple groups and result in event-free survival (EFS) rates of 15%–25% with treatment-related death rates approaching 50% or more [23]. More recently, reduced-intensity transplantation resulted in similar EFS rates with variably lower treatment-related mortality rates [15, 24–27]. For patients who fail to respond to salvage therapy, treatment is largely palliative and often incorporates agents that have not been used in prior regimens to minimize crossresistance. Presently, the available chemotherapeutic regimens are limited and often result in short-lived responses.
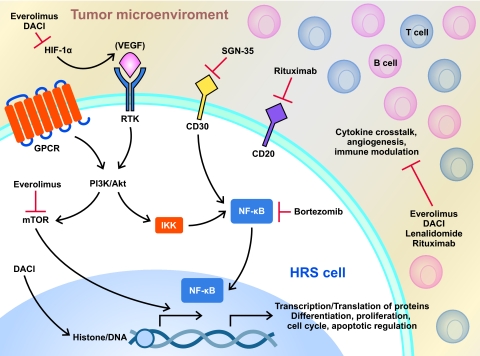
Despite the substantial success of ABVD, or possibly because of it, drug development for HL has remained static for >30 years. Clinical research has largely attempted to minimize toxicity from chemotherapy and radiation without sacrificing efficacy in early-stage patients, whereas for the high-risk population, the aim has been to improve relapse-free survival and OS rates. With the possible exception of escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP), attempts to improve outcomes for high-risk patients with more aggressive chemotherapy or upfront transplant regimens have not resulted in higher OS rates than those observed previously with ABVD [3, 9, 28]. The use of escalated BEACOPP over ABVD benefits a small percentage of patients with high-risk HL (freedom from treatment failure rate, 76% versus 68%; OS rate, 86% versus 75%) but is limited by substantial acute and long-term toxicities (infectious complications, infertility, and the risk for a second malignancy [29]). In addition, patient age and comorbidity status exclude many patients as candidates; consequently, this therapy has been slow to gain acceptance in the U.S., and ABVD continues to remain the standard of care [30]. Until recently, no new agents had been approved for the treatment of HL patients in over three decades. In the last few years, however, improvements in our understanding of epigenetic manipulation and intracellular signaling have resulted in the identification of several agents with the potential to capitalize on these advances. Moreover, advances in drug development have allowed researchers to evaluate a variety of targeted agents for HL (Fig. 1). The following pages detail several of the most promising of these agents.
Figure 1.
Targeted inhibition of HRS cells. Diagram depicts multiple biologically active aberrant pathways in HL, including mTOR, HIF-1α, deacetylation, and CD30. Included are many of the postulated sites of activity for the discussed agents. Of importance is the redundancy of the pathways. Novel therapeutic agents affect tumor pathways directly but also influence the microenvironment, DNA transcription and translation of antiapoptotic and antiangiogenic factors, and immune modulation of tumor cells.
Abbreviations: HL, Hodgkin's lymphoma; HIF, hypoxia-inducible factor; HRS, Hodgkin Reed–Sternberg; IκB, inhibitor of NF-κB; IKK, IκB kinase; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor κB; RANK, receptor activator of NF-κB.
Immunotherapy
HL is considered to be a B-cell malignancy, as identified by molecular genetic studies in the late 1990s [8]. Malignant HRS cells rarely express B-cell antigens and commonly acquire additional immunophenotypic markers, such as CD30, CD15, thymus and activation-regulated chemokine, and CD70, not commonly found on B cells. Moreover, HL is unique, with a relative scarcity of malignant cells within the tumor microenvironment, which is largely composed of non-neoplastic B and T lymphocytes, plasma cells, eosinophils, mast cells, histiocytes, and macrophages. These cells contribute to an abnormal network of cytokines and chemokines whose function and importance is only now beginning to be identified and understood. The relationship between HRS cells and the tumor microenvironment allows scientists and clinicians to target tumor cells directly or, alternatively, to disrupt the tumor–microenvironment interaction.
Anti-CD30
CD30, a member of the tumor necrosis factor (TNF) receptor superfamily, is an integral membrane glycoprotein present on HRS cells. CD30 signaling is thought to regulate the activation of nuclear factor κB (NF-κB), which is essential for HRS cell survival, through the induction of antiapoptotic genes. The high degree of expression in HL in combination with its limited expression in normal tissue makes CD30 an attractive target for antibody-based immunotherapy [21]. Phase I and phase II studies evaluating the activity of anti-CD30 antibodies, however, showed response rates of <5% and were clinically disappointing [24]. Attempts to improve on these results led to the development of CD30 antibody–chemotherapy conjugates [22–24].
Brentuximab Vedotin
Brentuximab vedotin (SGN-35) is a CD30-specific chimeric antibody–drug conjugate constructed from the variable regions of the anti-CD30 murine monoclonal AC10 and the human γ1 heavy chain and κ light chain constant region conjugated to a synthetic antimicrotubule agent, monomethyl auristatin E (MMAE) [12, 21, 25]. Once bound to the CD30+ cell, the antibody–MMAE complex is subsequently trafficked to the lysosome, where MMAE is released by lysosomal degradation within the cell. Subsequent binding of MMAE to tubulin disrupts the microtubule network, inducing cell cycle arrest and apoptosis.
Two phase I studies evaluating SGN-35 in HL T-cell lymphoma have been conducted [12, 25]. Both the weekly and every-3-week dosing regimens were similar in activity. Based on these findings, the maximum-tolerated dose was determined to be 1.8 mg/kg administered i.v. every 3 weeks. A phase II study evaluating this agent given for a maximum of 16 doses in 102 HL patients who failed salvage therapies and ASCT was recently completed [26]. Updated data presented at the American Society of Hematology (ASH) Annual Meeting in 2010 indicate that the agent is highly active, with 95% of patients achieving some reduction in tumor size. The overall response rate (ORR) was 75%, with an impressive 34% of patients achieving a CR in this highly refractory (39% of patients refractory to last therapy) and pretreated population. The agent was also relatively well tolerated with a 27-week median treatment duration. Grade 3 or 4 toxicities included neutropenia (18%), peripheral sensory neuropathy (5%), thrombocytopenia (4%), and hyperglycemia (3%) [26].
In the phase II study of SGN-35 presented at the ASH Annual Meeting in 2010, 55% of patients experienced peripheral neuropathy, with 12% of patients having motor neuropathy, all grades [26]. Neuropathy did not always occur immediately (median, 12 weeks, any grade), and the majority (68%) of patients had some improvement or resolution in their neuropathy upon discontinuation of SGN-35. Improvement was not immediate, however, with a median of 13.2 weeks. In addition to the aforementioned toxicities, tumor “flare” reactions presenting as positron emission tomography (PET)-avid, painful enlarging lymphadenopathy were noted. This reaction, while rare, is not limited to patients with HL, and occurred within the first few cycles of therapy and can be difficult to distinguish from progression. The mechanism and prognostic value of this phenomenon are unclear, and the flare reaction often improved with low-dose nonsteroidal anti-inflammatory drugs or steroid therapy.
Based on these impressive results, the U.S. Food and Drug Administration (FDA) approved the use of SGN-35 for patients with HL who have relapsed after ASCT and for those who are not eligible for transplantation after failure of two or more multiagent chemotherapeutic regimens. How to best to use this agent in the HL treatment algorithm, whether or not this drug has an acceptable toxicity profile in patients with preexisting neuropathy, and how resistance to this agent develops are questions that need to be answered. Additional studies evaluating the most appropriate setting for SGN-35 are already under way [27, 31–33]. These and other studies are expected to help define the most appropriate role for SGN-35 in HL treatment.
Rituximab
Rituximab is an anti-CD20 monoclonal antibody with single-agent activity in patients with relapsed or refractory classical HL, regardless of subtype or CD20 expression on HRS cells. Response rates to single-agent rituximab in patients relapsing post-transplant are in the range of 20%–30%, with a median duration of response of almost 8 months, with some patients achieving a CR [34]. Although the underlying mechanism of action is still being explored, a number of potential mechanistic effects have been postulated. For instance, rituximab is thought to interfere with HRS–B lymphocyte crosstalk and cytokine regulation, thereby resulting in the depletion of activated B lymphocytes and disruption of the tumor microenvironment. Consequently, it is postulated that survival and growth stimuli to putative malignant clones are inhibited, leading to tumor death. In addition, a subpopulation of HRS cells (20%–30%) is CD20+; therefore, anti-CD20 inhibition may result in direct cytotoxicity.
Interestingly, recent findings suggest the potential for a CD20+ stem cell that could be affected by exposure to an anti-CD20 agent such as rituximab, and although additional data are needed to further evaluate this, it may provide some insight into the activity of rituximab in HL patients [35]. Based on this rationale and the previously mentioned single-agent activity, several studies have been performed evaluating rituximab–chemotherapy combinations in the salvage and frontline settings. Rituximab administered weekly for 6 weeks in combination with ABVD was evaluated in 70 newly diagnosed patients at the MD Anderson Cancer Center. The EFS rate of the entire cohort was 87%, with a median follow-up of 5 years. When stratified by International Prognostic Score (IPS), the EFS rate of patients treated with rituximab plus ABVD (RABVD) compared favorably with those of historical and institutional ABVD treatment controls. The largest benefits in terms of the EFS rate at 5 years were noted in patients with higher risk IPS scores (>2)—80% for RABVD versus 55% for historical and institutional ABVD controls [36–38]. As a result of these findings, several phase II and phase III randomized studies incorporating rituximab with ABVD in the frontline setting or comparing this therapy with ABVD alone are currently under way [39–43].
Regulation of Epigenetic Modulation
Deacetylase Inhibitors
Histone modification has been implicated in the pathobiology of cancer and is mediated by acetylation or deacetylation of amino acid residues on the histone tail. Acetylation relaxes chromatin and leads to activation of RNA transcription, thus eliciting expression of several genes that can result in favorable biological responses, such as growth arrest, differentiation, and apoptosis of tumor cells. Deacetylases (DACs) alter the transcriptional activities of histones and nonhistone factors, including p53, 17-β-estradiol factor, c-Myc, NF-κB, hypoxia-inducible factor (HIF)-1α, heat shock protein 90, TNF-α, and TNF receptor apoptosis-inducing ligand. Through these factors, DACs mediate cell cycle regulation, suppression of apoptosis, modulation of the immune response, suppression of angiogenesis, and regulation of cell motility, proliferation, differentiation, and viability [44, 45]. Many of these pathways are aberrantly expressed in HL [46]; therefore, DAC inhibitors (DACIs) may provide a mechanism to inhibit tumor growth. Additionally, it is known that HRS cells, although of B-cell origin, rarely express B-cell antigens. It has been postulated that this phenotypic finding may be epigenetically regulated and therefore potentially reversible [47, 48].
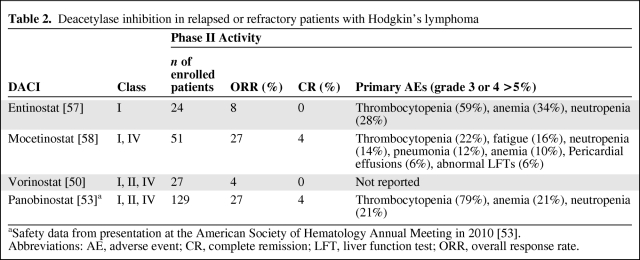
Inhibitors have been synthesized for the zinc-dependent DAC classes (I, II, and IV), with some agents inhibiting multiple classes and others affecting one individual class or subclass. Over 18 DACIs are currently in development, with several showing potential activity in HL patients. Both vorinostat and romidepsin are approved for the treatment of cutaneous T-cell lymphomas; however, to date no agent has been approved for the treatment of HL patients [46]. The following DACIs are currently being tested in heavily pretreated patients with HL.
Vorinostat
In vitro studies of the pan-DACI vorinostat demonstrated apoptosis and evidence of cell cycle arrest in HL cell lines [49]. These findings prompted a phase II trial in 27 heavily pretreated patients. In that study, only one patient had a partial response (PR); however, four others had stable disease (SD) lasting >1 year. Although the ORR was only 4% (Table 2), treatment was well tolerated and nearly 20% of patients maintained SD or better, leading to additional studies with DACIs [50].
Table 2.
Deacetylase inhibition in relapsed or refractory patients with Hodgkin's lymphoma
aSafety data from presentation at the American Society of Hematology Annual Meeting in 2010 [53].
Abbreviations: AE, adverse event; CR, complete remission; LFT, liver function test; ORR, overall response rate.
Panobinostat
Panobinostat is an oral pan-DACI found to be 10 times more potent than vorinostat in cell lines [51]. A phase I/II open-label trial evaluating panobinostat in advanced hematologic malignancies was conducted and antitumor activity was observed in a group of 13 response-evaluable patients with relapsed or refractory HL [52]. Consequently, a phase II study evaluating panobinostat in 129 highly pretreated patients with relapsed HL after ASCT was performed [53]. In total, 41% of patients were refractory to their last therapy and 10% of patients had received an allogeneic SCT. Furthermore, 66% of patients had relapsed within 12 months of their first ASCT. Responses, as measured by computed tomography (CT) or magnetic resonance imaging, were seen in 35 (27%) patients, with five (4%) patients achieving a CR (Table 2). In addition, 77% of patients had tumor reductions, and the overall duration of response approached 7 months. The median time to response was 7.4 weeks and the median progression-free survival interval was 5.7 months. The estimated 1-year OS rate was 78%, with the median OS time not yet reached. Panobinostat was well tolerated, with grade 3 and 4 toxicities largely confined to cytopenias, which were reversible upon drug interruption or dose reduction [53]. In particular, grade 3 or 4 thrombocytopenia and anemia occurred in 79% and 21% of patients, respectively (Table 2) [53]. Blood counts should initially be evaluated weekly because of the potential for rapid development of cytopenias [54]. The disease control rate of 82% (CR + PR + SD) and the duration of response in a highly treatment refractory population suggest that panobinostat has significant clinical activity in HL treatment. Studies identifying the optimal manner to use panobinostat are under way and include trials investigating the benefit of panobinostat in combination with chemotherapy in the salvage setting [55, 56].
Entinostat
Entinostat is an oral class I isoform-selective DACI currently being evaluated in an open-label phase II trial in patients with relapsed or refractory HL. Enrollment is ongoing; however, preliminary analysis of 24 patients identified two responding patients (8%) (Table 2). Consistent with findings with other DACIs, 65% of patients achieved disease control (PR + CR + SD) and toxicities were manageable, with cytopenias predominating [57].
Mocetinostat
Mocetinostat is a class I and class IV DACI. Preclinical activity was identified in HL and led to a phase II open-label study in patients with relapsed or refractory disease. In the most recent update, 51 patients were enrolled, with 14 (27%) (Table 2) achieving a PR (n = 12) or CR (n = 2) and one patient achieving durable SD. Grade 3 or higher adverse events (AEs) were notable for cytopenias, abnormal liver function studies, and pericardial effusions (Table 2) [58]. Although mocetinostat showed clinical activity, concerns surrounding the development of nonfatal pericardial effusions have precluded further clinical development in HL patients at this time [59].
Although the aforementioned agents are not the only DACIs showing activity in HL, they are the furthest along in development and, with the exception of pericardial effusions in the mocetinostat study, display similar activity and toxicity profiles (Table 2). Hematologic and gastrointestinal AEs (nausea, vomiting, and diarrhea) are common, and may be class effects. It must also be noted that, with the exception of panobinostat, the results use small sample sizes or are preliminary findings. Consequently, the effectiveness and toxicity profiles of these agents may continue to evolve [60]. Additionally, DACIs have shown preclinical synergism with a number of other antineoplastic drugs, including hypomethylating agents, conventional chemotherapeutic agents, mammalian target of rapamycin (mTOR) inhibitors, and bortezomib, resulting in several ongoing or planned trials aimed to evaluate combinations of DACIs in an effort to improve these results [61–63].
Additional Novel Agents
Immunomodulation: Lenalidomide
Lenalidomide is an analog of thalidomide and belongs to a novel class of immunomodulatory drugs. The mechanism of action of lenalidomide is incompletely understood. It is postulated to have multiple modes of action, including direct induction of apoptosis in tumor cells, antiangiogenic effects, and the modulation of immune cells, such as natural killer cells and T cells [64]. Given the interdependence of HL and its tumor environment and the understanding that apoptosis resistance, increased neoangiogenesis, and impaired immunity critically contribute to HL, lenalidomide was investigated as a potential therapeutic agent for this disease [65]. To date, three separate studies have investigated the activity of lenalidomide in heavily pretreated patients with HL at a dose of 25 mg daily for 21 of 28 consecutive days.
Fehniger et al. [66] evaluated 38 relapsed HL patients; 33 of 38 patients had prior SCTs. The ORR to lenalidomide in the 35 evaluable patients was 17%, with one CR. An additional six patients had SD lasting >6 months, resulting in an overall cytostatic response rate (CR + PR + SD >6 months) of 34% [66]. A second study by Kuruvilla et al. [67] evaluated lenalidomide in 14 patients with relapsed or refractory HL. Two patients achieved a PR (14%), with an additional seven patients having SD (50%). The median time to progression in that study was only 3.2 months, with a median OS time of 9.1 months [67]. Böll and colleagues subsequently investigated lenalidomide in 42 patients [68]. Preliminary results involving the first 24 patients have been reported. Twelve patients (50%) had an objective response (11 with a PR and one with a CR), with an additional eight patients achieving SD [68]. Grade 3 and 4 toxicities were largely hematologic, with neutropenia, thrombocytopenia, and anemia occurring in 40%, 16%, and 24% of patients, respectively, in the study by Fehniger et al. [66]. Dermatologic AEs such as rash were seen in a number of patients, a subset of which were grade ≥2. These studies show that lenalidomide has activity in HL patients with a relatively manageable toxicity profile. However, given the relatively small sample sizes and the variability in the ORR, further investigations evaluating lenalidomide are required.
Alkylating Agents: Bendamustine
Bendamustine is a purine analog–alkylator hybrid with a mechanism of action that is not completely understood. Antitumor profiles of bendamustine identify a unique mechanism of action when compared with other alkylators, with more extensive and durable DNA strand breaks. Moreover, bendamustine is active in alkylator-resistant cell lines and affects regulation of genes involved in apoptosis, DNA repair, and mitotic checkpoints [69]. In a recent study conducted by Moskowitz et al. [70] that evaluated a 120-mg/m2 dose of bendamustine as a bridge to allogeneic SCT in HL patients, 12 of 18 patients had a response, with six patients achieving a CR. The duration of response was only 2.6 months; however, many of these patients had multiple prior therapies, with the majority failing prior ASCT [59, 70]. Responses to bendamustine occurred quickly, with all responders identified by the second cycle. Treatment-related toxicities were largely hematologic and resulted in dose reductions or interruptions in seven patients. Given the relatively high response rate to this agent in a population of patients who had undergone multiple chemotherapies, combination studies designed to improve the duration of response and efficacy remain potential avenues of further investigation.
Everolimus
For a number of tumor types, mTOR signaling plays a key role in cell growth, protein translation, autophagy, and metabolism. Phosphatidylinositol-3-kinase (PI3K)–Akt signaling regulates mTOR through phosphorylation or inactivation of mTOR's negative regulator, tuberous sclerosis complex 2, thereby promoting mTOR activation. Upregulation of mTOR results in increased protein synthesis, translation, cell growth, ribosome biogenesis, metabolism, and proliferation and decreased autophagy [71]. PI3K and its targets, including the serine–threonine kinase Akt, are critical for B-cell survival and proliferation [59]. HRS cells contain active, phosphorylated Akt and display greater phosphorylation of known Akt target proteins. Inhibition of Akt in HL cell lines leads to apoptosis, suggesting that the PI3K–Akt–mTOR pathway has an essential role in the growth and survival of HRS cells [72].
Everolimus, an oral macrolide immunosuppressant, is one of several mTOR inhibitors currently being investigated as an anticancer agent in clinical trials [73]. In addition to inhibiting mTOR, everolimus inhibits vascular endothelial growth factor and HIF expression, resulting in antiangiogenic properties. A phase I trial of everolimus as a single agent in patients with relapsed or refractory hematologic malignancies showed that it was well tolerated [74], leading to a phase II study in 57 heavily pretreated HL patients [75]. The ORR was 35% with an additional 27% of patients achieving stable disease. A proportion of patients obtained durable responses lasting over one year and the median PFS was 7.2 months. The median time to response was 3.6 months; however, responses were seen after six cycles of therapy in four patients. Common grade 3 and grade 4 AEs included cytopenias such as thrombocytopenia (16%) neutropenia (8%) and anemia (8%) that were manageable with dose interruptions or reductions. In addition less common AEs such as dyspnea, pneumonits and hyperlipidemia were observed [75, 76]. In addition to single-agent activity, everolimus has shown synergy with a number of agents, including PI3K inhibitors, DACIs, bortezomib, and conventional chemotherapeutic agents. As a result, studies investigating everolimus in combination with these agents are currently ongoing or in preparation [59, 76–80].
Interestingly, in preclinical models, everolimus was shown to downregulate genes associated with glucose metabolism; various studies have shown that patients may develop hyperglycemia. This may be a result of reductions in (pro)-insulin secretion and downregulation of glucose transport into both tumor and normal cells [81]. This unique side effect was used advantageously in patients with insulinomas, in whom hypoglycemia is a concern [81, 82]. However, in lymphomas, for which PET imaging is heavily used, it is unclear what effect, if any, this phenomenon may have in assessing response. Given the uncertainty of PET findings when glucose metabolism is affected, it is notable that responses in current studies using everolimus have relied on CT imaging. This practice may underestimate the ORR of HL patients treated with everolimus because it is not uncommon to have metabolically inactive residual masses post-therapy. At the present time, it is unclear how best to use PET imaging in patients treated with everolimus, and it is important that future studies evaluate this question.
Proteasome Inhibitors: Bortezomib
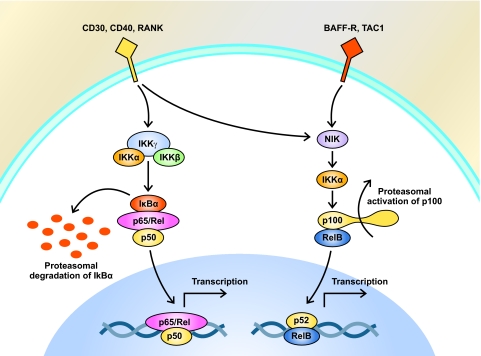
The NF-κB pathway is constitutively active in HRS cells (Fig. 2) and may play a critical role in gene upregulation, cell proliferation, and apoptotic evasion [83]. The proteasome inhibitor bortezomib inhibits proteasomal degradation of inhibitor of κB, thereby sequestering NF-κB in the cytoplasm and preventing its activity as a transcription factor. In addition, bortezomib, an inhibitor of NF-κB, has been demonstrated to have activity in HL cell lines [84] and has been evaluated in the clinical setting in patients with relapsed or refractory HL. In a pilot study of 14 patients at the MD Anderson Cancer Center, a maximum of six cycles of bortezomib (1.3 mg/m2) was administered on days 1, 4, 8, and 11 of a 21-day dosing schedule [85]. Despite promising preclinical findings, only one of the 14 patients had a PR. These findings were confirmed in a multi-institutional phase II trial performed by the Cancer and Leukemia Group B [86]. As a consequence, the investigators concluded that bortezomib administered using this schedule has no single-agent activity in patients with relapsed or refractory HL [86].
Figure 2.
Constitutive activation of the NF-κB pathway in HRS cells. Two distinct pathways of NF-κB activation have been identified in HRS cells. In the canonical pathway (left), stimulation of the B-cell receptor or TNF receptor superfamily members (such as CD30 or CD40) results in proteasomal degradation of IκB. The lack of IκB inhibition results in increased NF-κB nuclear transport and ultimately results in increased transcription of a number of target genes. In HL, these targets include a variety of proinflammatory cytokines and antiapoptotic factors. In the alternative pathway (right), ligand-mediated stimulation of a number of receptors, including BAFF-R, TAC1, CD30, CD40, and RANK, induces the proteasomal processing of the NF-κB precursor protein p100 into its active form, p52. This event permits heterodimers composed of p52 and another member of the NF-κB family, RelB, to move to the nucleus where they upregulate transcription of several target genes.
Abbreviations: cIAP2, cellular inhibitor of apoptosis 2; DACI, deacetylase inhibitor; FLIP, FLICE inhibitory protein; GPCR, G-protein-coupled receptor; HL, Hodgkin's lymphoma; HIF, hypoxia-inducible factor; HRS, Hodgkin Reed–Sternberg; IκB, inhibitor of NF-κB; IL, interleukin; IKK, IκB kinase; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor κB; PI3K, phosphatidylinositol-3-kinase; RANK, receptor activator of NF-κB; RANTES, regulated upon activation, normal T-cell expressed and presumably secreted; RTK, receptor tyrosine kinase; TAC1, transmembrane cyclophilin ligand interactor; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
A phase I combination of bortezomib with ifosfamide, carboplatin, and etoposide (ICE) was subsequently evaluated in 13 patients with relapsed or refractory HL [59, 87, 88]. ICE was given on days 1–3 and bortezomib was administered on day 1 and day 4 of a 2-week dosing regimen. This regimen was found to be well tolerated, with an ORR of 75% and a CR rate of 25% [88]. A randomized phase II study comparing bortezomib plus ICE (BICE) with ICE in the relapsed setting was then undertaken. To date, 12 patients have been enrolled, with reversible grade 4 neutropenia and thrombocytopenia occurring in both arms equally (50%). In total, 33% of BICE patients experienced grade 2 elevations in alanine aminotransferase or aspartate aminotransferase and only one patient had grade 1 neuropathy [89]. To date, there has been no clear clinical advantage identified by administering bortezomib as a single agent or in combination therapy, despite promising preclinical rationale and in vitro data. The reason for this is unclear; however, inherent mechanisms of in vivo resistance as well as an incomplete understanding of the role of the NF-κB pathway in HL may be factors contributing to the suboptimal results observed.
Summary and Conclusions
Despite the relative success in treating HL patients, a substantial proportion of patients ultimately relapse. In addition, although a proportion of patients are cured with salvage chemotherapy and ASCT, those who are not cured or who are ineligible for this procedure have extremely poor outcomes. Improvements in molecular biology and drug development have led to a number of promising drugs currently being evaluated for HL. Among these, SGN-35 and panobinostat have completed phase II trials and have been shown to produce substantial responses in high-risk, heavily pretreated populations. Despite the potential of these agents, it is important to note that SGN-35 is the only agent approved by the FDA for use in patients with relapsed or refractory HL. To date, there is no evidence to suggest that SGN-35 or any other of the discussed agents can be considered to be potentially curative when used alone. Therefore, it is vital that these agents continue to be evaluated and additional therapies be developed so that the momentum gained by these advances results in a sustainable benefit for future generations of HL patients.
References
- 1.Connors JM. State-of-the-art therapeutics: Hodgkin's lymphoma. J Clin Oncol. 2005;23:6400–6408. doi: 10.1200/JCO.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 2.Duggan DB, Petroni GR, Johnson JL, et al. Randomized comparison of ABVD and MOPP/ABV hybrid for the treatment of advanced Hodgkin's disease: Report of an intergroup trial. J Clin Oncol. 2003;21:607–614. doi: 10.1200/JCO.2003.12.086. [DOI] [PubMed] [Google Scholar]
- 3.Johnson PW, Radford JA, Cullen MH, et al. Comparison of ABVD and alternating or hybrid multidrug regimens for the treatment of advanced Hodgkin's lymphoma: Results of the United Kingdom Lymphoma Group LY09 Trial (ISRCTN97144519) J Clin Oncol. 2005;23:9208–9218. doi: 10.1200/JCO.2005.03.2151. [DOI] [PubMed] [Google Scholar]
- 4.Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin's disease. N Engl J Med. 1998;339:1506–1514. doi: 10.1056/NEJM199811193392104. [DOI] [PubMed] [Google Scholar]
- 5.Kuruvilla J. Standard therapy of advanced Hodgkin lymphoma. Hematology Am Soc Hematol Educ Program. 2009:497–506. doi: 10.1182/asheducation-2009.1.497. [DOI] [PubMed] [Google Scholar]
- 6.Oza AM, Ganesan TS, Leahy M, et al. Patterns of survival in patients with Hodgkin's disease: Long follow up in a single centre. Ann Oncol. 1993;4:385–392. doi: 10.1093/oxfordjournals.annonc.a058517. [DOI] [PubMed] [Google Scholar]
- 7.Quddus F, Armitage JO. Salvage therapy for Hodgkin's lymphoma. Cancer J. 2009;15:161–163. doi: 10.1097/PPO.0b013e3181a1438a. [DOI] [PubMed] [Google Scholar]
- 8.Marafioti T, Hummel M, Foss HD, et al. Hodgkin and Reed-Sternberg cells represent an expansion of a single clone originating from a germinal center B-cell with functional immunoglobulin gene rearrangements but defective immunoglobulin transcription. Blood. 2000;95:1443–1450. [PubMed] [Google Scholar]
- 9.Federico M, Luminari S, Iannitto E, et al. ABVD compared with BEACOPP compared with CEC for the initial treatment of patients with advanced Hodgkin's lymphoma: Results from the HD2000 Gruppo Italiano per lo Studio dei Linfomi Trial. J Clin Oncol. 2009;27:805–811. doi: 10.1200/JCO.2008.17.0910. [DOI] [PubMed] [Google Scholar]
- 10.Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin's disease: A randomised trial. Lancet. 2002;359:2065–2071. doi: 10.1016/S0140-6736(02)08938-9. [DOI] [PubMed] [Google Scholar]
- 11.Martin A, Fernàndez-Jiménez MC, Caballero MD, et al. Long-term follow-up in patients treated with mini-BEAM as salvage therapy for relapsed or refractory Hodgkin's disease. Br J Haematol. 2001;113:161–171. doi: 10.1046/j.1365-2141.2001.02714.x. [DOI] [PubMed] [Google Scholar]
- 12.Younes A, Bartlett NL, Leonard JP, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363:1812–1821. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 13.Salit RB, Bishop MR, Pavletic SZ. Allogeneic hematopoietic stem cell transplantation: Does it have a place in treating Hodgkin lymphoma? Curr Hematol Malig Rep. 2010;5:229–238. doi: 10.1007/s11899-010-0065-7. [DOI] [PubMed] [Google Scholar]
- 14.Varterasian M, Ratanatharathorn V, Uberti JP, et al. Clinical course and outcome of patients with Hodgkin's disease who progress after autologous transplantation. Leuk Lymphoma. 1995;20:59–65. doi: 10.3109/10428199509054754. [DOI] [PubMed] [Google Scholar]
- 15.Peggs KS, Anderlini P, Sureda A. Allogeneic transplantation for Hodgkin lymphoma. Br J Haematol. 2008;143:468–480. doi: 10.1111/j.1365-2141.2008.07349.x. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez I, Sureda A, Caballero MD, et al. Nonmyeloablative stem cell transplantation is an effective therapy for refractory or relapsed Hodgkin lymphoma: Results of a Spanish prospective cooperative protocol. Biol Blood Marrow Transplant. 2006;12:172–183. doi: 10.1016/j.bbmt.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Corradini P, Sarina B, Farina L. Allogeneic transplantation for Hodgkin's lymphoma. Br J Haematol. 2011;152:261–272. doi: 10.1111/j.1365-2141.2010.08492.x. [DOI] [PubMed] [Google Scholar]
- 18.Sarina B, Castagna L, Farina L, et al. Allogeneic transplantation improves the overall and progression-free survival of Hodgkin lymphoma patients relapsing after autologous transplantation: A retrospective study based on the time of HLA typing and donor availability. Blood. 2010;115:3671–3677. doi: 10.1182/blood-2009-12-253856. [DOI] [PubMed] [Google Scholar]
- 19.Sureda A, Robinson S, Canals C, et al. Reduced-intensity conditioning compared with conventional allogeneic stem-cell transplantation in relapsed or refractory Hodgkin's lymphoma: An analysis from the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2008;26:455–462. doi: 10.1200/JCO.2007.13.2415. [DOI] [PubMed] [Google Scholar]
- 20.Bierman PJ, Anderson JR, Freeman MB, et al. High-dose chemotherapy followed by autologous hematopoietic rescue for Hodgkin's disease patients following first relapse after chemotherapy. Ann Oncol. 1996;7:151–156. doi: 10.1093/oxfordjournals.annonc.a010542. [DOI] [PubMed] [Google Scholar]
- 21.Foyil KV, Bartlett NL. Anti-CD30 antibodies for Hodgkin lymphoma. Curr Hematol Malig Rep. 2010;5:140–147. doi: 10.1007/s11899-010-0053-y. [DOI] [PubMed] [Google Scholar]
- 22.Bartlett NL, Younes A, Carabasi MH, et al. A phase 1 multidose study of SGN-30 immunotherapy in patients with refractory or recurrent CD30+ hematologic malignancies. Blood. 2008;111:1848–1854. doi: 10.1182/blood-2008-01-127118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forero-Torres A, Leonard JP, Younes A, et al. A phase II study of SGN-30 (anti-CD30 mAb) in Hodgkin lymphoma or systemic anaplastic large cell lymphoma. Br J Haematol. 2009;146:171–179. doi: 10.1111/j.1365-2141.2009.07740.x. [DOI] [PubMed] [Google Scholar]
- 24.Ansell SM, Horwitz SM, Engert A, et al. Phase I/II study of an anti-CD30 monoclonal antibody (MDX-060) in Hodgkin's lymphoma and anaplastic large-cell lymphoma. J Clin Oncol. 2007;25:2764–2769. doi: 10.1200/JCO.2006.07.8972. [DOI] [PubMed] [Google Scholar]
- 25.Younes A, Forero-Torres A, Bartlett NL, et al. Multiple complete responses in a phase 1 dose-escalation study of the antibody-drug conjugate SGN-35 in patients with relapsed or refractory CD30-positive lymphomas. Blood. 2008;112 Abstract 1006. [Google Scholar]
- 26.Chen R, Gopal AK, Smith SE, et al. Results of a pivotal phase 2 study of brentuximab vedotin (SGN-35) in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2010;116 Abstract 283. [Google Scholar]
- 27.ClinicalTrials.gov. A Phase 3 Study of Brentuximab Vedotin (SGN-35) in Patients at High Risk of Residual Hodgkin Lymphoma Following Stem Cell Transplant (The AETHERA Trial) [accessed October, 2011]. Available at http://www.clinicaltrials.gov/ct2/show/NCT01100502.
- 28.Moskowitz CH, Nimer SD, Zelenetz AD, et al. A 2-step comprehensive high-dose chemoradiotherapy second-line program for relapsed and refractory Hodgkin disease: Analysis by intent to treat and development of a prognostic model. Blood. 2001;97:616–623. doi: 10.1182/blood.v97.3.616. [DOI] [PubMed] [Google Scholar]
- 29.Engert A, Diehl V, Franklin J, et al. Escalated-dose BEACOPP in the treatment of patients with advanced-stage Hodgkin's lymphoma: 10 years of follow-up of the GHSG HD9 study. J Clin Oncol. 2009;27:4548–4554. doi: 10.1200/JCO.2008.19.8820. [DOI] [PubMed] [Google Scholar]
- 30.Santoro A, Magagnoli M, Spina M, et al. Ifosfamide, gemcitabine, and vinorelbine: A new induction regimen for refractory and relapsed Hodgkin's lymphoma. Haematologica. 2007;92:35–41. doi: 10.3324/haematol.10661. [DOI] [PubMed] [Google Scholar]
- 31.ClinicalTrials.gov. A Phase 1 Study of Brentuximab Vedotin Combined With Multi-Agent Chemotherapy for Hodgkin Lymphoma. [accessed October, 2011]. Available at http://www.clinicaltrials.gov/ct2/show/NCT01060904.
- 32.ClinicalTrials.gov. Clinical Pharmacology Study of Brentuximab Vedotin (SGN-35) [accessed October, 2011]. Available at http://www.clinicaltrials.gov/ct2/show/NCT01026415.
- 33.ClinicalTrials.gov. An SGN-35 Trial for Patients who Have Previously Participated in an SGN-35 Study. [accessed October, 2011]. Available at http://www.clinicaltrials.gov/ct2/show/NCT00947856.
- 34.Younes A, Romaguera J, Hagemeister F, et al. A pilot study of rituximab in patients with recurrent, classic Hodgkin disease. Cancer. 2003;98:310–314. doi: 10.1002/cncr.11511. [DOI] [PubMed] [Google Scholar]
- 35.Jones RJ, Gocke CD, Kasamon YL, et al. Circulating clonotypic B cells in classic Hodgkin lymphoma. Blood. 2009;113:5920–5926. doi: 10.1182/blood-2008-11-189688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Younes A, Fayad LY, Goy A, et al. Results of rituximab plus ABVD in 65 newly diagnosed patients with classical Hodgkin lymphoma: Improvement of event free survival (EFS) in all International Prognostic Score (IPS) groups [abstract 2742] Blood. 2006;108:2742. [Google Scholar]
- 37.Wedgwood AR, Fanale MA, Fayad LE, et al. Rituximab + ABVD improves event-free survival (EFS) in patients with classical Hodgkin lymphoma in all International Prognostic Score (IPS) groups and in patients who have PET positive disease after 2–3 cycles of therapy [a] Blood. 2007;110:215. [Google Scholar]
- 38.Copeland AR, Cao Y, Fanale M, et al. Final report of a phase-II study of rituximab plus ABVD for patients with newly diagnosed advanced stage classical Hodgkin lymphoma: Results of long follow up and comparison to institutional historical data. Blood. 2009;114 Abstract 1680. [Google Scholar]
- 39.ClinicalTrials.gov. R-ABVD vs ABVD-RT in Early Stage Hodgkin's Lymphoma. [accessed October, 2011]. Available at http://www.clinicaltrials.gov/ct2/show/NCT00992030.
- 40.ClinicalTrials.gov. HD18 for Advanced Stages in Hodgkins Lymphoma. [accessed October, 2011]. Available at http://www.clinicaltrials.gov/ct2/show/NCT00515554.
- 41.ClinicalTrials.gov. Study Comparing R-mabHD and a Combination of ABVD in Hodgkin's Disease. [accessed October, 2011]. Available at http://www.clinicaltrials.gov/ct2/show/NCT00797472.
- 42.ClinicalTrials.gov. Rituximab and Combination Chemotherapy in Treating Patients With Newly Diagnosed Stage II, Stage III, or Stage IV Hodgkin's Lymphoma. [accessed October, 2011]. Available at http://www.clinicaltrials.gov/ct2/show/NCT00369681.
- 43.ClinicalTrials.gov. Phase II R-ABVD Versus ABVD for Advanced Stage Classical Hodgkin Lymphoma. [accessed October, 2011]. Available at http://www.clinicaltrials.gov/ct2/show/NCT00654732.
- 44.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 45.Cotto M, Cabanillas F, Tirado M, et al. Epigenetic therapy of lymphoma using histone deacetylase inhibitors. Clin Transl Oncol. 2010;12:401–409. doi: 10.1007/s12094-010-0527-3. [DOI] [PubMed] [Google Scholar]
- 46.Lane AA, Chabner BA. Histone deacetylase inhibitors in cancer therapy. J Clin Oncol. 2009;27:5459–5468. doi: 10.1200/JCO.2009.22.1291. [DOI] [PubMed] [Google Scholar]
- 47.Younes A. Novel treatment strategies for patients with relapsed classical Hodgkin lymphoma. Hematology Am Soc Hematol Educ Program. 2009:507–519. doi: 10.1182/asheducation-2009.1.507. [DOI] [PubMed] [Google Scholar]
- 48.Ushmorov A, Leithauser F, Sakk O, et al. Epigenetic processes play a major role in B-cell-specific gene silencing in classical Hodgkin lymphoma. Blood. 2006;107:2493–2500. doi: 10.1182/blood-2005-09-3765. [DOI] [PubMed] [Google Scholar]
- 49.Georgakis GV, Yazbeck VY, Li Y, et al. The histone deacetylase inhibitor vorinostat (SAHA) induces apoptosis and cell cycle arrest in Hodgkin lymphoma (HL) cell lines by altering several survival signaling pathways and synergizes with doxorubicin, gemcitabine and bortezomib. Blood. 2006;108 Abstract 2260. [Google Scholar]
- 50.Kirschbaum MH, Goldman BH, Zain JM, et al. Vorinostat (suberoylanilide hydroxamic acid) in relapsed or refractory Hodgkin lymphoma: SWOG 0517. Blood. 2007;110 Abstract 2574. [Google Scholar]
- 51.Smith JL, Patel A, Fan S, et al. Histone deacetylase inhibition using LBH589 is effective in lymphoma and results in down-regulation of the NF-KB pathway. Blood. 2009;114 Abstract 3730. [Google Scholar]
- 52.Ottmann OG, Spencer A, Prince HM, et al. Phase IA/II study of oral panobinostat (LBH589), a novel pan- deacetylase inhibitor (DACi) demonstrating efficacy in patients with advanced hematologic malignancies. Blood. 2008;112 Abstract 958. [Google Scholar]
- 53.Sureda A, Younes A, Ben-Yehuda D, et al. Final analysis: Phase II study of oral panobinostat in relapsed/refractory Hodgkin lymphoma patients following autologous hematopoietic stem cell transplant. Blood. 2010;116 Abstract 419. [Google Scholar]
- 54.Sureda A, Engert A, Browett PJ, et al. Interim results for the phase II study of panobinostat (LBH589) in patients (pts) with relapsed/refractory Hodgkin's lymphoma (HL) after autologous hematopoietic stem cell transplant (AHSCT) J Clin Oncol. 2010;28(15 suppl) Abstract 8007. [Google Scholar]
- 55.ClinicalTrials.gov. A Phase III Randomized, Double Blind, Placebo Controlled Multi-Center Study of Panobinostat for Maintenance of Response in Patients With Hodgkin's Lymphoma. [accessed October, 2011]. Available at http://www.clinicaltrials.gov/ct2/show/NCT01034163.
- 56.ClinicalTrials.gov. Panobinostat Plus Ifosfamide, Carboplatin, and Etoposide (ICE) Compared With ICE for Relapsed Hodgkin Lymphoma. [accessed October, 2011]. Available at http://www.clinicaltrials.gov/ct2/show/NCT01169636.
- 57.Younes A, Hernandez F, Bociek RG, et al. ENGAGE-501: Phase 2 study investigating the role of epigenetic therapy with entinostat (SNDX-275) in relapsed and refractory Hodgkin's lymphoma (HL), interim results. Blood. 2010;116 Abstract 3959. [Google Scholar]
- 58.Younes A, Bociek RG, Kuruvilla J, et al. Mocetinostat (MGCD0103), an isotype-selective histone deacetylase (HDAC) inhibitor, produces clinical responses in relapsed/refractory Hodgkin lymphoma (HL): Update from a phase II clinical study. Blood. 2010;116 Abstract 1763. [Google Scholar]
- 59.Blum KA. Upcoming diagnostic and therapeutic developments in classical Hodgkin's lymphoma. Hematology Am Soc Hematol Educ Program. 2010;2010:93–100. doi: 10.1182/asheducation-2010.1.93. [DOI] [PubMed] [Google Scholar]
- 60.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 61.Younes A, Copeland A, Fanale MA, et al. Phase I/II study of the novel combination of panobinostat (LBH589) and everolimus (RAD001) in relapsed/refractory Hodgkin and non-Hodgkin lymphoma. Blood. 2010;116 Abstract 3964. [Google Scholar]
- 62.Hernandez-Ilizaliturri FJ, Mavis C, Maraj I, et al. Panobinostat, a novel histone deacetylase (HiDAC) inhibitor enhances the anti-tumor activity of bortezomib (BTZ) in rituximab-chemotherapy sensitive and resistant lymphoma cell lines. Blood. 2010;116 Abstract 3936. [Google Scholar]
- 63.Lemoine M, Buglio D, Jona A, et al. The pan-deacetylase inhibitor panobinostat downregulates HIF-1α and VEGF and, synergizes with everolimus in Hodgkin lymphoma cell lines. Blood. 2010;116 doi: 10.1182/blood-2011-01-331421. Abstract 2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bartlett JB, Michael A, Clarke IA, et al. Phase I study to determine the safety, tolerability and immunostimulatory activity of thalidomide analogue CC-5013 in patients with metastatic malignant melanoma and other advanced cancers. Br J Cancer. 2004;90:955–961. doi: 10.1038/sj.bjc.6601579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Böll B, Borchmann P, Topp MS, et al. Lenalidomide in patients with refractory or multiple relapsed Hodgkin lymphoma. Br J Haematol. 2010;148:480–482. doi: 10.1111/j.1365-2141.2009.07963.x. [DOI] [PubMed] [Google Scholar]
- 66.Fehniger TA, Larson S, Trinkaus K, et al. A phase II multicenter study of lenalidomide in relapsed or refractory classical Hodgkin lymphoma. Blood. 2009;114 doi: 10.1182/blood-2011-07-362475. Abstract 3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kuruvilla J, Taylor D, Wang L, et al. Phase II trial of lenalidomide in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2008;112 Abstract 3052. [Google Scholar]
- 68.Böll B, Fuchs M, Reiners KS, et al. Lenalidomide in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2010;116 doi: 10.1111/j.1365-2141.2009.07963.x. Abstract 2828. [DOI] [PubMed] [Google Scholar]
- 69.Leoni LM, Bailey B, Reifert J, et al. Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents. Clin Cancer Res. 2008;14:309–317. doi: 10.1158/1078-0432.CCR-07-1061. [DOI] [PubMed] [Google Scholar]
- 70.Moskowitz AJ, Hamlin PA, Jr, Gerecitano J, et al. Bendamustine is highly active in heavily pre-treated relapsed and refractory Hodgkin lymphoma and serves as a bridge to allogeneic stem cell transplant. Blood. 2009;114 Abstract 720. [Google Scholar]
- 71.Meric-Bernstam F, Gonzalez-Angulo AM. Targeting the mTOR signaling network for cancer therapy. J Clin Oncol. 2009;27:2278–2287. doi: 10.1200/JCO.2008.20.0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schmitz R, Stanelle J, Hansmann ML, et al. Pathogenesis of classical and lymphocyte-predominant Hodgkin lymphoma. Annu Rev Pathol. 2009;4:151–174. doi: 10.1146/annurev.pathol.4.110807.092209. [DOI] [PubMed] [Google Scholar]
- 73.Vignot S, Faivre S, Aguirre D, et al. mTOR-targeted therapy of cancer with rapamycin derivatives. Ann Oncol. 2005;16:525–537. doi: 10.1093/annonc/mdi113. [DOI] [PubMed] [Google Scholar]
- 74.Yee KW, Zeng Z, Konopleva M, et al. Phase I/II study of the mammalian target of rapamycin inhibitor everolimus (RAD001) in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res. 2006;12:5165–5173. doi: 10.1158/1078-0432.CCR-06-0764. [DOI] [PubMed] [Google Scholar]
- 75.Johnston PB, Inwards DJ, Colgan JP, et al. A phase II trial of the oral mTOR inhibitor everolimus in relapsed Hodgkin lymphoma. Am J Hematol. 2010;85:320–324. doi: 10.1002/ajh.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.ClinicalTrials.gov. Study of RAD001 in Patients With Relapsed/Refractory Hodgkin Lymphoma That Has Progressed After High-Dose Chemotherapy and Autologous Stem Cell Transplant and/or After Gemcitabine- or Vinorelbine- or Vinblastine-Based Treatment. [accessed October, 2011]. Available at http://www.clinicaltrials.gov/ct2/show/NCT01022996.
- 77.von Tresckow B, Borchmann P, Fuchs M, et al. Current strategies in refractory or relapsed Hodgkin lymphoma. Haematologica. 2010;95 Abstract S31. [Google Scholar]
- 78.ClinicalTrials.gov. Panobinostat and Everolimus in Treating Patients With Relapsed or Refractory Lymphoma or Multiple Myeloma. [accessed October, 2011]. Available at http://www.clinicaltrials.gov/ct2/show/NCT00962507.
- 79.ClinicalTrials.gov. Everolimus and Lenalidomide in Treating Patients With Relapsed or Refractory Non-Hodgkin or Hodgkin Lymphoma. [accessed October, 2011]. Available at http://www.clinicaltrials.gov/ct2/show/NCT01075321.
- 80.Sweetenham J. Analyzing the use of CHOP-rituximab vs. dose-adjusted EPOCH-rituximab in the treatment of clinical stage IVB diffuse large B-cell lymphoma (DLBCL) ASH. 2011 Abstract 2717. [Google Scholar]
- 81.Fiebrich HB, Siemerink EJ, Brouwers AH, et al. Everolimus induces rapid plasma glucose normalization in insulinoma patients by effects on tumor as well as normal tissues. The Oncologist. 2011;16:783–787. doi: 10.1634/theoncologist.2010-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kulke MH, Bergsland EK, Yao JC. Glycemic control in patients with insulinoma treated with everolimus. N Engl J Med. 2009;360:195–197. doi: 10.1056/NEJMc0806740. [DOI] [PubMed] [Google Scholar]
- 83.Ghosh S, May MJ, Kopp EB. NF-κB and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 84.Georgakis GV, Li Y, Humphreys R, et al. Activity of selective fully human agonistic antibodies to the TRAIL death receptors TRAIL-R1 and TRAIL-R2 in primary and cultured lymphoma cells: Induction of apoptosis and enhancement of doxorubicin- and bortezomib-induced cell death. Br J Haematol. 2005;130:501–510. doi: 10.1111/j.1365-2141.2005.05656.x. [DOI] [PubMed] [Google Scholar]
- 85.Younes A, Pro B, Fayad L. Experience with bortezomib for the treatment of patients with relapsed classical Hodgkin lymphoma. Blood. 2006;107:1731–1732. doi: 10.1182/blood-2005-09-3731. [DOI] [PubMed] [Google Scholar]
- 86.Blum KA, Johnson JL, Niedzwiecki D, et al. Single agent bortezomib in the treatment of relapsed and refractory Hodgkin lymphoma: Cancer and Leukemia Group B protocol 50206. Leuk Lymphoma. 2007;48:1313–1319. doi: 10.1080/10428190701411458. [DOI] [PubMed] [Google Scholar]
- 87.Fanale MA, Fayad L, Pro B, et al. Safety and efficacy of bortezomib plus ICE (BICE) for the treatment of relapsed/refractory classical Hodgkin's lymphoma. Blood. 2007;110 Abstract 4506. [Google Scholar]
- 88.Fanale MA, Fayad LE, Pro B, et al. A phase I study of bortezomib in combination with ICE (BICE) in patients with relapsed/refractory classical Hodgkin lymphoma. Blood. 2008;112 Abstract 3048. [Google Scholar]
- 89.Fanale M, Fayad L, Kwak LW, et al. A randomized phase II study of bortezomib plus ICE (BICE) versus ICE for patients with relapsed or refractory Hodgkin lymphoma. Haematologica. 2010;95 Abstract S29. [Google Scholar]
- 90.Bartlett NL, Niedzwiecki D, Johnson JL, et al. Gemcitabine, vinorelbine, and pegylated liposomal doxorubicin (GVD), a salvage regimen in relapsed Hodgkin's lymphoma: CALGB 59804. Ann Oncol. 2007;18:1071–1079. doi: 10.1093/annonc/mdm090. [DOI] [PubMed] [Google Scholar]
- 91.Baetz T, Belch A, Couban S, et al. Gemcitabine, dexamethasone and cisplatin is an active and non-toxic chemotherapy regimen in relapsed or refractory Hodgkin's disease: A phase II study by the National Cancer Institute of Canada Clinical Trials Group. Ann Oncol. 2003;14:1762–1767. doi: 10.1093/annonc/mdg496. [DOI] [PubMed] [Google Scholar]
- 92.Stokoe CT, Ogden J, Jain VK. Activity of infusional etoposide, vincristine, and doxorubicin with bolus cyclophosphamide (EPOCH) in relapsed Hodgkin's disease. The Oncologist. 2001;6:428–434. doi: 10.1634/theoncologist.6-5-428. [DOI] [PubMed] [Google Scholar]
- 93.Aparicio J, Segura A, Garcerà S, et al. ESHAP is an active regimen for relapsing Hodgkin's disease. Ann Oncol. 1999;10:593–595. doi: 10.1023/a:1026454831340. [DOI] [PubMed] [Google Scholar]
- 94.Josting A, Rudolph C, Reiser M, et al. Time-intensified dexamethasone/cisplatin/cytarabine: An effective salvage therapy with low toxicity in patients with relapsed and refractory Hodgkin's disease. Ann Oncol. 2002;13:1628–1635. doi: 10.1093/annonc/mdf221. [DOI] [PubMed] [Google Scholar]
- 95.Ferme C, Bastion Y, Lepage E, et al. The MINE regimen as intensive salvage chemotherapy for relapsed and refractory Hodgkin's disease. Ann Oncol. 1995;6:543–549. doi: 10.1093/oxfordjournals.annonc.a059242. [DOI] [PubMed] [Google Scholar]