A systematic review of the published English-language literature was performed to evaluate evidenced-based practices for radiation-induced sarcoma treatment. Surgery with wide negative margins remains the primary treatment; the role of adjuvant and neoadjuvant chemotherapy is uncertain.
Keywords: Radiation-induced, Radiotherapy, Breast cancer, Second primary malignancy, Sarcoma, Angiosarcoma
Learning Objectives
After completing this course, the reader will be able to:
Evaluate the level of evidence to inform decision making for the treatment radiation induced sarcoma of the breast.
Explain diagnostic criteria for radiation-induced sarcoma.
Describe the effectiveness of surgery, chemotherapy and radiation therapy for radiation induced sarcoma of the breast.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Introduction.
Radiation-induced sarcoma (RIS) is a rare, aggressive malignancy. Breast cancer survivors treated with radiotherapy constitute a large fraction of RIS patients. To evaluate evidenced-based practices for RIS treatment, we performed a systematic review of the published English-language literature.
Methods.
We performed a systematic keyword search of PubMed for original research articles pertaining to RIS of the breast. We classified and evaluated the articles based on hierarchal levels of scientific evidence.
Results.
We identified 124 original articles available for analysis, which included 1,831 patients. No randomized controlled trials involving RIS patients were found. We present the best available evidence for the etiology, comparative biology to primary sarcoma, prognostic factors, and treatment options for RIS of the breast.
Conclusion.
Although the evidence to guide clinical practice is limited to single institutional cohort studies, registry studies, case–control studies, and case reports, we applied the available evidence to address clinically relevant questions related to best practice in patient management. Surgery with widely negative margins remains the primary treatment of RIS. Unfortunately, the role of adjuvant and neoadjuvant chemotherapy remains uncertain. This systematic review highlights the need for additional well-designed studies to inform the management of RIS.
Introduction
Radiotherapy is an integral component of the treatment of patients with primary breast cancer; in particular, prospective, randomized trials have established the safety of breast-conserving surgery (BCS) [1]. Radiation-induced sarcoma (RIS), a rare iatrogenic malignancy, can occur after radiotherapy and is associated with poor outcomes. After radiotherapy, the cumulative RIS incidence is 3.2 per 1,000 at 15 years (versus 2.3 per 1,000 for primary sarcoma in a population without radiotherapy) [2]. The occurrence rate of RIS is low: over a 10-year period, 0.03%–0.2% [3–8]. RIS comprises about 3% of all soft-tissue sarcomas [9].
The first reported case occurred in the early 1920s [10], yet little is known about the molecular biology of this disease; consequently, no targeted therapy is available. RIS typically occurs 10 years after the index breast cancer, but the latency period can be as long as 20 years [11–13] or even as short as 6 months [14]. Radiation-induced cutaneous angiosarcoma (RIA) typically has a shorter average latency period (4 years) [15, 16]. Establishing an accurate diagnosis of RIS is critical before embarking on treatment. To be classified as RIS, Cahan and colleagues proposed that: (a) there must be evidence of an initial malignant tumor of a different histology than the putative RIS, (b) development of the sarcoma must occur in an irradiated field, (3) there must be a prolonged latency period (typically >4 years) between the two malignancies, and (d) the second malignancy must histopathologically be a sarcoma [17–19].
Methods
To evaluate evidenced-based practices for RIS treatment, we performed a systematic review of the published English-language literature. Our PubMed search used the keywords “radiation-induced sarcoma of the breast,” “radiation-induced angiosarcoma of the breast,” “second primary malignancy of the breast,” “angiosarcoma and breast,” “radiotherapy and angiosarcoma of the breast,” “radiotherapy and sarcoma,” “radiation therapy and angiosarcoma,” and “malignant fibrous histiocytoma and radiation.” By searching references within those articles, we obtained further articles.
We identified a total of 124 original articles, which included 1,831 patients reported in 1970–2010. We excluded review articles that did not include original research, studies exclusively of sarcoma of the bone, and articles not specifically related to breast cancer.
Results
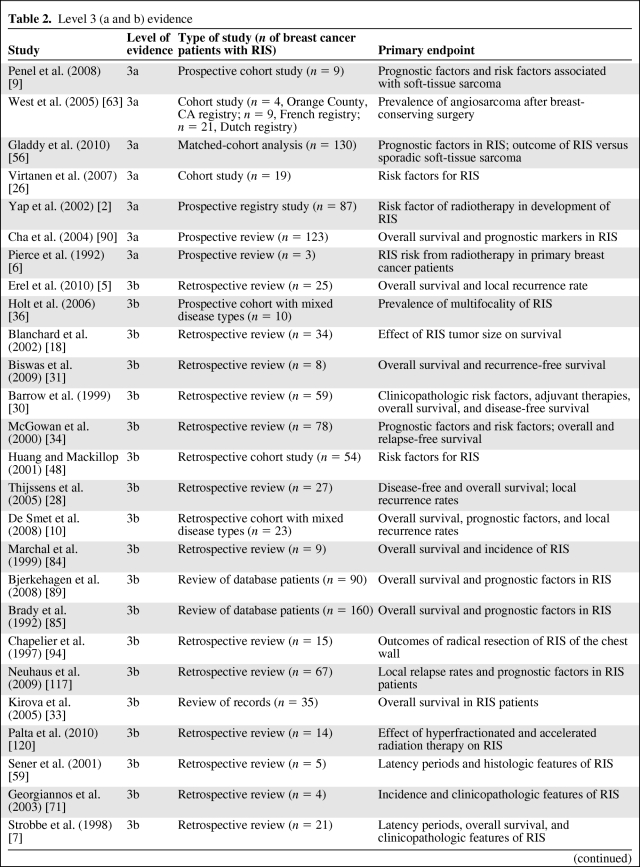
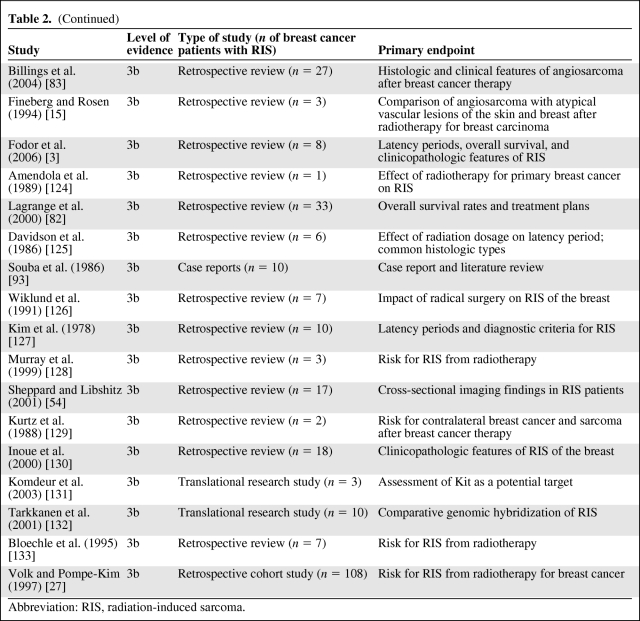
We found no prospective, randomized trials on RIS of the breast; therefore, no level 1 or level 2 evidence is available to guide treatment of RIS. Using the hierarchal levels of scientific evidence proposed by Hadorn et al. [20] (Table 1), we classified and evaluated the 124 original articles. We also subdivided the number of RIS patients (n = 1,827) and key clinical data by level 3 evidence (Table 2), level 4 evidence (Table 3), and level 5 evidence (Table 4).
Table 1.
Hierarchal levels of evidence
Table 2.
Level 3 (a and b) evidence
Table 2a.
(Continued)
Abbreviation: RIS, radiation-induced sarcoma.
Table 3.
Level 4 evidence
Abbreviation: RIS, radiation-induced sarcoma.
Table 4.
Level 5 evidence (a, b, and c)
Table 4a.
(Continued)
Abbreviation: RIS, radiation-induced sarcoma.
Etiology of RIS
Given the rarity of RIS of the breast, risk factors are difficult to elucidate. With the improved survival rates of patients with primary breast cancer, the incidence of RIS may be increasing. Breast cancer and non-Hodgkin's lymphoma were the most common antecedent malignancies [9, 21]. Cozen et al. [13] found a significantly elevated risk for RIA in breast cancer patients. Previous radiotherapy has always been considered the principle risk factor for RIS, although the incidence rate is only about 0.2% in breast cancer patients treated with radiation [6, 22–25]. Breast cancer patients treated with radiotherapy had a fivefold higher risk for RIA than controls who received no radiation [26]. They were also found to have a 33% higher risk for any second malignancy [27]. Both radiotherapy and chemotherapy for breast cancer were believed to increase the risk for RIS [28]. Only eight studies examined the risk for RIS after BCS versus mastectomy, but no apparent differences were found in the rates of RIS [18, 28–34].
In breast cancer patients, several studies found a dose–response effect between the amount of radiation and the risk for RIS [35, 36]. Karlsson et al. [37] reported a direct dose–response effect, with the risk for sarcoma increasing as the integral dose increased up to 150–200 joules; after that point, the relationship was unclear. A dose of 10 Gy was reported as the minimum total required to induce RIS [38]. Clarke et al. [32] noted a 20% higher risk for a second primary malignancy after radiotherapy. However, the overall risk for RIS was small compared with the potential benefit of radiotherapy [2, 39].
It is not known how new radiotherapy techniques, such as the delivery of intensity-modulated radiation therapy (IMRT), may influence the risk for RIS [40]. Whereas IMRT can increase the volume of tissue exposed to low doses of radiation [41], and thus increase the risk for radiation-induced carcinogenesis, it has also been shown to decrease the volume of tissue exposed to high doses of radiation [42], which could exert a beneficial impact on the risk for RIS. Further, it is not known if hypofractionated regimens, which administer a higher dose per fraction but a lower total dose, will modify the risk for RIS [43]. It is plausible that hypofractionation could impact the risk for RIS either favorably or unfavorably in comparison with conventional fractionation.
Other factors have been suggested in the pathogenesis of RIS, including having a deleterious BRCA-1 mutation [44]. This defect in the DNA repair mechanism may theoretically increase radiosensitivity, increasing susceptibility to carcinogenic effects in surviving cells. Other factors possibly associated with RIS include hereditary diseases (such as Li-Fraumeni syndrome), the radiation site for the initial tumor, and the use of alkylator-based chemotherapy [10].
Our systematic review found no evidence that chemotherapy is a contributing risk factor for RIS. A randomized study by Valagussa et al. [45] involving 845 women with resectable breast cancer found that chemotherapy (cyclophosphamide, methotrexate, and fluorouracil) alone—with no postoperative radiotherapy—was not associated with a higher risk for a second malignancy.
Comparative Biology of RIS Versus Primary Sarcoma of the Breast
Both RIS of the breast and primary sarcoma of the breast had a low incidence rate of <1% of the general population [35]. A primary sarcoma presents as a firm mass within the breast without a prior history of breast cancer; RIS is slightly more difficult to identify by physical examination because of changes after radiotherapy [7, 46]. Both varieties have a wide range of histopathologic subtypes, including leiomyosarcoma, malignant fibrous histiocytoma (the most common histopathologic subtype of RIS), liposarcoma, fibrosarcoma, and angiosarcoma [37, 47, 48]. Chondrosarcoma, osteosarcoma, and other less common mesenchymal neoplasms have also been described. Ancillary studies, including immunohistochemistry, are routinely performed, especially in patients with high-grade lesions whose tumor lineage is not histologically apparent. No studies directly compared the relative incidence of different histopathologic subtypes of RIS with those of primary sarcoma. Primary sarcoma and secondary sarcoma of the breast appear morphologically identical by histopathologic analysis [4, 22]. RIS is usually a high-grade tumor; its size at excision can vary widely [49–51]. The histopathologic features of RIS may consist of spindle-shaped tumor cells, hemorrhagic tumor nodules, conspicuous mitotic figures, and necrosis [49–52].
RIS frequently presents at an advanced clinical stage; the most common sites of metastasis include the lungs and the lymph nodes [53]. On imaging, the appearances of RIS and the primary sarcoma are similar, making diagnosis difficult [54]. The most common RIS location is the chest wall [9]. Sporadic sarcomas occur most commonly in the parenchyma [55].
Gladdy et al. [56] found that the disease-free survival rate was much lower in RIS patients than in sporadic soft-tissue sarcoma patients, independent of sarcoma histologic type. In addition, toxicity is much more of a concern in RIS patients because of their previous adjuvant therapy [56]. Because RIS patients may have already received the maximum safe dose of radiation (and may have received cytotoxic chemotherapy), fewer treatment options are typically available.
Typically, RIA of the breast appears in cutaneous areas [55, 57]. Secondary angiosarcoma is more difficult to identify clinically because it presents as skin thickening and discoloration, often causing a delay in diagnosis [58, 59]. The differential diagnosis for RIA also includes inflammatory breast carcinoma, edema of the breast, fibrous histiocytoma, and cellulitis [55, 60–62]. The most common RIA is hemangiosarcoma [13].
Diagnosis
The levels of evidence for RIS ranged from level 3a to level 5c (Tables 2–4). Like other breast neoplasms, RIS is diagnosed by physical examination of the patient and by appropriate imaging modalities, which guide the histologic confirmation of RIS by tissue diagnosis. For RIA, skin changes such as skin discoloration (ranging from red to purple), elevated skin, and skin thickening are common and indicative of disease [7, 16, 38, 55, 63–69]. During a skin biopsy, the specimen should be taken from the darkest and most infiltrated area [70]. Bruising of the skin may also be evident [71]. However, skin alterations and fibrosis are typical in radiated areas. During examination of the skin, the clinician must be alert to interval changes, which, unfortunately, are not typically documented systematically (as by serial photography). A high index of suspicion for the types of changes indicative of RIS is warranted, with a low threshold for biopsy.
In addition to physical examination, radiologic imaging is the backbone of longitudinal follow-up. Moore et al. [64] suggested comparing all prior mammograms and emphasized close attention to images of the quadrant of the breast where the patient has noticed skin changes. Of note, false-negative results of mammography make magnetic resonance imaging (MRI) and incisional biopsies valuable when evaluating RIS [31, 72]. Mammography results may be negative even after skin changes have been noticed [62, 67, 72, 73]. RIS can be distinguished from Stewart-Treves syndrome, which is the occurrence of angiosarcoma in patients with lymphedematous extremities after breast cancer surgery [74].
Ultimately, histologic evaluation confirms the diagnosis of RIS (Fig. 1). As noted above, skin biopsy may be sufficient for tumors with a predilection for cutaneous involvement, such as angiosarcoma. Deeper lesions require other techniques. RIS can be identified using fine-needle aspiration (FNA); however, RIS is difficult to distinguish from recurrent carcinoma given the typically small sample volume, its morphologic similarity to carcinoma cells, and the paucity of vasoformative elements in RIS [75]. FNA cannot be expected to provide definitive information about the histologic architecture of RIS nor can FNA provide adequate material for an immunohistochemical evaluation. Therefore, core needle or incisional biopsies are preferred: they provide accurate and satisfactory sampling [51, 58, 60, 65, 76, 77]. If FNA results are negative but clinical suspicion of RIS is strong, a core needle biopsy or even an incisional biopsy that includes skin and s.c. tissue may be required to achieve a tissue diagnosis [7, 16, 68].
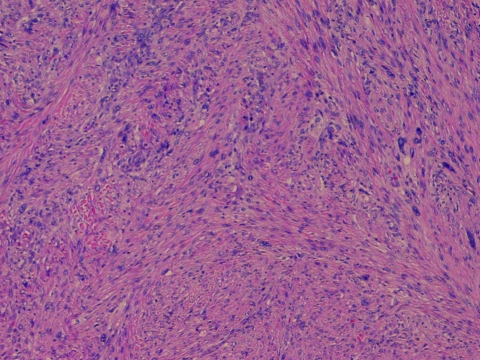
Figure 1.
Histologic micrograph of a radiation-induced sarcoma (RIS) of the breast. This image shows a radiation-induced malignant fibrous histiocytoma displaying a storiform growth pattern with highly atypical (often bizarre) neoplastic cell nuclei. This histologic image of RIS shows a striking low-power appearance as a result of cellularity, a vague whorled pattern, and marked nuclear atypia. The patient, a 52-year-old woman with ductal carcinoma in situ, was treated with breast-conserving surgery and adjuvant radiotherapy of 5,000 Gy. Six years later, she returned with a lump at her lumpectomy site; an incisional biopsy revealed atypical spindle-cell fibroblast proliferation consistent with a radiation-induced secondary malignancy.
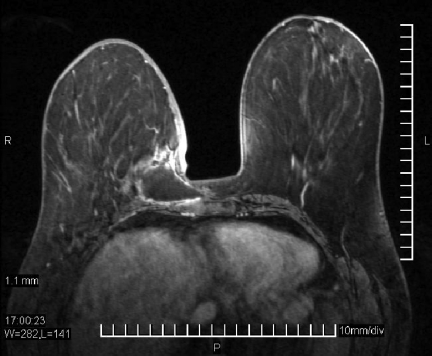
MRI as well as three-dimensional reconstructions can help facilitate excellent preoperative planning of the extent of resection [30, 78]. MRI scans can reveal the spread of a tumor and predict chest wall involvement, facilitating operative intervention (Fig. 2) [78, 79].
Figure 2.
Magnetic resonance imaging (MRI) scan of a radiation-induced sarcoma (RIS) of the breast. A breast MRI scan shows gadolinium in a patient with RIS. Tumor involvement with the chest wall is present. Bulky residual disease is demonstrated between 9 o'clock and 12 o'clock along the posterior margin of the seroma cavity and at 6 o'clock on the inferior margin of the seroma cavity 18 mm from the chest wall. The more posterior residual disease directly infiltrates the pectoralis major muscle.
Prognosis
The 5-year survival rate of RIS patients was in the range of 27%–48% [5, 80]. The 5-year disease-free survival rate was 35% [7]. The number of skin lesions was an important prognostic factor. For RIA patients with multiple skin lesions, the 2-year survival rate was 0% [81]; with a single lesion, it was 50% [3]. For RIS patients overall, the median survival time was 23 months [82].
Tumor size appeared to be an important prognostic factor. For RIS patients with tumors <2 cm, the median survival time was 80 months; with tumors >5 cm, the survival time was only 20 months [30]. Blanchard et al. [18] reported that patients with a smaller mean tumor size had fewer local recurrences than patients with larger tumors. Local recurrence rates after surgical therapy alone were high, in the range of 50%–68% [5].
Tumor grade is an important prognostic factor for RIS, as reflected by its inclusion in the seventh edition of the American Joint Committee on Cancer (AJCC) Staging Manual [81]. Most RIS tumors were high-grade, with some having heterogeneity in their grade [71]. De Smet et al. [10] reported that 80% of RIS tumors, but only 40% of primary sarcoma tumors, were high grade (grade 3 per the AJCC staging system). Low-grade tumors conferred better outcomes in terms of the disease-free survival interval [82, 83]. Patients with high-grade tumors had a higher stage (per the AJCC staging system) at presentation and a 5-year survival rate of only 18%; patients with lower stage tumors had a 5-year survival rate of 47%.
Many, but not all, studies noted no lymph node involvement in RIS patients [49, 68, 69, 84], underscoring that sarcoma does not tend to metastasize via a lymphatic route. Therefore, a surgical nodal evaluation procedure (such as sentinel lymph node biopsy) is not recommended for RIS patients. Brady et al. [85] noted three important factors that have an unfavorable impact on patient mortality rate: presentation with metastatic disease, incomplete or no surgical resection, and tumor size >5 cm.
Treatment
Although surgery remains the most effective treatment for RIS patients, surgery alone often was lacking in terms of overall survival and local recurrence rates, emphasizing the need for better multidisciplinary therapy [30, 86]. Many studies demonstrated that neither chemotherapy alone nor radiotherapy alone was sufficient therapy for RIS [18, 30, 87, 88]. Complete surgical excision was critical [89], specifically, wide local excision with negative surgical margins, either by segmental or total mastectomy. Positive margins had a significantly higher risk for local recurrence [28, 30, 90]. Thijssens et al. [28] found that R0 resections (microscopically free of tumor) conferred significantly higher survival rates than either R1 (microscopically positive for tumor) or R2 (macroscopically positive for tumor) resections. No significant differences in survival rates were found between patients with R1 and R2 resections. Retrospective studies noted that surgical margins of 2–4 cm were necessary for proper disease clearance in RIS patients [66, 91, 92], although another study reported that margins of 1 cm provided successful local control [73].
A proper surgical approach is crucial to decreasing the risk for recurrence; one study suggested that, for optimal margin control, the surgeon should extirpate one plane beyond the anatomic plane of known disease [5]. En bloc chest wall excision was necessary in some patients, such as those with tumors in previously radiated fields, with extensive disease infiltration [30]. Souba et al. [93] revealed good outcomes with complete excision and chest wall resection in RIS patients. Despite poor overall survival outcomes for patients undergoing chest wall resection, this may be justified given the low postoperative morbidity and the lack of other effective treatment options [94]. No reports were available regarding the appropriateness of BCS in RIS patients.
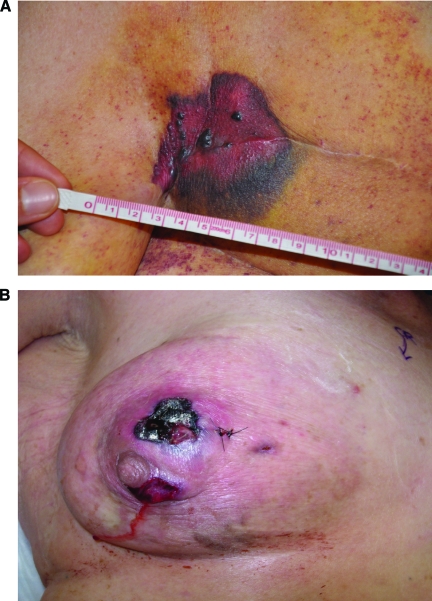
Physical exam findings for RIA are shown in Figure 3. In all RIA patients, a total mastectomy should be performed expediently after the diagnosis [63, 84, 89, 95, 96]. Colville et al. [97] recommended a margin of 5 cm. Turner and Greenall performed a radical mastectomy with axillary clearance [98]. Axillary dissection should be avoided to prevent lymphedema [30]. Sarcoma is not expected to metastasize to regional lymph nodes; therefore, axillary lymph node dissection has no role in RIS patients [30].
Figure 3.
Radiation-induced angiosarcoma. (A): Radiation-induced angiosarcoma occurring in a patient previously radiated after mastectomy. (B): Radiation-induced angiosarcoma occurring in a patient previously radiated after lumpectomy. Photos courtesy of Dr. Chan Raut, M.D., Department of Surgery, Brigham and Women's Hospital, Boston, Massachusetts.
Role of Chemotherapy
The role of chemotherapy for RIS remains ambiguous. No level 1 or 2 studies are available to address this question for RIS because of the rarity of this disease. Most retrospective studies addressing systemic therapy in RIS patients were thus subject to significant selection bias in their comparison and treatment groups. The literature on soft-tissue sarcoma treatment can be used for general guidance, although the assumption that RIS behaves the same as primary soft-tissue sarcoma might not be entirely accurate because RIS displays more aggressive biology.
A number of studies have reported poor survival and local recurrence rates even after combining surgery with standard adjuvant chemotherapy [28, 85, 87, 99]. Lagrange et al. [82] reported no difference in the survival rate between patients treated with surgery and those treated with surgery plus chemotherapy. However, other studies have reported that, following resection with widely negative margins, adjuvant therapy may be beneficial [29, 30, 56, 84, 86, 94, 100]. Barrow et al. [30] reported favorable results after adjuvant chemoradiotherapy. Angiosarcomas have also been shown to respond to the combination of radiation therapy and surgery [77, 100]. Rosen et al. [29] observed that adjuvant chemotherapy had a beneficial effect on the disease-free survival rate for high-grade angiosarcoma patients who received adjuvant therapy versus those who did not.
Kuten et al. [87] described treatment with cyclophosphamide, vincristine, doxorubicin, and DTIC (dacarbazine) therapy. Patients in that case series were treated with surgery with this combination chemotherapy or chemotherapy alone. Unfortunately, every patient died within 6–36 months from the time of their initial diagnosis, leading them to conclude that this regimen was ineffective [87]. Brady et al. [85] identified no difference in the 5-year survival rate between patients receiving chemotherapy as part of their treatment and patients not receiving chemotherapy. Yap et al. [101] revealed that agents such as methotrexate, 5-fluorouracil, and doxorubicin combined with DTIC may give a favorable response for lymphangiosarcoma. Another study reported the sensitivity of RIA to docetaxel and paclitaxel [102].
The use of adjuvant chemotherapy in the treatment of soft-tissue sarcoma is controversial. For certain sarcoma subtypes, such as Ewing's sarcoma, rhabdomyosarcoma, and osteosarcoma, chemotherapy plays a key role in disease management. For other subtypes, adjuvant therapy has not been proven to be reliably beneficial in randomized trials. Several meta-analyses have been conducted studying randomized trials of adjuvant chemotherapy for soft-tissue sarcomas. These suggest benefit for the addition of adjuvant chemotherapy, particularly for larger, high-grade soft-tissue sarcomas of the extremities [103]. The combination of doxorubicin and ifosfamide appears to be associated with the greatest benefit [104]. The overall efficacy of this approach is modest and it is associated with significant toxicity. Whether or not RIS patients would gain similar benefits from adjuvant chemotherapy is unknown because of the rarity of this disease, limiting the ability to perform a focused trial in RIS patients.
Neoadjuvant therapy in this setting may have several advantages over adjuvant administration. Primary tumors may regress, allowing a higher probability of R0 resection, less radical resection, or even resection of previously unresectable lesions. Chemosensitivity can be assessed, with early discontinuation of inactive therapy, sparing patients from potential treatment toxicity and allowing assessment of response to alternate agents. Micrometastatic disease may also be potentially treated using this approach, although no published reports are available regarding survival outcomes with this approach for RIS. The only randomized trial of neoadjuvant therapy for soft-tissue sarcoma was halted early as a result of slow accrual [105]. Among the 134 evaluable patients enrolled, there was no difference observed in the relapse-free or overall survival outcomes. At present, neoadjuvant therapy finds it primary use in patients with unresectable or marginally resectable disease. Quadros et al. [80] reported favorable outcomes for neoadjuvant chemotherapy followed by surgery to treat RIS. However, this was the only published article identified in our systematic literature search discussing outcomes of neoadjuvant chemotherapy in RIS patients. With neoadjuvant therapy, the tumor burden may be reduced such that surgical excision is facilitated, resulting in potentially less morbidity. However, complications with tissue quality may result because of radiation exposure. In this regard, such therapy may be relevant in the treatment of breast RIS, but decisions regarding the appropriateness of neoadjuvant chemotherapy should be made on an individual basis by the multidisciplinary team.
With adjuvant radiation therapy, histologic examination of surgical pathology specimens and the design of postoperative radiation therapy are more straightforward than after neoadjuvant radiation therapy. However, postoperative radiation therapy usually results in greater toxicities [106]. Additionally, radiation is believed to be less effective for RIS because of fibrotic changes resulting in an inadequate blood supply [87].
Treatment of metastatic or unresectable disease is, at present, inadequate. Such patients are routinely enrolled in trials of therapy for primary soft-tissue sarcomas. At present, single-agent anthracycline therapy is generally viewed as standard in patients with advanced soft-tissue sarcoma. A large meta-analysis of anthracycline-based therapy indicated a response rate of 26% and median overall survival time of 51 weeks [107].
Ifosfamide, an alkylating agent, may be used as salvage therapy or may be combined with doxorubicin. Ifosfamide-containing regimens yield results similar to those of single-agent doxorubicin, with a response rate of 25%, median progression-free survival time of 19 weeks, and median overall survival time of 54 weeks [108].
Considerable controversy exists regarding the benefit of combination therapy for advanced soft-tissue sarcomas with anthracyclines (primarily doxorubicin) and ifosfamide, but such regimens also have greater toxicity [108–110]. The studies published to date have been criticized for the relatively low doses of ifosfamide used.
Single-agent doxorubicin remains the standard first-line therapy for patients with advanced soft-tissue sarcomas. Ifosfamide may be added if a possible improvement in an individual patient's response justifies the greater toxicity and logistical complexity. Both drugs are associated with significant toxicity: dose-dependent cardiotoxicity, in the case of anthracyclines, and hemorrhagic cystitis and neurotoxicity, in the case of ifosfamide [109].
Several smaller, retrospective analyses indicated that breast RIS displays sensitivity to those agents [111–115]. RIA of the breast may be susceptible to taxane-based regimens, thus justifying their use in patients with metastatic disease [116]. Until more detailed molecular analysis allows personalized selection of therapy for breast RIS patients, the relative rarity of breast RIS suggests that treatment of advanced disease should be guided by general results obtained with soft-tissue sarcoma treatment. However, the overall role of chemotherapy in RIS treatment remains to be determined by additional clinical trials. With the development of novel chemotherapeutic agents, a combined approach may ultimately increase the probability of survival for RIS patients [5, 113, 117].
Role of Radiotherapy
Few studies evaluated radiotherapy and hyperthermia in RIS patients [118, 119]. Feigenberg et al. [100] reported that neoadjuvant hyperfractionated radiotherapy was successful in RIA patients, enhancing local control and allowing removal of as much twice-radiated tissue as possible. A recent study by Palta et al. [120] found similar results with hyperfractionated and accelerated radiation doses in RIA patients; the overall 5-year survival rate in 14 patients was 86%. Notably, radiotherapy in RIS patients had less impact than chemotherapy because of the patients' tolerance of the radiation dosage from their previous radiotherapy for their breast cancer [121, 122]. A second course of radiotherapy raises concerns about toxicities such as rib fracture, pneumonitis, and soft-tissue necrosis. We classified the evidence in support of radiotherapy in RIS patients as level 3a–5c.
Discussion
The optimal management of RIS—a rare and aggressive disease—is controversial [30, 88]. As with primary sarcoma patients, surgery remains crucial as the primary therapy for RIS [28]. For patients with nonmetastatic RIS, aggressive resection with widely negative margins is necessary [5, 30]. If the tumor infiltrates beyond the breast into the chest wall, then chest wall excision may also be required to achieve an R0 resection [30].
Low survival rates underscore the need for better detection and treatment. With regard to detection, the relatively long latency period for many RIS patients underscores the importance of long-term oncologic follow-up, exceeding 5 years after radiation, performed by skilled oncologists well versed in the evaluation of the radiated breast and chest wall. With regard to treatment, adverse prognostic variables suggesting the need for neoadjuvant or adjuvant therapy include tumor size, grade, and histologic type. Complete resection may not be possible because of extensive infiltration [28]. However, the effectiveness of chemotherapy in RIS patients is uncertain. We found no prospective randomized controlled trials of systemic therapy (radiotherapy or chemotherapy) in RIS patients. Long-term surveillance of skin changes (often sites of RIS) in the radiated field is important [123]. Much of the information on RIS that we found came from case reports and retrospective, single-institution cohort studies. Such evidence is low on the hierarchal levels of evidence, limiting our ability to reach a definitive conclusion on the best evidence-based practices in the treatment of RIS. Prospective, randomized trials, registry studies, and well-designed retrospective cohort studies are needed to better inform the treatment of RIS after breast cancer.
Conclusion
This systematic review of RIS of the breast evaluated evidenced-based treatment options for this rare disease. No published articles had level 1 or 2 evidence. Therefore, only level 3–5c evidence is available to guide clinicians. Patients with RIS have a worse prognosis than primary soft-tissue sarcoma patients. Surgery with widely negative margins remains the primary treatment of RIS. Unfortunately, the role of adjuvant and neoadjuvant chemotherapy remains uncertain; systemic therapy decision making for RIS is typically based on studies in primary sarcoma because of the rarity of this disease. Additionally, the role of reirradiation for RIS remains unclear because of the paucity of studies addressing this issue. This systematic review highlights the need for additional well-designed studies to inform the management of RIS.
Acknowledgment
The authors take full responsibility for the content of the paper but thank Mary Knatterud, Ph.D. (supported by the Department of Surgery of the University of Arizona), for her editorial assistance and copyediting.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Julie E. Lang
Provision of study material or patients: Julie E. Lang, Lee D. Cranmer
Collection and/or assembly of data: Julie E. Lang, Grishma R. Sheth, Lauren Grasso-LeBeau
Data analysis and interpretation: Julie E. Lang, Grishma R. Sheth, Lee D. Cranmer, Lauren Grasso-LeBeau
Manuscript writing: Julie E. Lang, Grishma R. Sheth, Lee D. Cranmer, Benjamin D. Smith, Lauren Grasso-LeBeau
Final approval of manuscript: Julie E. Lang, Grishma R. Sheth, Lee D. Cranmer, Benjamin D. Smith, Lauren Grasso-LeBeau
References
- 1.Fisher ER, Anderson S, Redmond C, et al. Ipsilateral breast tumor recurrence and survival following lumpectomy and irradiation: Pathological findings from NSABP protocol B-06. Semin Surg Oncol. 1992;8:161–166. [PubMed] [Google Scholar]
- 2.Yap J, Chuba PJ, Thomas R, et al. Sarcoma as a second malignancy after treatment for breast cancer. Int J Radiat Oncol Biol Phys. 2002;52:1231–1237. doi: 10.1016/s0360-3016(01)02799-7. [DOI] [PubMed] [Google Scholar]
- 3.Fodor J, Orosz Z, Szabó E, et al. Angiosarcoma after conservation treatment for breast carcinoma: Our experience and a review of the literature. J Am Acad Dermatol. 2006;54:499–504. doi: 10.1016/j.jaad.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 4.Velaj RH, DeLuca SA. Radiation-induced sarcoma. Am Fam Physician. 1987;36:129–130. [PubMed] [Google Scholar]
- 5.Erel E, Vlachou E, Athanasiadou M, et al. Management of radiation-induced sarcomas in a tertiary referral centre: A review of 25 cases. Breast. 2010;19:424–427. doi: 10.1016/j.breast.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Pierce SM, Recht A, Lingos TI, et al. Long-term radiation complications following conservative surgery (CS) and radiation therapy (RT) in patients with early stage breast cancer. Int J Radiat Oncol Biol Phys. 1992;23:915–923. doi: 10.1016/0360-3016(92)90895-o. [DOI] [PubMed] [Google Scholar]
- 7.Strobbe LJ, Peterse HL, van Tinteren H, et al. Angiosarcoma of the breast after conservation therapy for invasive cancer, the incidence and outcome. An unforseen sequela. Breast Cancer Res Treat. 1998;47:101–109. doi: 10.1023/a:1005997017102. [DOI] [PubMed] [Google Scholar]
- 8.Zucali R, Merson M, Placucci M, et al. Soft tissue sarcoma of the breast after conservative surgery and irradiation for early mammary cancer. Radiother Oncol. 1994;30:271–273. doi: 10.1016/0167-8140(94)90469-3. [DOI] [PubMed] [Google Scholar]
- 9.Penel N, Grosjean J, Robin YM, et al. Frequency of certain established risk factors in soft tissue sarcomas in adults: A prospective descriptive study of 658 cases. Sarcoma. 2008;2008:459386. doi: 10.1155/2008/459386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Smet S, Vandermeeren L, Christiaens MR, et al. Radiation-induced sarcoma: Analysis of 46 cases. Acta Chir Belg. 2008;108:574–579. doi: 10.1080/00015458.2008.11680288. [DOI] [PubMed] [Google Scholar]
- 11.Olcina M, Merck B, Giménez-Climent MJ, et al. Radiation-induced leiomyosarcoma after breast cancer treatment and TRAM flap reconstruction. Sarcoma. 2008;2008:456950. doi: 10.1155/2008/456950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Travis EL, Kreuther A, Young T, et al. Unusual postirradiation sarcoma of chest wall. Cancer. 1976;38:2269–2273. doi: 10.1002/1097-0142(197612)38:6<2269::aid-cncr2820380613>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 13.Cozen W, Bernstein L, Wang F, et al. The risk of angiosarcoma following primary breast cancer. Br J Cancer. 1999;81:532–536. doi: 10.1038/sj.bjc.6690726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chahin F, Paramesh A, Dwivedi A, et al. Angiosarcoma of the breast following breast preservation therapy and local radiation therapy for breast cancer. Breast J. 2001;7:120–123. doi: 10.1046/j.1524-4741.2001.007002120.x. [DOI] [PubMed] [Google Scholar]
- 15.Fineberg S, Rosen PP. Cutaneous angiosarcoma and atypical vascular lesions of the skin and breast after radiation therapy for breast carcinoma. Am J Clin Pathol. 1994;102:757–763. doi: 10.1093/ajcp/102.6.757. [DOI] [PubMed] [Google Scholar]
- 16.Sessions SC, Smink RD., Jr Cutaneous angiosarcoma of the breast after segmental mastectomy and radiation therapy. Arch Surg. 1992;127:1362–1363. doi: 10.1001/archsurg.1992.01420110114023. [DOI] [PubMed] [Google Scholar]
- 17.Pendlebury SC, Bilous M, Langlands AO. Sarcomas following radiation therapy for breast cancer: A report of three cases and a review of the literature. Int J Radiat Oncol Biol Phys. 1995;31:405–410. doi: 10.1016/0360-3016(95)93157-3. [DOI] [PubMed] [Google Scholar]
- 18.Blanchard DK, Reynolds C, Grant CS, et al. Radiation-induced breast sarcoma. Am J Surg. 2002;184:356–358. doi: 10.1016/s0002-9610(02)00943-1. [DOI] [PubMed] [Google Scholar]
- 19.Cahan WG, Woodard HQ, Higinbotham NL, et al. Sarcoma arising in irradiated bone; report of 11 cases. Cancer. 1948;1:3–29. doi: 10.1002/1097-0142(194805)1:1<3::aid-cncr2820010103>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 20.Hadorn DC, Baker D, Hodges JS, et al. Rating the quality of evidence for clinical practice guidelines. J Clin Epidemiol. 1996;49:749–754. doi: 10.1016/0895-4356(96)00019-4. [DOI] [PubMed] [Google Scholar]
- 21.Laskin WB, Silverman TA, Enzinger FM. Postradiation soft tissue sarcomas. An analysis of 53 cases. Cancer. 1988;62:2330–2340. doi: 10.1002/1097-0142(19881201)62:11<2330::aid-cncr2820621113>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 22.Chen KT, Hoffman KD, Hendricks EJ. Angiosarcoma following therapeutic irradiation. Cancer. 1979;44:2044–2048. doi: 10.1002/1097-0142(197912)44:6<2044::aid-cncr2820440614>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 23.Hardy TJ, An T, Brown PW, et al. Postirradiation sarcoma (malignant fibrous histiocytoma) of axilla. Cancer. 1978;42:118–124. doi: 10.1002/1097-0142(197807)42:1<118::aid-cncr2820420120>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 24.Tsuneyoshi M, Enjoji M. Postirradiation sarcoma (malignant fibrous histiocytoma) following breast carcinoma: An ultrastructural study of a case. Cancer. 1980;45:1419–1423. doi: 10.1002/1097-0142(19800315)45:6<1419::aid-cncr2820450620>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 25.Edeiken S, Russo DP, Knecht J, et al. Angiosarcoma after tylectomy and radiation therapy for carcinoma of the breast. Cancer. 1992;70:644–647. doi: 10.1002/1097-0142(19920801)70:3<644::aid-cncr2820700317>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 26.Virtanen A, Pukkala E, Auvinen A. Angiosarcoma after radiotherapy: A cohort study of 332,163 Finnish cancer patients. Br J Cancer. 2007;97:115–117. doi: 10.1038/sj.bjc.6603805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volk N, Pompe-Kirn V. Second primary cancers in breast cancer patients in Slovenia. Cancer Causes Control. 1997;8:764–770. doi: 10.1023/a:1018487506546. [DOI] [PubMed] [Google Scholar]
- 28.Thijssens KM, van Ginkel RJ, Suurmeijer AJ, et al. Radiation-induced sarcoma: A challenge for the surgeon. Ann Surg Oncol. 2005;12:237–245. doi: 10.1245/ASO.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 29.Rosen PP, Kimmel M, Ernsberger D. Mammary angiosarcoma. The prognostic significance of tumor differentiation. Cancer. 1988;62:2145–2151. doi: 10.1002/1097-0142(19881115)62:10<2145::aid-cncr2820621014>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 30.Barrow BJ, Janjan NA, Gutman H, et al. Role of radiotherapy in sarcoma of the breast—a retrospective review of the M.D. Anderson experience. Radiother Oncol. 1999;52:173–178. doi: 10.1016/s0167-8140(99)00070-5. [DOI] [PubMed] [Google Scholar]
- 31.Biswas T, Tang P, Muhs A, et al. Angiosarcoma of the breast: A rare clinicopathological entity. Am J Clin Oncol. 2009;32:582–586. doi: 10.1097/COC.0b013e3181967f09. [DOI] [PubMed] [Google Scholar]
- 32.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 33.Kirova YM, Vilcoq JR, Asselain B, et al. Radiation-induced sarcomas after radiotherapy for breast carcinoma: A large-scale single-institution review. Cancer. 2005;104:856–863. doi: 10.1002/cncr.21223. [DOI] [PubMed] [Google Scholar]
- 34.McGowan TS, Cummings BJ, O'Sullivan B, et al. An analysis of 78 breast sarcoma patients without distant metastases at presentation. Int J Radiat Oncol Biol Phys. 2000;46:383–390. doi: 10.1016/s0360-3016(99)00444-7. [DOI] [PubMed] [Google Scholar]
- 35.Rubino C, Shamsaldin A, Lê MG, et al. Radiation dose and risk of soft tissue and bone sarcoma after breast cancer treatment. Breast Cancer Res Treat. 2005;89:277–288. doi: 10.1007/s10549-004-2472-8. [DOI] [PubMed] [Google Scholar]
- 36.Holt GE, Thomson AB, Griffin AM, et al. Multifocality and multifocal postradiation sarcomas. Clin Orthop Relat Res. 2006;450:67–75. doi: 10.1097/01.blo.0000229301.75018.84. [DOI] [PubMed] [Google Scholar]
- 37.Karlsson P, Holmberg E, Samuelsson A, et al. Soft tissue sarcoma after treatment for breast cancer—a Swedish population-based study. Eur J Cancer. 1998;34:2068–2075. doi: 10.1016/s0959-8049(98)00319-0. [DOI] [PubMed] [Google Scholar]
- 38.Tahir M, Hendry P, Baird L, et al. Radiation induced angiosarcoma a sequela of radiotherapy for breast cancer following conservative surgery. Int Semin Surg Oncol. 2006;3:26. doi: 10.1186/1477-7800-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fisher B, Anderson SJ. The breast cancer alternative hypothesis: Is there evidence to justify replacing it? J Clin Oncol. 2010;28:366–374. doi: 10.1200/JCO.2009.26.8292. [DOI] [PubMed] [Google Scholar]
- 40.Smith BD, Pan IW, Shih YC, et al. Adoption of intensity-modulated radiation therapy for breast cancer in the United States. J Natl Cancer Inst. 2011;103:798–809. doi: 10.1093/jnci/djr100. [DOI] [PubMed] [Google Scholar]
- 41.Krueger EA, Fraass BA, McShan DL, et al. Potential gains for irradiation of chest wall and regional nodes with intensity modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2003;56:1023–1037. doi: 10.1016/s0360-3016(03)00183-4. [DOI] [PubMed] [Google Scholar]
- 42.Pignol JP, Olivotto I, Rakovitch E, et al. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol. 2008;26:2085–2092. doi: 10.1200/JCO.2007.15.2488. [DOI] [PubMed] [Google Scholar]
- 43.Smith BD, Bentzen SM, Correa CR, et al. Fractionation for whole breast irradiation: An American Society for Radiation Oncology (ASTRO) evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;81:59–68. doi: 10.1016/j.ijrobp.2010.04.042. [DOI] [PubMed] [Google Scholar]
- 44.de Bree E, van Coevorden F, Peterse JL, et al. Bilateral angiosarcoma of the breast after conservative treatment of bilateral invasive carcinoma: Genetic predisposition? Eur J Surg Oncol. 2002;28:392–395. doi: 10.1053/ejso.2001.1249. [DOI] [PubMed] [Google Scholar]
- 45.Valagussa P, Tancini G, Bonadonna G. Second malignancies after CMF for resectable breast cancer. J Clin Oncol. 1987;5:1138–1142. doi: 10.1200/JCO.1987.5.8.1138. [DOI] [PubMed] [Google Scholar]
- 46.Zelek L, Llombart-Cussac A, Terrier P, et al. Prognostic factors in primary breast sarcomas: A series of patients with long-term follow-up. J Clin Oncol. 2003;21:2583–2588. doi: 10.1200/JCO.2003.06.080. [DOI] [PubMed] [Google Scholar]
- 47.Karlsson P, Holmberg E, Johansson KA, et al. Soft tissue sarcoma after treatment for breast cancer. Radiother Oncol. 1996;38:25–31. doi: 10.1016/0167-8140(95)01663-5. [DOI] [PubMed] [Google Scholar]
- 48.Huang J, Mackillop WJ. Increased risk of soft tissue sarcoma after radiotherapy in women with breast carcinoma. Cancer. 2001;92:172–180. doi: 10.1002/1097-0142(20010701)92:1<172::aid-cncr1306>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 49.Moskaluk CA, Merino MJ, Danforth DN, et al. Low-grade angiosarcoma of the skin of the breast: A complication of lumpectomy and radiation therapy for breast carcinoma. Hum Pathol. 1992;23:710–714. doi: 10.1016/0046-8177(92)90331-v. [DOI] [PubMed] [Google Scholar]
- 50.Badwe RA, Hanby AM, Fentiman IS, et al. Angiosarcoma of the skin overlying an irradiated breast. Breast Cancer Res Treat. 1991;19:69–72. doi: 10.1007/BF01975207. [DOI] [PubMed] [Google Scholar]
- 51.Gherardi G, Rossi S, Perrone S, et al. Angiosarcoma after breast-conserving therapy: Fine-needle aspiration biopsy, immunocytochemistry, and clinicopathologic correlates. Cancer. 2005;105:145–151. doi: 10.1002/cncr.21035. [DOI] [PubMed] [Google Scholar]
- 52.Stokkel MP, Peterse HL. Angiosarcoma of the breast after lumpectomy and radiation therapy for adenocarcinoma. Cancer. 1992;69:2965–2968. doi: 10.1002/1097-0142(19920615)69:12<2965::aid-cncr2820691216>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 53.Otis CN, Peschel R, McKhann C, et al. The rapid onset of cutaneous angiosarcoma after radiotherapy for breast carcinoma. Cancer. 1986;57:2130–2134. doi: 10.1002/1097-0142(19860601)57:11<2130::aid-cncr2820571108>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 54.Sheppard DG, Libshitz HI. Post-radiation sarcomas: A review of the clinical and imaging features in 63 cases. Clin Radiol. 2001;56:22–29. doi: 10.1053/crad.2000.0599. [DOI] [PubMed] [Google Scholar]
- 55.Bolin DJ, Lukas GM. Low-grade dermal angiosarcoma of the breast following radiotherapy. Am Surg. 1996;62:668–672. [PubMed] [Google Scholar]
- 56.Gladdy RA, Qin LX, Moraco N, et al. Do radiation-associated soft tissue sarcomas have the same prognosis as sporadic soft tissue sarcomas? J Clin Oncol. 2010;28:2064–2069. doi: 10.1200/JCO.2009.25.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kunkel T, Mylonas I, Mayr D, et al. Recurrence of secondary angiosarcoma in a patient with post-radiated breast for breast cancer. Arch Gynecol Obstet. 2008;278:497–501. doi: 10.1007/s00404-008-0605-8. [DOI] [PubMed] [Google Scholar]
- 58.Soldic Z, Salopek D, Jazvic M, et al. Parenchymal post-irradiation angiosarcoma: A case report. Acta Clin Croat. 2009;48:433–437. [PubMed] [Google Scholar]
- 59.Sener SF, Milos S, Feldman JL, et al. The spectrum of vascular lesions in the mammary skin, including angiosarcoma, after breast conservation treatment for breast cancer. J Am Coll Surg. 2001;193:22–28. doi: 10.1016/s1072-7515(01)00863-8. [DOI] [PubMed] [Google Scholar]
- 60.Lamblin G, Oteifa M, Zinzindohoue C, et al. Angiosarcoma after conservative treatment and radiation therapy for adenocarcinoma of the breast. Eur J Surg Oncol. 2001;27:146–151. doi: 10.1053/ejso.2000.1073. [DOI] [PubMed] [Google Scholar]
- 61.Lucas DR. Angiosarcoma, radiation-associated angiosarcoma, and atypical vascular lesion. Arch Pathol Lab Med. 2009;133:1804–1809. doi: 10.5858/133.11.1804. [DOI] [PubMed] [Google Scholar]
- 62.Mermershtain W, Cohen AD, Koretz M, et al. Cutaneous angiosarcoma of breast after lumpectomy, axillary lymph node dissection, and radiotherapy for primary breast carcinoma: Case report and review of the literature. Am J Clin Oncol. 2002;25:597–598. doi: 10.1097/00000421-200212000-00014. [DOI] [PubMed] [Google Scholar]
- 63.West JG, Qureshi A, West JE, et al. Risk of angiosarcoma following breast conservation: A clinical alert. Breast J. 2005;11:115–123. doi: 10.1111/j.1075-122X.2005.21548.x. [DOI] [PubMed] [Google Scholar]
- 64.Moore A, Hendon A, Hester M, et al. Secondary angiosarcoma of the breast: Can imaging findings aid in the diagnosis? Breast J. 2008;14:293–298. doi: 10.1111/j.1524-4741.2008.00577.x. [DOI] [PubMed] [Google Scholar]
- 65.Hanasono MM, Osborne MP, Dielubanza EJ, et al. Radiation-induced angiosarcoma after mastectomy and TRAM flap breast reconstruction. Ann Plast Surg. 2005;54:211–214. doi: 10.1097/01.sap.0000134751.73260.3a. [DOI] [PubMed] [Google Scholar]
- 66.Rao J, Dekoven JG, Beatty JD, et al. Cutaneous angiosarcoma as a delayed complication of radiation therapy for carcinoma of the breast. J Am Acad Dermatol. 2003;49:532–538. doi: 10.1067/s0190-9622(03)00428-6. [DOI] [PubMed] [Google Scholar]
- 67.Deutsch M, Rosenstein MM. Angiosarcoma of the breast mimicking radiation dermatitis arising after lumpectomy and breast irradiation: A case report. Am J Clin Oncol. 1998;21:608–609. doi: 10.1097/00000421-199812000-00016. [DOI] [PubMed] [Google Scholar]
- 68.Majeski J, Austin RM, Fitzgerald RH. Cutaneous angiosarcoma in an irradiated breast after breast conservation therapy for cancer: Association with chronic breast lymphedema. J Surg Oncol. 2000;74:208–212. doi: 10.1002/1096-9098(200007)74:3<208::aid-jso10>3.0.co;2-2. discussion 212–213. [DOI] [PubMed] [Google Scholar]
- 69.Roukema JA, Leenen LP, Kuizinga MC, et al. Angiosarcoma of the irradiated breast: A new problem after breast conserving therapy? Neth J Surg. 1991;43:114–116. [PubMed] [Google Scholar]
- 70.Autio P, Kariniemi AL. Angiosarcoma. A rare secondary malignancy after breast cancer treatment. Eur J Dermatol. 1999;9:118–121. [PubMed] [Google Scholar]
- 71.Georgiannos SN, Sheaff M. Angiosarcoma of the breast: A 30 year perspective with an optimistic outlook. Br J Plast Surg. 2003;56:129–134. doi: 10.1016/s0007-1226(03)00025-0. [DOI] [PubMed] [Google Scholar]
- 72.Williams EV, Banerjee D, Dallimore N, et al. Angiosarcoma of the breast following radiation therapy. Eur J Surg Oncol. 1999;25:221–222. doi: 10.1053/ejso.1998.0631. [DOI] [PubMed] [Google Scholar]
- 73.Fant J, Grant M, May S, et al. Angiosarcoma of the breast: Mammographic, clinical, and pathologic correlation. Breast J. 2003;9:252–253. doi: 10.1046/j.1524-4741.2003.09316.x. [DOI] [PubMed] [Google Scholar]
- 74.Stewart FW, Treves N. Lymphangiosarcoma in postmastectomy lymphedema; a report of six cases in elephantiasis chirurgica. Cancer. 1948;1:64–81. doi: 10.1002/1097-0142(194805)1:1<64::aid-cncr2820010105>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 75.Vesoulis Z, Cunliffe C. Fine-needle aspiration biopsy of postradiation epithelioid angiosarcoma of breast. Diagn Cytopathol. 2000;22:172–175. doi: 10.1002/(sici)1097-0339(20000301)22:3<172::aid-dc8>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 76.Esler-Brauer L, Jaggernauth W, Zeitouni NC. Angiosarcoma developing after conservative treatment for breast carcinoma: Case report with review of the current literature. Dermatol Surg. 2007;33:749–755. doi: 10.1111/j.1524-4725.2007.33156.x. [DOI] [PubMed] [Google Scholar]
- 77.Nakamura R, Nagashima T, Sakakibara M, et al. Angiosarcoma arising in the breast following breast-conserving surgery with radiation for breast carcinoma. Breast Cancer. 2007;14:245–249. doi: 10.2325/jbcs.914. [DOI] [PubMed] [Google Scholar]
- 78.Mills TD, Vinnicombe SJ, Wells CA, et al. Angiosarcoma of the breast after wide local excision and radiotherapy for breast carcinoma. Clin Radiol. 2002;57:63–66. doi: 10.1053/crad.2001.0666. [DOI] [PubMed] [Google Scholar]
- 79.Marchant LK, Orel SG, Perez-Jaffe LA, et al. Bilateral angiosarcoma of the breast on MR imaging. AJR Am J Roentgenol. 1997;169:1009–1010. doi: 10.2214/ajr.169.4.9308452. [DOI] [PubMed] [Google Scholar]
- 80.Quadros CA, Vasconcelos A, Andrade R, et al. Good outcome after neoadjuvant chemotherapy and extended surgical resection for a large radiation-induced high-grade breast sarcoma. Int Semin Surg Oncol. 2006;3:18. doi: 10.1186/1477-7800-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Edge SB, Byrd DR, Compton CC, et al. AJCC: Soft Tissue Sarcoma. New York: Springer; 2010. pp. 291–298. [Google Scholar]
- 82.Lagrange JL, Ramaioli A, Chateau MC, et al. Sarcoma after radiation therapy: Retrospective multiinstitutional study of 80 histologically confirmed cases. Radiation Therapist and Pathologist Groups of the Fédération Nationale des Centres de Lutte Contre le Cancer. Radiology. 2000;216:197–205. doi: 10.1148/radiology.216.1.r00jl02197. [DOI] [PubMed] [Google Scholar]
- 83.Billings SD, McKenney JK, Folpe AL, et al. Cutaneous angiosarcoma following breast-conserving surgery and radiation: An analysis of 27 cases. Am J Surg Pathol. 2004;28:781–788. doi: 10.1097/01.pas.0000126055.33916.0b. [DOI] [PubMed] [Google Scholar]
- 84.Marchal C, Weber B, de Lafontan B, et al. Nine breast angiosarcomas after conservative treatment for breast carcinoma: A survey from French Comprehensive Cancer Centers. Int J Radiat Oncol Biol Phys. 1999;44:113–119. doi: 10.1016/s0360-3016(98)00537-9. [DOI] [PubMed] [Google Scholar]
- 85.Brady MS, Gaynor JJ, Brennan MF. Radiation-associated sarcoma of bone and soft tissue. Arch Surg. 1992;127:1379–1385. doi: 10.1001/archsurg.1992.01420120013002. [DOI] [PubMed] [Google Scholar]
- 86.Brenin CM, Small W, Jr, Talamonti MS, et al. Radiation-induced sarcoma following treatment of breast cancer. Cancer Control. 1998;5:425–432. doi: 10.1177/107327489800500505. [DOI] [PubMed] [Google Scholar]
- 87.Kuten A, Sapir D, Cohen Y, et al. Postirradiation soft tissue sarcoma occurring in breast cancer patients: Report of seven cases and results of combination chemotherapy. J Surg Oncol. 1985;28:168–171. doi: 10.1002/jso.2930280304. [DOI] [PubMed] [Google Scholar]
- 88.Khan M, Chandramala R, Sharma R, et al. Radiation-induced spindle cell sarcoma: A rare case report. Indian J Dent Res. 2009;20:380–384. doi: 10.4103/0970-9290.57366. [DOI] [PubMed] [Google Scholar]
- 89.Bjerkehagen B, Smeland S, Walberg L, et al. Radiation-induced sarcoma: 25-year experience from the Norwegian Radium Hospital. Acta Oncol. 2008;47:1475–1482. doi: 10.1080/02841860802047387. [DOI] [PubMed] [Google Scholar]
- 90.Cha C, Antonescu CR, Quan ML, et al. Long-term results with resection of radiation-induced soft tissue sarcomas. Ann Surg. 2004;239:903–909. doi: 10.1097/01.sla.0000128686.51815.8b. discussion 909–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Plotti F, Di Donato V, Zullo MA, et al. An unusual case of secondary fibrosarcoma after treatment for breast cancer. Gynecol Oncol. 2006;103:1133–1136. doi: 10.1016/j.ygyno.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 92.Borman H, Safak T, Ertoy D. Fibrosarcoma following radiotherapy for breast carcinoma: A case report and review of the literature. Ann Plast Surg. 1998;41:201–204. doi: 10.1097/00000637-199808000-00015. [DOI] [PubMed] [Google Scholar]
- 93.Souba WW, McKenna RJ, Jr, Meis J, et al. Radiation-induced sarcomas of the chest wall. Cancer. 1986;57:610–615. doi: 10.1002/1097-0142(19860201)57:3<610::aid-cncr2820570336>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 94.Chapelier AR, Bacha EA, de Montpreville VT, et al. Radical resection of radiation-induced sarcoma of the chest wall: Report of 15 cases. Ann Thorac Surg. 1997;63:214–219. doi: 10.1016/s0003-4975(96)00927-7. [DOI] [PubMed] [Google Scholar]
- 95.Adhikari D, Hajdu SI, Levine D. Post-radiation angiosarcoma and bilateral mastectomy. Ann Clin Lab Sci. 2002;32:428–433. [PubMed] [Google Scholar]
- 96.Wijnmaalen A, van Ooijen B, van Geel BN, et al. Angiosarcoma of the breast following lumpectomy, axillary lymph node dissection, and radiotherapy for primary breast cancer: Three case reports and a review of the literature. Int J Radiat Oncol Biol Phys. 1993;26:135–139. doi: 10.1016/0360-3016(93)90184-w. [DOI] [PubMed] [Google Scholar]
- 97.Colville RJ, Ramsden A, Malcolm A, et al. Angiosarcoma of the breast after quadrantectomy and postoperative radiotherapy for carcinoma. Br J Plast Surg. 2000;53:622–624. doi: 10.1054/bjps.2000.3406. [DOI] [PubMed] [Google Scholar]
- 98.Turner WH, Greenall MJ. Sarcoma induced by radiotherapy after breast conservation surgery. Br J Surg. 1991;78:1317–1318. doi: 10.1002/bjs.1800781113. [DOI] [PubMed] [Google Scholar]
- 99.Cafiero F, Gipponi M, Peressini A, et al. Radiation-associated angiosarcoma: Diagnostic and therapeutic implications—two case reports and a review of the literature. Cancer. 1996;77:2496–2502. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2496::AID-CNCR12>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 100.Feigenberg SJ, Mendenhall NP, Reith JD, et al. Angiosarcoma after breast-conserving therapy: Experience with hyperfractionated radiotherapy. Int J Radiat Oncol Biol Phys. 2002;52:620–626. doi: 10.1016/s0360-3016(01)02669-4. [DOI] [PubMed] [Google Scholar]
- 101.Yap BS, Yap HY, McBride CM, et al. Chemotherapy for postmastectomy lymphangiosarcoma. Cancer. 1981;47:853–856. doi: 10.1002/1097-0142(19810301)47:5<853::aid-cncr2820470507>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 102.Mano MS, Fraser G, Kerr J, et al. Radiation-induced angiosarcoma of the breast shows major response to docetaxel after failure of anthracycline-based chemotherapy. Breast. 2006;15:117–118. doi: 10.1016/j.breast.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 103.Pervaiz N, Colterjohn N, Farrokhyar F, et al. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008;113:573–581. doi: 10.1002/cncr.23592. [DOI] [PubMed] [Google Scholar]
- 104.Santoro A, Tursz T, Mouridsen H, et al. Doxorubicin versus CYVADIC versus doxorubicin plus ifosfamide in first-line treatment of advanced soft tissue sarcomas: A randomized study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol. 1995;13:1537–1545. doi: 10.1200/JCO.1995.13.7.1537. [DOI] [PubMed] [Google Scholar]
- 105.Gortzak E, Azzarelli A, Buesa J, et al. A randomised phase II study on neo-adjuvant chemotherapy for ‘high-risk’ adult soft-tissue sarcoma. Eur J Cancer. 2001;37:1096–1103. doi: 10.1016/s0959-8049(01)00083-1. [DOI] [PubMed] [Google Scholar]
- 106.DeLaney TF. Optimizing radiation therapy and post-treatment function in the management of extremity soft tissue sarcoma. Curr Treat Options Oncol. 2004;5:463–476. doi: 10.1007/s11864-004-0035-1. [DOI] [PubMed] [Google Scholar]
- 107.Van Glabbeke M, van Oosterom AT, Oosterhuis JW, et al. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: An analysis of 2,185 patients treated with anthracycline-containing first-line regimens—a European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group study. J Clin Oncol. 1999;17:150–157. doi: 10.1200/JCO.1999.17.1.150. [DOI] [PubMed] [Google Scholar]
- 108.Sleijfer S, Ouali M, van Glabbeke M, et al. Prognostic and predictive factors for outcome to first-line ifosfamide-containing chemotherapy for adult patients with advanced soft tissue sarcomas: An exploratory, retrospective analysis on large series from the European Organization for Research and Treatment of Cancer–Soft Tissue and Bone Sarcoma Group (EORTC-STBSG) Eur J Cancer. 2010;46:72–83. doi: 10.1016/j.ejca.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 109.Antman K, Crowley J, Balcerzak SP, et al. An intergroup phase III randomized study of doxorubicin and dacarbazine with or without ifosfamide and mesna in advanced soft tissue and bone sarcomas. J Clin Oncol. 1993;11:1276–1285. [Google Scholar]
- 110.Edmonson JH, Ryan LM, Blum RH, et al. Randomized comparison of doxorubicin alone versus ifosfamide plus doxorubicin or mitomycin, doxorubicin, and cisplatin against advanced soft tissue sarcomas. J Clin Oncol. 1993;11:1269–1275. [Google Scholar]
- 111.Mouridsen HT, Bastholt L, Somers R, et al. Adriamycin versus epirubicin in advanced soft tissue sarcomas. A randomized phase II/phase III study of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer Clin Oncol. 1987;23:1477–1483. doi: 10.1016/0277-5379(87)90089-7. [DOI] [PubMed] [Google Scholar]
- 112.Schlemmer M, Reichardt P, Verweij J, et al. Paclitaxel in patients with advanced angiosarcomas of soft tissue: A retrospective study of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer. 2008;44:2433–2436. doi: 10.1016/j.ejca.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 113.Okuno SH, Edmonson JH. Progress in the systemic treatment of advanced soft-tissue sarcomas. Cancer Control. 1998;5:34–39. [PubMed] [Google Scholar]
- 114.Skubitz KM, Haddad PA. Paclitaxel and pegylated-liposomal doxorubicin are both active in angiosarcoma. Cancer. 2005;104:361–366. doi: 10.1002/cncr.21140. [DOI] [PubMed] [Google Scholar]
- 115.Verweij J, Lee SM, Ruka W, et al. Randomized phase II study of docetaxel versus doxorubicin in first- and second-line chemotherapy for locally advanced or metastatic soft tissue sarcomas in adults: A study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol. 2000;18:2081–2086. doi: 10.1200/JCO.2000.18.10.2081. [DOI] [PubMed] [Google Scholar]
- 116.Gambini D, Visintin R, Locatelli E, et al. Paclitaxel-dependent prolonged and persistent complete remission four years from first recurrence of secondary breast angiosarcoma. Tumori. 2009;95:828–831. doi: 10.1177/030089160909500631. [DOI] [PubMed] [Google Scholar]
- 117.Neuhaus SJ, Pinnock N, Giblin V, et al. Treatment and outcome of radiation-induced soft-tissue sarcomas at a specialist institution. Eur J Surg Oncol. 2009;35:654–659. doi: 10.1016/j.ejso.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 118.Buatti JM, Harari PM, Leigh BR, et al. Radiation-induced angiosarcoma of the breast. Case report and review of the literature. Am J Clin Oncol. 1994;17:444–447. doi: 10.1097/00000421-199410000-00018. [DOI] [PubMed] [Google Scholar]
- 119.Taat CW, van Toor BS, Slors JF, et al. Dermal angiosarcoma of the breast: A complication of primary radiotherapy? Eur J Surg Oncol. 1992;18:391–395. [PubMed] [Google Scholar]
- 120.Palta M, Morris CG, Grobmyer SR, et al. Angiosarcoma after breast-conserving therapy: Long-term outcomes with hyperfractionated radiotherapy. Cancer. 2010;116:1872–1878. doi: 10.1002/cncr.24995. [DOI] [PubMed] [Google Scholar]
- 121.Schulz U, Gokel JM, Poleska W. Soft tissue sarcomas after radiation treatment for breast cancer. Three case studies and review of literature. Strahlenther Onkol. 2000;176:144–149. doi: 10.1007/pl00002340. [DOI] [PubMed] [Google Scholar]
- 122.Givens SS, Woo SY, Huang LY, et al. Non-metastatic Ewing's sarcoma: Twenty years of experience suggests that surgery is a prime factor for successful multimodality therapy. Int J Oncol. 1999;14:1039–1043. doi: 10.3892/ijo.14.6.1039. [DOI] [PubMed] [Google Scholar]
- 123.de Giorgi V, Santi R, Grazzini M, et al. Synchronous angiosarcoma, melanoma and morphea of the breast skin 14 years after radiotherapy for mammary carcinoma. Acta Derm Venereol. 2010;90:283–286. doi: 10.2340/00015555-0841. [DOI] [PubMed] [Google Scholar]
- 124.Amendola BE, Amendola MA, McClatchey KD, et al. Radiation-associated sarcoma: A review of 23 patients with postradiation sarcoma over a 50-year period. Am J Clin Oncol. 1989;12:411–415. [PubMed] [Google Scholar]
- 125.Davidson T, Westbury G, Harmer CL. Radiation-induced soft-tissue sarcoma. Br J Surg. 1986;73:308–309. doi: 10.1002/bjs.1800730420. [DOI] [PubMed] [Google Scholar]
- 126.Wiklund TA, Blomqvist CP, Räty J, et al. Postirradiation sarcoma. Analysis of a nationwide cancer registry material. Cancer. 1991;68:524–531. doi: 10.1002/1097-0142(19910801)68:3<524::aid-cncr2820680313>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 127.Kim JH, Chu FC, Woodard HQ, et al. Radiation-induced soft-tissue and bone sarcoma. Radiology. 1978;129:501–508. doi: 10.1148/129.2.501. [DOI] [PubMed] [Google Scholar]
- 128.Murray EM, Werner D, Greeff EA, et al. Postradiation sarcomas: 20 cases and a literature review. Int J Radiat Oncol Biol Phys. 1999;45:951–961. doi: 10.1016/s0360-3016(99)00279-5. [DOI] [PubMed] [Google Scholar]
- 129.Kurtz JM, Amalric R, Brandone H, et al. Contralateral breast cancer and other second malignancies in patients treated by breast-conserving therapy with radiation. Int J Radiat Oncol Biol Phys. 1988;15:277–284. doi: 10.1016/s0360-3016(98)90005-0. [DOI] [PubMed] [Google Scholar]
- 130.Inoue YZ, Frassica FJ, Sim FH, et al. Clinicopathologic features and treatment of postirradiation sarcoma of bone and soft tissue. J Surg Oncol. 2000;75:42–50. doi: 10.1002/1096-9098(200009)75:1<42::aid-jso8>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 131.Komdeur R, Hoekstra HJ, Molenaar WM, et al. Clinicopathologic assessment of postradiation sarcomas: KIT as a potential treatment target. Clin Cancer Res. 2003;9:2926–2932. [PubMed] [Google Scholar]
- 132.Tarkkanen M, Wiklund TA, Virolainen MJ, et al. Comparative genomic hybridization of postirradiation sarcomas. Cancer. 2001;92:1992–1998. doi: 10.1002/1097-0142(20011001)92:7<1992::aid-cncr1719>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 133.Bloechle C, Peiper M, Schwarz R, et al. Post-irradiation soft tissue sarcoma. Eur J Cancer. 1995;31:31–34. doi: 10.1016/0959-8049(94)00378-i. [DOI] [PubMed] [Google Scholar]
- 134.Perez-Ruiz E, Ribelles N, Sanchez-Muñoz A, et al. Response to paclitaxel in a radiotherapy-induced breast angiosarcoma. Acta Oncol. 2009;48:1078–1079. doi: 10.1080/02841860902777115. [DOI] [PubMed] [Google Scholar]
- 135.Lo TC, Silverman ML, Edelstein A. Postirradiation hemangiosarcoma of the chest wall. Report of a case. Acta Radiol Oncol. 1985;24:237–240. doi: 10.3109/02841868509134393. [DOI] [PubMed] [Google Scholar]
- 136.Benda JA, Al-Jurf AS, Benson AB., 3rd Angiosarcoma of the breast following segmental mastectomy complicated by lymphedema. Am J Clin Pathol. 1987;87:651–655. doi: 10.1093/ajcp/87.5.651. [DOI] [PubMed] [Google Scholar]
- 137.Hamels J, Blondiau P, Mirgaux M. Cutaneous angiosarcoma arising in a mastectomy scar after therapeutic irradiation. Bull Cancer. 1981;68:353–356. [PubMed] [Google Scholar]
- 138.Shaikh NA, Beaconsfield T, Walker M, et al. Postirradiation angiosarcoma of the breast—a case report. Eur J Surg Oncol. 1988;14:449–451. [PubMed] [Google Scholar]
- 139.Rubin E, Maddox WA, Mazur MT. Cutaneous angiosarcoma of the breast 7 years after lumpectomy and radiation therapy. Radiology. 1990;174:258–260. doi: 10.1148/radiology.174.1.2152984. [DOI] [PubMed] [Google Scholar]
- 140.Del Mastro L, Garrone O, Guenzi M, et al. Angiosarcoma of the residual breast after conservative surgery and radiotherapy for primary carcinoma. Ann Oncol. 1994;5:163–165. doi: 10.1093/oxfordjournals.annonc.a058770. [DOI] [PubMed] [Google Scholar]
- 141.Cwikiel M, Stene-Hurtzen C, Albertsson M, et al. Angiosarcoma as a sequela of breast cancer treatment with lumpectomy and radiotherapy. The Breast. 1997;6:307–309. [Google Scholar]
- 142.Roncadin M, Massarut S, Perin T, et al. Breast angiosarcoma after conservative surgery, radiotherapy and prosthesis implant. Acta Oncol. 1998;37:209–211. doi: 10.1080/028418698429801. [DOI] [PubMed] [Google Scholar]
- 143.Parker RG, Barsky SH, Bennion R. Angiosarcoma developing in a breast after conservation treatment for breast cancer. Am J Clin Oncol. 2003;26:486–488. doi: 10.1097/01.coc.0000037738.84777.E5. [DOI] [PubMed] [Google Scholar]
- 144.Weber B, Marchal C. Three cases of breast angiosarcomas after breast-conserving treatment for carcinoma. Radiother Oncol. 1995;37:250–252. doi: 10.1016/0167-8140(96)81281-3. [DOI] [PubMed] [Google Scholar]
- 145.Iwasaki K, Nagamitsu S, Tsuneyoshi M. Postirradiation fibrosarcoma following radical mastectomy. Jpn J Surg. 1978;8:73–77. doi: 10.1007/BF02469338. [DOI] [PubMed] [Google Scholar]
- 146.Arbabi L, Warhol MJ. Pleomorphic liposarcoma following radiotherapy for breast carcinoma. Cancer. 1982;49:878–880. doi: 10.1002/1097-0142(19820301)49:5<878::aid-cncr2820490510>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 147.Hatfield PM, Schulz MD. Postirradiation sarcoma. Including 5 cases after x-ray therapy of breast carcinoma. Radiology. 1970;96:593–602. doi: 10.1148/96.3.593. [DOI] [PubMed] [Google Scholar]
- 148.Moe M, Bertelli G. Breast angiosarcoma following lumpectomy and radiotherapy for breast cancer: A case with short latent period and false negative result on biopsies. Ann Oncol. 2007;18:801. doi: 10.1093/annonc/mdl482. [DOI] [PubMed] [Google Scholar]
- 149.Hunter TB, Martin PC, Dietzen CD, et al. Angiosarcoma of the breast. Two case reports and a review of the literature. Cancer. 1985;56:2099–2106. doi: 10.1002/1097-0142(19851015)56:8<2099::aid-cncr2820560836>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 150.Tomasini C, Grassi M, Pippione M. Cutaneous angiosarcoma arising in an irradiated breast. Case report and review of the literature. Dermatology. 2004;209:208–214. doi: 10.1159/000079891. [DOI] [PubMed] [Google Scholar]
- 151.Horii R, Fukuuchi A, Nishi T, et al. A case of malignant fibrous histiocytoma after breast conserving therapy for breast cancer. Breast Cancer. 2000;7:75–77. doi: 10.1007/BF02967192. [DOI] [PubMed] [Google Scholar]
- 152.Goette DK, Deffer TA. Postirradiation malignant fibrous histiocytoma. Arch Dermatol. 1985;121:535–538. [PubMed] [Google Scholar]
- 153.Luzzatto R, Grossmann S, Scholl JG, et al. Postradiation pleomorphic malignant fibrous histiocytoma of the breast. Acta Cytol. 1986;30:48–50. [PubMed] [Google Scholar]
- 154.Inui K, Morimoto T, Komaki K, et al. [postirradiation malignant fibrous histiocytoma following mastectomy in breast cancer] Nihon Geka Gakkai Zasshi. 1988;89:1722–1725. In Japanese. [PubMed] [Google Scholar]