Abstract
In solid organ transplantation, ischemia/reperfusion (IR) injury during organ procurement, storage and reperfusion is an unavoidable detrimental event for the graft, as it amplifies graft inflammation and rejection. Intracellular mitogen-activated protein kinase (MAPK) signaling pathways regulate inflammation and cell survival during IR injury. The four best-characterized MAPK subfamilies are the c-Jun NH2-terminal kinase (JNK), extracellular signal- regulated kinase-1/2 (ERK1/2), p38 MAPK, and big MAPK-1 (BMK1/ERK5). Here, we review the role of MAPK activation during myocardial IR injury as it occurs during heart transplantation. Most of our current knowledge regarding MAPK activation and cardioprotection comes from studies of preconditioning and postconditioning in nontransplanted hearts. JNK and p38 MAPK activation contributes to myocardial IR injury after prolonged hypothermic storage. p38 MAPK inhibition improves cardiac function after cold storage, rewarming and reperfusion. Small-molecule p38 MAPK inhibitors have been tested clinically in patients with chronic inflammatory diseases, but not in transplanted patients, so far. Organ transplantation offers the opportunity of starting a preconditioning treatment before organ procurement or during cold storage, thus modulating early events in IR injury. Future studies will need to evaluate combined strategies including p38 MAPK and/or JNK inhibition, ERK1/2 activation, pre- or postconditioning protocols, new storage solutions, and gentle reperfusion.
1. Introduction
Heart transplantation is the final therapeutic option for heart failure [1]. Over the past two decades, advances in immunosuppression and antimicrobial agents have improved outcomes after heart transplantation. An analysis of the UNOS database in 14,401 first-time orthotopic heart transplant recipients between the years 1999 and 2006 showed that the survival rate at 30 days, 1 year, and 5 years was 94%, 87%, and 75%, respectively, for the young group (<60 years of age) and 93%, 84%, and 69% for the older group [2]. Graft vasculopathy, a unique form of accelerated coronary artery disease, is a major cause of late graft failure [3]. The disease is characterized by intimal thickening mainly due to smooth muscle cell proliferation and fibrosis. Occlusive narrowing of the coronary vessels can develop within a few months and is not prevented by current treatments.
The pathogenesis of graft vasculopathy is complex and has been reviewed elsewhere [4–6]. The observation that, while graft coronary arteries develop lesions, the host's native arteries are spared suggests a major pathogenic role for immune rejection. Consistent with this, while hearts transplanted into a genetically different recipient are affected, those placed back in the original donor strain are spared [7]. Clinical data support a major role for chronic rejection in the development of graft vasculopathy and graft failure. Indeed, the degree of donor-recipient human leukocyte antigen (HLA) matching correlates significantly with graft survival [8–10]. Moreover, acute cellular rejection has been associated with an increased risk of developing graft vasculopathy [11–14].
Both the innate [15] and the adaptive immune system including B cells and antibody formation against graft antigens [16] play central roles in the development of graft vasculopathy. Nonimmunological factors such as dyslipidemia, hypertension, drug toxicity, and infections also play contributory roles. Accordingly, the current paradigm is that graft vasculopathy results from repeated immune and nonimmune-mediated insults to graft coronary endothelium leading to endothelial inflammation and dysfunction, vascular cell proliferation, fibrosis, and intimal thickening.
Extended cold ischemic times during heart transplantation have been associated with increased risk of developing graft vasculopathy and failure both in animal models [17, 18] and in humans [19]. Moreover, prolonged times between donor brain death and organ retrieval have been associated with increased mortality in cardiac transplant recipients [20]. Graft coronary microvascular dysfunction after ischemia and reperfusion can culminate in primary graft failure or untreatable chronic rejection [21].
Cold ischemia stimulates the expression of inflammatory mediators acting as “danger signals” and amplifying tissue injury and graft rejection. Toll-like receptors (TLRs) play a central role in this regard [22]. Consistent with this, systemic administration of anti-TLR-2 antibody reduces neutrophil, macrophage, and T-lymphocyte infiltration in mouse hearts after ischemia and reperfusion [23]. Multiple strategies applied at the time of organ transplantation have a potential for limiting cold ischemic organ damage, reperfusion injury, and graft immunogenicity [24, 25].
2. Myocardial Ischemia/Reperfusion (IR) Injury
Early observations in animal models of myocardial infarction indicated that ischemic cell death progresses as a “wavefront” phenomenon correlated to the duration of ischemia [26], and that early reperfusion can salvage reversibly injured ischemic myocardium [27]. Subsequently, morphological changes appearing during reperfusion, including cardiomyocyte swelling and loss of sarcomeric organization, were recognized [28]. Moreover, interventions applied at the onset of reperfusion were still able to limit infarct size, suggesting a contributory role for reperfusion in lethal cell injury.
A comprehensive discussion of the molecular mechanisms of myocardial IR injury is beyond the scope of the present paper. These mechanisms have been reviewed elsewhere [29, 30]. It is possible here to briefly mention the role of mitochondria as both a source and a target of IR injury [31, 32]. Under normoxic conditions, mitochondria use oxygen to synthesize adenosine triphosphate (ATP). Sustained hypoxia leads to ATP depletion, acidosis, intracellular calcium accumulation, mitochondrial swelling, and cell death [30]. Cold ischemia exacerbates swelling via inhibition of the Na+/K+ ATPase. At reperfusion, calcium is taken up into the sarcoplasmic reticulum (SR) by the SR calcium ATPase. Calcium overload then leads to calcium release into the cytosol, cardiomyocyte hypercontracture, membrane disruption, and cell death [30].
During ischemia, mitochondria produce reactive oxygen species (ROS). An extra burst of ROS generation takes place at reperfusion. ROS mediates opening of the mitochondrial permeability transition pore (MPTP) leading to increased inner mitochondrial membrane permeability, mitochondrial depolarization, ATP depletion, mitochondrial matrix swelling, outer mitochondrial membrane rupture, cytochrome c release, and apoptosis [30, 32]. In addition, ROS activates multiple molecular cascades of inflammation [33]. Proinflammatory cytokines, such as IL-1 and TNFα, and chemokines are produced within hours of reperfusion in allogeneic and syngeneic grafts alike. Chemokines mediate early migration of neutrophils and macrophages into the graft [34, 35]. Early T-cell reaction precedes alloantigen priming and induces graft necrosis [36, 37]. Inflammatory activation of graft endothelium [38], platelets, the coagulation cascade, and the complement system [39] plays important roles in early graft injury and subsequent graft vasculopathy.
A multitude of intracellular signal transduction pathways are activated during myocardial IR injury [29, 30]. Among them, mitogen-activated protein kinases (MAPKs) are key regulators of cell function and survival [40, 41]. The present paper aims to discuss the role of MAPK activation in myocardial IR injury and its potential implications for heart transplantation.
3. MAPK Subfamilies
The MAPK family includes four major serine/threonine protein kinase subfamilies. Each MAPK subfamily comprises successively acting kinases including an upstream MAPK kinase kinase, a MAPK kinase, and a MAPK (Figure 1) [40]. Distinct isoforms of a MAPK bind molecules with different affinities and can activate distinct signaling pathways. In response to a variety of stress stimuli, MAPKs convey extracellular signals to their intracellular targets, thereby regulating cell survival, function, growth, and differentiation [41]. The best characterized MAPK subfamilies are c-Jun NH2-terminal kinases (JNKs), extracellular signal-regulated kinase-1/2 (ERK1/2, also known as p42/p44 MAPK), p38 MAPKs, and the big MAPK-1 (BMK1/ERK5). The role of each MAPK subfamily in myocardial IR injury is discussed in the next sections.
Figure 1.
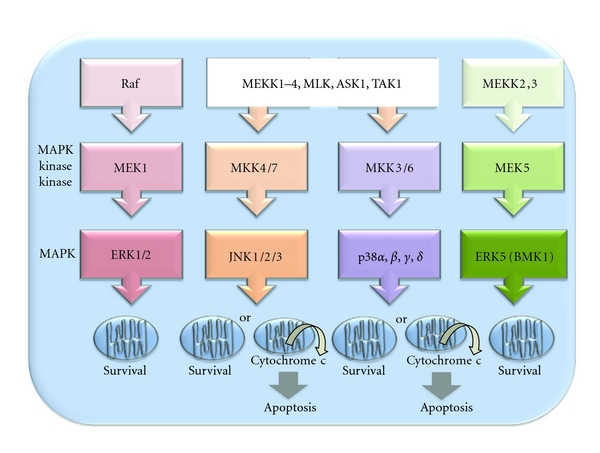
Schematic depicting the activation cascades of the four major MAPK subfamilies and corresponding effects on mitochondrial chromosome c release and apoptosis. ERK1/2 and ERK5/BMK1 have been associated with cell survival, whereas JNK and p38 MAPK have been predominantly associated with apoptosis.
3.1. ERK1/2 Activation during Myocardial IR Injury
ERK1/2 was discovered as the first member of the MAPK family in 1990 [42]. This serine/threonine protein kinase is tyrosine-phosphorylated in response to various extracellular signals. We observed a ≈2-fold increase in ERK1/2-specific in vitro kinase activity in isolated-perfused adult rat hearts subjected to 20 min of ischemia followed by 15 min of reperfusion [43]. Several studies support a protective role for the MEK1-ERK2 signaling pathway against IR injury [44–47]. Accordingly, this pathway has been identified as a central component of the so-called “Reperfusion Injury Salvage Kinase” (RISK) pathway [48].
3.2. JNK Activation during Myocardial IR Injury
JNK was discovered as the second member of the MAPK family in 1991 [49]. It is primarily activated by various cellular stresses such as heat, UV light, and cytokines. We observed a ≈6-fold increase in JNK-specific in vitro kinase activity and a ≈2-fold increase in phosphorylated c-Jun protein in nuclear extracts from isolated-perfused rat hearts subjected to 20 min of ischemia and 15 min of reperfusion [43]. JNK activation was increased during ischemia as well as reperfusion, in line with a limited number of previous studies [44, 50, 51]. In contrast, a larger number of studies reported JNK activation predominantly at reperfusion [52–56].
Dichotomous effects of JNK activation during IR injury including both cardioprotection [56–59] and myocardial damage [55, 60–64] have been reported. A potential mechanism of JNK-mediated protection is reactivation of Akt and enhanced cardiomyocyte survival after hypoxic injury [56]. Data in genetically modified mice show that JNK1/2 knockout mice and, paradoxically, transgenic mice overexpressing MKK7, the MAPK kinase upstream of JNK1/2, are each significantly protected from IR injury [65]. These findings illustrate the complexity of the biological effects of JNK activation.
A word of caution is warranted regarding the reliance on curcumin as a specific JNK inhibitor in early studies [66]. We therefore used a cell-penetrating peptide inhibitor of JNK, D-JNKI-1, as a more selective agent. In the isolated-perfused adult rat heart, D-JNKI-1 administered before the ischemic period selectively prevented JNK activation and improved post-ischemic cardiac function, cytochrome c release, caspase-3 activation, and apoptosis [43]. D-JNKI-1 administered at reperfusion failed to improve cardiac function but still prevented apoptosis. In vivo, D-JNKI-1 reduced myocardial infarct size by half after coronary artery occlusion and reperfusion in rats [43]. D-JNKI-1 similarly reduced cerebral infarct size after common carotid artery occlusion and reperfusion in adult rats [67].
Inconsistent findings from previous studies regarding the role of JNK activation during IR injury likely reflect differences in the experimental models and JNK inhibitors used, as well as JNK isoform-specific effects. It has been shown that inhibition of JNK1 isoform, but not of JNK2 isoform, prevents apoptosis induced by IR injury in rat cardiomyocytes [61].
3.3. p38 MAPK Activation during Myocardial IR Injury
The p38 MAPK subfamily comprises 4 main isoforms, p38α, p38β, p38γ, and p38δ, of which p38α and p38γ are most abundantly expressed within the myocardium. The role of p38 MAPK activation during myocardial IR remains controversial [68–70]. We observed a ≈2-fold increase in p38 MAPK-specific in vitro kinase activity in isolated-perfused rat hearts subjected to 20 min of ischemia and 15 min of reperfusion [43]. These results are in agreement with previous data [71]. p38 MAPK activation contributes to tissue injury induced by TNFα in response to hydrogen peroxide generated during reperfusion [33]. Moreover, p38 MAPK activation counteracts adenosine- or insulin-induced cardioprotection against IR injury [72, 73]. p38 MAPK inhibition limits infarct size and polymorphonuclear accumulation in mouse hearts subjected to IR injury [74]. Transgenic mice expressing a dominant-negative p38α mutant or a dominant-negative mutant of MKK6, a MAPK kinase upstream of p38 MAPK, are each significantly protected from IR injury [75]. These data suggest a potential role for p38α isoform as a mediator of myocardial IR injury.
Much of our current knowledge regarding cardioprotection comes from studies of preconditioning (PC) and postconditioning (PostC). Although a majority of these studies relate to nontransplanted hearts, they are relevant to heart transplantation.
4. Ischemic Preconditioning (IPC)
IPC was originally described as an experimental phenomenon whereby repeated episodes of brief, sublethal ischemia induced tolerance to a successive, prolonged period of lethal ischemia [30, 76, 77]. In the anesthetized dog, four 5 min periods of occlusion of the left coronary artery, interspersed with 5 min periods of rapid reflow, markedly attenuated infarct size after occlusion of the same artery for 40 min. Two distinct “windows” of IPC-mediated protection have been described [78, 79]. The first window of protection is induced within minutes, lasts for 1-2 h, is dependent on activation of MAPKs as well as of other signaling pathways, and attenuates infarct size but not contractile dysfunction nor myocardial stunning. The second window of protection takes place between 24 and 72 h after the triggering phase of IPC, requires synthesis of protective proteins within the heart, and limits cell death as well as contractile dysfunction [80]. IPC involves changes in energy metabolism, ionic homeostasis, and gene regulation as well as a decrease in ROS generation, neutrophil activation, and apoptosis [81]. Pharmacological agents such as opioids [82], inhalational anesthetics [83], adenosine, isoproterenol, and nitric oxide (NO) donors, [84] along with stress stimuli such as rapid cardiac pacing and thermal stress can precondition myocardial tissue to subsequent ischemia [30].
A comprehensive discussion of the molecular mechanisms of IPC is beyond the scope of the present paper. The interested reader is referred to recent reviews published elsewhere [30, 77, 85, 86]. It is possible here to merely mention a few molecular mechanisms. While the triggering phase of IPC requires NO and superoxide synthesis, IPC mitigates NO, superoxide, and peroxynitrite overproduction during subsequent IR [87]. Beside MAPKs, protein kinases activated by IPC include protein kinase C (PKC) isoforms [88, 89], phosphatidylinositol 3-kinase (PI3K) and its substrate kinase Akt [90, 91], receptor tyrosine kinases of the Src family [92, 93], the JAK/STAT pathway [94, 95], and glycogen synthase-3β (GSK-3β) [96]. The latter is a downstream kinase phosphorylated by other kinases such as ERK1/2 and Akt which has been implicated in cardioprotection including inhibition of MPTP opening at reperfusion. However, recent data suggest that decreased oxidative stress, rather than mitochondrial protein phosphorylation, is responsible for inhibition of MPTP opening in the context of IPC [97].
A number of studies have demonstrated MAPK activation during the triggering phase of IPC, at reperfusion, or both. In some cases, IPC has been associated with decreased MAPK activation during subsequent ischemia, suggesting a detrimental role for MAPK activation in this context. The activation of the different MAPK subfamilies in preconditioned hearts is discussed in the next sections.
4.1. ERK1/2 Activation during IPC
Both in vitro and in vivo studies have demonstrated ERK1/2 activation and cardioprotection after IPC [98–101], which was abolished by an ERK1/2 inhibitor in a pig model of IR injury [99]. In addition, hypoxic PC [102, 103] as well as delayed hypoxic PC [104, 105], adenosine-induced PC [106] as well as adenosine-induced delayed PC [107], isoflurane/desflurane-induced PC [83, 108], metabolic PC [109], and opioid-induced delayed PC are associated with increased ERK1/2 activation [110]. Moreover, mitochondrial KATP channel openers activate ERK1/2 by an oxidant-dependent mechanism [111].
Several studies reported biphasic ERK1/2 activation during IPC [82, 83]. The first phase of activation takes place immediately after the PC stimulus, and the second phase of activation occurs at reperfusion. Blocking the first phase of activation prevents the second one [83]. In response to IPC, PKCε induces the activation of ERK1/2 in the cytosol and its translocation to the nucleus, with increased activation of NF-kB and AP-1 transcription factors and protection against cardiomyocyte apoptosis [101]. Another mechanism by which ERK1/2 can impart protection to hypoxic myocardium involves phosphorylation of hypoxia-inducible factor (HIF)-1 [104].
A small number of studies either reported ERK1/2 activation during IPC [90] or metabolic preconditioning [112] without a contribution of it to the observed protection, or failed to detect ERK1/2 activation during IPC [113, 114].
4.2. JNK Activation during IPC
Several studies documented increased JNK activation during the triggering phase of IPC [55, 98, 100, 101, 113, 115–117] or, less frequently, during the sustained ischemic period after the IPC stimulus [113] or during reperfusion [55, 117]. Some studies suggested a potential role for JNK as a mediator of IPC-induced protection [100, 116], but this was not confirmed by other reports [117, 118]. Decreased JNK activation was observed in preconditioned brains, kidneys, and hepatocytes [119–121], suggesting that JNK activation may contribute to IR injury in these tissues.
4.3. p38 MAPK Activation during IPC
Several studies reported increased p38 MAPK activation during the triggering phase of IPC and reperfusion [83, 113, 116, 117, 122–132]. A limited number of studies showed p38 MAPK activation during the sustained ischemic period after the IPC stimulus [133–135]. p38 MAPK activation has also been observed in hypoxic PC [136, 137] and delayed hypoxic PC [138] as well as in NO, [139], angiotensin II [140], or adenosine-induced PC [115, 141–143].
The role of p38 MAPK as a potential mediator of protection in the preconditioned heart remains controversial. A majority of studies showed p38 MAPK activation during the triggering phase of PC [85, 110, 116, 122, 123, 125, 127–129, 131–138, 140, 141, 143–145]. IPC appears to require p38α but not p38β isoform activation [145]. Potential p38 MAPK-mediated protective mechanisms include phosphorylation of small heat shock protein (Hsp) 27, which stabilizes the actin cytoskeleton [146–148], and αβ crystalline [124].
A distinct group of studies failed to support a contributory role for p38 MAPK activation in IPC [50, 110, 149–152], hypoxic PC [140, 153], NO-induced PC [154], delayed metabolic PC [109, 112], and opioid-induced delayed PC [110]. A third group of studies showed reduced p38 MAPK activation during the sustained ischemic period after the PC stimulus [140, 150, 152–154], suggesting a detrimental role for p38 MAPK activation in this setting. Consistent with this, numerous studies demonstrated that a p38 MAPK inhibitor applied during the sustained ischemic period can protect the myocardium against IR injury [44, 116, 125, 126, 140, 147, 149–153, 155, 156].
These inconsistent findings from different studies are difficult to reconcile; however, it should be considered that the mechanism of p38 MAPK activation can differ by circumstance [70], and that distinct p38 MAPK isoforms activate different signaling pathways. Increased p38α isoform activation during sustained ischemia [50, 153] has been associated with cardiomyocyte apoptosis [157, 158], contractile dysfunction [158], and increased infarct size [159]. p38 MAPK has been shown to negatively regulate myocardial contractility [160–162].
4.4. IPC and BMK1/ERK5 Activation
The big MAP kinase 1 (BMK1/ERK5) pathway [163] is activated in the heart in response to IPC [164] and has been implicated as a potential mediator of cardioprotection [165]. BMK1/ERK5-induced phosphorylation of the mitochondrial protein BAD has been shown to attenuate endothelial cell and cardiomyocyte apoptosis [166–168]. Similarly, BMK1/ERK5 activation during cerebral IPC prevents apoptosis in the ischemic rat hippocampal CA1 region [169].
5. Remote Preconditioning (RPC)
RPC is a biological mechanism of interorgan protection against IR injury [170, 171]. Brief cycles of IR applied to a tissue remote from the heart, such as the small intestine [172] or the upper or lower limb [173], before the onset of myocardial ischemia limit myocardial infarct size. A comparison of RPC and IPC induced by occlusion of the superior mesenteric artery and the left coronary artery, respectively, in a rat model of myocardial IR injury showed a greater effect of IPC compared with RPC in terms of infarct size reduction [174]. In this study, IPC was associated with increased ERK1/2 and JNK1 activation but reduced p38 MAPK activation in the heart. In contrast, RPC triggered by occlusion of the superior mesenteric artery induced ERK1/2 and JNK1 activation in the small intestine without participation of MAPKs in the heart. Each of the applied ERK1/2, JNK, and p38 MAPK inhibitors abrogated RPC-mediated protection. An underlying mechanism may be PKCε isoform activation in the heart via remote ischemia-induced transmitter release [175]. A distinct study showed equivalent degrees of cardioprotection induced by IPC and RPC, while suggesting a role for bradykinin as a mediator of cardiac PC at a distance [176].
6. Postconditioning (PostC)
Ischemic PostC can be elicited by repetitive cycles of rapid reflow/reocclusion in the initial 2 min after release of a protracted coronary occlusion [29, 30, 177–181]. Because tissue injury is initiated within minutes of reperfusion, PostC must be applied at the onset of reperfusion [181]. PostC has limited infarct size in all species tested so far [177, 178, 182–184], including humans [185, 186]. The degree of PostC-mediated cardioprotection is comparable to that induced by IPC [177, 178, 186], or slightly lower than it [187]. PostC activates adenosine receptors and the NO/cGMP pathway [188, 189], mitochondrial KATP channels, PKC and protein kinase G (PKG) [190], and the RISK pathway including ERK1/2 [188] and PI3K/Akt [184, 189, 191]. In the rabbit model of myocardial IR injury, an ERK1/2 inhibitor abolished protection by brief episodes of coronary occlusion applied at reperfusion [188]. PostC has also been shown to reduce oxidative stress in a senescent mouse model [192] and to attenuate cardiomyocyte apoptosis after simulated ischemia via JNK and p38 MAPK inhibition [193]. Moreover, PostC has been shown to inhibit MPTP opening in the early minutes of reperfusion [194].
The RISK pathway is not the only cardioprotective pathway [195]. In mouse and rabbit hearts, protection after ischemic PostC was associated with increased activation of ERK, but not Akt [183, 196]. In pigs, ischemic PostC enhanced ERK and Akt activation during reperfusion without a decrease in infarct size [197]. A distinct study in anesthetized pigs demonstrated myocardial protection after PostC without an increase in Akt, ERK, and GSK-3β phosphorylation and with no effect of PI3K or ERK1/2 blockade [198]. Gentle reperfusion likewise reduced infarct size in pigs without activation of the RISK pathway [199]. The so-called “Survivor Activating Factor Enhancement” (SAFE) pathway [200] which includes the JAK-STAT signaling pathway [94, 95], may be responsible for cardioprotection in the absence of activation of the RISK pathway.
Pharmacological stimuli including inhalational anesthetics can replace the ischemic PostC stimulus applied at the onset of reperfusion [201–203]. While myocardial protection after ischemic PostC is not enhanced by IPC [187], pharmacological PostC and IPC or pharmacological PC may have additive effects.
7. IPC, RPC, and PostC for Protection against Myocardial IR Injury in Humans
Recently, IPC, RPC, and PostC strategies for attenuating myocardial IR injury have been tested in clinical trials in nontransplanted patients [77]. Both IPC and pharmacological PC reduced myocardial IR injury in patients undergoing coronary artery bypass graft surgery [204–207]. In a randomized controlled trial, RPC triggered by a simple noninvasive technique of four 5 min cycles of lower limb ischemia and reperfusion induced cardioprotection in children undergoing cardiac surgery for congenital heart disease [208]. In a distinct randomized controlled trial, RPC triggered by transient upper limb ischemia induced cardioprotection in adult patients undergoing coronary artery bypass graft surgery [209]. In the prospective randomized controlled cardiac remote ischemic preconditioning in coronary stenting (CRISP Stent) trial, RPC alleviated ischemic chest discomfort and myocardial injury during coronary stenting, while also reducing subsequent cardiovascular events [210]. In a randomised trial in patients with acute myocardial infarction undergoing angioplasty, ischemic RPC before hospital admission proved to be safe and appeared to salvage ischemic myocardium [211].
Ischemic PostC has been evaluated in patients with ST elevation myocardial infarction (STEMI) undergoing angioplasty [185]. Within the first minute after stent implantation, patients in the PostC group underwent four cycles of 1 min inflation and 1 min deflation of the coronary angioplasty balloon. Creatine kinase release, measured as a surrogate for infarct size, was significantly reduced by 36% in PostC versus control patients. Contractile function was still improved in the PostC group at 1 year following infarct [212]. Whether or not PostC protects against endothelial IR injury in humans remains unclear [213, 214].
To our knowledge, no data on IPC, RPC, or PostC in human heart transplantation have been published so far. Analogously, data on MAPK inhibitors in this setting are restricted to animal models, as discussed in the next section.
8. MAPK Inhibition in Experimental Heart Transplantation
ERK1/2, JNK, and p38 MAPK activation within cardiac grafts has been demonstrated in dogs [215]. MAPK activation can contribute to graft injury via multiple mechanisms including cytokine upregulation [216–219], immune cell activation, and apoptosis.
JNK promotes T-cell activation and differentiation. For instance, JNK and ERK1/2 have been shown to stimulate IL-2 production by Thy-1-activated mouse T lymphocytes in vitro [220]. JNK inhibition reduced histological rejection and improved graft survival in a rat model of heart transplantation [221].
p38 MAPK is involved in IL-2R signaling in T lymphocytes, while also stimulating cytokine release from human macrophages in vitro [222]. A p38 MAPK inhibitor administered at reperfusion improved functional recovery of rat hearts after prolonged hypothermic ischemia [223]. In a brain-dead donor model, a p38 MAPK inhibitor lowered systemic levels of proinflammatory cytokines while not affecting intracardiac cytokine levels [224]. Addition of a p38 MAPK inhibitor to the Celsior solution enhanced the viability of cardiac grafts from non-heart-beating donors in a canine model of heart transplantation [225]. Moreover, p38 MAPK blockade attenuated the release of proinflammatory IL-6 by human endothelial cells in vitro after cooling and rewarming [226]. p38 MAPK inhibition similarly prevented endothelial adhesion molecule expression and polymorphonuclear accumulation after myocardial IR injury in rats [74]. p38 MAPK blockade markedly reduced vascular smooth muscle cell proliferation in aortic grafts and the development of graft vasculopathy [227]. Finally, addition of a p38 MAPK inhibitor to the Euro-Collins and University of Wisconsin solutions mitigated IR injury in lung [228] and liver [229] grafts, respectively, as well as in kidney grafts from non-heart-beating donors [230]. Thus, a p38 MAPK inhibitor applied during organ procurement and storage can protect the graft against IR injury.
9. PC and PostC in Experimental Heart Transplantation
The potential relevance of PC and PostC strategies to organ transplantation has been reviewed elsewhere [231–233]. Proof-of-principle studies in animal models have demonstrated that IPC can impart protection on cardiac grafts [234–236]. Pretreatment of rat hearts with an adenosine analog prior to harvesting and storage in the Euro-Collins solution for 8 hours improved functional recovery at reperfusion [237]. In another study, IPC combined with Na+/H+ antiporter inhibition improved cardiac function in rat hearts after 4 hours of storage at 4°C in Celsior solution and extracorporeal reperfusion [238]. KATP channel activation mimicked the protective effect of IPC in hearts after prolonged hypothermic storage [239–241]. However, one study showed IPC-induced cardioprotection after global ischemia, but not after cold cardioplegia [242]. Also, brain death completely abolished PC-mediated protection in ischemic rabbit hearts [243]. This finding might be explained by catecholamine storm after brain death, since norepinephrine injection before IPC abolished protection in the absence of brain death [244]. AMP-activated protein kinase (AMPK) is emerging as a target for PC in transplantation medicine [245].
PC induced by sildenafil administration to the donor 30 min before the onset of ischemia improved the function of cardiac grafts after 3 h of hypothermic cardioplegic arrest [246]. In contrast, PostC induced by sildenafil administration 5 min before reperfusion in the recipient was ineffective.
PKCδ inhibition improved cardiac contractile performance and coronary perfusion after cold cardioplegic arrest in isolated rat hearts [247]. This approach similarly attenuated heart transplant injury and graft coronary vasculopathy after prolonged organ ischemia [248]. Isoflurane as well as inhaled hydrogen or carbon monoxide has been shown to alter energy substrate metabolism to preserve mechanical function in isolated rat hearts after extended no-flow hypothermic storage [249, 250].
Ischemic RPC was tested in a pig model of orthotopic heart transplantation from brain-dead donors [251]. RPC of the recipient by four 5 min cycles of lower limb ischemia attenuated IR injury of the denervated donor heart via a KATP channel-dependent mechanism.
Ischemic PostC was tested in isolated working rat hearts after global total ischemia (4 h/4°C) and 45 min of reperfusion [252]. Three brief episodes of total global ischemia applied at the onset of reperfusion reduced myocardial injury and postischemic dysfunction. In another study, both PostC and remote PostC attenuated tissue damage in warm ischemic rat cardiac grafts [253].
The first clinical application of IPC in solid organ transplantation concerned liver transplantation [254]. Although IPC mitigated inflammatory responses [255], it was associated with initial poor function. It did neither improve nor compromise the outcome of cadaver liver transplantation [254].
10. Concluding Remarks and Perspectives
Proof-of-principle studies have provided evidence that therapeutic manipulation of the donor heart at the time of transplantation can mitigate graft injury, immunogenicity, and rejection. A possibility is that molecular events during the triggering phase of PC, which induce protection, can be applied to the donor heart before transplantation. A preconditioning drug (e.g., sildenafil) can be administered to the donor before organ retrieval and/or 5 min before reperfusion in the recipient [246]. The clinical efficacy of ischemic PostC in STEMI patients [185] suggests that this approach might be beneficial in heart-transplanted patients as well. A p38 MAPK inhibitor can be added to an organ preservation solution or administered at reperfusion [223, 225]. A p38 MAPK inhibitor administered to the recipient markedly inhibited the development of aortic graft vasculopathy in an experimental model [227]. Small-molecule inhibitors of p38 MAPK have been developed [256] and tested in initial clinical trials in patients with active rheumatoid arthritis or neuropathic pain [257, 258]. Further preclinical studies are needed, however, before these drugs can be tested in heart transplant recipients. In principle, extended p38 MAPK inhibitor administration during several weeks or months after transplantation might protect against graft vasculopathy.
Because distinct MAPK isoforms have different substrate affinities and functions [61, 145, 159], the precise identification of MAPK isoforms that contribute to IR injury would allow for the development of targeted therapies. Avoiding indiscriminate MAPK blockade is important because MAPK activates signaling pathways participating in host defense against infection and tumors.
Despite promising results obtained with MAPK inhibitors as well as PC and PostC in animal models, it should be noted that clinical trials of cardioprotective agents successfully tested in animal models have been largely negative so far [259]. However, a recent trial suggested a protective effect of cyclosporine, a MPTP opening inhibitor, against reperfusion injury in patients with acute myocardial infarction [260]. In transplantation medicine, MAPK inhibitors will need to be tested in combination with other PC and PostC strategies, as well as with improved organ preservation solutions and reperfusion protocols (e.g., continuous myocardial perfusion and controlled initial reperfusion) [261–263].
Acknowledgment
Financial support by the Cecilia-Augusta Foundation, Lugano, Switzerland, and by the Swiss National Science Foundation is gratefully acknowledged.
References
- 1.Koerner MM, Durand JB, Lafuente JA, Noon GP, Torre-Amione G. Cardiac transplantation: the final therapeutic option for the treatment of heart failure. Current Opinion in Cardiology. 2000;15(3):178–182. doi: 10.1097/00001573-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Weiss ES, Nwakanma LU, Patel ND, Yuh DD. Outcomes in patients older than 60 years of age undergoing orthotopic heart transplantation: an analysis of the UNOS database. Journal of Heart and Lung Transplantation. 2008;27(2):184–191. doi: 10.1016/j.healun.2007.11.566. [DOI] [PubMed] [Google Scholar]
- 3.Christie JD, Edwards LB, Kucheryavaya AY, et al. The registry of the international society for heart and lung transplantation: twenty-seventh official adult lung and heart-lung transplant report 2010. Journal of Heart and Lung Transplantation. 2010;29(10):1104–1118. doi: 10.1016/j.healun.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Rahmani M, Cruz RP, Granville DJ, McManus BM. Allograft vasculopathy versus atherosclerosis. Circulation Research. 2006;99(8):801–815. doi: 10.1161/01.RES.0000246086.93555.f3. [DOI] [PubMed] [Google Scholar]
- 5.Vassalli G, Gallino A, Weis M, et al. Alloimmunity and nonimmunologic risk factors in cardiac allograft vasculopathy. European Heart Journal. 2003;24(13):1180–1188. doi: 10.1016/s0195-668x(03)00237-9. [DOI] [PubMed] [Google Scholar]
- 6.Schmauss D, Weis M. Cardiac allograft vasculopathy: recent developments. Circulation. 2008;117(16):2131–2141. doi: 10.1161/CIRCULATIONAHA.107.711911. [DOI] [PubMed] [Google Scholar]
- 7.Fiedel S, Bayer J, Schaub M, et al. The influence of antigen-dependent and antigen-independent factors on the development of graft vasculopathy in a fully allogeneic cardiac allograft model in the rat. Transplantation Proceedings. 2009;41(6):2625–2627. doi: 10.1016/j.transproceed.2009.06.111. [DOI] [PubMed] [Google Scholar]
- 8.Hosenpud JD, Edwards EB, Lin HM, Daily OP. Influence of HLA matching on thoracic transplant outcomes: an analysis from the UNOS/ISHLT thoracic registry. Circulation. 1996;94(2):170–174. doi: 10.1161/01.cir.94.2.170. [DOI] [PubMed] [Google Scholar]
- 9.Opelz G, Wujciak T, Döhler B, Scherer S, Mytilineos J. HLA compatibility and organ transplant survival. Collaborative Transplant Study. Reviews in immunogenetics. 1999;1(3):334–342. [PubMed] [Google Scholar]
- 10.Kaczmarek I, Deutsch MA, Rohrer ME, et al. HLA-DR matching improves survival after heart transplantation: is it time to change allocation policies? Journal of Heart and Lung Transplantation. 2006;25(9):1057–1062. doi: 10.1016/j.healun.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Jimenez J, Kapadia SR, Yamani MH, et al. Cellular rejection and rate of progression of transplant vasculopathy: a 3-year serial intravascular ultrasound study. Journal of Heart and Lung Transplantation. 2001;20(4):393–398. doi: 10.1016/s1053-2498(00)00249-7. [DOI] [PubMed] [Google Scholar]
- 12.Yamani MH, Yousufuddin M, Starling RC, et al. Does acute cellular rejection correlate with cardiac allograft vasculopathy? Journal of Heart and Lung Transplantation. 2004;23(3):272–276. doi: 10.1016/S1053-2498(03)00189-X. [DOI] [PubMed] [Google Scholar]
- 13.Brunner-La Rocca HP, Schneider J, Künzli A, Turina M, Kiowski W. Cardiac allograft rejection late after transplantation is a risk factor for graft coronary artery disease. Transplantation. 1998;65(4):538–543. doi: 10.1097/00007890-199802270-00015. [DOI] [PubMed] [Google Scholar]
- 14.Dandel M, Hummel M, Wellnhofer E, Kapell S, Lehmkuhl HB, Hetzer R. Association between acute rejection and cardiac allograft vasculopathy. Journal of Heart and Lung Transplantation. 2003;22(9):1064–1065. doi: 10.1016/s1053-2498(02)01152-x. [DOI] [PubMed] [Google Scholar]
- 15.Millington TM, Madsen JC. Innate immunity in heart transplantation. Current Opinion in Organ Transplantation. 2009;14(5):571–576. doi: 10.1097/MOT.0b013e32832e7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gareau A, Hirsch GM, Lee TDG, Nashan B. Contribution of b cells and antibody to cardiac allograft vasculopathy. Transplantation. 2009;88(4):470–477. doi: 10.1097/TP.0b013e3181b076cc. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka M, Mokhtari GK, Terry RD, et al. Prolonged cold ischemia in rat cardiac allografts promotes ischemia-reperfusion injury and the development of graft coronary artery disease in a linear fashion. Journal of Heart and Lung Transplantation. 2005;24(11):1906–1914. doi: 10.1016/j.healun.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Schneeberger S, Amberger A, Mandl J, et al. Cold ischemia contributes to the development of chronic rejection and mitochondrial injury after cardiac transplantation. Transplant International. 2010;23(12):1282–1292. doi: 10.1111/j.1432-2277.2010.01126.x. [DOI] [PubMed] [Google Scholar]
- 19.Gaudin PB, Rayburn BK, Hutchins GM, et al. Peritransplant injury to the myocardium associated with the development of accelerated arteriosclerosis in heart transplant recipients. American Journal of Surgical Pathology. 1994;18(4):338–346. doi: 10.1097/00000478-199404000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Ramjug S, Hussain N, Yonan N. Prolonged time between donor brain death and organ retrieval results in an increased risk of mortality in cardiac transplant recipients. Interactive Cardiovascular and Thoracic Surgery. 2011;12(6):938–942. doi: 10.1510/icvts.2010.252809. [DOI] [PubMed] [Google Scholar]
- 21.Tuuminen R, Syrjälä S, Krebs R, et al. Donor simvastatin treatment abolishes rat cardiac allograft ischemia/reperfusion injury and chronic rejection through microvascular protection. Circulation. 2011;124(10):1138–1150. doi: 10.1161/CIRCULATIONAHA.110.005249. [DOI] [PubMed] [Google Scholar]
- 22.Kaczorowski DJ, Nakao A, Vallabhaneni R, et al. Mechanisms of toll-like receptor 4 (TLR4)-mediated inflammation after cold ischemia/reperfusion in the heart. Transplantation. 2009;87(10):1455–1463. doi: 10.1097/TP.0b013e3181a36e5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arslan F, Smeets MB, O’Neill LAJ, et al. Myocardial ischemia/reperfusion injury is mediated by leukocytic toll-like receptor-2 and reduced by systemic administration of a novel anti-toll-like receptor-2 antibody. Circulation. 2010;121(1):80–90. doi: 10.1161/CIRCULATIONAHA.109.880187. [DOI] [PubMed] [Google Scholar]
- 24.Van Der Woude FJ, Schnuelle P, Yard BA. Preconditioning strategies to limit graft immunogenicity and cold ischemic organ injury. Journal of Investigative Medicine. 2004;52(5):323–329. doi: 10.1136/jim-52-05-32. [DOI] [PubMed] [Google Scholar]
- 25.Lutz J, Thürmel K, Heemann U. Anti-inflammatory treatment strategies for ischemia/reperfusion injury in transplantation. Journal of Inflammation. 2010;7, article 27 doi: 10.1186/1476-9255-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reimer KA, Lowe JE, Rasmussen MM, Jennings RB. The wavefront phenomenon of ischemic cell death—1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation. 1977;56(5):786–794. doi: 10.1161/01.cir.56.5.786. [DOI] [PubMed] [Google Scholar]
- 27.Maroko PR, Libby P, Ginks WR, et al. Coronary artery reperfusion—I. Early effects on local myocardial function and the extent of myocardial necrosis. Journal of Clinical Investigation. 1972;51(10):2710–2716. doi: 10.1172/JCI107090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jennings RB, Sommers HM, Smyth GA, Flack HA, Linn H. Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Archives of Pathology. 1960;70:68–78. [PubMed] [Google Scholar]
- 29.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. New England Journal of Medicine. 2007;357(11):1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 30.Ferdinandy P, Schulz R, Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacological Reviews. 2007;59(4):418–458. doi: 10.1124/pr.107.06002. [DOI] [PubMed] [Google Scholar]
- 31.Boengler K, Heusch G, Schulz R. Nuclear-encoded mitochondrial proteins and their role in cardioprotection. Biochimica et Biophysica Acta. 2011;1813(7):1286–1294. doi: 10.1016/j.bbamcr.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 32.Murphy E, Steenbergen C. What makes the mitochondria a killer? Can we condition them to be less destructive? Biochimica et Biophysica Acta. 2011;1813(7):1302–1308. doi: 10.1016/j.bbamcr.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meldrum DR, Dinarello CA, Cleveland JC, et al. Hydrogen peroxide induces tumor necrosis factor α-mediated cardiac injury by a P38 mitogen-activated protein kinase-dependent mechanism. Surgery. 1998;124(2):291–297. doi: 10.1067/msy.1998.90570. [DOI] [PubMed] [Google Scholar]
- 34.Ishii D, Schenk AD, Baba S, Fairchild RL. Role of TNFα in early chemokine production and leukocyte infiltration into heart allografts. American Journal of Transplantation. 2010;10(1):59–68. doi: 10.1111/j.1600-6143.2009.02921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jordan JE, Zhao ZQ, Vinten-Johansen J. The role of neutrophils in myocardial ischemia-reperfusion injury. Cardiovascular Research. 1999;43(4):860–878. doi: 10.1016/s0008-6363(99)00187-x. [DOI] [PubMed] [Google Scholar]
- 36.Morita K, Miura M, Paolone DR, et al. Early chemokine cascades in murine cardiac grafts regulate T cell recruitment and progression of acute allograft rejection. Journal of Immunology. 2001;167(5):2979–2984. doi: 10.4049/jimmunol.167.5.2979. [DOI] [PubMed] [Google Scholar]
- 37.El-Sawy T, Miura M, Fairchild R. Early T cell response to allografts occuring prior to alloantigen priming up-regulates innate-mediated inflammation and graft necrosis. American Journal of Pathology. 2004;165(1):147–157. doi: 10.1016/s0002-9440(10)63283-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mikalsen B, Fosby B, Wang J, et al. Genome-wide transcription profile of endothelial cells after cardiac transplantation in the rat. American Journal of Transplantation. 2010;10(7):1534–1544. doi: 10.1111/j.1600-6143.2010.03157.x. [DOI] [PubMed] [Google Scholar]
- 39.Diepenhorst GMP, Van Gulik TM, Hack CE. Complement-mediated ischemia-reperfusion injury: lessons learned from animal and clinical studies. Annals of Surgery. 2009;249(6):889–899. doi: 10.1097/SLA.0b013e3181a38f45. [DOI] [PubMed] [Google Scholar]
- 40.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiological Reviews. 1999;79(1):143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 41.Gerits N, Kostenko S, Moens U. In vivo functions of mitogen-activated protein kinases: conclusions from knock-in and knock-out mice. Transgenic Research. 2007;16(3):281–314. doi: 10.1007/s11248-006-9052-0. [DOI] [PubMed] [Google Scholar]
- 42.Boulton TG, Yancopoulos GD, Gregory JS, et al. An insulin-stimulated protein kinase similar to yeast kinases involved in cell cycle control. Science. 1990;249(4964):64–67. doi: 10.1126/science.2164259. [DOI] [PubMed] [Google Scholar]
- 43.Milano G, Morel S, Bonny C, et al. A peptide inhibitor of c-Jun NH2-terminal kinase reduces myocardial ischemia-reperfusion injury and infarct size in vivo. American Journal of Physiology. 2007;292(4):H1828–H1835. doi: 10.1152/ajpheart.01117.2006. [DOI] [PubMed] [Google Scholar]
- 44.Yue TL, Wang C, Gu JL, et al. Inhibition of extracellular signal-regulated kinase enhances ischemia/reoxygenation-induced apoptosis in cultured cardiac myocytes and exaggerates reperfusion injury in isolated perfused heart. Circulation Research. 2000;86(6):692–699. doi: 10.1161/01.res.86.6.692. [DOI] [PubMed] [Google Scholar]
- 45.Lips DJ, Bueno OF, Wilkins BJ, et al. MEK1-ERK2 signaling pathway protects myocardium from ischemic injury in vivo. Circulation. 2004;109(16):1938–1941. doi: 10.1161/01.CIR.0000127126.73759.23. [DOI] [PubMed] [Google Scholar]
- 46.Das A, Salloum FN, Xi L, Rao YJ, Kukreja RC. ERK phosphorylation mediates sildenafil-induced myocardial protection against ischemia-reperfusion injury in mice. American Journal of Physiology. 2009;296(5):H1236–H1243. doi: 10.1152/ajpheart.00100.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang X, Liu Y, Yang X-M, et al. Cardioprotection by mild hypothermia during ischemia involves preservation of ERK activity. Basic Research in Cardiology. 2011;106(3):421–430. doi: 10.1007/s00395-011-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovascular Research. 2004;61(3):448–460. doi: 10.1016/j.cardiores.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 49.Kyriakis JM, Banerjee P, Nikolakaki E, et al. The stress-activated protein kinase subfamily of c-jun kinases. Nature. 1994;369(6476):156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 50.Ping P, Zhang J, Huang S, et al. PKC-dependent activation of p46/p54 JNKs during ischemic preconditioning in conscious rabbits. American Journal of Physiology. 1999;277(5):H1771–H1785. doi: 10.1152/ajpheart.1999.277.5.H1771. [DOI] [PubMed] [Google Scholar]
- 51.Shimizu N, Yoshiyama M, Omura T, et al. Activation of mitogen-activated protein kinases and activator protein- 1 in myocardial infarction in rats. Cardiovascular Research. 1998;38(1):116–124. doi: 10.1016/s0008-6363(97)00327-1. [DOI] [PubMed] [Google Scholar]
- 52.Knight RJ, Buxton DB. Stimulation of c-Jun kinase and mitogen-activated protein kinase by ischemia and reperfusion in the perfused rat heart. Biochemical and Biophysical Research Communications. 1996;218(1):83–88. doi: 10.1006/bbrc.1996.0016. [DOI] [PubMed] [Google Scholar]
- 53.Mizukami Y, Yoshioka K, Morimoto S, Yoshida KI. A novel mechanism of JNK1 activation. Nuclear translocation and activation of JNK1 during ischemia and reperfusion. Journal of Biological Chemistry. 1997;272(26):16657–16662. doi: 10.1074/jbc.272.26.16657. [DOI] [PubMed] [Google Scholar]
- 54.Yin T, Sandhu G, Wolfgang CD, et al. Tissue-specific pattern of stress kinase activation in ischemic/reperfused heart and kidney. Journal of Biological Chemistry. 1997;272(32):19943–19950. doi: 10.1074/jbc.272.32.19943. [DOI] [PubMed] [Google Scholar]
- 55.Fryer RM, Patel HH, Hsu AK, Gross GJ. Stress-activated protein kinase phosphorylation during cardioprotection in the ischemic myocardium. American Journal of Physiology. 2001;281(3):H1184–H1192. doi: 10.1152/ajpheart.2001.281.3.H1184. [DOI] [PubMed] [Google Scholar]
- 56.Shao Z, Bhattacharya K, Hsich E, et al. c-Jun N-terminal kinases mediate reactivation of Akt and cardiomyocyte survival after hypoxic injury in vitro and in vivo. Circulation Research. 2006;98(1):111–118. doi: 10.1161/01.RES.0000197781.20524.b9. [DOI] [PubMed] [Google Scholar]
- 57.Andreka P, Zang J, Dougherty C, Slepak TI, Webster KA, Bishopric NH. Cytoprotection by Jun kinase during nitric oxide-induced cardiac myocyte apoptosis. Circulation Research. 2001;88(3):305–312. doi: 10.1161/01.res.88.3.305. [DOI] [PubMed] [Google Scholar]
- 58.Dougherty CJ, Kubasiak LA, Prentice H, Andreka P, Bishopric NH, Webster KA. Activation of c-Jun N-terminal kinase promotes survival of cardiac myocytes after oxidative stress. Biochemical Journal. 2002;362(3):561–571. doi: 10.1042/0264-6021:3620561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Engelbrecht AM, Niesler C, Page C, Lochner A. p38 and JNK have distinct regulatory functions on the development of apoptosis during simulated ischaemia and reperfusion in neonatal cardiomyocytes. Basic Research in Cardiology. 2004;99(5):338–350. doi: 10.1007/s00395-004-0478-3. [DOI] [PubMed] [Google Scholar]
- 60.Aoki H, Kang PM, Hampe J, et al. Direct activation of mitochondrial apoptosis machinery by c-Jun n-terminal kinase in adult cardiac myocytes. Journal of Biological Chemistry. 2002;277(12):10244–10250. doi: 10.1074/jbc.M112355200. [DOI] [PubMed] [Google Scholar]
- 61.Hreniuk D, Garay M, Gaarde W, Monia BP, Mckay RA, Cioffi CL. Inhibition of C-Jun N-terminal kinase 1, but not c-Jun N-terminal kinase 2, suppresses apoptosis induced by ischemia/reoxygenation in rat cardiac myocytes. Molecular Pharmacology. 2001;59(4):867–874. doi: 10.1124/mol.59.4.867. [DOI] [PubMed] [Google Scholar]
- 62.Li WG, Coppey L, Weiss RM, Oskarsson HJ. Antioxidant therapy attenuates JNK activation and apoptosis in the remote noninfarcted myocardium after large myocardial infarction. Biochemical and Biophysical Research Communications. 2001;280(1):353–357. doi: 10.1006/bbrc.2000.4134. [DOI] [PubMed] [Google Scholar]
- 63.Kwon SH, Pimentel DR, Remondino A, Sawyer DB, Colucci WS. H2O2 regulates cardiac myocyte phenotype via concentration-dependent activation of distinct kinase pathways. Journal of Molecular and Cellular Cardiology. 2003;35(6):615–621. doi: 10.1016/s0022-2828(03)00084-1. [DOI] [PubMed] [Google Scholar]
- 64.Remondino A, Kwon SH, Communal C, et al. β-adrenergic receptor-stimulated apoptosis in cardiac myocytes is mediated by reactive oxygen species/c-Jun NH2-terminal kinase-dependent activation of the mitochondrial pathway. Circulation Research. 2003;92(2):136–138. doi: 10.1161/01.res.0000054624.03539.b4. [DOI] [PubMed] [Google Scholar]
- 65.Kaiser RA, Liang Q, Bueno O, et al. Genetic inhibition or activation of JNK1/2 protects the myocardium from ischemia-reperfusion-induced cell death in vivo. Journal of Biological Chemistry. 2005;280(38):32602–32608. doi: 10.1074/jbc.M500684200. [DOI] [PubMed] [Google Scholar]
- 66.Chen YR, Tan TH. Inhibition of the c-Jun N-terminal kinase (JNK) signaling pathway by curcumin. Oncogene. 1998;17(2):173–178. doi: 10.1038/sj.onc.1201941. [DOI] [PubMed] [Google Scholar]
- 67.Borsellol T, Clarkel PGH, Hirt L, et al. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nature Medicine. 2003;9(9):1180–1186. doi: 10.1038/nm911. [DOI] [PubMed] [Google Scholar]
- 68.Steenbergen C. The role of p38 mitogen-activated protein kinase in myocardial ischemia/reperfusion injury; Relationship to ischemic preconditioning. Basic Research in Cardiology. 2002;97(4):276–285. doi: 10.1007/s00395-002-0364-9. [DOI] [PubMed] [Google Scholar]
- 69.Ping P, Murphy E. Role of p38 mitogen-activated protein kinases in preconditioning: a detrimental factor or a protective kinase? Circulation Research. 2000;86(9):921–922. doi: 10.1161/01.res.86.9.921. [DOI] [PubMed] [Google Scholar]
- 70.Bassi R, Heads R, Marber MS, Clark JE. Targeting p38-MAPK in the ischaemic heart: kill or cure? Current Opinion in Pharmacology. 2008;8(2):141–146. doi: 10.1016/j.coph.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 71.Kumar D, Menon V, Ford WR, Clanachan AS, Jugdutt BI. Effect of angiotensin II type 2 receptor blockade on mitogen activated protein kinase during myocardial ischemia-reperfusion. Molecular and Cellular Biochemistry. 2004;258(1-2):211–218. doi: 10.1023/b:mcbi.0000012857.06723.81. [DOI] [PubMed] [Google Scholar]
- 72.Jaswal JS, Gandhi M, Finegan BA, Dyck JRB, Clanachan AS. Inhibition of p38 MAPK and AMPK restores adenosine-induced cardioprotection in hearts stressed by antecedent ischemia by altering glucose utilization. American Journal of Physiology. 2007;293(2):H1107–H1114. doi: 10.1152/ajpheart.00455.2007. [DOI] [PubMed] [Google Scholar]
- 73.Chai W, Wu Y, Li G, Cao W, Yang Z, Liu Z. Activation of p38 mitogen-activated protein kinase abolishes insulin-mediated myocardial protection against ischemia-reperfusion injury. American Journal of Physiology-Endocrinology and Metabolism. 2008;294(1):E183–E189. doi: 10.1152/ajpendo.00571.2007. [DOI] [PubMed] [Google Scholar]
- 74.Gao F, Yue TL, Shi DW, et al. p38 MAPK inhibition reduces myocardial reperfusion injury via inhibition of endothelial adhesion molecule expression and blockade of PMN accumulation. Cardiovascular Research. 2002;53(2):414–422. doi: 10.1016/s0008-6363(01)00488-6. [DOI] [PubMed] [Google Scholar]
- 75.Kaiser RA, Bueno OF, Lips DJ, et al. Targeted inhibition of p38 mitogen-activated protein kinase antagonizes cardiac injury and cell death following ischemia-reperfusion in vivo. Journal of Biological Chemistry. 2004;279(15):15524–15530. doi: 10.1074/jbc.M313717200. [DOI] [PubMed] [Google Scholar]
- 76.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 77.Granfeldt A, Lefer DJ, Vinten-Johansen J. Protective ischaemia in patients: preconditioning and postconditioning. Cardiovascular Research. 2009;83(2):234–246. doi: 10.1093/cvr/cvp129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kuzuya T, Hoshida S, Yamashita N, et al. Delayed effects of sublethal ischemia on the acquisition of tolerance to ischemia. Circulation Research. 1993;72(6):1293–1299. doi: 10.1161/01.res.72.6.1293. [DOI] [PubMed] [Google Scholar]
- 79.Marber MS, Latchman DS, Walker JM, Yellon DM. Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation. 1993;88(3):1264–1272. doi: 10.1161/01.cir.88.3.1264. [DOI] [PubMed] [Google Scholar]
- 80.Bolli R, Li QH, Tang XL, et al. The late phase of preconditioning and its natural clinical application—gene therapy. Heart Failure Reviews. 2007;12(3-4):189–199. doi: 10.1007/s10741-007-9031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Piot CA, Padmanaban D, Ursell PC, Sievers RE, Wolfe CL. Ischemic preconditioning decreases apoptosis in rat hearts in vivo. Circulation. 1997;96(5):1598–1604. doi: 10.1161/01.cir.96.5.1598. [DOI] [PubMed] [Google Scholar]
- 82.Fryer RM, Pratt PF, Hsu AK, Gross GJ. Differential activation of extracellular signal regulated kinase isoforms in preconditioning and opioid-induced cardioprotection. Journal of Pharmacology and Experimental Therapeutics. 2001;296(2):642–649. [PubMed] [Google Scholar]
- 83.Da Silva R, Grampp T, Pasch T, Schaub MC, Zaugg M. Differential activation of mitogen-activated protein kinases in ischemic and anesthetic preconditioning. Anesthesiology. 2004;100(1):59–69. doi: 10.1097/00000542-200401000-00013. [DOI] [PubMed] [Google Scholar]
- 84.Bolli R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. Journal of Molecular and Cellular Cardiology. 2001;33(11):1897–1918. doi: 10.1006/jmcc.2001.1462. [DOI] [PubMed] [Google Scholar]
- 85.Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. American Journal of Physiology. 2005;288(2):H971–H976. doi: 10.1152/ajpheart.00374.2004. [DOI] [PubMed] [Google Scholar]
- 86.Hausenloy DJ, Yellon DM. Survival kinases in ischemic preconditioning and postconditioning. Cardiovascular Research. 2006;70(2):240–253. doi: 10.1016/j.cardiores.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 87.Ferdinandy P, Schulz R. Nitric oxide, superoxide, and peroxynitrite in myocardial ischaemia-reperfusion injury and preconditioning. British Journal of Pharmacology. 2003;138(4):532–543. doi: 10.1038/sj.bjp.0705080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu Y, Ytrehus K, Downey JM. Evidence that translocation of protein kinase C is a key event during ischemic preconditioning of rabbit myocardium. Journal of Molecular and Cellular Cardiology. 1994;26(5):661–668. doi: 10.1006/jmcc.1994.1078. [DOI] [PubMed] [Google Scholar]
- 89.Meldrum DR, Cleveland JC, Meng X, et al. Protein kinase C isoform diversity in preconditioning. Journal of Surgical Research. 1997;69(1):183–187. doi: 10.1006/jsre.1997.5072. [DOI] [PubMed] [Google Scholar]
- 90.Tong H, Chen W, Steenbergen C, Murphy E. Ischemic preconditioning activates phosphatidylinositol-3-kinase upstream of protein kinase C. Circulation Research. 2000;87(4):309–315. doi: 10.1161/01.res.87.4.309. [DOI] [PubMed] [Google Scholar]
- 91.Mocanu MM, Bell RM, Yellon DM. PI3 kinase and not p42/p44 appears to be implicated in the protection conferred by ischemic preconditioning. Journal of Molecular and Cellular Cardiology. 2002;34(6):661–668. doi: 10.1006/jmcc.2002.2006. [DOI] [PubMed] [Google Scholar]
- 92.Vahlhaus C, Schulz R, Post H, Rose J, Heusch G. Prevention of ischemic preconditioning only by combined inhibition of protein kinase C and protein tyrosine kinase in pigs. Journal of Molecular and Cellular Cardiology. 1998;30(2):197–209. doi: 10.1006/jmcc.1997.0609. [DOI] [PubMed] [Google Scholar]
- 93.Oldenburg O, Critz SD, Cohen MV, Downey JM. Acetylcholine-induced production of reactive oxygen species in adult rabbit ventricular myocytes is dependent on phosphatidylinositol 3- and Src-kinase activation and mitochondrial KATP channel opening. Journal of Molecular and Cellular Cardiology. 2003;35(6):653–660. doi: 10.1016/s0022-2828(03)00083-x. [DOI] [PubMed] [Google Scholar]
- 94.Hattori R, Maulik N, Otani H, et al. Role of STAT3 in ischemic preconditioning. Journal of Molecular and Cellular Cardiology. 2001;33(11):1929–1936. doi: 10.1006/jmcc.2001.1456. [DOI] [PubMed] [Google Scholar]
- 95.Barry SP, Townsend PA, Latchman DS, Stephanou A. Role of the JAK-STAT pathway in myocardial injury. Trends in Molecular Medicine. 2007;13(2):82–89. doi: 10.1016/j.molmed.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 96.Juhaszova M, Zorov DB, Kim SH, et al. Glycogen synthase kinase-3β mediates convergence of protection signalling to inhibit the mitochondrial permeability transition pore. Journal of Clinical Investigation. 2004;113(11):1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Clarke SJ, Khaliulin I, Das M, Parker JE, Heesom KJ, Halestrap AP. Inhibition of mitochondrial permeability transition pore opening by ischemic preconditioning is probably mediated by reduction of oxidative stress rather than mitochondrial protein phosphorylation. Circulation Research. 2008;102(9):1082–1090. doi: 10.1161/CIRCRESAHA.107.167072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ping P, Zhang J, Cao X, et al. PKC-dependent activation of p44/p42 MAPKs during myocardial ischemia- reperfusion in conscious rabbits. American Journal of Physiology. 1999;276(5):H1468–H1481. doi: 10.1152/ajpheart.1999.276.5.H1468. [DOI] [PubMed] [Google Scholar]
- 99.Strohm C, Barancik M, Brühl MLV, Kilian SAR, Schaper W. Inhibition of the ER-kinase cascade by PD98059 and UO126 counteracts ischemic preconditioning in pig myocardium. Journal of Cardiovascular Pharmacology. 2000;36(2):218–229. doi: 10.1097/00005344-200008000-00012. [DOI] [PubMed] [Google Scholar]
- 100.Strohm C, Barancík M, Von Bruehl ML, et al. Transcription inhibitor actinomycin-D abolishes the cardioprotective effect of ischemic reconditioning. Cardiovascular Research. 2002;55(3):602–618. doi: 10.1016/s0008-6363(02)00453-4. [DOI] [PubMed] [Google Scholar]
- 101.Li RCX, Ping P, Zhang J, et al. PKCε modulates NF-κB and AP-1 via mitogen-activated protein kinases in adult rabbit cardiomyocytes. American Journal of Physiology. 2000;279(4):H1679–H1689. doi: 10.1152/ajpheart.2000.279.4.H1679. [DOI] [PubMed] [Google Scholar]
- 102.Button L, Mireylees SE, Germack R, Dickenson JM. Phosphatidylinositol 3-kinase and ERK1/2 are not involved in adenosine A1, A2A or A3 receptor-mediated preconditioning in rat ventricle strips. Experimental Physiology. 2005;90(5):747–754. doi: 10.1113/expphysiol.2005.030635. [DOI] [PubMed] [Google Scholar]
- 103.Solenkova NV, Solodushko V, Cohen MV, Downey JM. Endogenous adenosine protects preconditioned heart during early minutes of reperfusion by activating Akt. American Journal of Physiology. 2006;290(1):H441–H449. doi: 10.1152/ajpheart.00589.2005. [DOI] [PubMed] [Google Scholar]
- 104.Liu X, Wu X, Cai L, Tang C, Su J. Hypoxic preconditioning of cardiomyocytes and cardioprotection: phophorylation of HIF-1α induced by p42/p44 mitogen-activated protein kinases is involved. Pathophysiology. 2003;9(4):201–205. doi: 10.1016/s0928-4680(03)00006-3. [DOI] [PubMed] [Google Scholar]
- 105.Gong KZ, Zhang ZG, Li AH, et al. ROS-mediated ERK activation in delayed protection from anoxic preconditioning in neonatal rat cardiomyocytes. Chinese Medical Journal. 2004;117(3):395–400. [PubMed] [Google Scholar]
- 106.Reid EA, Kristo G, Yoshimura Y, et al. In vivo adenosine receptor preconditioning reduces myocardial infarct size via subcellular ERK signaling. American Journal of Physiology. 2005;288(5):H2253–H2259. doi: 10.1152/ajpheart.01009.2004. [DOI] [PubMed] [Google Scholar]
- 107.Lasley RD, Keith BJ, Kristo G, Yoshimura Y, Mentzer RM., Jr. Delayed adenosine A1 receptor preconditioning in rat myocardium is MAPK dependent but iNOS independent. American Journal of Physiology. 2005;289(2):H785–H791. doi: 10.1152/ajpheart.01008.2004. [DOI] [PubMed] [Google Scholar]
- 108.Toma O, Weber NC, Wolter JI, Obal D, Preckel B, Schlack W. Desflurane preconditioning induces time-dependent activation of protein kinase C epsilon and extracellular signal-regulated kinase 1 and 2 in the rat heart in vivo. Anesthesiology. 2004;101(6):1372–1380. doi: 10.1097/00000542-200412000-00018. [DOI] [PubMed] [Google Scholar]
- 109.Punn A, Mockridge JW, Farooqui S, Marber MS, Heads RJ. Sustained activation of p42/p44 mitogen-activated protein kinase during recovery from simulated ischaemia mediates adaptive cytoprotection in cardiomyocytes. Biochemical Journal. 2000;350(3):891–899. [PMC free article] [PubMed] [Google Scholar]
- 110.Fryer RM, Hsu AK, Gross GJ. ERK and p38 MAP kinase activation are components of opioid-induced delayed cardioprotection. Basic Research in Cardiology. 2001;96(2):136–142. doi: 10.1007/s003950170063. [DOI] [PubMed] [Google Scholar]
- 111.Samavati L, Monick MM, Sanlioglu S, Buettner GR, Oberley LW, Hunninghake GW. Mitochondrial KATP channel openers activate the ERK kinase by an oxidant-dependent mechanism. American Journal of Physiology. 2002;283(1):C273–C281. doi: 10.1152/ajpcell.00514.2001. [DOI] [PubMed] [Google Scholar]
- 112.Mockridge JW, Punn A, Latchman DS, Marber MS, Heads RJ. PKC-dependent delayed metabolic preconditioning is independent of transient MAPK activation. American Journal of Physiology. 2000;279(2):H492–H501. doi: 10.1152/ajpheart.2000.279.2.H492. [DOI] [PubMed] [Google Scholar]
- 113.Takeishi Y, Huang Q, Wang T, et al. Src family kinase and adenosine differentially regulate multiple MAP kinases in ischemic myocardium: modulation of MAP kinases activation by ischemic preconditioning. Journal of Molecular and Cellular Cardiology. 2001;33(11):1989–2005. doi: 10.1006/jmcc.2001.1463. [DOI] [PubMed] [Google Scholar]
- 114.Kim SO, Baines CP, Critz SD, et al. Ischemia induced activation of heat shock protein 27 kinases and casein kinase 2 in the preconditioned rabbit heart. Biochemistry and Cell Biology. 1999;77(6):559–567. [PubMed] [Google Scholar]
- 115.Haq SEA, Clerk A, Sugden PH. Activation of mitogen-activated protein kinases (p38-MAPKs, SAPKs/JNKs and ERKs) by adenosine in the perfused rat heart. FEBS Letters. 1998;434(3):305–308. doi: 10.1016/s0014-5793(98)01000-x. [DOI] [PubMed] [Google Scholar]
- 116.Sato M, Cordis GA, Maulik N, Das DK. SAPKs regulation of ischemic preconditioning. American Journal of Physiology. 2000;279(3):H901–H907. doi: 10.1152/ajpheart.2000.279.3.H901. [DOI] [PubMed] [Google Scholar]
- 117.Behrends M, Schulz R, Post H, et al. Inconsistent relation of MAPK activation to infarct size reduction by ischemic preconditioning in pigs. American Journal of Physiology. 2000;279(3):H1111–H1119. doi: 10.1152/ajpheart.2000.279.3.H1111. [DOI] [PubMed] [Google Scholar]
- 118.Iliodromitis EK, Gaitanaki C, Lazou A, et al. Dissociation of stress-activated protein kinase (p38-MAPK and JNKs) phosphorylation from the protective effect of preconditioning in vivo. Journal of Molecular and Cellular Cardiology. 2002;34(8):1019–1028. doi: 10.1006/jmcc.2002.2039. [DOI] [PubMed] [Google Scholar]
- 119.Gu Z, Jiang Q, Zhang G. Extracellular signal-regulated kinase and c-Jun N-terminal protein kinase in ischemic tolerance. NeuroReport. 2001;12(16):3487–3491. doi: 10.1097/00001756-200111160-00023. [DOI] [PubMed] [Google Scholar]
- 120.Crenesse D, Laurens M, Gugenheim J, et al. Intermittent ischemia reduces warm hypoxia-reoxygenation-induced JNK1/SAPK1 activation and apoptosis in rat hepatocytes. Hepatology. 2001;34(5):972–978. doi: 10.1053/jhep.2001.28709. [DOI] [PubMed] [Google Scholar]
- 121.Park KM, Chen A, Bonventre JV. Prevention of kidney ischemia/reperfusion-induced functional injury and JNK, p38, and MAPK kinase activation by remote ischemic pretreatment. Journal of Biological Chemistry. 2001;276(15):11870–11876. doi: 10.1074/jbc.M007518200. [DOI] [PubMed] [Google Scholar]
- 122.Nakano A, Baines CP, Kim SO, et al. Ischemic preconditioning activates MAPKAPK2 in the isolated rabbit heart: evidence for involvement of p38 MAPK. Circulation Research. 2000;86(2):144–151. doi: 10.1161/01.res.86.2.144. [DOI] [PubMed] [Google Scholar]
- 123.Sakamoto K, Urushidani T, Nagao T. Translocation of HSP27 to sarcomere induced by ischemic preconditioning in isolated rat hearts. Biochemical and Biophysical Research Communications. 2000;269(1):137–142. doi: 10.1006/bbrc.2000.2233. [DOI] [PubMed] [Google Scholar]
- 124.Eaton P, Fuller W, Bell JR, Shattock MJ. αB crystallin translocation and phosphorylation: signal transduction pathways and preconditioning in the isolated rat heart. Journal of Molecular and Cellular Cardiology. 2001;33(9):1659–1671. doi: 10.1006/jmcc.2001.1418. [DOI] [PubMed] [Google Scholar]
- 125.Sanada S, Kitakaze M, Papst PJ, et al. Role of phasic dynamism of p38 mitogen-activated protein kinase activation in ischemic preconditioning of the canine heart. Circulation Research. 2001;88(2):175–180. doi: 10.1161/01.res.88.2.175. [DOI] [PubMed] [Google Scholar]
- 126.Marais E, Genade S, Huisamen B, Strijdom JG, Moolman JA, Lochner A. Activation of p38 MAPK induced by a multi-cycle ischaemic preconditioning protocol is associated with attenuated p38 MAPK activity during sustained ischaemia and reperfusion. Journal of Molecular and Cellular Cardiology. 2001;33(4):769–778. doi: 10.1006/jmcc.2001.1347. [DOI] [PubMed] [Google Scholar]
- 127.Schulz R, Belosjorow S, Gres P, Jansen J, Michel MC, Heusch G. p38 MAP kinase is a mediator of ischemic preconditioning in pigs. Cardiovascular Research. 2002;55(3):690–700. doi: 10.1016/s0008-6363(02)00319-x. [DOI] [PubMed] [Google Scholar]
- 128.Schulz R, Gres P, Skyschally A, et al. Ischemic preconditioning preserves connexin 43 phosphorylation during sustained ischemia in pig hearts in vivo. The FASEB Journal. 2003;17(10):1355–1357. doi: 10.1096/fj.02-0975fje. [DOI] [PubMed] [Google Scholar]
- 129.Nakamura Y, Miura T, Nakano A, et al. Role of microtubules in ischemic preconditioning against myocardial infarction. Cardiovascular Research. 2004;64(2):322–330. doi: 10.1016/j.cardiores.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 130.Marais E, Genade S, Salie R, et al. The temporal relationship between p38 MAPK and HSP27 activation in ischaemic and pharmacological preconditioning. Basic Research in Cardiology. 2005;100(1):35–47. doi: 10.1007/s00395-004-0495-7. [DOI] [PubMed] [Google Scholar]
- 131.Ballard-Croft C, Kristo G, Yoshimura Y, et al. Acute adenosine preconditioning is mediated by p38 MAPK activation in discrete subcellular compartments. American Journal of Physiology. 2005;288(3):H1359–H1366. doi: 10.1152/ajpheart.01006.2004. [DOI] [PubMed] [Google Scholar]
- 132.Moolman JA, Hartley S, Van Wyk J, Marais E, Lochner A. Inhibition of myocardial apoptosis by ischaemic and beta-adrenergic preconditioning is dependent on p38 MAPK. Cardiovascular Drugs and Therapy. 2006;20(1):13–25. doi: 10.1007/s10557-006-6257-7. [DOI] [PubMed] [Google Scholar]
- 133.Weinbrenner C, Liu GS, Cohen MV, Downey JM. Phosphorylation of tyrosine 182 of p38 mitogen-activated protein kinase correlates with the protection of preconditioning in the rabbit heart. Journal of Molecular and Cellular Cardiology. 1997;29(9):2383–2391. doi: 10.1006/jmcc.1997.0473. [DOI] [PubMed] [Google Scholar]
- 134.Maulik N, Sato M, Price BD, Das DK. An essential role of NFκB in tyrosine kinase signaling of p38 MAP kinase regulation of myocardial adaptation to ischemia. FEBS Letters. 1998;429(3):365–369. doi: 10.1016/s0014-5793(98)00632-2. [DOI] [PubMed] [Google Scholar]
- 135.Maulik N, Yoshida T, Zu YL, Sato M, Banerjee A, Das DK. Ischemic preconditioning triggers tyrosine kinase signaling: a potential role for MAPKAP kinase 2. American Journal of Physiology. 1998;275(5):H1857–H1864. doi: 10.1152/ajpheart.1998.275.5.H1857. [DOI] [PubMed] [Google Scholar]
- 136.Armstrong SC, Delacey M, Ganote CE. Phosphorylation state of hsp27 and p38 MAPK during preconditioning and protein phosphatase inhibitor protection of rabbit cardiomyocytes. Journal of Molecular and Cellular Cardiology. 1999;31(3):555–567. doi: 10.1006/jmcc.1998.0891. [DOI] [PubMed] [Google Scholar]
- 137.Loubani M, Galiñanes M. Pharmacological and ischemic preconditioning of the human myocardium: mitoK(ATP) channels are upstream and p38MAPK is downstream of PKC. BMC Physiology. 2002;2(1, article 10):p. 10. doi: 10.1186/1472-6793-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Loubani M, Hassouna A, Galiñanes M. Delayed preconditioning of the human myocardium: signal transduction and clinical implications. Cardiovascular Research. 2004;61(3):600–609. doi: 10.1016/j.cardiores.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 139.Lochner A, Marais E, Toit EDU, Moolman J. Nitric oxide triggers classic ischemic preconditioning. Annals of the New York Academy of Sciences. 2002;962:402–414. doi: 10.1111/j.1749-6632.2002.tb04084.x. [DOI] [PubMed] [Google Scholar]
- 140.Kimura S, Zhang GX, Nishiyama A, et al. Role of NAD(P)H oxidase- and mitochondria-derived reactive oxygen species in cardioprotection of ischemic reperfusion injury by angiotensin II. Hypertension. 2005;45(5):860–866. doi: 10.1161/01.HYP.0000163462.98381.7f. [DOI] [PubMed] [Google Scholar]
- 141.Nagarkatti DS, Sha’afi RI. Role of p38 MAP kinase in myocardial stress. Journal of Molecular and Cellular Cardiology. 1998;30(8):1651–1664. doi: 10.1006/jmcc.1998.0733. [DOI] [PubMed] [Google Scholar]
- 142.Dana A, Skarli M, Papakrivopoulou J, Yellon DM. Adenosine A1 receptor induced delayed preconditioning in rabbits: induction of p38 mitogen-activated protein kinase activation and Hsp27 phosphorylation via a tyrosine kinase- and protein kinase C-dependent mechanism. Circulation Research. 2000;86(9):989–997. doi: 10.1161/01.res.86.9.989. [DOI] [PubMed] [Google Scholar]
- 143.Zhao TC, Hines DS, Kukreja RC. Adenosine-induced late preconditioning in mouse hearts: role of p38 MAP kinase and mitochondrial KATP channels. American Journal of Physiology. 2001;280(3):H1278–H1285. doi: 10.1152/ajpheart.2001.280.3.H1278. [DOI] [PubMed] [Google Scholar]
- 144.Mocanu MM, Baxter GF, Yue Y, Critz SD, Yellon DM. The p38 MAPK inhibitor, SB203580, abrogates ischaemic preconditioning in rat heart but timing of administration is critical. Basic Research in Cardiology. 2000;95(6):472–478. doi: 10.1007/s003950070023. [DOI] [PubMed] [Google Scholar]
- 145.Sicard P, Clark JE, Jacquet S, et al. The activation of p38alpha, and not p38beta, mitogen-activated protein kinase is required for ischemic preconditioning. Journal of Molecular and Cellular Cardiology. 2010;48(6):1324–1328. doi: 10.1016/j.yjmcc.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rouse J, Cohen P, Trigon S, et al. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78(6):1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 147.Li G, Ali IS, Currie RW. Insulin-induced myocardial protection in isolated ischemic rat hearts requires p38 MAPK phosphorylation of Hsp27. American Journal of Physiology. 2008;294(1):H74–H87. doi: 10.1152/ajpheart.00675.2007. [DOI] [PubMed] [Google Scholar]
- 148.Clements RT, Feng J, Cordeiro B, Bianchi C, Sellke FW. P38 MAPK-dependent small HSP27 and αB-crystallin phosphorylation in regulation of myocardial function following cardioplegic arrest. American Journal of Physiology. 2011;300(5):H1669–H1677. doi: 10.1152/ajpheart.00272.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Barancik M, Htun P, Strohm C, Kilian S, Schaper W. Inhibition of the cardiac p38-MAPK pathway by SB203580 delays ischemic cell death. Journal of Cardiovascular Pharmacology. 2000;35(3):474–483. doi: 10.1097/00005344-200003000-00019. [DOI] [PubMed] [Google Scholar]
- 150.Gysembergh A, Simkhovich BZ, Kloner RA, Przyklenk K. p38 MAPK activity is not increased early during sustained coronary artery occlusion in preconditioned versus control rabbit heart. Journal of Molecular and Cellular Cardiology. 2001;33(4):681–690. doi: 10.1006/jmcc.2000.1331. [DOI] [PubMed] [Google Scholar]
- 151.Schneider S, Chen W, Hou J, Steenbergen C, Murphy E. Inhibition of p38 MAPK α/β reduces ischemic injury and does not block protective effects of preconditioning. American Journal of Physiology. 2001;280(2):H499–H508. doi: 10.1152/ajpheart.2001.280.2.H499. [DOI] [PubMed] [Google Scholar]
- 152.Lochner A, Genade S, Hattingh S, Marais E, Huisamen B, Moolman JA. Comparison between Ischaemic and Anisomycin-Induced Preconditioning: role of p38 MAPK. Cardiovascular Drugs and Therapy. 2003;17(3):217–230. doi: 10.1023/a:1026116022552. [DOI] [PubMed] [Google Scholar]
- 153.Saurin AT, Martin JL, Heads RJ, et al. The role of differential activation of p38-mitogen-activated protein kinase in preconditioned ventricular myocytes. FASEB Journal. 2000;14(14):2237–2246. doi: 10.1096/fj.99-0671com. [DOI] [PubMed] [Google Scholar]
- 154.Rakhit RD, Kabir ANM, Mockridge JW, Saurin A, Marber MS. Role of G proteins and modulation of p38 MAPK activation in the protection by nitric oxide against ischemia-reoxygenation injury. Biochemical and Biophysical Research Communications. 2001;286(5):995–1002. doi: 10.1006/bbrc.2001.5477. [DOI] [PubMed] [Google Scholar]
- 155.Mackay K, Mochly-Rosen D. An inhibitor of p38 mitogen-activated protein kinase protects neonatal cardiac myocytes from ischemia. Journal of Biological Chemistry. 1999;274(10):6272–6279. doi: 10.1074/jbc.274.10.6272. [DOI] [PubMed] [Google Scholar]
- 156.Ma XL, Kumar S, Gao F, et al. Inhibition of p38 mitogen-activated protein kinase decreases cardiomyocyte apoptosis and improves cardiac function after myocardial ischemia and reperfusion. Circulation. 1999;99(13):1685–1691. doi: 10.1161/01.cir.99.13.1685. [DOI] [PubMed] [Google Scholar]
- 157.Nemoto S, Xiang J, Huang S, Lin A. Induction of apoptosis by SB202190 through inhibition of p38β mitogen- activated protein kinase. Journal of Biological Chemistry. 1998;273(26):16415–16420. doi: 10.1074/jbc.273.26.16415. [DOI] [PubMed] [Google Scholar]
- 158.Li Z, Jing YM, Kerr I, et al. Selective inhibition of p38α MAPK improves cardiac function and reduces myocardial apoptosis in rat model of myocardial injury. American Journal of Physiology. 2006;291(4):H1972–H1977. doi: 10.1152/ajpheart.00043.2006. [DOI] [PubMed] [Google Scholar]
- 159.Kumphune S, Bassi R, Jacquet S, et al. A chemical genetic approach reveals that p38alpha MAPK activation by diphosphorylation aggravates myocardial infarction and is prevented by the direct binding of SB203580. Journal of Molecular and Cellular Cardiology. 2007;42(5):972–980. doi: 10.1074/jbc.M109.079228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Liao P, Wang SQ, Wang S, et al. p38 mitogen-activated protein kinase mediates a negative inotropic effect in cardiac myocytes. Circulation Research. 2002;90(2):190–196. doi: 10.1161/hh0202.104220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chen F, Kan H, Hobbs G, Finkel MS. P38 MAP kinase inhibitor reverses stress-induced myocardial dysfunction in vivo. Journal of Applied Physiology. 2009;106(4):1132–1141. doi: 10.1152/japplphysiol.90542.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Szokodi I, Kerkelä R, Kubin AM, et al. Functionally opposing roles of extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase in the regulation of cardiac contractility. Circulation. 2008;118(16):1651–1658. doi: 10.1161/CIRCULATIONAHA.107.758623. [DOI] [PubMed] [Google Scholar]
- 163.Chao TH, Hayashi M, Tapping RI, Kato Y, Lee JD. MEKK3 directly regulates MEK5 activity as part of the big mitogen- activated protein kinase 1 (BMK1) signaling pathway. Journal of Biological Chemistry. 1999;274(51):36035–36038. doi: 10.1074/jbc.274.51.36035. [DOI] [PubMed] [Google Scholar]
- 164.Takeishi Y, Abe JI, Lee JD, Kawakatsu H, Walsh RA, Berk BC. Differential regulation of p90 ribosomal S6 kinase and big mitogen- activated protein kinase 1 by ischemia/reperfusion and oxidative stress in perfused guinea pig hearts. Circulation Research. 1999;85(12):1164–1172. doi: 10.1161/01.res.85.12.1164. [DOI] [PubMed] [Google Scholar]
- 165.Cameron SJ, Itoh S, Baines CP, et al. Activation of big MAP kinase 1 (BMK1/ERK5) inhibits cardiac injury after myocardial ischemia and reperfusion. FEBS Letters. 2004;566(1–3):255–260. doi: 10.1016/j.febslet.2004.03.120. [DOI] [PubMed] [Google Scholar]
- 166.Pi X, Yan C, Berk BC. Big Mitogen-Activated Protein Kinase (BMK1)/ERK5 protects endothelial cells from apoptosis. Circulation Research. 2004;94(3):362–369. doi: 10.1161/01.RES.0000112406.27800.6F. [DOI] [PubMed] [Google Scholar]
- 167.Yan C, Ding B, Shishido T, et al. Activation of extracellular signal-regulated kinase 5 reduces cardiac apoptosis and dysfunction via inhibition of a phosphodiesterase 3A/inducible cAMP early repressor feedback loop. Circulation Research. 2007;100(4):510–519. doi: 10.1161/01.RES.0000259045.49371.9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Qi SH, Guan QH, Wang M, Zhang GY. Action of ERK5 in ischemic tolerance suggests its probable participation in the signaling mechanism ERK5 in the signaling mechanism S.-H. Qi et al. Journal of Receptors and Signal Transduction. 2009;29(1):38–43. doi: 10.1080/10799890802675767. [DOI] [PubMed] [Google Scholar]
- 169.Wang RM, Zhang QG, Li J, Yang LC, Yang F, Brann DW. The ERK5-MEF2C transcription factor pathway contributes to anti-apoptotic effect of cerebral ischemia preconditioning in the hippocampal CA1 region of rats. Brain Research. 2009;1255:32–41. doi: 10.1016/j.brainres.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 170.Hausenloy DJ, Yellon DM. Remote ischaemic preconditioning: underlying mechanisms and clinical application. Cardiovascular Research. 2008;79(3):377–386. doi: 10.1093/cvr/cvn114. [DOI] [PubMed] [Google Scholar]
- 171.Przyklenk K, Darling CE, Dickson EW, Whittaker P. Cardioprotection “outside the box”: the evolving paradigm of remote preconditioning. Basic Research in Cardiology. 2003;98(3):149–157. doi: 10.1007/s00395-003-0406-y. [DOI] [PubMed] [Google Scholar]
- 172.Gho BCG, Schoemaker RG, Van den Doel MA, Duncker DJ, Verdouw PD. Myocardial protection by brief ischemia in noncardiac tissue. Circulation. 1996;94(9):2193–2200. doi: 10.1161/01.cir.94.9.2193. [DOI] [PubMed] [Google Scholar]
- 173.Kharbanda RK, Mortensen UM, White PA, et al. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation. 2002;106(23):2881–2883. doi: 10.1161/01.cir.0000043806.51912.9b. [DOI] [PubMed] [Google Scholar]
- 174.Heidbreder M, Naumann A, Tempel K, Dominiak P, Dendorfer A. Remote vs. ischaemic preconditioning: the differential role of mitogen-activated protein kinase pathways. Cardiovascular Research. 2008;78(1):108–115. doi: 10.1093/cvr/cvm114. [DOI] [PubMed] [Google Scholar]
- 175.Wolfrum S, Schneider K, Heidbreder M, Nienstedt J, Dominiak P, Dendorfer A. Remote preconditioning protects the heart by activating myocardial PKCε-isoform. Cardiovascular Research. 2002;55(3):583–589. doi: 10.1016/s0008-6363(02)00408-x. [DOI] [PubMed] [Google Scholar]
- 176.Schoemaker RG, van Heijningen CL. Bradykinin mediates cardiac preconditioning at a distance. American Journal of Physiology. 2000;278(5):H1571–H1576. doi: 10.1152/ajpheart.2000.278.5.H1571. [DOI] [PubMed] [Google Scholar]
- 177.Zhao Z-Q, Corvera JS, Halkos ME, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: Comparison with ischemic preconditioning. American Journal of Physiology. 2003;285(2 54-2):H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 178.Zhao ZQ, Corvera JS, Halkos ME, et al. Erratum: Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. American Journal of Physiology. 2004;286(1):p. H477. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 179.Valen G, Vaage J. Pre- and postconditioning during cardiac surgery. Basic Research in Cardiology. 2005;100(3):179–186. doi: 10.1007/s00395-005-0517-8. [DOI] [PubMed] [Google Scholar]
- 180.Yellon DM, Opie LH. Postconditioning for protection of the infarcting heart. Lancet. 2006;367(9509):456–458. doi: 10.1016/S0140-6736(06)68157-9. [DOI] [PubMed] [Google Scholar]
- 181.Ovize M, Baxter GF, Di Lisa F, et al. Postconditioning and protection from reperfusion injury: where do we stand: position Paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovascular Research. 2010;87(3):406–423. doi: 10.1093/cvr/cvq129. [DOI] [PubMed] [Google Scholar]
- 182.Tsang A, Hausenloy DJ, Mocanu MM, Yellon DM. Postconditioning: a form of “modified reperfusion” protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circulation Research. 2004;95(3):230–232. doi: 10.1161/01.RES.0000138303.76488.fe. [DOI] [PubMed] [Google Scholar]
- 183.Darling CE, Jiang K, Maynard M, Whittaker P, Vinten-Johansen J, Przyklenk K. Postconditioning via stuttering reperfusion limits myocardial infarct size in rabbit hearts: role of ERK1/2. American Journal of Physiology. 2005;289(4):H1618–H1626. doi: 10.1152/ajpheart.00055.2005. [DOI] [PubMed] [Google Scholar]
- 184.Iliodromitis EK, Georgiadis M, Cohen MV, Downey JM, Bofilis E, Kremastinos DT. Protection from postconditioning depends on the number of short ischemic insults in anesthetized pigs. Basic Research in Cardiology. 2006;101(6):502–507. doi: 10.1007/s00395-006-0606-3. [DOI] [PubMed] [Google Scholar]
- 185.Staat P, Rioufol G, Piot C, et al. Postconditioning the human heart. Circulation. 2005;112(14):2143–2148. doi: 10.1161/CIRCULATIONAHA.105.558122. [DOI] [PubMed] [Google Scholar]
- 186.Darling CE, Solari PB, Smith CS, Furman MI, Przyklenk K. “Postconditioning” the human heart: multiple balloon inflations during primary angioplasty may confer cardioprotection. Basic Research in Cardiology. 2007;102(3):274–278. doi: 10.1007/s00395-007-0643-6. [DOI] [PubMed] [Google Scholar]
- 187.Halkos ME, Kerendi F, Corvera JS, et al. Myocardial protection with postconditioning is not enhanced by ischemic preconditioning. Annals of Thoracic Surgery. 2004;78(3):961–969. doi: 10.1016/j.athoracsur.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 188.Yang XM, Proctor JB, Cui L, Krieg T, Downey JM, Cohen MV. Multiple, brief coronary occlusions during early reperfusion protect rabbit hearts by targeting cell signaling pathways. Journal of the American College of Cardiology. 2004;44(5):1103–1110. doi: 10.1016/j.jacc.2004.05.060. [DOI] [PubMed] [Google Scholar]
- 189.Yang XM, Philipp S, Downey JM, Cohen MV. Postconditioning’s protection is not dependent on circulating blood factors or cells but involves adenosine receptors and requires PI3-kinase and guanylyl cyclase activation. Basic Research in Cardiology. 2005;100(1):57–63. doi: 10.1007/s00395-004-0498-4. [DOI] [PubMed] [Google Scholar]
- 190.Burley DS, Ferdinandy P, Baxter GF. Cyclic GMP and protein kinase-G in myocardial ischaemia-reperfusion: opportunities and obstacles for survival signaling. British Journal of Pharmacology. 2007;152(6):855–869. doi: 10.1038/sj.bjp.0707409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sivaraman V, Mudalgiri NR, Di Salvo C, et al. Postconditioning protects human atrial muscle through the activation of the RISK pathway. Basic Research in Cardiology. 2007;102(5):453–459. doi: 10.1007/s00395-007-0664-1. [DOI] [PubMed] [Google Scholar]
- 192.Lauzier B, Delemasure S, Debin R, et al. Beneficial effects of myocardial postconditioning are associated with reduced oxidative stress in a senescent mouse model. Transplantation. 2008;85(12):1802–1808. doi: 10.1097/TP.0b013e3181775367. [DOI] [PubMed] [Google Scholar]
- 193.Sun HY, Wang NP, Halkos M, et al. Postconditioning attenuates cardiomyocyte apoptosis via inhibition of JNK and p38 mitogen-activated protein kinase signaling pathways. Apoptosis. 2006;11(9):1583–1593. doi: 10.1007/s10495-006-9037-8. [DOI] [PubMed] [Google Scholar]
- 194.Cohen MV, Yang XM, Downey JM. Acidosis, oxygen, and interference with mitochondrial permeability transition pore formation in the early minutes of reperfusion are critical to postconditioning’s success. Basic Research in Cardiology. 2008;103(5):464–471. doi: 10.1007/s00395-008-0737-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Heusch G. No RISK, no...cardioprotection? A critical perspective. Cardiovascular Research. 2009;84(2):173–175. doi: 10.1093/cvr/cvp298. [DOI] [PubMed] [Google Scholar]
- 196.Przyklenk K, Maynard M, Darling CE, Whittaker P. Aging mouse hearts are refractory to infarct size reduction with post-conditioning. Journal of the American College of Cardiology. 2008;51(14):1393–1398. doi: 10.1016/j.jacc.2007.11.070. [DOI] [PubMed] [Google Scholar]
- 197.Schwartz LM, Lagranha CJ. Ischemic postconditioning during reperfusion activates Akt and ERK without protecting against lethal myocardial ischemia-reperfusion injury in pigs. American Journal of Physiology. 2006;290(3):H1011–H1018. doi: 10.1152/ajpheart.00864.2005. [DOI] [PubMed] [Google Scholar]
- 198.Skyschally A, van Caster P, Boengler K, et al. Ischemic postconditioning in pigs: no causal role for risk activation. Circulation Research. 2009;104(1):15–18. doi: 10.1161/CIRCRESAHA.108.186429. [DOI] [PubMed] [Google Scholar]
- 199.Musiolik J, Van Caster P, Skyschally A, et al. Reduction of infarct size by gentle reperfusion without activation of reperfusion injury salvage kinases in pigs. Cardiovascular Research. 2010;85(1):110–117. doi: 10.1093/cvr/cvp271. [DOI] [PubMed] [Google Scholar]
- 200.Lecour S. Activation of the protective Survivor Activating Factor Enhancement (SAFE) pathway against reperfusion injury: does it go beyond the RISK pathway? Journal of Molecular and Cellular Cardiology. 2009;47(1):32–40. doi: 10.1016/j.yjmcc.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 201.Chiari PC, Bienengraeber MW, Pagel PS, Krolikowski JG, Kersten JR, Warltier DC. Isoflurane protects against myocardial infarction during early reperfusion by activation of phosphatidylinositol-3-kinase signal transduction: evidence for anesthetic-induced postconditioning in rabbits. Anesthesiology. 2005;102(1):102–109. doi: 10.1097/00000542-200501000-00018. [DOI] [PubMed] [Google Scholar]
- 202.Tessier-Vetzel D, Tissier R, Waintraub X, Ghaleh B, Berdeaux A. Isoflurane inhaled at the onset of reperfusion potentiates the cardioprotective effect of ischemic postconditioning through a NO-dependent mechanism. Journal of Cardiovascular Pharmacology. 2006;47(3):487–492. doi: 10.1097/01.fjc.0000211731.69045.fe. [DOI] [PubMed] [Google Scholar]
- 203.Obal D, Dettwiler S, Favoccia C, Scharbatke H, Preckel B, Schlack W. The influence of mitochondrial KATP-channels in the cardioprotection of preconditioning and postconditioning by sevoflurane in the rat in vivo. Anesthesia and Analgesia. 2005;101(5):1252–1260. doi: 10.1213/01.ANE.0000181336.96511.32. [DOI] [PubMed] [Google Scholar]
- 204.Yellon DM, Alkhulaifi AM, Pugsley WB. Preconditioning the human myocardium. Lancet. 1993;342(8866):276–277. doi: 10.1016/0140-6736(93)91819-8. [DOI] [PubMed] [Google Scholar]
- 205.Jenkins DP, Pugsley WB, Alkhulaifi AM, Kemp M, Hooper J, Yellon DM. Ischaemic preconditioning reduces troponin T release in patients undergoing coronary artery bypass surgery. Heart. 1997;77(4):314–318. doi: 10.1136/hrt.77.4.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lu EX, Chen SX, Yuan MD, et al. Preconditioning improves myocardial preservation in patients undergoing open heart operations. Annals of Thoracic Surgery. 1997;64(5):1320–1324. doi: 10.1016/S0003-4975(97)00838-2. [DOI] [PubMed] [Google Scholar]
- 207.Teoh LK, Grant R, Hulf JA, Pugsley WB, Yellon DM. The effect of preconditioning (ischemic and pharmacological) on myocardial necrosis following coronary artery bypass graft surgery. Cardiovascular Research. 2002;53(1):175–180. doi: 10.1016/s0008-6363(01)00435-7. [DOI] [PubMed] [Google Scholar]
- 208.Cheung MMH, Kharbanda RK, Konstantinov IE, et al. Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery. First clinical application in humans. Journal of the American College of Cardiology. 2006;47(11):2277–2282. doi: 10.1016/j.jacc.2006.01.066. [DOI] [PubMed] [Google Scholar]
- 209.Hausenloy DJ, Mwamure PK, Venugopal V, et al. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet. 2007;370(9587):575–579. doi: 10.1016/S0140-6736(07)61296-3. [DOI] [PubMed] [Google Scholar]
- 210.Hoole SP, Heck PM, Sharples L, et al. Cardiac remote ischemic preconditioning in coronary stenting (CRISP stent) study. A prospective, randomized control trial. Circulation. 2009;119(6):820–827. doi: 10.1161/CIRCULATIONAHA.108.809723. [DOI] [PubMed] [Google Scholar]
- 211.Bøtker HE, Kharbanda R, Schmidt MR, et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. The Lancet. 2010;375(9716):727–734. doi: 10.1016/S0140-6736(09)62001-8. [DOI] [PubMed] [Google Scholar]
- 212.Thibault H, Piot C, Staat P, et al. Long-term benefit of postconditioning. Circulation. 2008;117(8):1037–1044. doi: 10.1161/CIRCULATIONAHA.107.729780. [DOI] [PubMed] [Google Scholar]
- 213.Loukogeorgakis SP, Panagiotidou AT, Yellon DM, Deanfield JE, MacAllister RJ. Postconditioning protects against endothelial ischemia-reperfusion injury in the human forearm. Circulation. 2006;113(7):1015–1019. doi: 10.1161/CIRCULATIONAHA.105.590398. [DOI] [PubMed] [Google Scholar]
- 214.Dragoni S, Di Stolfo G, Sicuro S, et al. Postconditioning fails to prevent radial artery endothelial dysfunction induced by ischemia and reperfusion: evidence from a human in vivo study. Canadian Journal of Physiology and Pharmacology. 2006;84(6):611–615. doi: 10.1139/y05-160. [DOI] [PubMed] [Google Scholar]
- 215.Koike N, Takeyoshi I, Ohki S, Tokumine M, Morishita Y. The comparison of mitogen-activated protein kinases that become activated within the left ventricular and right atrial tissues following heart transplantation in canine model. Journal of Investigative Surgery. 2007;20(2):105–111. doi: 10.1080/08941930701235502. [DOI] [PubMed] [Google Scholar]
- 216.Ballard-Croft C, White DJ, Maass DL, Hybki DP, Horton JW. Role of p38 mitogen-activated protein kinase in cardiac myocyte secretion of the inflammatory cytokine TNF-α . American Journal of Physiology. 2001;280(5):H1970–H1981. doi: 10.1152/ajpheart.2001.280.5.H1970. [DOI] [PubMed] [Google Scholar]
- 217.Li M, Georgakopoulos D, Lu G, et al. p38 MAP kinase mediates inflammatory cytokine induction in cardiomyocytes and extracellular matrix remodeling in heart. Circulation. 2005;111(19):2494–2502. doi: 10.1161/01.CIR.0000165117.71483.0C. [DOI] [PubMed] [Google Scholar]
- 218.Bellahcene M, Jacquet S, Cao XB, et al. Activation of p38 mitogen-activated protein kinase contributes to the early cardiodepressant action of tumor necrosis factor. Journal of the American College of Cardiology. 2006;48(3):545–555. doi: 10.1016/j.jacc.2006.02.072. [DOI] [PubMed] [Google Scholar]
- 219.Yin H, Zhang J, Lin H, et al. p38 mitogen-activated protein kinase inhibition decreases TNFalpha secretion and protects against left ventricular remodeling in rats with myocardial ischemia. Inflammation. 2008;31(2):65–73. doi: 10.1007/s10753-007-9050-2. [DOI] [PubMed] [Google Scholar]
- 220.Conrad DM, Furlong SJ, Doucette CD, Boudreau RTM, Hoskin DW. Role of mitogen-activated protein kinases in Thy-1-induced T-lymphocyte activation. Cellular Signalling. 2009;21(8):1298–1307. doi: 10.1016/j.cellsig.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 221.Tabata A, Morikawa M, Miyajima M, et al. Suppression of alloreactivity and allograft rejection by SP600125, a small molecule inhibitor of c-Jun N-terminal kinase. Transplantation. 2007;83(10):1358–1364. doi: 10.1097/01.tp.0000264196.23944.90. [DOI] [PubMed] [Google Scholar]
- 222.Smith SJ, Fenwick PS, Nicholson AG, et al. Inhibitory effect of p38 mitogen-activated protein kinase inhibitors on cytokine release from human macrophages. British Journal of Pharmacology. 2006;149(4):393–404. doi: 10.1038/sj.bjp.0706885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Clanachan AS, Jaswal JS, Gandhi M, et al. Effects of inhibition of myocardial extracellular-responsive kinase and p38 mitogen-activated protein kinase on mechanical function of rat hearts after prolonged hypothermic ischemia. Transplantation. 2003;75(2):173–180. doi: 10.1097/01.TP.0000040429.40245.3A. [DOI] [PubMed] [Google Scholar]
- 224.Oto T, Calderone A, Li Z, Rosenfeldt FL, Pepe S. p38 Mitogen-activated protein kinase inhibition reduces inflammatory cytokines in a brain-dead transplant donor animal model. Heart, lung & circulation. 2009;18(6):393–400. doi: 10.1016/j.hlc.2009.05.706. [DOI] [PubMed] [Google Scholar]
- 225.Koike N, Takeyoshi I, Ohki S, Tokumine M, Matsumoto K, Morishita Y. Effects of adding p38 mitogen-activated protein-kinase inhibitor to celsior solution in canine heart transplantation from non-heart-beating donors. Transplantation. 2004;77(2):286–292. doi: 10.1097/01.TP.0000101039.12835.A4. [DOI] [PubMed] [Google Scholar]
- 226.Diestel A, Roessler J, Pohl-Schickinger A, et al. Specific p38 inhibition in stimulated endothelial cells: a possible new anti-inflammatory strategy after hypothermia and rewarming. Vascular Pharmacology. 2009;51(4):246–252. doi: 10.1016/j.vph.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 227.Öllinger R, Thomas M, Kogler P, et al. Blockade of p38 MAPK inhibits chronic allograft vasculopathy. Transplantation. 2008;85(2):293–297. doi: 10.1097/TP.0b013e318160130f. [DOI] [PubMed] [Google Scholar]
- 228.Hashimoto N, Takeyoshi I, Yoshinari D, et al. Effects of a p38 mitogen-activated protein kinase inhibitor as an additive to euro-collins solution on reperfusion injury in canine lung transplantation. Transplantation. 2002;74(3):320–326. doi: 10.1097/00007890-200208150-00006. [DOI] [PubMed] [Google Scholar]
- 229.Yoshinari D, Takeyoshi I, Kobayashi M, et al. Effects of a p38 mitogen-activated protein kinase inhibitor as an additive to University of Wisconsin solution on reperfusion injury in liver transplantation. Transplantation. 2001;72(1):22–27. doi: 10.1097/00007890-200107150-00007. [DOI] [PubMed] [Google Scholar]
- 230.Doucet C, Milin S, Favreau F, et al. A p38 mitogen-activated protein kinase inhibitor protects against renal damage in a non-heart-beating donor model. American Journal of Physiology. 2008;295(1):F179–F191. doi: 10.1152/ajprenal.00252.2007. [DOI] [PubMed] [Google Scholar]
- 231.Kosieradzki M. Mechanisms of ischemic preconditioning and its application in transplantation. Annals of Transplantation. 2002;7(3):12–20. [PubMed] [Google Scholar]
- 232.Ambros JT, Herrero-Fresneda I, Borau OG, Boira JMG. Ischemic preconditioning in solid organ transplantation: from experimental to clinics. Transplant International. 2007;20(3):219–229. doi: 10.1111/j.1432-2277.2006.00418.x. [DOI] [PubMed] [Google Scholar]
- 233.Selzner N, Boehnert M, Selzner M. Preconditioning, postconditioning, and remote conditioning in solid organ transplantation: basic mechanisms and translational applications. doi: 10.1016/j.trre.2011.07.003. Transplantation Reviews. In press. [DOI] [PubMed] [Google Scholar]
- 234.Karck M, Rahmanian P, Haverich A. Ischemic preconditioning enhances donor heart preservation. Transplantation. 1996;62(1):17–22. doi: 10.1097/00007890-199607150-00004. [DOI] [PubMed] [Google Scholar]
- 235.Cope JT, Mauney MC, Banks D, et al. Intravenous phenylephrine preconditioning of cardiac grafts from non- heart-beating donors. Annals of Thoracic Surgery. 1997;63(6):1664–1668. doi: 10.1016/s0003-4975(97)00092-1. [DOI] [PubMed] [Google Scholar]
- 236.Landymore RW, Bayes AJ, Murphy JT, Fris JH. Preconditioning prevents myocardial stunning after cardiac transplantation. Annals of Thoracic Surgery. 1998;66(6):1953–1957. doi: 10.1016/s0003-4975(98)00902-3. [DOI] [PubMed] [Google Scholar]
- 237.Ahmet I, Sawa Y, Nishimura M, Kitakaze M, Matsuda H. Cardioprotective effect of diadenosine tetraphosphate (AP4A) preservation in hypothermic storage and its relation with mitochondrial ATP- sensitive potassium channels. Transplantation. 2000;69(1):16–20. doi: 10.1097/00007890-200001150-00004. [DOI] [PubMed] [Google Scholar]
- 238.Kevelaitis E, Oubénaissa A, Mouas C, Peynet J, Menasché P. Ischemic preconditioning with opening of mitochondrial adenosine triphosphate-sensitive potassium channels or Na+/H+ exchange inhibition: which is the best protective strategy for heart transplants? Journal of Thoracic and Cardiovascular Surgery. 2001;121(1):155–162. doi: 10.1067/mtc.2001.111417. [DOI] [PubMed] [Google Scholar]
- 239.Kevelaitis E, Oubénaïssa A, Peynet J, Mouas C, Menasché P. Preconditioning by mitochondrial ATP-sensitive potassium channel openers: an effective approach for improving the preservation of heart transplants. Circulation. 1999;100(19, supplement):II345–II350. doi: 10.1161/01.cir.100.suppl_2.ii-345. [DOI] [PubMed] [Google Scholar]
- 240.Hicks M, Du ZY, Jansz P, Rainer S, Spratt P, Macdonald PS. ATP-sensitive potassium channel activation mimics the protective effect of ischaemic preconditioning in the rat isolated working heart after prolonged hypothermic storage. Clinical and Experimental Pharmacology and Physiology. 1999;26(1):20–25. doi: 10.1046/j.1440-1681.1999.02985.x. [DOI] [PubMed] [Google Scholar]
- 241.Kevelaitis E, Peynet J, Mouas C, Launay JM, Menasché P. Opening of potassium channels the common cardioprotective link between preconditioning and natural hibernation? Circulation. 1999;99(23):3079–3085. doi: 10.1161/01.cir.99.23.3079. [DOI] [PubMed] [Google Scholar]
- 242.Valen G, Takeshima S, Vaage J. Preconditioning improves cardiac function after global ischemia, but not after cold cardioplegia. Annals of Thoracic Surgery. 1996;62(5):1397–1403. doi: 10.1016/0003-4975(96)00499-7. [DOI] [PubMed] [Google Scholar]
- 243.Kirsch M, Farhat F, Garnier JP, Loisance D. Acute brain death abolishes the cardioprotective effects of ischemic preconditioning in the rabbit. Transplantation. 2000;69(10):2013–2019. doi: 10.1097/00007890-200005270-00007. [DOI] [PubMed] [Google Scholar]
- 244.Farhat F, Loisance D, Garnier JP, Kirsch M. Norepinephrine release after acute brain death abolishes the cardioprotective effects of ischemic preconditioning in rabbit. European Journal of Cardio-thoracic Surgery. 2001;19(3):313–320. doi: 10.1016/s1010-7940(00)00659-x. [DOI] [PubMed] [Google Scholar]
- 245.Bouma HR, Ketelaar ME, Yard BA, Ploeg RJ, Henning RH. AMP-activated protein kinase as a target for preconditioning in transplantation medicine. Transplantation. 2010;90(4):353–358. doi: 10.1097/TP.0b013e3181e7a3aa. [DOI] [PubMed] [Google Scholar]
- 246.Botha P, MacGowan GA, Dark JH. Sildenafil citrate augments myocardial protection in heart transplantation. Transplantation. 2010;89(2):169–177. doi: 10.1097/TP.0b013e3181c42b22. [DOI] [PubMed] [Google Scholar]
- 247.Clements RT, Cordeiro B, Feng J, Bianchi C, Sellke FW. Rottlerin increases cardiac contractile performance and coronary perfusion through BKca++ channel activation after cold cardioplegic arrest in isolated hearts. Circulation. 2011;124(11, supplement 1):S55–S61. doi: 10.1161/CIRCULATIONAHA.110.012112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Tanaka M, Gunawan F, Terry RD, et al. Inhibition of heart transplant injury and graft coronary artery disease after prolonged organ ischemia by selective protein kinase C regulators. Journal of Thoracic and Cardiovascular Surgery. 2005;129(5):1160–1167. doi: 10.1016/j.jtcvs.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 249.Finegan BA, Gandhi M, Cohen MR, Legatt D, Clanachan AS. Isoflurane alters energy substrate metabolism to preserve mechanical function in isolated rat hearts following prolonged no-flow hypothermic storage. Anesthesiology. 2003;98(2):379–386. doi: 10.1097/00000542-200302000-00018. [DOI] [PubMed] [Google Scholar]
- 250.Nakao A, Kaczorowski DJ, Wang Y, et al. Amelioration of rat cardiac cold ischemia/reperfusion injury with inhaled hydrogen or carbon monoxide, or both. Journal of Heart and Lung Transplantation. 2010;29(5):544–553. doi: 10.1016/j.healun.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 251.Konstantinov IE, Li J, Cheung MM, et al. Remote ischemic preconditioning of the recipient reduces myocardial ischemia-reperfusion injury of the denervated donor heart via a Katp channel-dependent mechanism. Transplantation. 2005;79(12):1691–1695. doi: 10.1097/01.tp.0000159137.76400.5d. [DOI] [PubMed] [Google Scholar]
- 252.Lauzier B, Sicard P, Bouchot O, et al. After four hours of cold ischemia and cardioplegic protocol, the heart can still be rescued with postconditioning. Transplantation. 2007;84(11):1474–1482. doi: 10.1097/01.tp.0000288637.18796.0e. [DOI] [PubMed] [Google Scholar]
- 253.Tang Y, Mennander A, Oksala N, et al. Postconditioning and remote postconditioning of ischemic rat cardiac grafts. European Surgical Research. 2010;45(1):1–8. doi: 10.1159/000315479. [DOI] [PubMed] [Google Scholar]
- 254.Azoulay D, Del Gaudio M, Andreani P, et al. Effects of 10 minutes of ischemic preconditioning of the cadaveric liver on the graft’s preservation and function: the Ying and the Yang. Annals of Surgery. 2005;242(1):133–139. doi: 10.1097/01.sla.0000167848.96692.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Jassem W, Fuggle SV, Cerundolo L, Heaton ND, Rela M. Ischemic preconditioning of cadaver donor livers protects allografts following transplantation. Transplantation. 2006;81(2):169–174. doi: 10.1097/01.tp.0000188640.05459.37. [DOI] [PubMed] [Google Scholar]
- 256.Marber MS, Molkentin JD, Force T. Developing small molecules to inhibit kinases unkind to the heart: P38 MAPK as a case in point. Drug Discovery Today. 2010;7(2):e123–e127. doi: 10.1016/j.ddmec.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Genovese MC, Cohen SB, Wofsy D, et al. A 24-week, randomized, double-blind, placebo-controlled, parallel group study of the efficacy of oral SCIO-469, a p38 mitogen-activated protein kinase inhibitor, in patients with active rheumatoid arthritis. Journal of Rheumatology. 2011;38(5):846–854. doi: 10.3899/jrheum.100602. [DOI] [PubMed] [Google Scholar]
- 258.Anand P, Shenoy R, Palmer JE, et al. Clinical trial of the p38 MAP kinase inhibitor dilmapimod in neuropathic pain following nerve injury. European Journal of Pain. 2011;15(10):1040–1048. doi: 10.1016/j.ejpain.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 259.Hausenloy DJ, Baxter G, Bell R, et al. Translating novel strategies for cardioprotection: the Hatter Workshop Recommendations. Basic Research in Cardiology. 2010;105(6):677–686. doi: 10.1007/s00395-010-0121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Piot C, Croisille P, Staat P, et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. New England Journal of Medicine. 2008;359(5):473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 261.Jamieson RW, Friend PJ. Organ reperfusion and preservation. Frontiers in Bioscience. 2008;13(1):221–235. doi: 10.2741/2672. [DOI] [PubMed] [Google Scholar]
- 262.Jacobs S, Rega F, Meyns B. Current preservation technology and future prospects of thoracic organs—part 2: heart. Current Opinion in Organ Transplantation. 2010;15(2):156–159. doi: 10.1097/MOT.0b013e328337343f. [DOI] [PubMed] [Google Scholar]
- 263.Osaki S, Ishino K, Kotani Y, et al. Resuscitation of non-beating donor hearts using continuous myocardial perfusion: the importance of controlled initial reperfusion . Annals of Thoracic Surgery. 2006;81(6):2167–2171. doi: 10.1016/j.athoracsur.2006.01.066. [DOI] [PubMed] [Google Scholar]


