Brainstem gliomas are uncommon in adults and account for only 1%–2% of intracranial gliomas. In adults, a low-grade phenotype predominates, which is a feature that likely explains their better prognosis compared to that in children. A better understanding of the biology of these tumors is of primary importance for identifying homogeneous subgroups and for improving therapy options and outcomes.
Keywords: Brainstem glioma, Classification, MRI, Treatment, Adults
Learning Objectives
After completing this course, the reader will be able to:
Identify the different types of brainstem glioma in adults and their radiological features.
Select the most accurate diagnostic test and propose options for treatment in patients suffering from brainstem gliomas.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Brainstem gliomas are uncommon in adults and account for only 1%–2% of intracranial gliomas. They represent a heterogeneous group of tumors that differ from those found in their pediatric counterparts. In adults, a low-grade phenotype predominates, which is a feature that likely explains their better prognosis compared to that in children. Because biopsies are rarely performed, classifications based on the radiological aspect of magnetic resonance imaging results have been proposed to establish treatment strategies and to determine outcomes: (a) diffuse intrinsic low-grade, (b) enhancing malignant glioma, (c) focal tectal gliomas, and (d) exophytic gliomas. Despite significant advances in neuroradiology techniques, a purely radiological classification remains imperfect in the absence of a histological diagnosis. Whereas a biopsy may often be reasonably avoided in the diffuse nonenhancing forms, obtaining histological proof seems necessary in many contrast-enhanced brainstem lesions because of the wide variety of differential diagnoses in adults. Conventional radiotherapy is the standard treatment for diffuse intrinsic low-grade brainstem gliomas in adults (the median survival is 5 years). In malignant brainstem gliomas, radiotherapy is the standard treatment. However, the possible benefit of combined radiotherapy and chemotherapy (temozolomide or other agents) has not been thoroughly evaluated in adults. The role of anti-angiogenic therapies in brainstem gliomas remains to be defined. A better understanding of the biology of these tumors is of primary importance for identifying homogeneous subgroups and for improving therapy options and outcomes.
Introduction
Brainstem tumors are defined as lesions occurring in the midbrain, the pons, or the medulla oblongata. This definition excludes patients with tumors originating in the thalamus and hypothalamus or lesions originating in the cerebellum, cerebellar peduncles, or upper cervical spinal cord. The tumor's extension is considered focal when it occupies <50% of the axial brainstem diameter, and the extension is considered diffuse when the lesion is poorly demarcated and is >50% of the brainstem diameter [1–3]. Brainstem glioma is the most frequent tumor of the region. A clear bimodal age distribution supports the distinction between brainstem gliomas in children and adults. In contrast with the pediatric population in which brainstem gliomas represent up to 20% of brain tumors and exhibit a rather homogeneous and unfavorable course, adult brainstem gliomas are rare (accounting for only 1%–2% of adult brain gliomas) and heterogeneous with varying radiological patterns and variable prognoses [4, 5]. In this review, we review the literature on adult brainstem gliomas, while acknowledging the problems that we encountered in extracting comparable data from the many reports because of the heterogeneity of the terms used and the difficulties of evaluating treatments and courses in individual patients. Because biopsies are too rare to allow for an analysis based on histological criteria alone, we used an MRI (magnetic resonance imaging)-based radiological classification that considered the following four major groups: (a) diffuse intrinsic or diffusely infiltrative low-grade brainstem glioma, (b) enhancing focal malignant brainstem glioma, (c) tectal gliomas, and (d) exophytic gliomas [4].
Adult Diffuse Intrinsic Low-Grade Brainstem Glioma
Definition
This type of tumor is defined radiologically as a poorly demarcated and nonenhancing lesion affecting >50% of the brainstem diameter [1–3]. Commonly, adult diffuse intrinsic brainstem gliomas are low-grade tumors at the onset in contrast with those in children where a similar nonenhancing MRI aspect often indicates a high-grade glioma associated with rapidly progressive clinical impairment and shorter survival. The reason for this striking pathoradiological difference between adults and children at the onset is still unknown [6, 7]. Moreover, the delayed development of contrast-enhancing lesions on MRI in adults suggests a late evolution toward a higher grade tumor (reported in 27% of patients in one study) [4]. At present, it is still uncertain if this presumed anaplastic transformation is the natural long-term evolution for all diffuse intrinsic brainstem gliomas.
Epidemiology
Diffuse intrinsic low-grade brainstem gliomas usually occur in young adults between the ages of 20 and 50 years (median age of 34 years at diagnosis) and represent the most frequent type of brainstem glioma in adults, accounting for 45%–50% of cases [4, 5, 8, 9].
Pathology
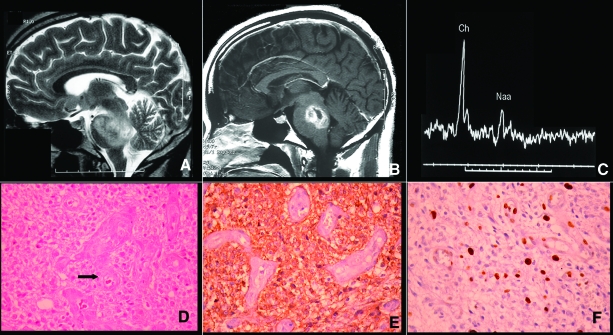
A biopsy is rarely performed in typical intrinsic diffuse glioma in adults. When it is performed, a low-grade histology (grade II glioma) is found in up to 80% of cases, whereas in children a grade IV glioma is the most frequent phenotype reported in 50%–60% of cases (Fig. 1) [8–10]. This feature probably accounts for the better prognosis of the adult form [4, 5, 8, 11]. However, periodically adult patients present a rapidly growing tumor similar to the diffuse intrinsic brainstem gliomas found in the pediatric population [4, 12].
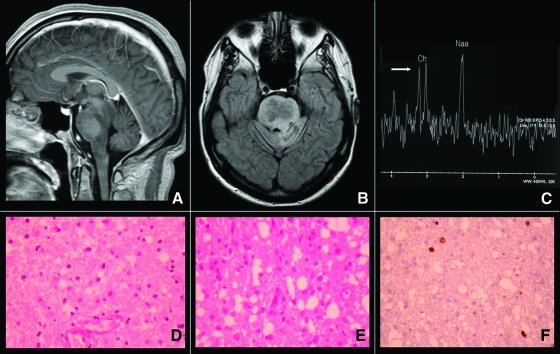
Figure 1.
Low-grade oligoastrocytoma in a 19-year-old man presenting a progressive cerebellar syndrome and dysphagia. Upper panel: (A, B): Evocative radiologic pattern in magnetic resonance showing a diffuse intrinsic brainstem hypointense lesion without contrast enhancement as seen in T1 sequences and T2-Fluid Attenuation Inversion Recovery (FLAIR). (C): Magnetic resonance spectroscopy showing only a mild increase of the choline/N-acetyl aspartate ratio without lipids/lactates peaks modification (white arrow). Lower panel: (D, E): Histologic sample obtained after biopsy showing a low-grade glioma with an astrocytic component and oligodendroglial differentiation. (F): Immunohistochemistry showing a low MIB-1 proliferation index.
The low frequency of biopsies and the subsequent paucity of tissues obtained for examination are the reasons that the distinct molecular biological patterns of diffuse brainstem gliomas remain almost unknown. A prospective multicenter study in children, which aims to analyze tissue obtained at autopsies of patients with brainstem gliomas, has recently been proposed [13]. It is hoped that a better knowledge of the profile of molecular alterations in the tumor tissue will help to classify and predict tumor behavior as previously shown in supratentorial gliomas.
Clinical Features
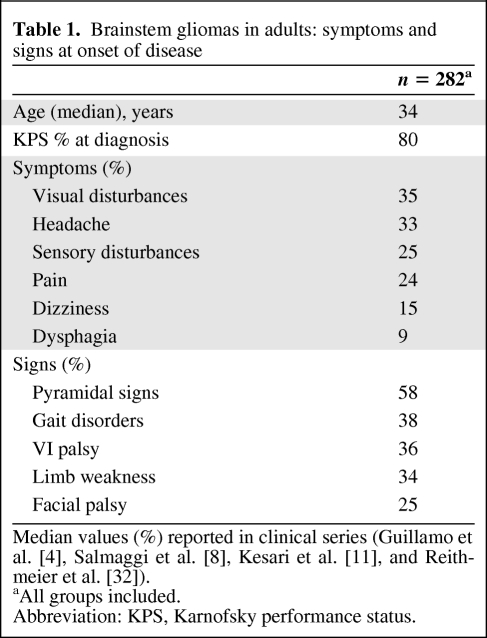
Table 1 illustrates the main presenting symptoms in four large clinical series. Usually, brainstem gliomas in adults are discovered in patients with a Karnofsky performance status >80% at the time of diagnosis. The clinical symptoms and signs are closely linked to the localization of the tumor. Visual disturbances, limb weakness, and gait disorders are the main symptoms in the majority of cases [4, 8, 11]. Typically, a combination of cranial nerve dysfunction and long-tract signs is considered suggestive of a brainstem lesion. Patients may present with a long history of facial palsy sometimes associated with facial myokymia or hemifacial spasm [14]. Pyramidal weakness, cerebellar signs, or involvement of other cranial nerves (V, VI, VIII, palatal palsy) have also been commonly found during medical examinations.
Table 1.
Brainstem gliomas in adults: symptoms and signs at onset of disease
Rare presentations include snoring associated with Ondine's curse in the case of medulla oblongata gliomas [15], central neurogenic hyperventilation [16], tongue tremors [17], hemiparkinsonism [18], visual migraine auras [19], and pseudomyasthenic syndrome [20]. Other clinical manifestations, such as headaches and raised intracranial pressure, may be present at the onset when the tumor obstructs cerebrospinal fluid drainage, causing the hydrocephalus reported in 23% of patients [4].
Imaging
Currently, a brain MRI is the most useful radiological tool. It usually reveals a diffuse enlargement of the brainstem with hyperintense areas on T2/Fluid Attentuation Inversion Recovery (FLAIR) weighted sequences and hypointense signals on T1-weighted images. These tumors are diffusely infiltrative and do not enhance after the administration of gadolinium [21]. In some cases, pontine lesions present a ventral exophytic growth surrounding the basilar artery (Fig. 2). Lesions may be limited to a brainstem segment (midbrain, pons, or medulla oblongata), or they may infiltrate other brainstem segments or adjacent regions, such as the cerebellum or thalamus. In one case series, the pons was affected in 64% of patients, followed by the medulla in 51% and the midbrain in 43% [4]. As a group, diffuse intrinsic brainstem gliomas involve the ponto-medullary structures in 80% of cases [11].
Figure 2.
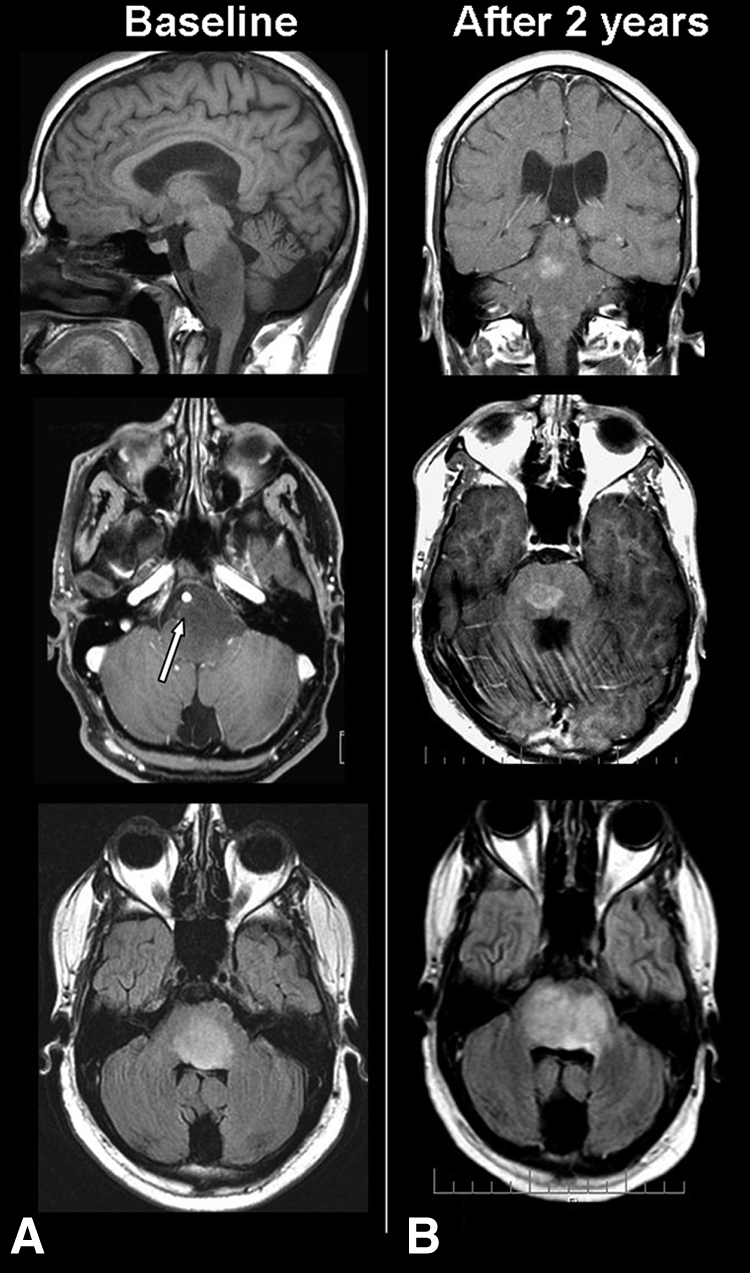
Diffuse intrinsic “low-grade” brainstem glioma (without biopsy) in a 24-year-old man suffering from facial palsy, dysarthia, and gait imbalance. Left panel (A): Magnetic resonance imaging showing a diffuse nonenhancing lesion in T1 sequences (top and middle) and hyperintensity in T2-Fluid Attenuation Inversion Recovery (FLAIR) sequences. Note the ventral extension of the lesion surrounding the basilar artery (white arrow). Right panel (B): Follow-up MRI showing multiple nodular enhancing brainstem lesions in T1 (top, middle) and increased T2-FLAIR hyperintensity (bottom).
Magnetic resonance spectroscopy (MRS) is a complementary tool used in the differential diagnosis of a brainstem lesion [10, 21, 22]. However, compared to its application in supratentorial lesions, the use of MRS is more limited in the brainstem because of the technical difficulties related to the small size of the anatomical structures and the proximity of bone and fatty tissue of the skull base. Currently, single-voxel MRS is the technique used to study diffuse brainstem lesions, particularly in pontine lesions greater than 2 cm. More recently, the application of the 3T multivoxel MRS has improved the spatial resolution of this technique when acquisition times of 12–15 minutes are used [21]. The role of MRS is still limited in identifying lesions in the medulla oblongata.
In one retrospective study, nine adult patients suffering from brainstem gliomas (histologically confirmed in four cases) were evaluated with single-voxel MRS before treatment [8]. All patients showed an elevation in the choline/N-acetyl aspartate ratio (Cho/NAA ratio) from 1.08 to 3.32 (normal is 0.6–0.8), an elevation in the Cho/creatine (Cr) ratio (range 1.89–1.01) in eight patients, and an elevation in the lactate signal in three patients. The creatine/NAA ratio was abnormal in all patients because of the loss of the NAA signal. Thus, the changes observed using MRS appear similar to those observed in supratentorial gliomas.
In the pediatric population, MRS has been shown to detect progression (decrease in NAA, elevation of Cho, and decrease in the NAA/Cho and Cr/Cho ratios) before radiological or clinical deterioration [22]. Experience in children also suggests that MRS may contribute to differential diagnoses with infectious and demyelinating diseases [23]. Diffusion tensor imaging and white matter fiber tractography could also help to differentiate diffuse brainstem gliomas (deflected fibers) from demyelinating disease (lack of distortion) [24].
An arteriovenous fistula has been reported as a rare but reversible cause of brainstem dysfunction. MRI findings may be similar to a low-grade tumor including T2 hyperintensity signal without contrast enhancement [25]. Additional diagnostic procedures (for example, cerebral angiography) are recommended if an intracranial fistula with brainstem involvement is suspected.
Neurofibromatosis type 1 (NF1) is a well-known risk factor for brainstem gliomas in children, and very rare cases have been reported in adults [26]. MRI in NF1 patients should be carefully interpreted because T2 hyperintensities in the brainstem, so-called “unidentified bright objects”, are frequent and must be differentiated from neoplastic lesions [27].
Principles of Treatment
Symptomatic Treatment
Glucocorticoids are frequently administered to patients with brainstem tumors. As in supratentorial gliomas, their beneficial effects are attributed to a reduction in tumor-associated edema and an improvement in symptoms related to the compression of long-tract white fibers [28]. Despite their wide use in patients with diffuse intrinsic brainstem gliomas, systematic reviews are lacking. In a retrospective series, glucocorticoids were administrated to 73% of 82 brainstem glioma patients during radiotherapy [11]. Approximately half of those patients needed long-term glucocorticoid therapy to obtain symptomatic relief, and consequently, multiple side effects such as myopathy, gastritis, osteonecrosis, and psychosis appeared. Glucocorticoids should be used sparingly, at the lowest necessary dosage, to avoid these complications. Patients with difficulties in swallowing may need a gastrostomy, such as percutaneous esophagogastrostomy, to guarantee their daily nutritional requirements.
Surgery
The resection of diffuse intrinsic brainstem gliomas is impossible, and a surgical approach is justified only in the case of shunt placement for symptomatic relief [29, 30]. It is often considered that adult patients exhibiting the classic radiological picture of a diffusely infiltrating nonenhancing lesion, which enlarges the brainstem, do not require a biopsy because of a perceived unfavorable risk/benefit ratio of a biopsy [4]. One prospective study, which included 46 adult patients with suspected brainstem glioma, compared the preoperative diagnosis assessed by conventional MRI to the histological result obtained by stereotactic biopsy [31]. For 26 patients with presumed low-grade lesions based on the MRI, the histological assessment found a high-grade glioma in 27% of patients as well as different types of tumors such as metastases (in 23% of cases) and lymphomas (in 7% of cases). Another study estimated a sensitivity of 41% for preoperative MRI to distinguish a low-grade tumor in 110 adult patients with brainstem lesions [32].
MRI-guided stereotactic brainstem biopsy in adults is considered a relatively safe procedure with a perioperative morbidity of ca. 2.5% and a rare mortality rate [31]. An accurate diagnosis should be recommended in all cases and not only when atypical clinical or imaging features suggest a non-neoplastic lesion. Moreover, a recent survey among pediatric neurosurgeons has underlined the considerable interobserver discordance in interpreting a typical MRI image of brainstem glioma, and it found an agreement in only 43.8% of cases [33].
Radiotherapy
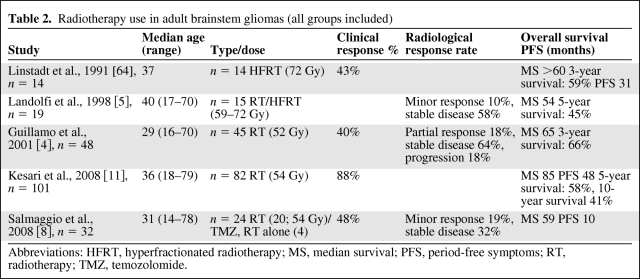
Radiotherapy is the standard treatment for adult diffuse intrinsic brainstem gliomas and can improve or stabilize patients for years (Table 2). The conventional median dose of radiotherapy is 50–55 Gy, using fractions of 1.8–2 Gy. Clinical improvement occurs in the majority of patients; however, there is a substantial discrepancy between the clinical and radiological response [4, 34]. In one series of 48 adult patients (including 46% of intrinsic diffuse gliomas), radiotherapy improved clinical status in 61%, whereas a partial radiological response (a decrease of 50% in the T2 hyperintensity in the greatest axial cross-sectional area) was noted in only 19% [4]. This discordance suggests that specific criteria to evaluate tumor response to treatment in brainstem gliomas could be different than the criteria used for supratentorial glioma and that they need to be defined.
Table 2.
Radiotherapy use in adult brainstem gliomas (all groups included)
Abbreviations: HFRT, hyperfractionated radiotherapy; MS, median survival; PFS, period-free symptoms; RT, radiotherapy; TMZ, temozolomide.
The optimal time of treatment remains unknown. Indeed, some patients may have mild symptoms during extended periods without any treatment, suggesting that, as in low-grade supratentorial glioma, radiotherapy may sometimes be deferred until clear evidence of symptomatic tumor progression is confirmed [8].
Chemotherapy
The data on chemotherapy are scarce. In a series of 34 patients with all groups of brainstem tumors (50% nonenhancing diffusely infiltrative), 22 patients were treated by conformal radiotherapy (48–58 Gy in 1.8 Gy per fraction) along with upfront concomitant chemotherapy of temozolomide at 75 mg/m2 per day followed by 200 mg/m2 per day at 5 days per month after radiotherapy [8]. The median time to tumor progression was 10 months, and the overall survival (OS) was 59 months (all patients included). Unfortunately, the fact that different types of brainstem gliomas (particularly low- and high-grade tumors) were analyzed together limits interpretation of these data, and it remains unknown whether concomitant or adjuvant chemotherapy has a role in the management of the diffuse intrinsic form.
A retrospective study evaluated chemotherapy with various agents (nitrosoureas or platinum-based) at relapse after radiotherapy in 22 patients. A modest rate (10%) of a radiological response was observed, whereas clinical improvement was noted in 18% of cases [4].
Thus, the efficacy of chemotherapy in adult diffuse brainstem gliomas remains unproven, and adjuvant chemotherapy cannot be recommended currently. Moreover, the effectiveness of chemotherapy on relapse is unsettled, although some patients clearly benefit, either with distinct clinical and radiological improvement or with an isolated clinical improvement contrasting with a lack of a radiological response.
Prognosis and Follow-up
The growth pattern of the intrinsic classic low-grade glioma in adults is slow and progressive. The median survival of diffuse intrinsic low-grade gliomas in adults ranges between 4.9 and 7.3 years as noted by three recent retrospective European and U.S. studies focused on adult brainstem gliomas [4, 5, 11]. In rare cases, a more aggressive diffuse intrinsic form similar to children appears in adults (that is, corresponding to a malignant glioma) and shares a similar poor prognosis (ca. 1 year OS).
The pattern of radiologic evolution is characterized by an infiltration and enlargement of brainstem volume taking place over months to years (Fig. 3). Objective radiological deterioration may be substantially delayed as compared with clinical deterioration. Sometimes, this gradual progression pattern is supplanted by the rapid development of a contrast-enhancing mass within the diffusely infiltrated areas, suggesting a transformation to a higher grade glioma (Fig. 2). In ca. 15% of cases, leptomeningeal dissemination manifested as locoregional and/or distant lesions has been observed in advanced stages of the disease usually associated with clinical deterioration (Fig. 4), as previously reported in children [35]. Eventually, patients develop tetraplegia, severe dysarthria, difficulties swallowing, and multiple cranial nerve palsies, sometimes culminating in a locked-in syndrome. The immediate cause of death is often a systemic complication (pulmonary embolism, aspiration pneumonia).
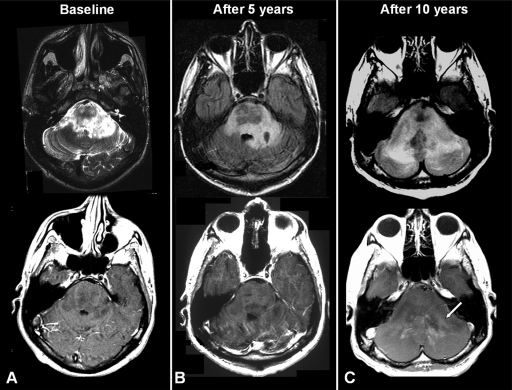
Figure 3.
Low-grade oligoastrocytoma. (A): Follow-up magnetic resonance image of the patient in Figure 1 showing an infiltrative lesion seen as T2 hyperintensity without contrast enhancement at baseline. (B): Five years later, the tumor remains stable as seen in Fluid Attenuation Inversion Recovery (FLAIR) and T1 sequences. (C): Tumor progression seen as an increase of hyperintensity signal in FLAIR sequence, significant mass effect, and contrast-enhanced lesion (white arrow).
Figure 4.
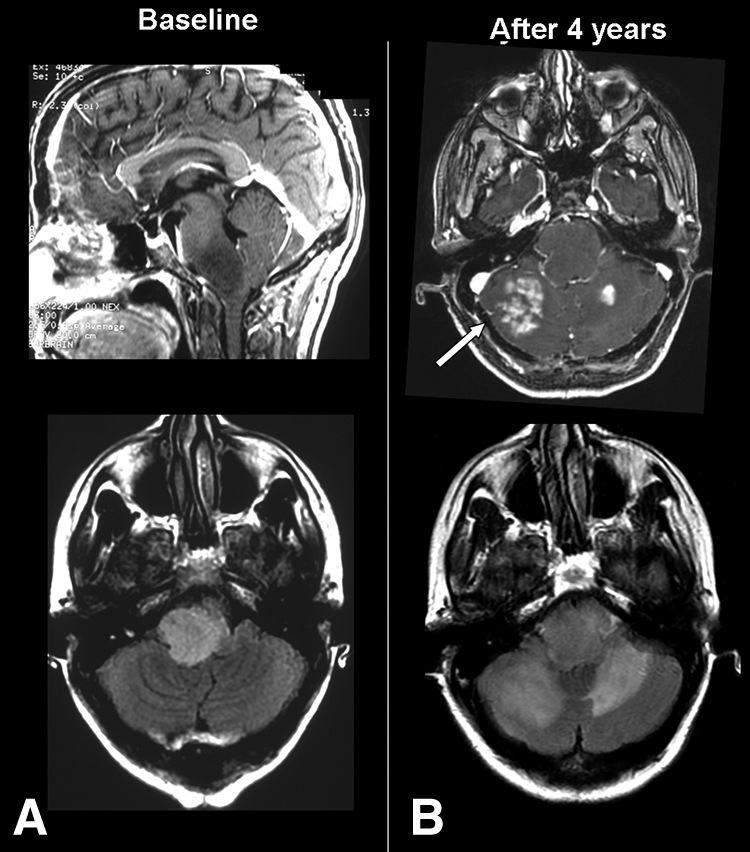
Oligodendroglioma grade II in a 42-year-old woman presenting an ataxic gait and vertigo. Left panel: (A): Diffuse intrinsic lesion involving the pons and medulla oblongata. T1-weighted sequence (top) shows a nonenhancing lesion, and T2- Fluid Attenuation Inversion Recovery (FLAIR) sequences (bottom) show a hyperintensity of the brainstem. Right panel: (B): Four years later follow-up magnetic resonance imaging shows a locoregional progression seen as multiple tumoral enhancing lesions in the cerebellum (top, white arrow) and extension of T2-FLAIR hyperintensity.
No distinct prognostic factors have been identified in diffuse low-grade brainstem gliomas in adults. The available literature cannot be reliably interpreted because the data often aggregate adults, children, and various subtypes of brainstem gliomas. Suspected negative prognostic factors include increasing age at diagnosis (>45 years), tumor location (diffuse pontine), and ethnicity (non-Caucasian) [9, 11].
Enhancing Malignant Brainstem Gliomas
Epidemiology
Malignant gliomas account for ca. 30% of brainstem tumors in adults [10]. Compared to diffuse intrinsic low-grade gliomas, malignant gliomas generally appear in patients older than 40 years [4, 5].
Pathology
Histological examination is more frequently available in malignant brainstem gliomas compared to the diffuse intrinsic group because the MRI pattern is not specific. In these patients biopsies are performed more frequently to confirm the diagnosis. They correspond mainly to anaplastic astrocytomas and glioblastomas. Guillamo et al. [4] reported 14 malignant gliomas (12 anaplastic astrocytomas and 2 anaplastic oligodendrogliomas). There are limited data evaluating possible histological differences between brainstem and supratentorial malignant gliomas, but no striking differences have been noted [36].
Clinical Features
The clinical evolution is subacute, consisting of cranial nerve palsies and long-tract signs, rapidly leading to compromised performance status. Symptoms at presentation are dependent on the localization of the lesion. Compared to patients with diffuse intrinsic low-grade brainstem gliomas, patients with malignant brainstem gliomas have a much shorter evolution of symptoms before diagnosis and a poorer clinical condition at onset [11].
Imaging
Typically, malignant focal brainstem gliomas present as a contrast-enhancing mass surrounded by an area of edema. Contrast enhancement was found in 100% of cases upon initial MRI in one series, of which 66% of patients exhibited a ringlike pattern, suggesting central necrosis (Fig. 5) [4, 12]. Despite the fact that MRS may be helpful, it is important to underline that, in contrast to children, the radiological pattern of malignant brainstem gliomas in adults is nonspecific [37]. In previous studies, preoperative radiological diagnoses were found to be incorrect in 10%–25% of cases in patients over 20 years of age who presented with a contrast-enhancing lesion in the brainstem. One of those series found benign non-neoplastic pathologies in 17% of adults with a suspected malignant brainstem mass [38]. There are many causes of contrast-enhancing lesions in the brainstem, particularly inflammatory (for example, neuro-Behçet, sarcoidosis, and demyelinating diseases), infectious (for example, tuberculomas or pyogenic abscess), or vascular diseases (for example, hematomas, vasculitis, arteriovenous malformations, or cavernous angiomas) as well as other non-malignant (for example, pilocytic astrocytoma, epidermoid cysts, or acoustic neurinoma) or malignant tumors (for example, lymphomas or metastasis) [39–43]. Thus, although a careful systemic and neurological work-up and a complete radiological evaluation may lead to the correct diagnosis, a biopsy should be considered when these tests are inconclusive. In this situation, positron emission tomography (PET) using [18F] fluorodeoxyglucose and [11C] methionine may be helpful to increase the diagnostic yield by defining a biopsy target [44, 45].
Figure 5.
Anaplastic oligodendroglioma in a 25-year-old woman suffering from headache, ataxic gait, diplopia, and facial paresthesia. Upper panel: (A): Magnetic resonance image showing a hyperintensity in T2 sequences involving the pons. (B): T1-weighted sequence showing a contrast-enhanced pontine tumor with “ringlike” pattern. (C): Magnetic resonance spectroscopy showing an increase in Cho peak and notable reduction in Naa (Cho/Naa = 3.5). Lower panel: (D, E): Histologic sample obtained after biopsy showing a microvascular proliferation (black arrow) and diffuse expression of glial fibrillary acidic protein. (F): Immunohistochemistry showing a high MIB-1 proliferation index.
Abbreviations: Cho, choline; Naa, N-acetyl aspartate.
Principles of Treatment
Surgery
As noted above, the surgical approach is limited to a biopsy or shunt placement in case of a secondary hydrocephalus. The risk of severe complications of a stereotactic biopsy for a suspected malignant brainstem glioma is low. In one series, out of 71 patients who underwent computed-tomography-guided biopsy, a permanent neurological deficit occurred in 1.4% (1 patient) and transitory worsening occurred in 5.6% (4 patients) [38]. Surgical morbidity is usually minimal and temporary, consisting of transient neurological deterioration or obstructive hydrocephalus. Operative mortality is exceedingly rare [38–40].
In general, the lower in the brainstem that the lesion is located, the greater is the risk involved. Stereotactic surgery of the brainstem guided by computed tomography, MRI, and if possible PET appears safe and reliable, and it provides a high yield (ca. 95%) of positive histological diagnosis [38–42]. However, the optimal methods and routes of biopsies remain a matter of debate [46, 47].
Radiotherapy
Malignant brainstem gliomas are highly resistant to treatment by radiotherapy. Indeed, after radiotherapy, only 13% (2 out of 15 patients) showed clinical and radiological improvement [4]. Gamma knife radiosurgery has been reported in some cases of high-grade brainstem gliomas, but the published data are limited and preliminary [48].
Chemotherapy
Chemotherapy has been occasionally used in this subgroup of tumors, but the data are too scant to draw any recommendations on this issue. As for the diffuse intrinsic form, it is unknown, despite encouraging case reports, whether the concomitant and adjuvant administration of temozolomide (TMZ) at diagnosis provides the benefit demonstrated in supratentorial glioblastomas. Occasionally, patients may respond to chemotherapy alone as illustrated by the report of a patient with a pathologically confirmed glioblastoma who received TMZ without radiotherapy at 150–200 mg/m2 every 28 days (a total of 17 cycles) and showed a complete radiological response and clinical improvement with an overall survival of 37 months [49].
At relapse, response to chemotherapy after radiotherapy failure has been reported with various agents in a minority of patients (ca. 15%), as noted above. Prospective studies on this issue, as well as the evaluation of new therapies, such as anti-angiogenic drugs, are clearly needed. The use of bevacizumab in adults with recurrent diffuse brainstem gliomas has not been clearly established. In the literature, there are only some case reports showing a clinical and radiological response to bevacizumab in recurrent disease after radiotherapy and TMZ failure [50, 51]. A phase II study that included 16 children with recurrent brainstem gliomas treated by radiotherapy found that bevacizumab plus irinotecan had minimal efficacy in clinical and radiological outcomes [52].
Prognosis
In contrast with low-grade gliomas, particularly the diffuse intrinsic forms, the prognosis of malignant brainstem gliomas is very poor, with a median survival of ca. 1 year [9, 11]. As with supratentorial high-grade gliomas, older age (>50 years) appears to be a pejorative prognostic factor [9].
Focal Tectal Brainstem Gliomas
Epidemiology
Focal tectal gliomas are well-defined anatomical and clinical entities in children generally associated with a favorable outcome [53–55]. The tumor is located in the posterior part of the midbrain, behind the aqueduct of Sylvius, without evident infiltration of the adjacent tissues. Focal tectal gliomas constitute a small subgroup of brainstem tumors in adults (8% of cases) and seem identical to the subtype found in children.
Pathology
In the few cases where it could be obtained, the histopathology most often revealed grade II oligoastrocytomas [4]. However, some cases of pilocytic astrocytomas have also been described, as well as rare cases of glioblastoma [56, 57].
Clinical and Radiological Presentation
Tumors of the tectal plate are usually well-defined intrinsic lesions. The periaqueductal location and long periods of stability are classic features of focal tectal gliomas. The clinical circumstances leading to the diagnoses are variable. An incidental finding is possible. Usually, the progressive signs and symptoms of obstructive hydrocephalus herald the disease and require placement of a shunt [53]. On rare occasions, the obstructive hydrocephalus is acute, and an intracranial hemorrhage constitutes the first clinical manifestation. Focal neurological findings such as cranial nerve paralysis are less frequent and may resolve after the normalization of intracranial pressure [57].
Treatment and Follow-up
Tectal gliomas are considered a different subset of brainstem tumors and have been associated with longer median survival (exceeding 10 years) following ventriculoperitoneal shunting and in some cases focal radiotherapy [53, 54]. Because simple observation after shunting may be an appropriate option in children, the decision to treat adult patients with radiotherapy is questionable for some authorities who propose a similar conservative approach [4].
Exophytic Brainstem Gliomas
Exophytic contrast-enhancing gliomas, which are well known in children (up to 10% of cases) and are associated with good prognoses, are extremely rare in adults, perhaps because most exophytic gliomas are pilocytic astrocytomas, which are rare tumor types in adults [4, 5]. In adults great caution is needed to attribute an exophytic contrast-enhancing brainstem mass to this type of benign lesion because malignant gliomas may have a similar radiographic appearance, underlining the importance of histological confirmation [58–60]. Surgical resection is recommended in some cases including dorsal exophytic tumors protruding into the fourth ventricle. Improvement in neurosurgical techniques (particularly the use of intraoperative ultrasound, intraoperative neurophysiological mapping, and computer reconstruction techniques) has facilitated partial resection of tumors previously considered inoperable, or even gross total removal in some cases, without affecting the functional status [30]. A stereotaxic approach can be a rapid and safe method for evacuation of the contents of cysts, providing a neurological benefit in most cases [29].
Conclusion
Despite improvements in the clinical and radiological understanding of brainstem gliomas, the primary challenge remains: to obtain a better knowledge of these tumors, particularly their classification and management. These tumors are not so rare, and every neuro-oncologist follows several of these unfortunate patients. In contrast with supratentorial gliomas, little progress has been made over the last 2 decades. Improvement will require advances in the understanding of the biology of these tumors and innovative approaches, possibly based on the analysis of the molecular profile of the tissue. Dedicated and careful pathological studies should also help to complete and enrich the current radiological classification. Experimental animal models of brainstem gliomas and preclinical human studies to investigate new agents such as signal transduction inhibitors are actively being studied in children and could subsequently benefit adult patients [61–63]. Finally, major multicenter efforts are needed to define standard treatment and refine criteria to evaluate tumor response.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Provision of study material or patients: German A. Reyes-Botero
Collection and/or assembly of data: German A. Reyes-Botero
Manuscript writing: Karima Mokhtari, German A. Reyes-Botero, Florence Laigle-Donadey, Jean-Yves Delattre, Nadine Martin-Duverneuil
Final approval of manuscript: Jean-Yves Delattre
References
- 1.Barkovich AJ, Krischer J, Kun LE, et al. Brain stem gliomas: a classification system based on magnetic resonance imaging. Pediatr Neurosurg. 1990;16:73–83. doi: 10.1159/000120511. [DOI] [PubMed] [Google Scholar]
- 2.Donaldson SS, Laningham F, Fisher P, et al. Advances toward an understanding of brainstem gliomas. J Clin Oncol. 2006;24:1266–1272. doi: 10.1200/JCO.2005.04.6599. [DOI] [PubMed] [Google Scholar]
- 3.Fischbein NJ, Prados MD, Wara W, et al. Radiologic classification of brain stem tumors: correlation of magnetic resonance imaging appearance with clinical outcome. Pediatr Neurosurg. 1996;24:9–23. doi: 10.1159/000121010. [DOI] [PubMed] [Google Scholar]
- 4.Guillamo JS, Monjour A, Taillandier L, et al. Brainstem gliomas in adults: prognostic factors and classification. Brain. 2001;124:2528–2539. doi: 10.1093/brain/124.12.2528. [DOI] [PubMed] [Google Scholar]
- 5.Landolfi JC, Thaler HT, DeAngelis LM. Adult brainstem gliomas. Neurology. 1998;51:1136–1139. doi: 10.1212/wnl.51.4.1136. [DOI] [PubMed] [Google Scholar]
- 6.Grill J, Puget S, Andreiuolo F, et al. Critical oncogenic mutations in newly diagnosed pediatric diffuse intrinsic pontine glioma. Pediatr Blood Cancer. 2011 doi: 10.1002/pbc.24060. doi: 10.1002/pbc.24060 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Shumacher M, Schulte-Monting J, Stoeter P, et al. Magnetic resonance imaging compared with biopsy in the diagnosis of brainstem diseases of childhood: a multicenter review. J Neurosurg. 2007;106(2 Suppl):111–119. doi: 10.3171/ped.2007.106.2.111. [DOI] [PubMed] [Google Scholar]
- 8.Salmaggi A, Fariselli L, Milanesi I, et al. Natural history and management of brainstem gliomas in adults: a retrospective Italian study. J Neurol. 2008;255:171–177. doi: 10.1007/s00415-008-0589-0. [DOI] [PubMed] [Google Scholar]
- 9.Rineer J, Schreiber D, Choi K, et al. Characterization and outcomes of infratentorial malignant glioma: a population-based study using the Surveillance Epidemiology and End-Results database. Radiother Oncol. 2010;95:321–326. doi: 10.1016/j.radonc.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Martin-Duverneuil N, Mothkari K. Gliomes du tronc cérébral. In: Martin-Duverneuil N, Mothkari K, editors. Les tumeurs intracraniennes de l'adulte. First edition. Paris: Sauramps Medical editorial; 2009. pp. 114–116. [Google Scholar]
- 11.Kesari S, Kim RS, Markos V, et al. Prognostic factors in adult brainstem gliomas: a multicenter, retrospective analysis of 101 cases. J Neurooncol. 2008;88:175–183. doi: 10.1007/s11060-008-9545-1. [DOI] [PubMed] [Google Scholar]
- 12.Guillamo JS, Doz F, Delattre JY. Brain stem gliomas. Curr Opin Neurol. 2001;14:711–715. doi: 10.1097/00019052-200112000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Broniscer A, Baker J, Baker S, et al. Prospective collection of tissue samples at autopsy in children with diffuse intrinsic pontine glioma. Cancer. 2010;116:4632–4637. doi: 10.1002/cncr.25405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selvapandian S, Rajshekhar V, Chandy MJ. Brainstem glioma: comparative study of clinico-radiological presentation, pathology and outcome in children and adults. Acta Neurochir (Wien) 1999;141:721–726. doi: 10.1007/s007010050367. discussion 726–727. [DOI] [PubMed] [Google Scholar]
- 15.Marin-Sanabria EA, Kobayashi N, Miyake S, et al. Snoring associated with Ondine's curse in a patient with brainstem glioma. J Clin Neurosci. 2006;13:370–373. doi: 10.1016/j.jocn.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 16.Gaviani P, Gonzalez R, Zhu J. Brainstem glioma central neurogenic hyperventilation and lactate production. Neurology. 2005;64:166. doi: 10.1212/01.WNL.0000148579.80486.F1. [DOI] [PubMed] [Google Scholar]
- 17.Saka E, Ozkaynak S, Tuncer R. Tongue tremor in brainstem pilocytic astrocytoma. J Clin Neurosci. 2006;13:503–506. doi: 10.1016/j.jocn.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimura M, Yamamoto T, Iso-o N, et al. Hemiparkinsonism associated with a mesencephalic tumor. J Neurol Sci. 2002;197:89–92. doi: 10.1016/s0022-510x(02)00042-4. [DOI] [PubMed] [Google Scholar]
- 19.Lim EC, Wilder-Smith EP, Chong JL, et al. Seeing the light: brainstem glioma causing visual auras and migraine. Cephalalgia. 2005;25:154–156. doi: 10.1111/j.1468-2982.2004.00833.x. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda K, Baba S, Iwasaki Y. Pseudomyasthenia in a patient with pontine glioma. Clin Neurol Neurosurg. 2006;108:809–810. doi: 10.1016/j.clineuro.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez-Linera J. Magnetic resonance techniques for the brainstem. Semin Ultrasound CT MR. 2010;31:230–245. doi: 10.1053/j.sult.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Panigrahy A, Nelson M, Finlay J, et al. Metabolism of diffuse intrinsic brainstem gliomas in children. Neuro-Oncology. 2008;10:32–44. doi: 10.1215/15228517-2007-042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porto L, Hattingen E, Pilatus U, et al. Proton magnetic resonance spectroscopy in childhood brainstem lesions. Childs Nerv Syst. 2007;23:305–314. doi: 10.1007/s00381-006-0221-5. [DOI] [PubMed] [Google Scholar]
- 24.Giussani C, Poliakov A, Ferri RT. DTI fiber tracking to differentiate demyelinating diseases from diffuse brain stem glioma. NeuroImage. 2010;52:217–223. doi: 10.1016/j.neuroimage.2010.03.079. [DOI] [PubMed] [Google Scholar]
- 25.Kiyosue H, Tanoue S, Sagara Y. The anterior medullary–anterior pontomesencephalic venous system and its bridging veins communicating to the dural sinuses: normal anatomy and drainage routes from dural arteriovenous fistulas. Neuroradiology. 2008;50:1013–1023. doi: 10.1007/s00234-008-0433-3. [DOI] [PubMed] [Google Scholar]
- 26.Guillamo JS, Créange A, Kalifa C, et al. Prognostic factors of CNS tumours in Neurofibromatosis 1 (NF1): a retrospective study of 104 patients. Brain. 2003;126:152–160. doi: 10.1093/brain/awg016. [DOI] [PubMed] [Google Scholar]
- 27.Ullrich NJ, Raja AI, Irons MB, et al. Brainstem lesions in neurofibromatosis type 1. Neurosurgery. 2007;61:762–766. doi: 10.1227/01.NEU.0000298904.63635.2D. [DOI] [PubMed] [Google Scholar]
- 28.Piette C, Munaut C, Foidart JM. Treating gliomas with glucocorticoids: from bedside to bench. Acta Neuropathol. 2006;112:651–664. doi: 10.1007/s00401-006-0100-x. [DOI] [PubMed] [Google Scholar]
- 29.Mursch K, Halatsch ME, Markakis E, et al. Intrinsic brainstem tumours in adults: results of microneurosurgical treatment of 16 consecutive patients. Br J Neurosurg. 2005;19:128–136. doi: 10.1080/02688690500145530. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka S, Kobayashi I, Utsuki S, et al. Biopsy of brain stem glioma using motor-evoked potential mapping by direct peduncular stimulation and individual adjuvant therapy. Case report. Neurol Med Chir (Tokyo) 2005;45:49–55. doi: 10.2176/nmc.45.49. [DOI] [PubMed] [Google Scholar]
- 31.Rachinger W, Grau S, Holtmannspötter M, et al. Serial stereotactic biopsy of brainstem lesions in adults improves diagnostic accuracy compared with MRI only. J Neurol Neurosurg Psychiatry. 2009;80:1134–1139. doi: 10.1136/jnnp.2009.174250. [DOI] [PubMed] [Google Scholar]
- 32.Reithmeier T, Kuzeawu A, Berger H, et al. Adult brainstem gliomas: a retrospective analysis of 110 cases within the German Glioma Network 60th Annual Meeting of the German Society of Neurosurgery (DGNC); May 24–27, 2009; Münster, Germany. [Google Scholar]
- 33.Hankinson TC, Campagna EJ, Forem NK, et al. Interpretation of magnetic resonance images in diffuse intrinsic pontine glioma: a survey of pediatric neurosurgeons. J Neurosurg Pediatr. 2011;8:97–102. doi: 10.3171/2011.4.PEDS1180. [DOI] [PubMed] [Google Scholar]
- 34.Mandell LR, Kadota R, Freeman C, et al. There is no role for hyperfractionated radiotherapy in the management of children with newly diagnosed diffuse intrinsic brainstem tumors: results of a Pediatric Oncology Group phase III trial comparing conventional vs. hyperfractionated radiotherapy. Int J Radiat Oncol Biol Phys. 1999;43:959–964. doi: 10.1016/s0360-3016(98)00501-x. [DOI] [PubMed] [Google Scholar]
- 35.Gururangan S, McLaughlin CA, Brashears J, et al. Incidence and patterns of neuraxis metastases in children with diffuse pontine glioma. J Neurooncol. 2006;77:207–212. doi: 10.1007/s11060-005-9029-5. [DOI] [PubMed] [Google Scholar]
- 36.Stark AM, Maslehaty H, Hugo HH, et al. Glioblastoma of the cerebellum and brainstem. J Clin Neurosci. 2010;17:1248–125130. doi: 10.1016/j.jocn.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 37.Kwon J, Kim I, Cheon J, et al. Paediatric brain-stem gliomas: MRI, FDG-PET and histological grading correlation. Pediatr Radiol. 2006;36:959–964. doi: 10.1007/s00247-006-0256-5. [DOI] [PubMed] [Google Scholar]
- 38.Rajshekhar V, Chandy MJ. Computerized tomography-guided stereotactic surgery for brainstem masses: a risk-benefit analysis in 71 patients. J Neurosurg. 1995;82:976–981. doi: 10.3171/jns.1995.82.6.0976. [DOI] [PubMed] [Google Scholar]
- 39.Boviatsis EJ, Kouyialis AT, Stranjalis G, et al. CT-guided stereotactic biopsies of brain stem lesions: personal experience and literature review. Neurol Sci. 2003;24:97–102. doi: 10.1007/s10072-003-0093-3. [DOI] [PubMed] [Google Scholar]
- 40.Friedman WA, Sceats J, Nestok B, et al. The incidence of unexpected pathological findings in an image-guided biopsy series: a review of 100 consecutive cases. Neurosurgery. 1989;25:180–184. doi: 10.1097/00006123-198908000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Gonçalves-Ferreira AJ, Herculano-Carvalho M, Pimentel J. Stereotactic biopsies of focal brainstem lesions. Surg Neurol. 2003;60:311–320. doi: 10.1016/s0090-3019(03)00379-3. [DOI] [PubMed] [Google Scholar]
- 42.Swinson BM, Friedman W, Yachnis AT. Pontine atypical neurocytoma: cAse report. Neurosurgery. 2006;58:E990. doi: 10.1227/01.NEU.0000210213.12847.1E. [DOI] [PubMed] [Google Scholar]
- 43.Shenoy SN, Raja A. Cystic trochlear nerve neurinoma mimicking intrinsic brainstem tumour. Br J Neurosurg. 2004;18:183–186. doi: 10.1080/02688690410001681073. [DOI] [PubMed] [Google Scholar]
- 44.Massager N, David P, Goldman S, et al. Combined magnetic resonance imaging-and positron emission tomography-guided stereotactic biopsy in brainstem mass lesions: diagnostic yield in a series of 30 patients. J Neurosurg. 2000;93:951–957. doi: 10.3171/jns.2000.93.6.0951. [DOI] [PubMed] [Google Scholar]
- 45.Pirotte JM, Luba A, Massager N, et al. Clinical impact of integrating positron emission tomography during surgery in 85 children with brain tumors. J Neurosurg Pediatr. 2010;5:486–499. doi: 10.3171/2010.1.PEDS09481. [DOI] [PubMed] [Google Scholar]
- 46.Mehta VS, Chandra PS, Kumar Singh Pankaj, et al. Surgical considerations for ‘intrinsic’ brainstem gliomas: proposal of a modification in classification. Neurol India. 2009;57:274–281. doi: 10.4103/0028-3886.53272. [DOI] [PubMed] [Google Scholar]
- 47.Abernathey CD, Camacho A, Kelly PJ. Stereotaxic suboccipital transcerebellar biopsy of pontine mass lesions. J Neurosurg. 1989;70:195–200. doi: 10.3171/jns.1989.70.2.0195. [DOI] [PubMed] [Google Scholar]
- 48.Fuchs I, Kreil W, Sutter B, et al. Gamma knife radiosurgery of brainstem gliomas. Acta Neurochir Suppl. 2002;84:85–90. doi: 10.1007/978-3-7091-6117-3_10. [DOI] [PubMed] [Google Scholar]
- 49.Zhang C, Yao Y, Wang Y, et al. Temozolomide for adult brain stem glioblastoma: case report of a long-term survivor. Int J Neurosci. 2010;120:787–791. doi: 10.3109/00207454.2010.520377. [DOI] [PubMed] [Google Scholar]
- 50.Torcuator R, Zuniga R, Loutfi R, et al. Bevacizumab and irinotecan treatment for progressive diffuse brainstem glioma: case report. J Neurooncol. 2009;93:409–412. doi: 10.1007/s11060-008-9782-3. [DOI] [PubMed] [Google Scholar]
- 51.Raza S, Donach M. Bevacizumab in adult malignant brainstem gliomas. J Neurooncol. 2009;95:299–300. doi: 10.1007/s11060-009-9933-1. [DOI] [PubMed] [Google Scholar]
- 52.Gururangan S, Chi S, Poussaint TY, et al. Lack of efficacy of bevacizumab plus irinotecan in children with recurrent malignant glioma and diffuse brainstem glioma: a pediatric brain tumor consortium study. J Clin Oncol. 2010;28:3069–3075. doi: 10.1200/JCO.2009.26.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamilton MG, Lauryssen C, Hagen N. Focal midbrain glioma: long term survival in a cohort of 16 patients and the implications for management. Can J Neurol Sci. 1996;23:204–207. doi: 10.1017/s031716710003852x. [DOI] [PubMed] [Google Scholar]
- 54.Bowers DC, Georgiades C, Aronson LJ, et al. Tectal gliomas: natural history of an indolent lesion in pediatric patients. Pediatr Neurosurg. 2000;32:24–29. doi: 10.1159/000028893. [DOI] [PubMed] [Google Scholar]
- 55.Dağlioğlu E, Cataltepe O, Akalan N. Tectal gliomas in children: the implications for natural history and management strategy. Pediatr Neurosurg. 2003;38:223–231. doi: 10.1159/000069823. [DOI] [PubMed] [Google Scholar]
- 56.Chaddad Neto F, Lopes A, Alberto Filho M, et al. Tectal glioblastoma. Arq Neuropsiquiatr. 2007;65:996–999. doi: 10.1590/s0004-282x2007000600015. [DOI] [PubMed] [Google Scholar]
- 57.Oka F, Yamashita Y, Kumabe T, et al. Total resection of a hemorrhagic tectal pilocytic astrocytoma–case report. Neurol Med Chir (Tokyo) 2007;47:219–221. doi: 10.2176/nmc.47.219. [DOI] [PubMed] [Google Scholar]
- 58.Lee T, Galarza M, Petito C, et al. Exophytic malignant brainstem mixed glioma in an adult. J Neurooncol. 1998;37:123–129. doi: 10.1023/a:1005969429013. [DOI] [PubMed] [Google Scholar]
- 59.Luetjens G, Mirzayan MJ, Brandis A, et al. Exophytic giant cell glioblastoma of the medulla oblongata. J Neurosurg. 2009;110:589–593. doi: 10.3171/2008.8.JNS17644. [DOI] [PubMed] [Google Scholar]
- 60.Swaroop GR, Whittle IR. Exophytic pontine glioblastoma mimicking acoustic neuroma. J Neurosurg Sci. 1997;41:409–411. [PubMed] [Google Scholar]
- 61.Fouladi M, Nicholson HS, Zhou T, et al. A phase II study of the farnesyl transferase inhibitor, tipifarnib, in children with recurrent or progressive high-grade glioma, medulloblastoma/primitive neuroectodermal tumor, or brainstem glioma: a Children's Oncology Group study. Cancer. 2007;110:2535–2541. doi: 10.1002/cncr.23078. [DOI] [PubMed] [Google Scholar]
- 62.Pollack IF, Jakacki RI, Blaney SM, et al. Phase I trial of imatinib in children with newly diagnosed brainstem and recurrent malignant gliomas: a Pediatric Brain Tumor Consortium report. Neuro Oncol. 2007;9:145–160. doi: 10.1215/15228517-2006-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pollack IF, Stewart CF, Kocak M, et al. A phase II study of gefitinib and irradiation in children with newly diagnosed brainstem gliomas: a report from the Pediatric Brain Tumor Consortium. Neuro Oncol. 2011;13:290–297. doi: 10.1093/neuonc/noq199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Linstadt DE, Edwards MS, Prados M, et al. Hyperfractionated irradiation for adults with brainstem gliomas. Int J Radiat Oncol Biol Phys. 1991;20:757–760. doi: 10.1016/0360-3016(91)90019-z. [DOI] [PubMed] [Google Scholar]