Abstract
Protein quality control (QC) in the endoplasmic reticulum (ER) comprises many steps, including folding and transport of nascent proteins as well as degradation of misfolded proteins. Recent studies have revealed that high-mannose-type glycans play a pivotal role in the QC process. To gain knowledge about the molecular basis of this process with well-defined homogeneous compounds, we achieved a convergent synthesis of high-mannose-type glycans and their functionalized derivatives. We focused on analyses of UDP-Glc: glycoprotein glucosyltransferase (UGGT) and ER Glucosidase II, which play crucial roles in glycoprotein QC; however, their specificities remain unclear. In addition, we established an in vitro assay system mimicking the in vivo condition which is highly crowded because of the presence of various biomacromolecules.
Keywords: protein quality control, high-mannose-type glycans, UDP-Glc: glycoprotein glucosyltransferase (UGGT), glucosidase II, macromolecular crowding
Introduction
Oligosaccharides or glycan chains are important constituents of intra- and extracellularly distributing glycoconjugates such as glycoproteins, glycolipids, and proteoglycans. Their roles are numerous, and mostly essential for normal cellular activities. The most prominent among them are cell development, immune response,1,2) infection,3,4) and malignant transformation.5,6) However, all in all, detailed understanding on molecular basis of their functions has been limited.
In order to advance the study of biological roles of glycan chains, chemical synthesis has been expected to play a pivotal role. Compared to other classes of biooligomers, such as peptides (proteins)7,8) and oligonucleotides (DNA and RNA),9,10) synthesis of glycan chain is less straightforward due to several reasons. Firstly, formation of glycosidic linkages that connect sugar residues generates two isomers, namely α- and β-glycosides. Additionally, biologically interesting glycan chains often comprise branching structure, instead of being linear oligomers. By contrast, since peptide and nucleotide chains are linear and their structures are solely defined by sequence or alignment of respective components, synthesis of these oligomers is not associated with isomer formation.
In order for proteins to function normally, structural modifications are often indispensable. Among numerous types of post- or co-translational modifications of proteins, introduction of asparagines (Asn)-linked (or N-linked) glycan chains is one of the most prominent. Introduction of N-linked glycans takes place in the endoplasmic reticulum (ER) of the cells in a co-translational manner.11,12) This type of glycans is added to Asn residues embedded in the “consensus” triad Asn-X-Ser/Thr (Ser: serine, Thr: threonine, X: any amino acid except proline).13)
Glycan chains are characterized by their steric bulkiness as well as the presence of a large number of hydroxyl groups which makes them highly hydrophilic. Consequently, they are able to give a significant impact to physicochemical properties of proteins, conferring stability, water solubility, and resistance to protease cleavage.14–17)
Glycoconjugates, including N-glycosylated proteins, mostly exist on the cell-surface and play pivotal roles in intercellular recognition such as signal transduction, cell adhesion and immune response. More recent studies have provided convincing evidence on their roles in protein quality control (QC) in the ER.18–20) It is a wide-ranging process, including folding and transport of nascent proteins as well as degradation of incurably misfolded proteins. In this context, pivotal roles of N-linked glycans as key signals in glycoprotein QC have attracted growing attention. To gain molecular basis of this process, establishment of reliable in vitro experimental systems is essential. However, in many cases, complexity originating from structural heterogeneity of naturally occurring glycoproteins has been difficult to be removed and hampered unambiguous interpretation of experimental results.
Our study summarized in this article has aimed to reduce aforementioned ambiguity by using chemically synthesized glycans and their protein conjugates, and gain clear understanding of glycan-protein interplays in the ER.
1. Biosynthesis and processing of N-glycosylated proteins and their functions in the ER
Modification of N-glycosylated proteins occurs co-translationally in the ER. This reaction is catalyzed by a multisubunit enzyme, oligosaccharyltransferase (OST).11,12) Amazingly, a lipid-linked oligosaccharide consisting of as many as 14 sugar residues is transferred en bloc by this enzyme.21,22)
Studies have indicated that oligosaccharides attached to proteins are key signals in glycoprotein QC system. It ensures that only glycoproteins that have attained correct 3D structure are transported to Golgi apparatus for further processing and eventual secretion. On the other hand, those that have failed to achieve proper folding are transported to cytosol for ubiquitination followed by degradation by 26S proteasomes.
In most eukaryotes such as mammals, oligosaccharide consists of 14 sugar residues of three d-glucose (Glc), nine d-mannose (Man), and two N-acetyl-d-glucosamine (GlcNAc) residues (Glc3Man9GlcNAc2; G3M9). It is firstly assembled on a lipid carrier, dolichylphosphate (Dol-P), by successive action of glycosyltransferases in the lumen of the ER. Subsequently, the tetradecasaccharide G3M9 is transferred by the action of OST, as a block to Asn residues in the consensus of nascent polypeptides.
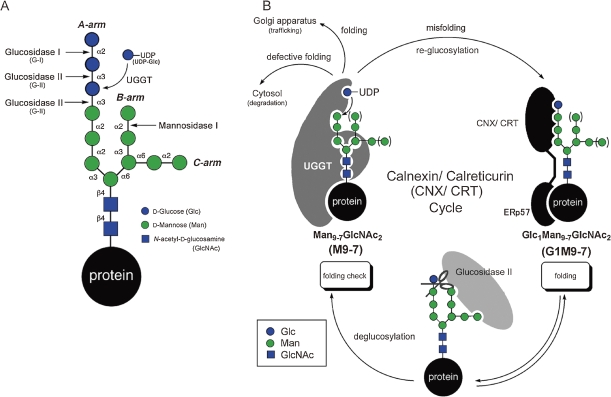
Thus introduced G3M9 is firstly processed by glucosidase I (G-I), which removes the outermost Glc residues to produce diglucosylated tridecasaccharide (Glc2Man9GlcNAc2; G2M9). The latter in turn is digested by glucosidase II (G-II), a dual activity enzyme, which is able to cleave Glc residues linked to Glc (Glcα1→3Glc) and Man (Glcα1→3Man) residues and generate dodeca- (Glc1Man9GlcNAc2; G1M9) and undecasaccharide (Man9GlcNAc2; M9), successively. The dodecasaccharide G1M9 formed by the first activity of G-II enters the glycoprotein-specific folding process, called calnexin/calreticulin (CNX/CRT) cycle.18,19,23) (Fig. 1)
Figure 1.
(A) N-glycan processing in ER. (B) Glycoprotein folding and quality control machineries in the ER.
In this cycle, CNX and CRT function as chaperones. They also possess a nearly identical property as lectin, and specifically bind Glcα1→3Man containing glycans such as G1M9. Both of them are proposed to exist as complexes with ERp57,24,25) a member of peptide disulfide isomerase superfamily. By virtue of their lectin activity, these chaperons are able to capture G1M9 containing glycoproteins and facilitate their folding. Subsequent trimming by G-II completely removes the Glc residue, leading guest glycoproteins into the non-glucosylated glycoform M9, which will be liberated from CNX/CRT.
Intriguingly, there is a proof-reading system in the CNX/CRT cycle, which is conducted by UDP-Glc: glycoprotein glucosyltransferase (UGGT). This enzyme functions as a “folding sensor” in the ER. Namely, only when protein folding is incomplete, glycoproteins processed to M9 are re-glucosylated to G1M9 by UGGT. By regenerating G1M9, this enzyme enables repeated interaction of glycoproteins with CNX/CRT and maximizes their folding. Substrate recognizing mode of UGGT is highly subtle. In addition to glycan structures, it discriminates folding state of protein backbones.26–28) Previous study29) has shown that its reactivity toward glycopeptides clearly correlates with the proportion of hydrophobic amino acids, suggesting that exposed hydrophobic patch of misfolded protein is the key element in UGGT recognition.
As for glycan specificity, our study clarified that M9 was the most reactive, while depletion of Man residue resulted in marked reduction of reactivity. T of Man8GlcNAc2 (M8) and Man7GlcNAc2 (M7) being ca. 1/2 and 1/5 of G9, respectively (vide infra).
An isoform of UGGT (HUGT2) has been identified in human.30,31) HUGT2 shares 55% identity with human UGGT (HUGT1). HUGT2 and HUGT1 mRNA are broadly expressed ubiquitously in every tissue. However, no glucose transfer activity has been identified for this protein and, consequently, its physiological role has yet to be identified.
UGGT is a widely conserved protein. Studies with mouse embryonic stem cells have shown that its deficiency is lethal in most cases.32) On the other hand, it has been shown to be an activation factor of BiP (GRP78), a molecular chaperone in the ER, and overexpression of UGGT was reported to enhance the expression level of medicinally important recombinant proteins including erythropoietin and interferon γ.33) Glycoproteins that have not achieved correct folding are slowly processed by ER mannosidase I, and partially demannosylated glycoproteins are transported to cytosol for degradation, while a possibility that they are partially rescued by BiP has been put forward, suggesting that an intricate fail-safe mechanism is provided in the quality control system.34)
2. Functional analysis of glycoprotein glycans using chemically synthesized probes
2.1. Synthesis of ER-related high-mannose-type glycans.
Despite the wealth of information provided by recent studies, fine picture of glycoprotein QC has been unclear. Because most of previous studies have employed substrates derived from naturally occurring glycoproteins, results have been complicated by heterogeneity of glycans.
Primary aim of our study was to systematically synthesize N-linked glycans, which were expected to be powerful in removing such difficulty.
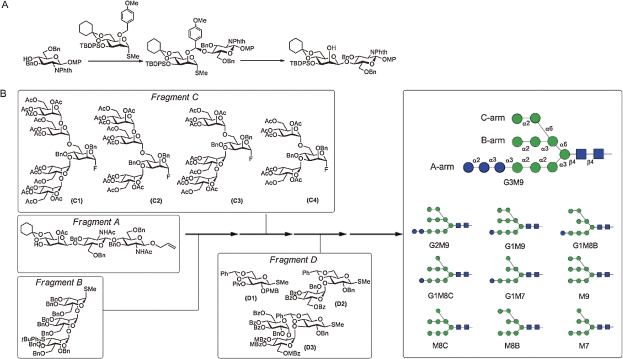
However, fully chemical synthesis of biologically relevant glycans consisting of more than ten sugar residues is not a trivial task. Among several problems associated with stereochemical control, certain type of so-called 1,2-cis glycosides such as β-linked mannose (β-Man) are difficult to construct selectively.35) Our study established a strategy which realized completely selective and efficient synthesis of β-Man glycosides, by using a concept dubbed “intramolecular aglycon delivery” (IAD).36) (Fig. 2A) Our approach employed a p-methoxybenzyl (PMB) group introduced to 2-position of mannose. Under oxidative conditions, the PMB group was converted to mixed acetal-type tethered intermediate, from which exclusive formation of 1,2-cis glycoside was realized in high yield (>80%).37) More recent studies have shown that efficiency as well as generality of the IAD was further enhanced by using 2-naphthylmethyl (NAP) group in place of PMB.38)
Figure 2.
Systematic synthesis of high-mannose-type oligosaccharide. (A) Stereoselective construction of β-mannoside linkage with intramolecular aglycon delivery (IAD). (B) Convergent synthesis of high-mannose-type oligosaccharide.
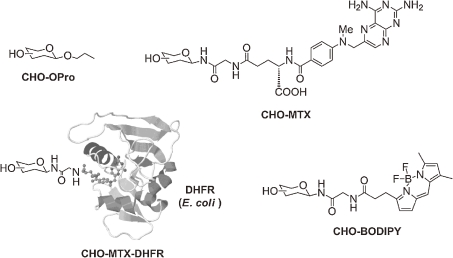
Systematic synthesis of high-mannose-type glycans was achieved in a convergent manner. (Fig. 2B) Combination of fragments corresponding to core trisaccharide (ManGlcNAc2; A), trimannose (Man3; B), branched mannose (Man5-3; C1,2,3,4) and glucose (Glc3-1; D1,2,3) components allowed us to prepare all possible high-mannose-type glycans.39) Furthermore, standardized protocol to functionalize these glycans with a variety of aglycons has been established. For instance, our study was directed to modification of glycans with methotrexate (MTX), which turned out to be highly versatile. Namely, MTX conjugated glycans (CHO-MTX) were easily detectable because of intense UV absorbance of MTX. In addition, they can be easily grafted to dihydrofolate reductase (DHFR), providing glycoprotein mimetic (CHO-MTX-DHFR), by virtue of a strong affinity between MTX and DHFR.40) (Fig. 3)
Figure 3.
Synthetic N-glycan molecular probes by introducing various aglycons. The term “CHO” means carbohydrate. MTX bind tightly with DHFR to form glycan-attached proteins (CHO-MTX-DHFR).
2.2. Analysis of UGGT, a folding sensor in the ER.
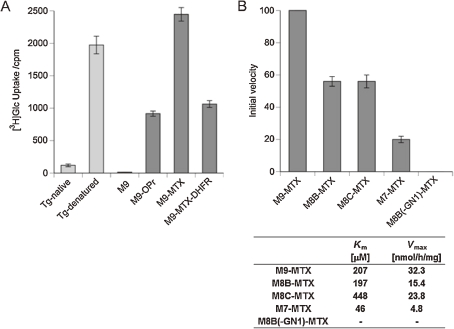
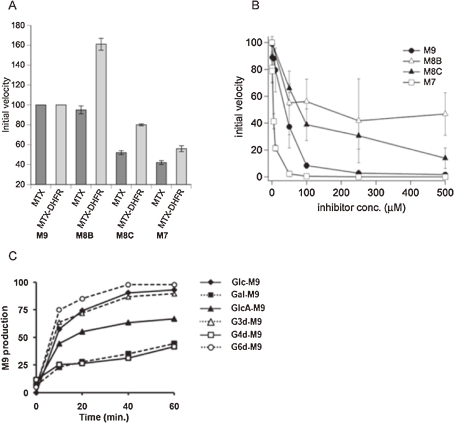
As was discussed in Chapter 1, UGGT recognizes glycoproteins that have not achieved correct folding as substrates and incorporates Glc residue to the terminal Man of the A-arm. As a matter of fact, denatured thyroglobulin (Tg) has been employed as a benchmark substrate of this enzyme, while native Tg is a poor substrate.41,42) However, precise specificity of UGGT has been unclear. Fortuitously, our experiments revealed that UGGT smoothly glucosylates MTX-modified glycan (CHO-MTX; CHO=M9), which became a well-defined non-peptidic synthetic substrate of UGGT.43) (Fig. 4A)
Figure 4.
Analysis of UGGT. (A) UGGT activity against protein substrate and synthetic M9-derivatives. (B) Glycan specificity analysis of UGGT and kinetics of CHO-MTX.
Subsequently, specificity of UGGT in terms of glycan structure was revealed to be M9>M8>M7. Intriguingly, although the reactivity of M7-MTX was low, its affinity to UGGT was the highest (Km), indicating that UGGT strongly recognizes the inner region of high-mannose-type glycans. In support of this notion, a glycan lacking the innermost GlcNAc was completely devoid of activity. (Fig. 4B)
Further study has shown that other types of glycan modification with fluorescently active substituents, such as BODIPY, or Fmoc, a widely used amino protecting group, were fruitful providing high reactivity substrates of UGGT.44) In particular, BODIPY modified glycans were preferable to high-sensitivity detection of the enzymatic activity, because of their high reactivity and strongly fluorescent activity of BODIPY.
A common feature of misfolded proteins is speculated to be the presence of hydrophobic patch exposed on their surface, which is likely to be an element recognized by UGGT. This speculation has been supported by several lines of evidence, such as 1) unmodified glycans are poorly glucosylated by UGGT, 2) M9 containing n-propyl, a modestly hydrophobic aglycon, was marginally reactive, and 3) the reactivity of M9-MTX was markedly attenuated when it was conjugated with DHFR. (Fig. 4A)
2.3. Analysis of glucosidase II (G-II).
G-II successively cleaves Glcα1-3Glc (cleavage-1) and Glcα1-3Man (cleavage-2) linkages. (Fig. 1A) In order to evaluate the magnitude of these activities, we monitored the Glc trimming of G2M9-MTX. Our analysis unambiguously confirmed that the cleavage-1 is by far faster than the cleavage-2.45) In addition, the observation that the cleavage-2 was strongly suppressed by the addition of CRT provided an implication that glycoproteins enter the CNX/CRT cycle immediately after they are converted to G1M9 by cleavage-1 of G-II.
In the ER, combined action of UGGT and G-II (cleavage-2) interconverts M9 and G1M9 glycoforms. Simultaneously, trimming by mannosidase(s) occurs, giving G0 and G1 glycans that have less than 9 Man residues. Studies in 1980s concluded that G-II cleaves G1M9 exceedingly faster than other monoglucosylated glycoforms.21,46,47) However, our study using structurally defined substrates (CHO-MTX; CHO = G1M9-7) revealed that the difference of reactivity was not as large as has been believed. Most noticeably, the reactivity of G1M8B-MTX which lacks a Man residue of the B-arm, was nearly identical with G1M9-MTX, while reaction rate of its regioisomer (G1M8C-MTX) was reduced to ca. 50%. (Fig. 5A) Interestingly, when conjugated to DHFR, the activity of G1M8B was markedly higher than G1M9. These results implicate that the specificity of G-II is sensitive to nature of aglycon, and possibly, to folding state of proteins.
Figure 5.
Substrate specificity of G-II. (A) G-II (cleavage-2) activity against various glycoprobes having different oligosaccharide or aglycone structure. (B) Inhibitory activity of various glycoprobes toward Glc trimming from G2M9. (C) Cleavage of various G1M9 analogues by G-II. Gal, Galactose; GlcA, Glucuronic acid; G3d, 3-Deoxyglucose; G4d, 4-Deoxyglucose; G6d, 6-Deoxyglucose.
Furthermore, inhibition experiments using several glycans indicated that G-II strongly recognizes the terminal Man of the C-arm. Among M9, M8(B), M8(C) and M7, inhibitory activity of M7 was the strongest.48) Since, in the ER, misfolded glycoproteins may be major targets of mannosidase(s), it would be tempting to speculate that accumulation of demannosylated glycoforms would suppress the G-II activity and decelerate the entry of newly generated glycoproteins into CNX/CRT. (Fig. 5B)
Interestingly, non-natural dodecasaccharides that had d-galactose, d-glucuronic acid, or 3-, 4-, 6-deoxy-d-glucose residue in place of Glc were all smoothly converted to M9.49) Taken together, G-II, while stringently recognizing mannose residues of the B- and C-arms, is quite tolerant to structure perturbation of departing sugar residues. (Fig. 5C)
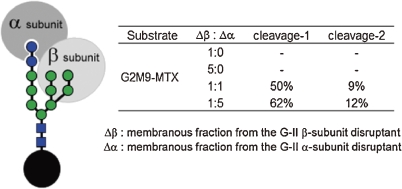
G-II is a heterodimeric protein, consisting of α- and β-subunit. While the α-subunit comprises a catalytic domain, precise function of the other subunit has been obscure.50) To clarify the issue, we prepared strains of Aspergillus oryzae in which either α- or β-subunit was disrupted, and G-II activity of their microsomal fraction was tested, by using G2M9- and G1M9-MTX. Our analysis showed 1) not only the α-subunit disruptant, but the β-subunit disruptant was inactive toward all of these glycans, although the former (but not the latter) was fully active toward a small molecule substrate pNP-Glc, and 2) mixed microsomal fractions of both disruptans exhibited the activity to digest both G2M9 and G1M9.51) Together, these results provide a clear indication that the presence of the β-subunit is essential in order for G-II to exhibit hydrolytic activity in the ER, possibly by virtue of its ability to recognize high-mannose-type glycans. (Fig. 6)
Figure 6.
G-II activity toward G2M9-MTX using the membranous fraction of gene disruptants lacking either the G-II α-subunit or β-subunit. The membranous fraction lacking the β-subunit was inactive against high mannose-type oligosaccharide.
2.4. Macromolecular crowding conditions as pseudo-intracellular environments.
While in vitro biochemical assays are typically performed in dilute buffer solutions containing less than 1% of proteins, intracellular environments are known to be highly congested. They consist of various macromolecules whose total concentration may reach 30–40%, creating “macromolecular crowding” conditions.52) Under such conditions, diffusion of bulky molecules such as proteins will be retarded, while their association may be enhanced and proteins tend to have more compact conformation. These considerations raise the possibility that intracellular behavior of proteins may not be estimated directly from conventional in vitro experiments. Accordingly, we became interested in re-investigating ER related glycan processing reactions under crowded conditions.53) Interestingly, we observed that the cleavage-2 of G-II was dramatically enhanced in high concentrations of bovine serum albumin (BSA), while the cleavage-1 was not affected. Similar effects were observed in the presence of other macromolecules such as polyethylene glycol or RNase A. On the other hand, the effect of macromolecular crowding on glucosylation by UGGT was negligible, and trimming by α-mannosidase was markedly retarded.
Although biological relevance of the effects exerted by macromolecular crowding must be addressed carefully, these results implicate that kinetics as well as specificities of glycoprotein processing enzymes may be quite different between intracellular and dilute buffer conditions. Previous studies have lead researchers to believe that the cleavage-1 of G-II trimming is overwhelmingly faster than the cleavage-2. However, the observation that the cleavage-2, but not cleavage-1, of G-II trimming was accelerated under macromolecular crowding conditions suggests that, in the ER, difference of velocities between them may be much smaller. In fact, recent study that analyzed in vivo trimming of glycoproteins in S. cerevisiae supports this prediction.
3. Conclusion
In glycobiology, researches have been challenged by the difficulty in obtaining well-defined homogeneous compounds, especially due to microheterogeneity of glycoprotein glycans. Furthermore, the glycoprotein quality control system discriminates guest proteins according to their folding state in addition to their glycoforms. In spite of quite extensive studies conducted using substrates of biological origin, more precise and quantitative studies, particularly in terms of specificity to discriminate subtly different glycans, have been limited. Our study stemmed from systematic synthesis of high-mannose-type glycans proven to be powerful in revealing specificities of key players in glycoprotein quality control such as CRT, UGGT, and G-II.
The research filed called “chemical biology” aims to clarify various biological events by exploitating chemical means, such as organic synthesis. Obviously, glycobiolgy is not an exception. Because of glycoconjugate glycans’ structural complexity and diversity, it will provide exciting research opportunities for synthetic chemists. We predict the importance of organic synthesis will be more firmly established in this area.
Acknowledgement
We thank Drs Kiichiro Totani, Ichiro Matsuo, Atsushi Tatami, Taisuke Watanabe, Atsushi Miyagawa, and Takashi Tsujimoto for their contributions to the works conducted in our laboratory. We also thank Dr. Yoshito Ihara for G-II and UGGT, and Ms. Akemi Takahashi and Ms. Satoko Shirahata for technical assistance. Financial supports from Ministry of Education, Culture, Sports, Science, and Technology [Grant-in-Aid for Creative Scientific Research (No. 17GS0420)] and Human Frontier Science Program (RGP0031/2005-C), and RIKEN Chemical Genomics Program are acknowledged.
Biographies
Profile
Yukishige Ito graduated the University of Tokyo in 1977 and obtained Ph.D. degree in Pharmaceutical Sciences (Research Advisor: Prof. Masaji Ohno) from the same university in 1982. After two years postdoctoral work at Department of Chemistry, Massachusetts Institute of Technology (Research Advisor: Prof. Satoru Masamune), he joined the laboratory of Dr. Tomoya Ogawa at RIKEN. He was promoted to Senior Scientist (Associated Chief Scientist) in 1996 and then to Chief Scientist in 1998. From 1991 to 1993, he was a Visiting Scientist in James C. Paulson’s laboratory at the Scripps Research Institute and CYTLE Corporation in San Diego, California. Currently, he is directing Synthetic Cellular Chemistry Laboratory at RIKEN Advanced Science Institute. Since 2009, he has also been the Research Director of JST ERATO Glycotrilogy Project. He received Japan Society for Bioscience, Biotechnology, and Agrochemistry Award for the Encouragement of Young Scientists (1993), Roy L. Whistler International Award in Carbohydrate Chemistry (2008), RIKEN Significant Achievement Award (S) (2010), and Takeda Award for International Achievement (2010). His research interest covers development of methodologies for oligosaccharide synthesis, synthesis of glycoconjugate related compounds, and analysis of glycoprotein processing in intracellular compartments.
Yoichi Takeda received his Ph.D. degree in Engineering from Tokyo University of Science in 2005 under the supervision of Professor Shigeomi Horito. After working as a postdoctoral fellow from 2005 to 2006 in the JST SORST Project involving sugar-based gene manipulators and as a NEDO fellow from 2006 to 2007 at the University of Kitakyushu with Professor Kazuo Sakurai, he joined the laboratory of Dr. Yukishige Ito at RIKEN as a contract researcher. Since 2009, he has worked as a group leader in the JST ERATO Glycotrilogy Project (Research Director: Dr. Yukishige Ito). His research interests include analysis of glycoprotein folding and processing in the endoplasmic reticulum by chemical approaches.
References
- 1).Rabinovich G.A., Toscano M.A. (2009) Turning ‘sweet' on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat. Rev. Immunol. 9, 338–352 [DOI] [PubMed] [Google Scholar]
- 2).van Kooyk Y., Rabinovich G.A. (2008) Protein-glycan interactions in the control of innate and adaptive immune responses. Nat. Immunol. 9, 593–601 [DOI] [PubMed] [Google Scholar]
- 3).Guerry P., Szymanski C.M. (2008) Campylobacter sugars sticking out. Trends Microbiol. 16, 428–435 [DOI] [PubMed] [Google Scholar]
- 4).Vigerust D.J., Shepherd V.L. (2007) Virus glycosylation: role in virulence and immune interactions. Trends Microbiol. 15, 211–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Kirmiz C., Li B., An H.J., Clowers B.H., Chew H.K., Lam K.S., Ferrige A., Alecio R., Borowsky A.D., Sulaimon S., Lebrilla C.B., Miyamoto S. (2007) A serum glycomics approach to breast cancer biomarkers. Mol. Cell. Proteomics 6, 43–55 [DOI] [PubMed] [Google Scholar]
- 6).Yin J., Miyazaki K., Shaner R.L., Merrill A.H., Jr., Kannagi R. (2010) Altered sphingolipid metabolism induced by tumor hypoxia—new vistas in glycolipid tumor markers. FEBS Lett. 584, 1872–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Atherton, E. and Sheppard, R.C. (1989) Solid phase peptide synthesis: a practical approach. IRL Press at Oxford University Press, New York. [Google Scholar]
- 8).Wellings D.A., Atherton E. (1997) Standard Fmoc protocols. Methods Enzymol. 289, 44–67 [DOI] [PubMed] [Google Scholar]
- 9).Caruthers M.H. (1985) Gene synthesis machines: DNA chemistry and its uses. Science 230, 281–285 [DOI] [PubMed] [Google Scholar]
- 10).Caruthers M.H. (1991) Chemical synthesis of DNA and DNA analogs. Acc. Chem. Res. 24, 278–284 [Google Scholar]
- 11).Daniels R., Kurowski B., Johnson A.E., Hebert D.N. (2003) N-linked glycans direct the cotranslational folding pathway of influenza hemagglutinin. Mol. Cell 11, 79–90 [DOI] [PubMed] [Google Scholar]
- 12).Whitley P., Nilsson I.M., von Heijne G. (1996) A nascent secretory protein may traverse the ribosome/endoplasmic reticulum translocase complex as an extended chain. J. Biol. Chem. 271, 6241–6244 [DOI] [PubMed] [Google Scholar]
- 13).Bause E. (1983) Structural requirements of N-glycosylation of proteins. Studies with proline peptides as conformational probes. Biochem. J. 209, 331–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Schulke N., Schmid F.X. (1988) Effect of glycosylation on the mechanism of renaturation of invertase from yeast. J. Biol. Chem. 263, 8832–8837 [PubMed] [Google Scholar]
- 15).Wang C., Eufemi M., Turano C., Giartosio A. (1996) Influence of the carbohydrate moiety on the stability of glycoproteins. Biochemistry 35, 7299–7307 [DOI] [PubMed] [Google Scholar]
- 16).Falgout B., Markoff L. (1995) Evidence that flavivirus Ns1-Ns2a cleavage is mediated by a membrane-bound host protease in the endoplasmic-reticulum. J. Virol. 69, 7232–7243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Olden K., Bernard B.A., Humphries M.J., Yeo T.K., Yeo K.T., White S.L., Newton S.A., Bauer H.C., Parent J.B. (1985) Function of glycoprotein glycans. Trends Biochem. Sci. 10, 78–82 [Google Scholar]
- 18).Helenius A., Aebi M. (2004) Roles of N-linked glycans in the endoplasmic reticulum. Annu. Rev. Biochem. 73, 1019–1049 [DOI] [PubMed] [Google Scholar]
- 19).Ruddock L.W., Molinari M. (2006) N-glycan processing in ER quality control. J. Cell Sci. 119, 4373–4380 [DOI] [PubMed] [Google Scholar]
- 20).Lederkremer G.Z. (2009) Glycoprotein folding, quality control and ER-associated degradation. Curr. Opin. Struct. Biol. 19, 515–523 [DOI] [PubMed] [Google Scholar]
- 21).Hubbard S.C., Robbins P.W. (1979) Synthesis and processing of protein-linked oligosaccharides in vivo. J. Biol. Chem. 254, 4568–4576 [PubMed] [Google Scholar]
- 22).Kornfeld R., Kornfeld S. (1985) Assembly of asparagine-linked oligosaccharides. Annu. Rev. Biochem. 54, 631–664 [DOI] [PubMed] [Google Scholar]
- 23).Caramelo J.J., Parodi A.J. (2008) Getting in and out from calnexin/calreticulin cycles. J. Biol. Chem. 283, 10221–10225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Russell S.J., Ruddock L.W., Salo K.E., Oliver J.D., Roebuck Q.P., Llewellyn D.H., Roderick H.L., Koivunen P., Myllyharju J., High S. (2004) The primary substrate binding site in the b' domain of ERp57 is adapted for endoplasmic reticulum lectin association. J. Biol. Chem. 279, 18861–18869 [DOI] [PubMed] [Google Scholar]
- 25).Kozlov G., Maattanen P., Schrag J.D., Pollock S., Cygler M., Nagar B., Thomas D.Y., Gehring K. (2006) Crystal structure of the bb' domains of the protein disulfide isomerase ERp57. Structure 14, 1331–1339 [DOI] [PubMed] [Google Scholar]
- 26).Caramelo J.J., Castro O.A., Alonso L.G., de Prat-Gay G., Parodi A.J. (2003) UDP-Glc:glycoprotein glucosyltransferase recognizes structured and solvent accessible hydrophobic patches in molten globule-like folding intermediates. Proc. Natl. Acad. Sci. U.S.A. 100, 86–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Caramelo J.J., Castro O.A., de Prat-Gay G., Parodi A.J. (2004) The endoplasmic reticulum glucosyltransferase recognizes nearly native glycoprotein folding intermediates. J. Biol. Chem. 279, 46280–46285 [DOI] [PubMed] [Google Scholar]
- 28).Taylor S.C., Ferguson A.D., Bergeron J.J., Thomas D.Y. (2004) The ER protein folding sensor UDP-glucose glycoprotein-glucosyltransferase modifies substrates distant to local changes in glycoprotein conformation. Nat. Struct. Mol. Biol. 11, 128–134 [DOI] [PubMed] [Google Scholar]
- 29).Taylor S.C., Thibault P., Tessier D.C., Bergeron J.J., Thomas D.Y. (2003) Glycopeptide specificity of the secretory protein folding sensor UDP-glucose glycoprotein:glucosyltransferase. EMBO Rep. 4, 405–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Arnold S.M., Fessler L.I., Fessler J.H., Kaufman R.J. (2000) Two homologues encoding human UDP-glucose:glycoprotein glucosyltransferase differ in mRNA expression and enzymatic activity. Biochemistry 39, 2149–2163 [DOI] [PubMed] [Google Scholar]
- 31).Arnold S.M., Kaufman R.J. (2003) The noncatalytic portion of human UDP-glucose: glycoprotein glucosyltransferase I confers UDP-glucose binding and transferase function to the catalytic domain. J. Biol. Chem. 278, 43320–43328 [DOI] [PubMed] [Google Scholar]
- 32).Molinari M., Galli C., Vanoni O., Arnold S.M., Kaufman R.J. (2005) Persistent glycoprotein misfolding activates the glucosidase II/UGT1-driven calnexin cycle to delay aggregation and loss of folding competence. Mol. Cell 20, 503–512 [DOI] [PubMed] [Google Scholar]
- 33).Ku S.C., Lwa T.R., Giam M., Yap M.G., Chao S.H. (2009) Identification of HUGT1 as a potential BiP activator and a cellular target for improvement of recombinant protein production using a cDNA screening system. Mol. Cells 27, 577–582 [DOI] [PubMed] [Google Scholar]
- 34).Moremen K.W., Molinari M. (2006) N-linked glycan recognition and processing: the molecular basis of endoplasmic reticulum quality control. Curr. Opin. Struct. Biol. 16, 592–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Gridley J., Osborn H. (2000) Recent advances in the construction of β-D-mannose and β-D-mannosamine linkages. J. Chem. Soc., Perkin Trans. 1, 1471–1491 [Google Scholar]
- 36).Barresi F., Hindsgaul O. (1991) Synthesis of β-mannopyranosides by intramolecular aglycon delivery. J. Am. Chem. Soc. 113, 9376–9377 [Google Scholar]
- 37).Ito Y., Ohnishi Y., Ogawa T., Nakahara Y. (1998) Highly optimized β-mannosylation via p-methoxybenzyl assisted intramolecular aglycon delivery. Synlett, 1102–1104 [Google Scholar]
- 38).Ishiwata A., Munemura Y., Ito Y. (2008) NAP ether mediated intramolecular aglycon delivery: A unified strategy for 1,2-cis-glycosylation. Eur. J. Org. Chem., 4250–4263 [Google Scholar]
- 39).Matsuo I., Totani K., Tatami A., Ito Y. (2006) Comprehensive synthesis of ER related high-mannose-type sugar chains by convergent strategy. Tetrahedron 62, 8262–8277 [Google Scholar]
- 40).Totani K., Matsuo I., Ito Y. (2004) Tight binding ligand approach to oligosaccharide-grafted protein. Bioorg. Med. Chem. Lett. 14, 2285–2289 [DOI] [PubMed] [Google Scholar]
- 41).Banerjee S., Vishwanath P., Cui J., Kelleher D.J., Gilmore R., Robbins P.W., Samuelson J. (2007) The evolution of N-glycan-dependent endoplasmic reticulum quality control factors for glycoprotein folding and degradation. Proc. Natl. Acad. Sci. U.S.A. 104, 11676–11681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Trombetta S.E., Bosch M., Parodi A.J. (1989) Glucosylation of glycoproteins by mammalian, plant, fungal, and trypanosomatid protozoa microsomal membranes. Biochemistry 28, 8108–8116 [DOI] [PubMed] [Google Scholar]
- 43).Totani K., Ihara Y., Matsuo I., Koshino H., Ito Y. (2005) Synthetic substrates for an endoplasmic reticulum protein-folding sensor, UDP-glucose: glycoprotein glucosyltransferase. Angew. Chem. Int. Ed. Engl. 44, 7950–7954 [DOI] [PubMed] [Google Scholar]
- 44).Totani K., Ihara Y., Matsuo I., Tsujimoto T., Ito Y. (2009) The recognition motif of the glycoprotein-folding sensor enzyme, UDP-Glc: glycoprotein glucosyltransferase. Biochemistry 48, 2933–2940 [DOI] [PubMed] [Google Scholar]
- 45).Totani K., Ihara Y., Matsuo I., Ito Y. (2006) Substrate specificity analysis of endoplasmic reticulum glucosidase II using synthetic high mannose-type glycans. J. Biol. Chem. 281, 31502–31508 [DOI] [PubMed] [Google Scholar]
- 46).Burns D.M., Touster O. (1982) Purification and characterization of glucosidase II, an endoplasmic reticulum hydrolase involved in glycoprotein biosynthesis. J. Biol. Chem. 257, 9990–10000 [PubMed] [Google Scholar]
- 47).Michael J.M., Kornfeld S. (1980) Partial purification and characterization of the glucosidases involved in the processing of asparagine-linked oligosaccharides. Arch. Biochem. Biophys. 199, 249–258 [DOI] [PubMed] [Google Scholar]
- 48).Bosis E., Nachliel E., Cohen T., Takeda Y., Ito Y., Bar-Nun S., Gutman M. (2008) Endoplasmic reticulum glucosidase II is inhibited by its end products. Biochemistry 47, 10970–10980 [DOI] [PubMed] [Google Scholar]
- 49).Miyagawa A., Totani K., Matsuo I., Ito Y. (2010) Promiscuous activity of ER glucosidase II discovered through donor specificity analysis of UGGT. Biochem. Biophys. Res. Commun. 403, 322–328 [DOI] [PubMed] [Google Scholar]
- 50).Trombetta E.S., Simons J.F., Helenius A. (1996) Endoplasmic reticulum glucosidase II is composed of a catalytic subunit, conserved from yeast to mammals, and a tightly bound noncatalytic HDEL-containing subunit. J. Biol. Chem. 271, 27509–27516 [DOI] [PubMed] [Google Scholar]
- 51).Watanabe T., Totani K., Matsuo I., Maruyama J., Kitamoto K., Ito Y. (2009) Genetic analysis of glucosidase II beta-subunit in trimming of high-mannose-type glycans. Glycobiology 19, 834–840 [DOI] [PubMed] [Google Scholar]
- 52).Ellis R.J., Minton A.P. (2003) Cell biology: join the crowd. Nature 425, 27–28 [DOI] [PubMed] [Google Scholar]
- 53).Totani K., Ihara Y., Matsuo I., Ito Y. (2008) Effects of macromolecular crowding on glycoprotein processing enzymes. J. Am. Chem. Soc. 130, 2101–2107 [DOI] [PubMed] [Google Scholar]