Patients treated in a palliative care unit were compared with those treated by a palliative care consultation service at the same center. The coexistence of the two institutions at one hospital contributed to the goal of ensuring optimal palliative care for patients in complex and challenging clinical situations.
Keywords: Palliative care, Terminal care, Organization and administration, Health planning guidelines, Institutionalization
Abstract
Background.
Palliative care (PC) infrastructure has developed differently around the globe. Whereas some institutions consider the palliative care unit (PCU) a valuable component, others report that the sole provision of a state-of-the art palliative care consultation service (PCCS) suffices to adequately care for the severely ill and dying.
Objective.
To aid institutional planning, this study aimed at gathering patient data to distinguish assignments of a concomitantly run PCU and PCCS at a large hospital and academic medical center.
Methods.
Demographics, Eastern Cooperative Oncology Group performance status, symptom/problem burden, discharge modality, and team satisfaction with care for all 601 PCU and 851 PCCS patients treated in 2009 and 2010 were retrospectively analyzed.
Results.
Patients admitted to the PCU versus those consulted by the PCCS: (a) had a significantly worse performance status (odds ratio [OR], 1.48); (b) were significantly more likely to suffer from severe symptoms and psychosocial problems (OR, 2.05), in particular concerning physical suffering and complexity of care; and (c) were significantly much more likely to die during hospital stay (OR, 11.03). For patients who were dying or in other challenging clinical situations (suffering from various severe symptoms), self-rated team satisfaction was significantly higher for the PCU than the PCCS.
Conclusion.
This study presents a direct comparison between patients in a PCU and a PCCS. Results strongly support the hypothesis that the coexistence of both institutions in one hospital contributes to the goal of ensuring optimal high-quality PC for patients in complex and challenging clinical situations.
Background
The implementation of a palliative care consultation service (PCCS) [1] is emerging rapidly, especially in large academic centers [1]. For example, in the U.S., the proportion of academic medical centers providing a PCCS increased fivefold (from 15% to 75%) during the last decade [2, 3]. At present, nine of 10 U.S. medical centers supported by the National Cancer Institute provide a PCCS [1]. In contrast, the provision of inpatient palliative care units (PCUs) is not well established in the U.S. though there is a comparatively long tradition in western Europe [4]. Whereas some institutions consider the PCU as a valuable component of specialized palliative care (PC) infrastructure [1, 4, 5], others report that the sole provision of a state-of-the-art PCCS suffices to adequately care for the severely ill and dying [6]. Yet data assessing either one of these assumptions are not available so far.
Aim of the Study
In our own medical center, both a PCCS and a PCU are run in parallel. Our clinical experience from the last 5 years has shown that it is often difficult to ensure optimal care for PC patients on non-PC wards, especially if the patients are suffering from severe or complex symptoms or are imminently dying. As perceived by our team, the care of these patients is sometimes too complex and challenging, and the provision of specialized PC expertise and infrastructure as available in the PCU is inevitable. Therefore, this study intended to gather basic information for the hypothesis that running a PCU in addition to a PCCS might be a valuable component of high-quality patient PC, in particular for patients who are dying or suffering from a severe or complex symptom burden.
By identifying specific criteria that distinguish patients needing PC support by the PCCS from those cared for in the PCU, the objective of this study was to provide information to aid institutional planning for the concomitant implementation of both a PCU and a PCCS at an academic medical center or larger hospital.
Methods
Institutional Background
The Department of Palliative Medicine of the University Hospital of Cologne is an academically and clinically independent department as a part of one of the major comprehensive cancer centers in Germany. It aims at providing (a) cross-sectoral and (b) integrated PC by providing (a) Germany's first specialized PC home care team (founded in 1984), (b) outpatient care, (c) Germany's first PCU (founded in 1983), and (d) a PCCS that was established in 2006 [7]. The PCCS integrates specialized PC into the care of patients with life-limiting and incurable diseases in addition to the routine management of the disease in the primary treating department (e.g., oncology, radiotherapy, neurology, etc.) [8–10]. The PCCS consists of a senior PC physician and a specialized PC nurse in close cooperation with a social worker, a psychologist, and a chaplain in accordance with the European Commission's recommendations for PCCS [11]. Its assignments and mission statement are communicated throughout the institution [9, 12]. Patients can also be admitted to the PCU [7], where PC is provided by a multiprofessional team in a specialized 15-bed unit.
Study Design and Data Collection
Routine electronic patient data from all inpatient admissions in 2009 and 2010 to the PCU and the PCCS were reviewed retrospectively. On admission and discharge, the PC team performed a thorough medical and psychosocial assessment; routine documentation for each patient was performed using the Hospice and Palliative Care Evaluation (HOPE). The PC team documented the patient's data using the online interface of the HOPE database [13]. These data are saved anonymously and stored securely; the ethics committee of our institution did not have any objections concerning data processing and evaluation. HOPE is a nationwide, multisectoral, and multiprofessional core documentation system that provides standardized online documentation of patient-related data as a tool for quality assurance, benchmarking, and scientific evaluation [14, 15]. Therefore, it has been implemented in many PC studies in Germany concerning epidemiology [16], symptom control [17], drug use [18, 19], PC interventions [20], and the comparison of different PC populations (e.g., cancer versus noncancer) [21, 22].
For this study, the following data from each patient were retrieved:
Demographics. Age, gender, underlying disease, and the presence of metastases were assessed to minimize confounders in multivariate analyses.
Performance status on admission. Performance status was rated according to the Eastern Cooperative Oncology Group (ECOG) scale: 0, fully active; 1, restricted strenuous activity; 2, ambulatory, unable to work; 3, limited self-care, confined to bed or chair >50 waking hours per week; 4, confined to bed or chair, no self-care [23].
Symptom and problem checklist [24] on admission. The documentation form includes a checklist of the most relevant symptoms and problems of PC patients identified in a survey in 2001 [17] that is documented by professionals from PC teams. This checklist was recently validated [24]. Its characteristics and content are similar to those of widely accepted and validated PC self-assessment questionnaires such as the Edmonton Symptom Assessment System [25, 26]. This checklist consists of 17 items including: (a) eight physical symptoms (pain, nausea, vomiting, dyspnea, constipation, weakness, loss of appetite, tiredness), (b) four psychological symptoms (feeling depressed, anxiety, tension, disorientation/confusion), (c) two nursing issues (wound care, assistance with activities of daily living), (d) two social problems (organization of care, overburdening of family), and (e) one free-text entry [17, 24, 27]. The 17 items are rated on a four-point grading scale: 0, no; 1, mild; 2, moderate; 3, severe. A global sum score was calculated for each patient, ranging from a minimum of 0 (all 17 items rated as 0) to a theoretically possible maximum of 51 (all 17 items rated as 3) [24]. Additionally, the following categories were subsumed [24]: (a) physical suffering (nausea, vomiting, constipation, and pain), (b) psychological burden (anxiety, feeling depressed, and tension), and (c) complexity of patient care (organization of care, overburdening of family, disorientation/confusion, and wound care).
Discharge modality. The end of PC treatment was retrieved from the database as (a) death during hospital stay, (b) discharge to ambulatory/home care, (c) transfer to a hospice, or (d) transfer to the PCU (for PCCS patients). For the latter, a separate analysis of these specific patient characteristics of care in the PCU was not feasible because the HOPE databases of the PCU and PCCS are organized as two independent and anonymized files.
Satisfaction of the PC team with patient care on discharge. Data on PCCS and PCU team satisfaction with patient care were analyzed. Rating was performed by a team member (nurse or physician) on a five-point grading scale (1, very bad; 2, bad; 3, moderate; 4, good; 5, very good).
Statistical Analysis
Data were evaluated with PASW Statistics 18 (SPSS Inc., Chicago, IL) and Microsoft Excel 2007 (Microsoft, Inc., Redmond, WA). Summary statistics were used to describe the demographic and clinical characteristics of each group: age, gender, underlying disease, presence of metastases, and the prevalence of symptoms and problems. For comparisons between the two groups, statistical significance was set at a p-value <.01 to minimize type I errors for each test. Ninety-five percent confidence intervals (CIs) were calculated if appropriate.
Comparisons of proportions (e.g., the number of patients with an ECOG performance status score of 4 per group) were conducted using the Pearson χ2 test of independence. Relative differences were calculated as ratios of the absolute difference in percentage points of the two groups and the respective proportion of the PCCS group. CI analysis of these differences was performed using Newcombe's method [28].
The number of severe symptoms or problems per patient was calculated. Because this continuous outcome was not distributed normally, the nonparametric Mann-Whitney U test (Wilcoxon rank-sum test) was used for statistical analysis. The median was considered to describe this outcome most suitably and relative differences in the medians were calculated. The global sum score described above was distributed approximately normally as tested with the Kolmogorov–Smirnov test; the means of both groups were compared using Student's t test.
To assess the hypothesis that dying patients and those suffering from a complex physical and/or psychosocial symptom burden or who have a very reduced performance status are more challenging for PC teams than others, and therefore might be better cared for in the PCU, the following conditions were specifically evaluated: (a) having an ECOG score of 4, (b) having a global sum score >20 (explanation: a high global sum score is a surrogate for complex symptom burden; a score of 20 represents the third quartile of the score in this population), (c) having more than three severe symptoms or problems, (d) suffering from weakness, (e) suffering from pain, (f) suffering from tension, (g) overburdening of the family, and (g) death during hospital stay.
Binary logistic regression was used to identify the association between the group assignment, either PCU or PCCS, and the conditions mentioned above (a-g). Thus, the independent variable was whether the patients were part of the PCU or the PCCS group; dependent variables were the conditions (binary variables). The model was adjusted in the multivariate analysis by the independent variables age (continuous), gender (binary), diagnosis (nominal), and presence of metastases (binary). The PCCS group was chosen as the reference group for the resulting odds ratio (OR) of the PCU group. Hence, the resulting OR describes the ratio of the likelihood of an event occurring in the PCU group to the likelihood of it occurring in the PCCS group.
Missing data were assumed to be missing completely at random because no obvious confounding factors could be identified. Yet because of the retrospective study design, confounders cannot be totally excluded.
Results
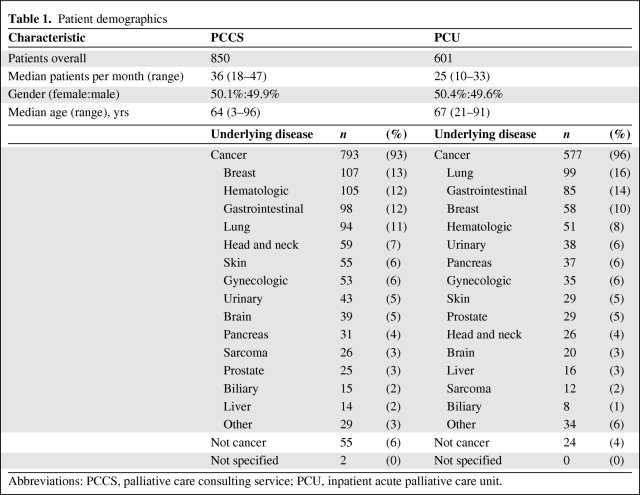
A total of 1,451 PC patients could be assessed. In 2009 and 2010, 850 patients were referred to the PCCS and 601 were admitted to the PCU. Of the latter, 226 (27%) were referred to the PCU by the PCCS. Cancer was the most common underlying disease (94%). The PCU and PCCS groups did not exhibit obvious demographic differences (Table 1).
Table 1.
Patient demographics
Abbreviations: PCCS, palliative care consulting service; PCU, inpatient acute palliative care unit.
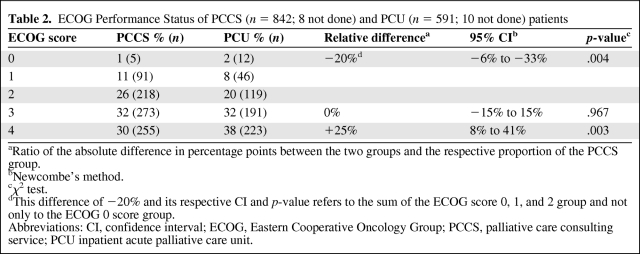
Performance Status
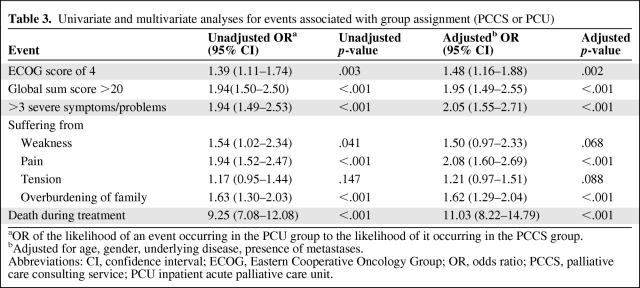
More patients cared for in the PCU had a more severely reduced performance status (ECOG score, 4) (Table 2). Logistic regression suggested that the likelihood of patients having an ECOG score of 4 was significantly higher in the PCU group (OR, 1.39; 95% CI, 1.11–1.74; p = .003). In an adjusted analysis, this finding became even more obvious (OR, 1.48; 95% CI, 1.16–1.88; p = .002) (Table 3).
Table 2.
ECOG Performance Status of PCCS (n = 842; 8 not done) and PCU (n = 591; 10 not done) patients
aRatio of the absolute difference in percentage points between the two groups and the respective proportion of the PCCS group.
bNewcombe's method.
cχ2 test.
dThis difference of −20% and its respective CI and p-value refers to the sum of the ECOG score 0, 1, and 2 group and not only to the ECOG 0 score group.
Abbreviations: CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; PCCS, palliative care consulting service; PCU inpatient acute palliative care unit.
Table 3.
Univariate and multivariate analyses for events associated with group assignment (PCCS or PCU)
aOR of the likelihood of an event occurring in the PCU group to the likelihood of it occurring in the PCCS group.
bAdjusted for age, gender, underlying disease, presence of metastases.
Abbreviations: CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; OR, odds ratio; PCCS, palliative care consulting service; PCU inpatient acute palliative care unit.
Symptom Burden and Problems
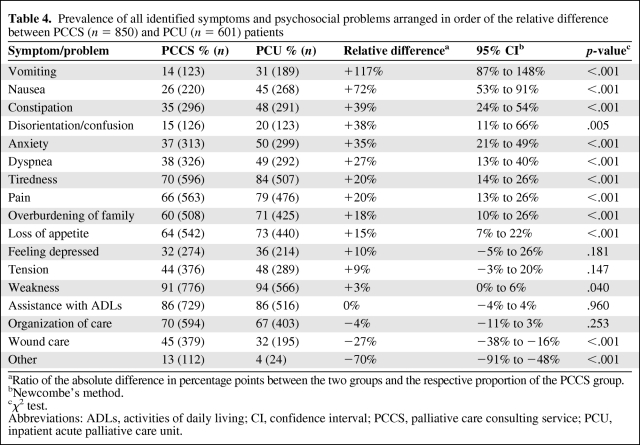
PCU patients presented with a significantly more complex symptom and problem burden than PCCS patients. The median number of severe (rated 3) symptoms or problems per patient was twice as high in the PCU group (relative difference, +100%; p < .001), and the PCU group had a mean global sum score per patient of 17.0, whereas the PCCS group had a mean global sum score per patient of only 14.4 (difference, 2.6; 95% CI, 2.0–3.3; p < .001). Correspondingly, the adjusted ORs of patients in the PCU group compared with those in the PCCS group for the events “having a global sum score >20” and “having more than three severe symptoms or problems” were 1.95 (p < .001) and 2.05 (p < .001), respectively (Table 3). Detailed analysis of the specific symptoms and problems identified in the two groups showed statistically and clinically significant differences, in particular for physical symptoms (e.g., vomiting, nausea, and constipation) (Table 4).
Table 4.
Prevalence of all identified symptoms and psychosocial problems arranged in order of the relative difference between PCCS (n = 850) and PCU (n = 601) patients
aRatio of the absolute difference in percentage points between the two groups and the respective proportion of the PCCS group.
bNewcombe's method.
cχ2 test.
Abbreviations: ADLs, activities of daily living; CI, confidence interval; PCCS, palliative care consulting service; PCU, inpatient acute palliative care unit.
Additionally, adjusted logistic regression revealed that the likelihood of having at least one severe symptom or problem in the categories physical suffering (OR, 1.63, 95% CI, 1.30–2.05; p < .001) and complexity of patient care (OR, 1.43; 95% CI, 1.15–1.79; p = .001) was significantly higher in the PCU group. In contrast, the category psychological burden (OR, 1.02; 95% CI, 0.79–1.31; p = .899) showed no significant difference. Correspondingly, for the most frequent symptom or problem in each of these three categories (physical suffering: pain, complexity of patient care: overburdening of family, psychological burden: tension) elevated ORs could be demonstrated for pain and overburdening of family but not for tension (Table 3).
Discharge Modality
In the PCCS group, the number of patients who were discharged to a hospice (n = 23; 3%) or died during their hospital stay (n = 106; 12%) was far less than in the PCU group, with 64 patients (11%) discharged to hospice and 349 patients (58%) dying. Thus, with the use of adjusted logistic regression, the group assignment highly significantly predicted the likelihood of death during the hospital stay (OR, 11.03; 95% CI, 8.22–14.79; p < .001) (Table 3). Two hundred twenty-six (27%) patients of the PCCS group were admitted to the PCU after PCCS consultation.
Team Satisfaction
If patients were dying or needed PC support because of a severe or complex symptom burden, the PC team was significantly more satisfied with patient care if these patients were cared for in the PCU. Specifically, if patients died in the hospital, team satisfaction was rated as at least “good” for 61% of the PCCS patients versus 77% of the PCU group (relative difference, +27%; 95% CI, 10%–44%; p = .001). Accordingly, if patients had a global sum score > 20, team satisfaction was significantly higher for patients treated in the PCU: 77%, versus 62% for the PCCS group (relative difference, +25%; 95% CI, 8%–42%; p = .004).
Discussion
PC infrastructure has developed differently around the globe [5]. Whereas for outpatients, PC home care teams, telephone consultations, and outpatient clinics have been implemented and evaluated [5], this publication takes a specific look at the PC infrastructure established to deliver PC for inpatients. This study provides four major insights: patients admitted to the PCU, compared with those consulted by the PCCS, (a) had a significantly worse performance status, (b) were more likely to suffer from severe symptoms and psychosocial problems (in particular concerning physical suffering and complexity of care), and (c) were much more likely to die during their hospital stay, whereas (d) team satisfaction with patient care for patients in challenging clinical situations (e.g., dying patients) was higher if patients were cared for in the PCU. Because data directly comparing a PCCS with a PCU were not yet available, this is the first study that provides this comparison to gather information on the extent to which the coexistence of both institutions in one hospital reasonably adds to the goal of ensuring optimal PC support for dying or severely ill PC patients.
PCCS
In many countries, the most common PC concept for hospitalized PC patients is the implementation of a PCCS [1–3]. The majority of publications uniformly reported the following professional assignments for PCCSs: (a) symptom control (e.g., pain and dyspnea); (b) quality of life (QoL) improvement; (c) psychosocial, emotional, and spiritual support; (d) dealing with uncertainty about the goals of care (improve communication); and (e) coordination (“navigation through the health care system”) [1–3, 8–10].
A recent review showed that, so far, only two randomized controlled trials (RCTs) evaluated this concept with respect to the evidence of outcomes for patients [29]. One of those RCTs focused on patients with dementia [30] and the other one focused on a population of mostly cancer patients (90%) [31]. Those RCTs and other studies that published data from noncontrolled studies [8–10, 32] reported that the PCCS generally met the five assignments listed above, but most publications assessed a sole PCCS concept because their institution did not additionally provide a PCU. No publication provided a detailed analysis of unsuccessfully treated patients (e.g., bad symptom control despite PCCS treatment). Taking a closer look at publications from institutions with both a PCU and a PCCS, it becomes obvious that a proportion of patients could not be treated successfully by the PCCS and had to be admitted to the PCU (34%–60%) [32, 33]. This is in line with the findings of the study presented here. As in those publications, we were unable to provide a specific analysis of why some patients had to be referred to the PCU despite the provision of PC support by the PCCS in the primary treating department [32, 33]. Correspondingly, in a previous publication, we reported that 237 (27%) PCCS patients had to be admitted to the PCU despite the support of our PCCS, but we were unable to identify specific reasons for this [9]. In the study presented here, it became obvious that patients admitted to the PCU generally had a more reduced performance status and the clinical and psychosocial condition of these patients was more complex and challenging. Yet some institutions successfully supply state-of-the-art PC by the sole provision of a PCCS that specifically aims at care of the dying [6]. Before discussing our findings, it is necessary to clarify the terminology and assignments for the PCU [34].
PCU
When reporting findings from a PCU, von Gunten pointed out that it is indispensable to supply information that empowers the reader to understand the clinical assignment of the PCU to distinguish it from inpatient hospice facilities to avoid terminological confusion and misunderstandings related to various PC traditions in different health care systems [34].
Different PCU Concepts
The primary goal of care for PCU patients is improvement in QoL and not prolongation of life [1–3, 5, 12, 17, 24]. As in our institution, this is mainly achieved by mere symptomatic treatment [18]. In general, patients admitted to PCUs are in refractory stages of their disease; thus, antineoplastic agents are seldom administered and cardiopulmonary resuscitation is no longer indicated [7, 12]. This clinical practice therefore differs from some PCUs [4, 35], where, in some centers, disease-modifying therapy (e.g., systemic i.v. chemotherapy) plays a significant role. In Germany, patients are admitted to the PCU for either symptom control or a complex psychosocial situation that cannot be managed at home with the available resources [18]. A large proportion of patients do not die in the PCU but are discharged home again after several days of PC treatment [18].
PCU Versus Inpatient Hospice Facilities
In Germany and large parts of western Europe, hospices are separate institutions [36]. Patients are admitted because they cannot be cared for at home. They stay until death and receive professional support from PC nurses who are accompanied by general practitioners who come on rounds to see patients as demanded by the clinical situation of the patients.
Principle Findings: PCU Versus PCCS
Though this is the first study presenting a direct comparison of the two PC services, the results can be indirectly compared with the findings of Bruera and colleagues, whose institution provides a PCU in addition to a PCCS. They separately reported on patients cared for in their PCU and by their PCCS in a number of different publications [4, 35, 37]. As in previous studies by our own group, many patients had to be admitted to the PCU even though they had already received PC support from the PCCS [9]. Moreover, a main finding of the study presented here was in line with a comparison of the three articles of Bruera and colleagues [4, 35, 37]. PCU patients in our institution also had a more reduced performance status or were suffering from a more complex and challenging symptom burden. Moreover, dying patients were more likely to be cared for in the PCU than by the PCCS. Still, other working groups argue that it is not necessary to provide a PCU for these patients in addition to a state-of-the-art PCCS [6, 38]. Though our PCCS meets the requirements of the European Commission [11], and meets a number of additional prerequisites [12], our experience questions this assumption.
This is supported by the recently published findings of Casarett et al. [39], who reported the results of telephone interviews with family members of patients who died in a PCU or a “usual” ward that was supported by a PCCS. Family members of patients who died in a PCU were significantly more likely to report “excellent care.” Moreover, Bruera et al. [40] reported that patients with clinical indicators for high distress were more likely to require care in a PCU.
In light of all these considerations, another finding of our study was that the satisfaction of the PC team with patient care was significantly higher for those treated in the PCU than for those treated by the PCCS for those patients who were dying or suffering from a complex symptom burden. Reasons for the team's perception that severely ill PC patients might be cared for more adequately in a PCU than a non-PC ward might be (a) the comparably lower time and staff resources of non-PC wards and (b) the superiority of the medical and communicative PC expertise in PC wards [5].
Limitations
Though this is the first study providing the information discussed above, some limitations have to be considered.
Satisfaction of the PC team with patient care is a surrogate measure. We were unable to provide patient-reported outcomes, which should be the gold standard when assessing the quality of PC services [41]. Moreover, the validity of a one-item five-point grading scale is considerably restricted, and the findings may be influenced by other circumstances such as a lack of confidence in the PCCS team outside their own institution.
In a current review, El-Jawahri et al. [29] pointed out that the PC infrastructure should suit the institution where it is implemented. This could mean that, because of limited resources (e.g., funding, available expertise), the concomitant provision of both a PCCS and PCU in all hospitals is not feasible.
Conclusion
This study supports the hypothesis that PC patients with complex and challenging clinical situations might especially benefit from the provision of a specialized PCU in addition to a PCCS. Further studies providing patient-reported outcomes on this topic are required.
Acknowledgments
The scientific work of the Department of Palliative Care, University Clinic of Cologne, is supported by the Federal Ministry for Education and Science (BMBF 01KN1106). The clinical and academic activities of the Department of Palliative Medicine, University Clinic of Cologne, are substantially supported by the German Cancer Aid (Deutsche Krebshilfee.V.). The authors would like to thank Dr. Julia Strupp for careful proofreading and her scientific feedback on the manuscript.
Jan Gaertner and Sebastian Frechen contributed equally to the manuscript.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Sebastian Frechen, Jan Gaertner, Raymond Voltz
Provision of study material or patients: Jan Gaertner, Christoph Ostgathe
Collection and/or assembly of data: Sebastian Frechen, Jan Gaertner, Markus Sladek
Data analysis and interpretation: Sebastian Frechen, Jan Gaertner, Markus Sladek, Raymond Voltz
Manuscript writing: Sebastian Frechen, Jan Gaertner, Raymond Voltz
Final approval of manuscript: Sebastian Frechen, Jan Gaertner, Christoph Ostgathe, Raymond Voltz
References
- 1.Norton SA, Powers BA, Schmitt MH, et al. Navigating tensions: Integrating palliative care consultation services into an academic medical center setting. J Pain Symptom Manage. 2011;42:680–690. doi: 10.1016/j.jpainsymman.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Center to Advance Palliative Care (CAPC) Hospital Palliative Care Programs Continue Rapid Growth: New Data Show Fifth Consecutive Annual Increase. [accessed June 9, 2011]. Available at http://www.capc.org/news-and-events/releases/items/december-2006-release.
- 3.Morrison RS, Maroney-Galin C, Kralovec PD, et al. The growth of palliative care programs in United States hospitals. J Palliat Med. 2005;8:1127–1134. doi: 10.1089/jpm.2005.8.1127. [DOI] [PubMed] [Google Scholar]
- 4.Elsayem A, Calderon BB, Camarines EM, et al. A month in an acute palliative care unit: Clinical interventions and financial outcomes. Am J Hosp Palliat Care. 2011;28:550–555. doi: 10.1177/1049909111404024. [DOI] [PubMed] [Google Scholar]
- 5.Higginson IJ. End-of-life care: Lessons from other nations. J Palliat Med. 2005;8(suppl 1):S161–S173. doi: 10.1089/jpm.2005.8.s-161. [DOI] [PubMed] [Google Scholar]
- 6.Ellershaw J, Dewar S, Murphy D. Achieving a good death for all. BMJ. 2010;341:c4861. doi: 10.1136/bmj.c4861. [DOI] [PubMed] [Google Scholar]
- 7.Ostgathe C, Walshe R, Wolf J, et al. A cost calculation model for specialist palliative care for patients with non-small cell lung cancer in a tertiary centre. Support Care Cancer. 2008;16:501–506. doi: 10.1007/s00520-007-0337-5. [DOI] [PubMed] [Google Scholar]
- 8.Gaertner J, Wolf J, Scheicht D, et al. Implementing WHO recommendations for palliative care into routine lung cancer therapy: A feasibility project. J Palliat Med. 2010;13:727–732. doi: 10.1089/jpm.2009.0399. [DOI] [PubMed] [Google Scholar]
- 9.Gaertner J, Wolf J, Frechen S, et al. Recommending early integration of palliative care—does it work? Support Care Cancer. 2012;20:507–513. doi: 10.1007/s00520-011-1111-2. [DOI] [PubMed] [Google Scholar]
- 10.Gaertner J, Wuerstlein R, Klein U, et al. Integrating palliative medicine into comprehensive breast cancer therapy—a pilot project. Breast Care (Basel) 2011;6:215–220. doi: 10.1159/000328162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.European Commission. Luxembourg: Office for Official Publications of the European Communities; 2004. Promoting the Development and Integration of Palliative Care Mobile Support Teams in the Hospital—Quality of Life and Management of Living Resources; pp. 1–23. [Google Scholar]
- 12.Gaertner J, Wolf J, Hallek M, et al. Standardizing integration of palliative care into comprehensive cancer therapy—a disease specific approach. Support Care Cancer. 2011;19:1037–1043. doi: 10.1007/s00520-011-1131-y. [DOI] [PubMed] [Google Scholar]
- 13.Hospice and Palliative Care Evaluation (HOPE) [online user interface] [accessed October 25, 2011]. Available at https://www.hope-clara.de/
- 14.Hospice and Palliative Care Evaluation (HOPE) Concept HOPE 2011. [accessed May 25, 2011]. Available at https://www.hope-clara.de/download/Konzept_HOPE_2011.pdf.
- 15.Stiel S, Pulst K, Krumm N, et al. The development of palliative care over the time—Comparison of results from the representative surveys of 2004 and 2009. Z Palliativmed. 2010;11:78–84. [Google Scholar]
- 16.Radbruch L, Nauck F, Fuchs M, et al. What is palliative care in Germany? Results from a representative survey. J Pain Symptom Manage. 2002;23:471–483. doi: 10.1016/s0885-3924(02)00408-6. [DOI] [PubMed] [Google Scholar]
- 17.Radbruch L, Nauck F, Ostgathe C, et al. What are the problems in palliative care? Results from a representative survey. Support Care Cancer. 2003;11:442–451. doi: 10.1007/s00520-003-0472-6. [DOI] [PubMed] [Google Scholar]
- 18.Nauck F, Ostgathe C, Klaschik E, et al. Drugs in palliative care: Results from a representative survey in Germany. Palliat Med. 2004;18:100–107. doi: 10.1191/0269216304pm852oa. [DOI] [PubMed] [Google Scholar]
- 19.Stiel S, Krumm N, Pestinger M, et al. Antibiotics in palliative medicine—results from a prospective epidemiological investigation from the HOPE survey. Support Care Cancer. 2012;20:325–333. doi: 10.1007/s00520-011-1084-1. [DOI] [PubMed] [Google Scholar]
- 20.Radbruch L, Ostgathe C, Elsner F, et al. [What is the profile of palliative care in Germany. Results of a representative survey.] Schmerz. 2004;18:179–188. doi: 10.1007/s00482-003-0276-0. In German. [DOI] [PubMed] [Google Scholar]
- 21.Ostgathe C, Gaertner J, Kotterba M, et al. Differential palliative care issues in patients with primary and secondary brain tumours. Support Care Cancer. 2010;18:1157–1163. doi: 10.1007/s00520-009-0735-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ostgathe C, Alt-Epping B, Golla H, et al. Non-cancer patients in specialized palliative care in Germany: What are the problems? Palliat Med. 2010;25:148–152. doi: 10.1177/0269216310385370. [DOI] [PubMed] [Google Scholar]
- 23.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 24.Stiel S, Pollok A, Elsner F, et al. Validation of the symptom and problem checklist of the German Hospice and Palliative Care Evaluation (HOPE) J Pain Symptom Manage. 2012;3:593–605. doi: 10.1016/j.jpainsymman.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 25.Bruera E, Kuehn N, Miller MJ, et al. The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. J Palliat Care. 1991;7:6–9. [PubMed] [Google Scholar]
- 26.Ströomgren AS, Groenvold M, Pedersen L, et al. Symptomatology of cancer patients in palliative care: Content validation of self-assessment questionnaires against medical records. Eur J Cancer. 2002;38:788–794. doi: 10.1016/s0959-8049(01)00470-1. [DOI] [PubMed] [Google Scholar]
- 27.Hospice and Palliative Care Evaluation (HOPE) 2011. Core data set. [accessed May 25, 2011]. Available at https://www.hope-clara.de/download/HOPE2011Basisbogen.pdf.
- 28.Newcombe RG, Altman DG. Proportions and their differences. In: Altman D, Machin D, Bryant T, et al., editors. Statistics With Confidence: Confidence Intervals and Statistical Guidelines. Second Edition. London: BMJ Books; 2000. pp. 45–56. [Google Scholar]
- 29.El-Jawahri A, Greer JA, Temel JS. Does palliative care improve outcomes for patients with incurable illness? A review of the evidence. J Support Oncol. 2011;9:87–94. doi: 10.1016/j.suponc.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Ahronheim JC, Morrison RS, Morris J, et al. Palliative care in advanced dementia: A randomized controlled trial and descriptive analysis. J Palliat Med. 2000;3:265–273. doi: 10.1089/jpm.2000.3.265. [DOI] [PubMed] [Google Scholar]
- 31.Hanks GW, Robbins M, Sharp D, et al. The imPaCT study: A randomised controlled trial to evaluate a hospital palliative care team. Br J Cancer. 2002;87:733–739. doi: 10.1038/sj.bjc.6600522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dhillon N, Kopetz S, Pei BL, et al. Clinical findings of a palliative care consultation team at a comprehensive cancer center. J Palliat Med. 2008;11:191–197. doi: 10.1089/jpm.2007.0094. [DOI] [PubMed] [Google Scholar]
- 33.Santa-Emma PH, Roach R, Gill MA, et al. Development and implementation of an inpatient acute palliative care service. J Palliat Med. 2002;5:93–100. doi: 10.1089/10966210252785051. [DOI] [PubMed] [Google Scholar]
- 34.von Gunten CF. Humpty-Dumpty Syndrome. Palliat Med. 2007;21:461–462. doi: 10.1177/0269216307081943. [DOI] [PubMed] [Google Scholar]
- 35.Hui D, Elsayem A, Li Z, et al. Antineoplastic therapy use in patients with advanced cancer admitted to an acute palliative care unit at a comprehensive cancer center: A simultaneous care model. Cancer. 2010;116:2036–2043. doi: 10.1002/cncr.24942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salloch S, Breitsameter C. Morality and moral conflicts in hospice care: Results of a qualitative interview study. J Med Ethics. 2010;36:588–592. doi: 10.1136/jme.2009.034462. [DOI] [PubMed] [Google Scholar]
- 37.Fadul N, Elsayem A, Palmer JL, et al. Predictors of access to palliative care services among patients who died at a Comprehensive Cancer Center. J Palliat Med. 2007;10:1146–1152. doi: 10.1089/jpm.2006.0259. [DOI] [PubMed] [Google Scholar]
- 38.Ellershaw J. Care of the dying: What a difference an LCP makes! Palliat Med. 2007;21:365–368. doi: 10.1177/0269216307081117. [DOI] [PubMed] [Google Scholar]
- 39.Casarett D, Johnson M, Smith D, et al. The optimal delivery of palliative care: A national comparison of the outcomes of consultation teams vs inpatient units. Arch Intern Med. 2011;171:649–655. doi: 10.1001/archinternmed.2011.87. [DOI] [PubMed] [Google Scholar]
- 40.Bruera E, Neumann C, Brenneis C, et al. Frequency of symptom distress and poor prognostic indicators in palliative cancer patients admitted to a tertiary palliative care unit, hospices, and acute care hospitals. J Palliat Care. 2000;16:16–21. [PubMed] [Google Scholar]
- 41.Bausewein C, Simon ST, Benalia H, et al. Implementing patient reported outcome measures (PROMs) in palliative care—users' cry for help. Health Qual Life Outcomes. 2011;9:27. doi: 10.1186/1477-7525-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]