Abstract
The aim of the present study was to explore the hedonic effects of D2 receptor agonist, quinpirole and D2 receptor antagonist, and sulpiride alone or in combination with a low dose of 17β-E2-estradiol (17β-E2) in the adult ovariectomized female rats (OVX). OVX rats of Wistar strain were used in all experiments. Two weeks after surgery rats were chronically treated with vehicle, a low dose of 17β-E2 (5.0 μg/rat), quinpirole (0.1 mg/kg), sulpiride (10.0 mg/kg), quinpirole plus 17β-E2, or sulpiride plus 17β-E2 for 14 days before the forced swimming test. We found that sulpiride significantly decreased immobility time in the OVX females. A combination of sulpiride with a low dose of 17β-E2 induced more profound decrease of immobility time in the OVX rats compared to the rats treated with sulpiride alone. On the contrary, quinpirole failed to modify depression-like behavior in the OVX rats. In addition, quinpirole significantly blocked the antidepressant-like effect of 17β-E2 in OVX rats. Thus, the D2 receptor antagonist sulpiride alone or in combination with a low dose of 17β-E2 exerted antidepressant-like effect in OVX female rats, while the D2 receptor agonist quinpirole produced depressant-like profile on OVX rats.
1. Introduction
Depressive disorders belong to the most frequent diseases worldwide showing a lifetime prevalence of up to 20% [1, 2]. The traditional focus on a prominent participation of the central serotonergic and noradrenergic systems in the mechanisms of depression is generally accepted [3, 4]. However, recent evidences suggest that the dysfunction of the central dopaminergic pathways may be a critical component of the neurobiological basis of depression [5–8]. The mainfold effects of dopamine (DA) are mediated by two structurally and functionally different classes of G protein-coupled receptors, termed D1 and D2, originally distinguished by their ability to activate (D1 class) or inhibit (D2 class) adenylate cyclase [9]. The D2 receptors play important role in regulating DA transmission [9]. Together, D2 receptors and the DA transporter (DAT) proteins provide control over synaptic DA signaling, as evidenced by behavioral abnormalities that accompany D2 receptors and DAT gene ablation [10, 11]. These findings underscore the coordinated nature of DA homeostasis that, once perturbed, may lead to multiple neuropsychiatric disorders. Behavioral and biochemical data indicate involvement of a dopaminergic neurotransmitter system in the pathophysiology of depression, as well as in the mechanism of action of antidepressant drugs [12–16]. It has been shown that mesolimbic dopamine pathways are important to goal-directed behaviors, and that the hypofunction of this system may mediate anhedonia and a motivational apathy [17], that is, behaviors which are the key features of depression. Because at least some of the D2-receptor ligands investigated have selectivity for dopamine autoreceptors, it is speculated that the dopamine autoreceptor may be a target for the action of psychotropic drugs [18].
A number of clinical studies have shown a therapeutic efficacy of D2 antagonists (sulpiride and raclopride) in different types of depression. In fact, various studies suggest that sulpiride given in low doses (100–300 mg/day) may be an appropriate treatment of slightly depressed patients, including those suffering from endogenous depression [19, 20]. Therapeutic effect of sulpiride was also evaluated in patients with major depression [21]. Furthermore, the D2 receptors have been implicated in major depression by clinical evidence from neuroimaging and postmortem and studies [4, 22, 23]. Also, low-dose atypical antipsychotics (which increase extracellular levels of dopamine and norepinephrine, but not serotonin, in the prefrontal cortex) appear effective in the pharmacotherapy of treatment resistant depression [24, 25]. Although effective antidepressants tend to be useful across the range of depressive symptoms, there is some evidence that dopaminergic agents are particularly useful for reduced positive affect and for psychomotor symptoms [12, 17]. Nevertheless, there are many controversies about the exact role of DA in depression [6, 7].
On the other hand, estrogens have powerful effects beyond their role in reproduction. Estrogens have been suggested to affect several psychiatric and neurodegenerative diseases including anxiety, depression, schizophrenia, and Alzheimer's disease [26, 27]. In female rodents, naturally occurring changes in experimental depression-like behavior have been observed [28–30]. In our recent studies we reported that depression-like behavior increases during the proestrous and estrous [31]. In addition to the physiological actions of endogenous steroid secretions on the female depression state, our experiments have also demonstrated that the antidepressant effect of clomipramine varies through the estrous cycle [31, 32]. Furthermore, experimental evidences have shown that estradiol exerts antidepressant-like effect in paradigms used to assess the antidepressant potency of drugs [29, 30, 33]. Human studies have demonstrated that the lifetime prevalence rate of mood disorders is approximately two times more frequent in women than in men [28, 34]. The increased prevalence of mood disruptions during the reproductive years and the potential for mood disorders to occur at times when estrogen levels are changing [35]. Indeed, increased risk of affective disorders in women is related to hormonal changes who are premenstrual, postpartum, and hypoestrogenic due to the medical surgery or menopause [36, 37]. In spite of this, preclinical data examining the effects of antidepressants on either behavioral, physiological, or neurochemical parameters, have mainly used males subjects until recently.
Moreover, it is well established that estrogens may also modulate the central DA functions. Prolonged estrogen administration induces downregulation of presynaptic dopamine activity and produces a dopamine receptor supersensitivity that results in a release from the inhibitory action of these receptors and enhancement of stimulated dopamine release [38]. On the other hand, DA also can influence ovary hormonal system. So, estrogen receptors (ERs) can be activated (in the absence of cognate ligand) by the neurotransmitter DA [39] and by dopaminergic ligands [40, 41]. This implies that ligand-independent activation of ERs by DA might organize a unique set of behavior in contrast to the ligand-dependent activation of ERs by estrogen. Thus, such close interactions between ovary hormonal and dopaminergic neurotransmitter systems then it is of interest to elucidate the involvement of D2 receptors in depression-like state under conditions of estrogen deficiency, that is, under such critical conditions that are contributed to produce the profound mood impairments in females.
In our preliminary experiments, we were not able to find any effects for D2-receptor agonist quinpirole and D2-receptor antagonist sulpiride after acute administration alone or in combination with 17β-estradiol (17β-E2) on depression-like behavior in adult ovariectomized (OVX) rats (data are not shown). However, we assumed that effects of DA drugs can be different after acute and chronic administration in OVX rats. In order to study the behavioral effects of DA drugs alone or in combination with 17β-E2 after repeated treatment, the present study was created.
The aim of this work was to study the effects of administration of D2 receptor agonist quinpirole and D2 receptor antagonist sulpiride injected chronically for 14 days alone or in combination with 17β-E2 on depression-like behavior in adult OVX rats. The forced swimming test used in the present study has been behaviorally, physiologically, and pharmacologically validated as an animal test model of depression in rodents [42]. In the experiment OVX rats and OVX rats with administration of 17β-E2 were used.
2. Methods
2.1. Subjects
A total number of 60 adult female rats of Wistar strain (purchased from Rappolovo, Russia) weighing 180–200 g were used. For at least a week prior to the experiments, the rats were housed six to a cage under standard environmental conditions: constant temperature of 23 ± 1°C, 60% humidity, 12 h light/dark cycle (light on 8:00 a.m.), food and water ad libitum. All experiments were carried out in accordance with the guide for care and use of Laboratory Animals published by the national Institute of Health (National Research council, publication no. 85-23, revised in 1996), and the Animal Welfare Assurance Renewal for Pavlov Institute of Physiology. The rationale, design, and methods of this study have been approved by the Ethical Committee for Animal Research, Institute of Experimental Medicine. All animals were gently handled by experienced keepers from the facility each day for a week prior to experimental procedures. Any environmental or physical stress was avoided in order to habituate the rats to manipulation. Animals were randomly assigned to experimental groups and were used only once in the behavioral experiments. The behavioral tests were conducted between 10:00 AM and 2:00 PM. Experiments were carried out in a soundproof and air-regulated experimental room, to which animals were habituated at least 30 min before each test.
2.2. Surgery
Ovariectomy was performed through an abdominal ventral incision under ethylic ether anesthesia. The complete extraction of the ovaries was corroborated by visual inspection. After the surgery the rats were retrieved in a community cage with other rats. Efficiency of 17β-E2 administration to OVX females was controlled by vaginal smears. OVX animals were allowed to have 14 days for postoperative recovery before administration of drugs. After two weeks, all OVX rats were randomly assigned to each of the experimental groups and subjected to treatments and behavioral tests. We used 14 days for postoperative recovery before administration of drugs because of estrogen depletion induced by ovariectomy results in a marked decrease in dopaminergic cell density in the brain [43], an effect that can be reversed by estrogen administration 14 days after ovariectomy. Thus, 17β-E2 or dopaminergic drugs administration in 14 days after ovariectomy would still be able to change the activity of dopaminergic neurons, whereas it looses this capability after longer period of estrogen depletion when at least part of the mesocortical dopamine neurons might be inactive or dead.
2.3. Drugs
D2 receptor agonist (−)-quinpirole hydrochloride (Q-102, Sigma Chemical Co) was dissolved in sterile saline (0.9%). D2 receptor antagonist (S)-(−)-sulpiride (S-7771, Sigma Chemical Co) was dissolved in a constant volume of 0.1 NHCl, then neutralized with 0.1 M NaHCO3 (pH 6.9) and brought up to volume. The estrogen, 17β-E2 (E-8875, Sigma Chemical Co.) was dissolved in sterile sesame oil. All solutions were freshly prepared before each experimental series. Drugs were administered during 14 days after postoperative period following ovariectomy. Dopaminergic chemicals were injected s.c. or i.p. in volume of 0.1 mL. Control OVX rats received oil solvent injection in the same volume.
2.4. Experimental Groups
For performance of behavioral tests OVX rats were divided into six groups of 10 animals in each. The rats used for forced swimming test and locomotor test were the same. The first group was constituted of OVX rats treated with oil solvent subcutaneously (control). The five other groups were of OVX rats treated with 17β-E2 (5.0 μkg/rat, s.c.) daily; OVX rats injected with quinpirole (0.1 mg/kg, i.p.) daily; OVX females injected with sulpiride (10.0 mg/kg, i.p.) daily; OVX rats treated with quinpirole in combination with 17β-E2 in the same dose which was given to OVX rats; OVX females with sulpiride daily in combination with 17β-E2 in the same dose which was applied to OVX females. All preparations were chronically injected for 14 days once daily through all behavioral sessions (i.e., in the forced swimming test treatment was made on the first day and the second day too). All behavioral experiments were done in 45 min after the last injection of the drugs.
2.5. Determining Optimal Dosing for Sulpiride and Quinpirole
The motor behavior can be attributed to altered immobility, swimming and struggling observed in the forced swimming test. Because of it, we used locomotor activity test for determination of optimal dosing for dopaminergic drugs, since behavioral studies indicated that DA drugs may influence on motor activity of rats [44–46], and we did not have any way to predict the doses of DA drugs that failed to induce deeply marked changes of the locomotor activity. In a preliminary experiment the dose-effect relationship of DA drugs after acute administration compared with saline in the OVX rats was studied in the locomotor activity test. We injected different doses of sulpiride (1.0–10.0 mg/kg, i.p.), quinpirole (0.05–0.2 mg/kg, i.p.), or saline 45 min before testing. We found that the 10.0 mg/kg dose of sulpiride and the 0.1 mg/kg dose did not change locomotor activity. These doses of DA drugs were selected for the succeeding forced swimming test. Furthermore, these doses were considered as the minimum behavioral doses for the OVX rats that failed to induce any alteration in the motor behavior. The low dose of 17β-E2 (5.0 μg/rat) was chosen from the studies performed by [47, 48].
2.6. Behavioral Tests
2.6.1. Forced Swimming Test
Rats were individually forced to swim inside vertical Plexiglas cylinders containing water maintained at 25°C (modified from Porsolt et al. [42]). The behavioral cylinder was 60 cm high and 20 cm wide maintained at 25°C, filled with 30 cm of water, so that rats could not support themselves by touching the bottom with their paws or tail. The FST included 2 phases: an initial 15 min pretest followed by a 5 min test 24 h later. After each session, the rats were removed from the cylinders, dried with towels, and placed into heated cages for 10 min, and then returned to their home cages. Total duration of immobility, total duration of struggling, and total duration of swimming were measured during a 5 min test. A rat was judged to be immobile whenever it remained passively floating in the water in a slightly hunched but upright position, its head just above the surface. Struggling was considered when the rats make active movements with their forepaws in and out of the water along the side of the swim chamber. Swimming was considered when the rats make active swimming or circular movements. A video camera was installed above the model to recode the rat activity in the test.
2.6.2. Locomotor Activity Test
To evaluate the effect of treatments on spontaneous motor activity all experimental groups were submitted to the locomotor activity test. Horizontal activity was measured for 5 min using Plexiglas cage (20 cm × 40 × 23 cm) placed into a photobeam apparatus (Opto-Varimex, Columbus, OH): 15 photobeams cross the width of the cage 2.5 cm apart and 8 cm above the cage floor. The locomotor activity was recorded by a video-camera and subsequently evaluated by two distinct observers unaware of treatment assignment of experimental groups. After each test session, the apparatus was carefully cleaned and deodorized with a cleaning solution (ammonia 0.5%, ethanol 15%, extran 10%, isopropyl alcohol 5%, antiseptic with aromatizing 19%, and distillated water 50.5%).
2.7. Statistical Analysis
All values were expressed as mean ± S.E.M. Comparisons between values were performed using two-way ANOVA test with between-subject factors of hormone condition (OVX or OVX plus 17β-E2) and drug treatment (vehicle, quinpirole, or sulpiride) followed by Dunnett's test for multiple comparisons post hoc test. Statistical analysis was performed using SPSS version 11.5. Differences with P < 0.05 were considered significant.
3. Results
3.1. Forced Swimming Test
The two-way ANOVA revealed significant differences in immobility time between hormone conditions (F4,30 = 7.39, P < 0.0004), between drug treatments (F3,90 = 12.05, P < 0.0003), and an interaction between hormone condition and treatments (F1,56 = 2.87, P < 0.01). The post-hoc test revealed differences among the groups for depression-like behavior in the FST (P < 0.0001). Repeated 17β-E2 administration in low dose to the OVX rats significantly reduced immobility time compared to the control (Figure 1(a)). After repeated treatment, neither quinpirole (0.1 mg/kg) alone nor quinpirole combined with 17β-E2 had any significant effect on immobility behavior in the OVX rats (post hoc versus control group, both P > 0.05). Interestingly, the administration of quinpirole together with 17β-E2 in OVX rats completely abolished the reduction of immobility behavior induced by 17β-E2 (post hoc versus OVX group treated with 17β-E2, P < 0.05; Figure 1(a)). The D2 receptor antagonist, sulpiride (0.1 mg/kg) significantly decreased immobility time compared to OVX rats (P < 0.05; Figure 1(a)). Combined administration of sulpiride with 17β-E2 in the OVX rats resulted in a profound reduction of immobility behavior (post hoc versus control group and OVX group treated with 17β-E2, P < 0.05). It should be noted that the repeated administration of this combination potentiated the positive effects of sulpiride and 17β-E2 on immobility behavior in the OVX rats.
Figure 1.
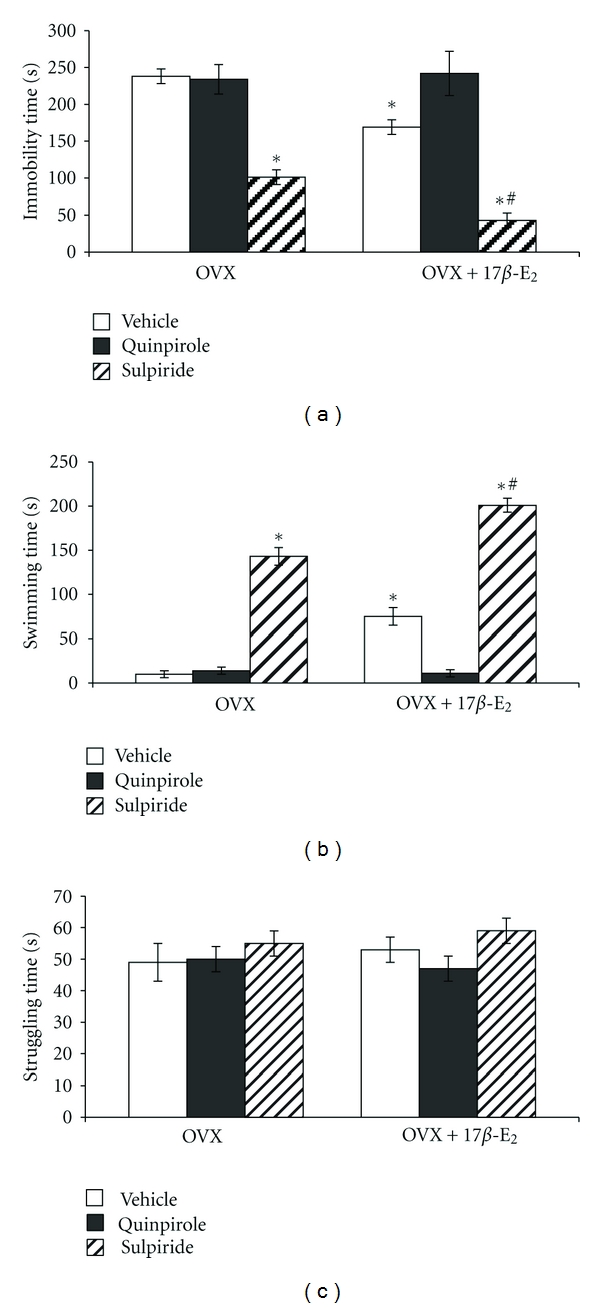
Effects of dopaminergic drugs alone or in combination with 17β-E2 on depressive-like behavior of the OVX rats. Columns represent the immobility time (a), struggling duration (b), and swimming duration (c) in the forced swimming test mean ± SEM (in sec). Each group comprised of ten rats. *P < 0.05 versus control group of rats, #P < 0.05 versus OVX rats treated with 17β-E2.
The two-way ANOVA revealed significant differences in swimming time between hormone conditions (F3,90 = 15.77, P < 0.01), between drug treatments (F2,80 = 9.44, P < 0.05), and an interaction between hormone condition and treatments (F2,54 = 7.43, P < 0.001). Repeated 17β-E2 administration in low dose to the OVX rats significantly increased swimming time compared to the control (Figure 1(b)). After repeated treatment, neither quinpirole (0.1 mg/kg) alone nor quinpirole combined with 17β-E2 had any significant effect on swimming time in the OVX rats (Post hoc, versus control group, both P > 0.05). Interestingly, the administration of quinpirole together with 17β-E2 in OVX rats completely inhibited the increase of swimming time induced by 17β-E2 (post hoc versus OVX group treated with 17β-E2, P < 0.05; Figure 1(b)). The D2 receptor antagonist, sulpiride (0.1 mg/kg) significantly increased swimming time compared to OVX rats (P < 0.05; Figure 1(b)). Combined administration of sulpiride with 17β-E2 in the OVX rats resulted in a profound enhancement of swimming time (post hoc versus control group and OVX group treated with 17β-E2, P < 0.05). It should be noted that the repeated administration of this combination potentiated the positive effects of sulpiride and 17β-E2 on swimming behavior in the OVX rats. No significant differences due to the repeated drug treatments or hormonal condition were observed for struggling time (post hoc versus control group, both P > 0.05, Figure 1(c)).
3.2. Locomotor Activity Test
To determine whether quinpirole- or sulpiride-induced changes in behavior of the OVX rats in the FST could be due to nonspecific effects of dopaminergic ligands and 17β-E2 on locomotor activity, spontaneous locomotor activity was assessed in a separate groups of the OVX rats treated with 17β-E2 (5.0 μkg/rat, s.c.) daily, OVX rats injected with quinpirole (0.1 mg/kg, i.p.) daily, OVX females injected with sulpiride (0.1 mg/kg, i.p.) daily, OVX rats treated with quinpirole in combination with 17β-E2 in the same dose which was given to OVX rats, and OVX females with quinpirole daily in combination with 17β-E2. The two-way ANOVA failed to reveal any significant differences in the locomotor activity between hormone conditions (F3,14 = 29.41, P > 0.05; Figure 2), between drug treatments (F3,14 = 12.78, P > 0.01; Figure 2), and an interaction between hormone condition and treatments (F1,54 = 11.56, P > 0.05; Figure 2). The post hoc test failed to reveal differences among the groups for this parameter in the test (P > 0.05).
Figure 2.
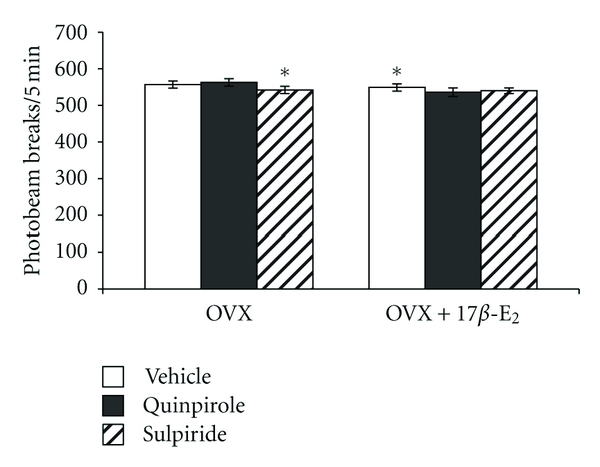
Effects of dopaminergic drugs alone or in combination with 17β-E2 on the locomotor activity of the OVX rats. Data are presented as total horizontal activity counts (photobeam breaks) for the 5 min test (mean ± SEM). Each group comprised of ten rats.
4. Discussion
Results of the present study support previous evidence that depression-like behavior is influenced by estrogen depletion and by low dose of 17β-E2 administration to OVX rats [23, 33]. We found that ovariectomy produced an increase of immobility time and substitution treatment with low dose of 17β-E2 in OVX rats partially attenuated this parameter. The data received in the present study are in a good agreement with our previous data [31, 32]. We found previously that ovariectomy and phases of ovary cycle affect the behavioral response in a model of behavioral despair, exhibiting a longer immobility time as compared to diestrous animals [31, 32]. Also, our previous data have suggested that 17β-E2 administration produced an antidepressant-like effect in ovariectomized rats [32, 49].
However, the major finding of the present study is that drugs influencing DA transmission can also modify depression-like behavior in female animals with different hormone levels occurring after ovariectomy and after 17β-E2 administration. In our experiments chronic administration of selective D2 receptor antagonist sulpiride in OVX females decreased immobility time in the FST, that is, sulpiride exerted definite antidepressant-like action. Also, chronic treatment with sulpiride and low dose of 17β-E2 induced a more relevant antidepressant-like effect in OVX rats. These effects were similar to those of the classical antidepressant clomipramine in OVX rats as we demonstrated in our previous study [32]. The data from the present study suggest that sulpiride and 17β-E2 exert synergic positive effects on depression-like behavior and can potentiate effects of each other. Some reports have also showed that repeated administration of 17β-E2 in low dose with serotonergic drugs may have the same synergic positive effects on depression-like behavior [47, 48] as we found using combination of dopaminergic drugs with 17β-E2 in low dose. On the other hand, chronic administration of selective D2 receptor agonist quinpirole failed to modify the depression-like behavior of OVX rats in the FST. Interestingly, quinpirole significantly blocked the antidepressant-like effect of 17β-E2. Pharmacological studies have demonstrated that the central DA neuronal system may functionally be involved in depression-like behavior [5–7]. Some data suggest that selective D2 agonists have antidepressant effects in both learned helplessness procedure and the forced swimming test in male rats, while blockade of D2 receptors prevents the antidepressant effect of imipramine as well as other agents [15, 24, 25, 50]. However, our present results are in good agreement with data of others [51–53], showing that some dopaminergic antipsychotic drugs acting as DA receptor antagonists are also active as antidepressants. In our experimental conditions there was no direct relation between the effects of D2 receptor agonist or antagonist on depression-like behavior and behavioral changes observed in the locomotor activity test. Since neither 17β-E2 nor sulpiride significantly influenced behavior in the locomotor activity test, the antidepressant synergy observed in the FST—decreased immobility and increased swimming—cannot be attributed to increased locomotor activation. Instead, results from the FST and locomotor behavioral tests considered together indicate that the interaction between 17β-E2 and sulpiride is a hedonic rather that a motor effect. Also, the data of literature suggest that D2 receptor antagonist sulpiride was able to increase the immobility time in the FST in mice at dose (20.0 mg/kg, i.p.) which increased motor activity [54]. Here, in our experimental conditions we used sulpiride in a dose of 10.0 mg/kg for OVX females which failed to change locomotor activity. Thus, our results demonstrated that action of D2 receptor antagonist on depression-like is dependent from the used dose and gender of animals and D2 receptor antagonist may possess antidepressant activity as it was shown in some reports [55, 56].
Sulpiride is the oldest and clinically most established member of the substituted benzamides. It binds to D2/D3 receptors that may be located on the presynaptic membrane [57]. Furthermore, sulpiride has been found to increase brain dopamine synthesis [58]. The mechanism of the antidepressant action of sulpiride seems to involve the block of the dopaminergic system and a possible D1 and D2 receptors sensitization in the mesolimbic area [21]. The effectiveness of sulpiride in experimental models of depression confirm its beneficial effect in various types of depression in men [59, 60]. Since an antidepressant action has been described for dopamine agonists such as bromocriptine and piribedil [55, 60], it may be surprising that under certain circumstances neuroleptics acting as dopamine receptor antagonists are also active as antidepressants. This has been proved not only for sulpiride but also for other neuroleptics [61] that may act preferentially as presynaptic dopamine receptor antagonists, thus increasing dopamine turnover. In our preliminary experiments, we were not able to find any effects for D2 receptor agonist quinpirole and D2 receptor antagonist sulpiride after acute administration alone or in combination with 17β-E2 on depression-like behavior in adult OVX rats (data are not shown). However, based on the results of the present study, it can be assumed that effects of DA drugs are different after acute and chronic administration in OVX rats. Thus, the behavioral effects of DA drugs alone or in combination with 17β-E2 after repeated treatment may due to any adaptive changes in the brain.
It should be noted that one limitation in interpretation of the present findings is that sulpiride, in common with virtually all “selective” D2 receptor antagonists and agonists, exhibits affinity for the D3 as well as the D2 receptor [62]. Although D3 receptors appear to be preferentially located to the ventral rather than the dorsal striatum, there is evidence of some expression of D3 receptors in the putamen [63] and so it cannot be excluded that some of the effects of sulpiride described here are due its antagonism of that receptor. An alternative explanation for the improvement in despair behavior after sulpiride is that it is actually due to a drug-induced increase in extracellular DA. Animal studies have shown that relatively low single doses of DA antagonists such as sulpiride and haloperidol increase levels of striatal DA [62, 63] by inhibiting the presynaptic activity of DA autoreceptors [62]. This increased DA will hit D1 receptors and thus behavioural improvements after sulpiride may in fact be attributable to the increase in extracellular striatal DA induced by downregulation of presynaptic activity. Also, it should be noted that ovariectomy of female rats results in a decrease in dopamine-specific proteins, including dopamine receptors and dopamine transporters. 17β-E2 administration can restore these functional responses [63–65]. In this connection, it can be assumed that profound positive effect of sulpiride in a combination with 17β-E2 on despair behavior in OVX rats may due to their mutual positive actions on dopaminergic system. Thus, the depression-like state, which was prevented by sulpiride in OVX rats could be considered as part of an estrogen withdrawal syndrome, or the expression of genomic actions of this hormone. The precise mechanism by which sulpiride alone or in a combination with 17β-E2 induced positive effect on depression-like behavior in the present study remains to be established.
There is no doubt that female gonadal hormones may act directly or indirectly to have influence on the different neurotransmitter systems, especially serotonergic system, which in turn, modulate DA activity [28, 29]. Considering that sulpiride is a selective D2 receptor antagonist, one may suppose that its antidepressant effect in OVX rats with or without 17β-E2 treatment could be correlated with a previous hyperactivity of different neurotransmitter systems. The main interesting result from this study is that quinpirole, selective D2 receptor agonist, inhibited antidepressant-like effect of 17β-E2 in the FST. Some evidence has shown that ERs can be activated by dopaminergic ligands acting on D2 receptors [40]. One may assume that quinpirole could be present to binding of 17β-E2 with ERs resulting in blockade of positive action of 17β-E2 on depression-like behavior in OVX rats. However, it seems to be very difficult to draw clear-cut conclusion on the exact nature interactions between DA and estrogens in the mechanisms of mood disorders.
It should be emphasized that in studies dealing with dopamine-affective interactions only male animals were usually used as subjects. Also, no comparative analysis of effects of selective agonists or antagonists of D2 receptors on despair behavior in OVX females has been performed so far. Diverging effects of quinpirole and sulpiride in OVX females in our experiments can be related to changed metabolism of DA, expression of D2 receptors, expression of 17β-E2 receptors, and to their binding ability in the brain structures directly related to mood functions. In summary, the D2 receptor antagonist, sulpiride administered alone, or in a combination with a low dose of 17β-E2 resulted in an antidepressant-like effect in the OVX rats. Repeated treatment with sulpiride and a low dose of 17β-E2 profoundly enhanced antidepressant-like effects the single substances exert per se. The D2 receptor agonist, quinpirole failed to modify the depression-like behavior of OVX rats in the FST. In addition, quinpirole blocked the antidepressant-like effect of 17β-E2 in OVX rats. Further research is needed to elucidate the detailed mechanisms by which sulpiride and 17β-E2 exert synergic effect and affect the dopaminergic system. This study provides additional evidence to support the role of dopaminergic mechanisms in depression-like behavior and contributes to the better understanding of the complex interaction between estrogens and DA neurotransmitter systems.
References
- 1.Stoppel C, Bielau H, Bogerts B, Northoff G. Neurobiological basis of depressive disorders. Fortschritte der Neurologie Psychiatrie. 2006;74(12):696–705. doi: 10.1055/s-2006-932192. [DOI] [PubMed] [Google Scholar]
- 2.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455(7215):894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olie JP, Costa E, Silva J, Macher JP. Neuroplasticity: A New Approach to the Pathophysiology of Depression. London, UK: Science Press; 2004. [Google Scholar]
- 4.Slattery DA, Hudson AL, Nutt DJ. Invited review: the evolution of antidepressant mechanisms. Fundamental and Clinical Pharmacology. 2004;18(1):1–21. doi: 10.1111/j.1472-8206.2004.00195.x. [DOI] [PubMed] [Google Scholar]
- 5.Nestler EJ, Carlezon WA. The mesolimbic dopamine reward circuit in depression. Biological Psychiatry. 2006;59(12):1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 6.Nutt DJ. The role of dopamine and norepinephrine in depression and antidepressant treatment. Journal of Clinical Psychiatry. 2006;67(supplement 6):3–8. [PubMed] [Google Scholar]
- 7.Nutt DJ, Baldwin DS, Clayton AH, et al. The role of dopamine and norepinephrine in depression and antidepressant treatment. Journal of Clinical Psychiatry. 2006;67(supplement 6):46–49. [PubMed] [Google Scholar]
- 8.Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Archives of General Psychiatry. 2007;64(3):327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- 9.Kebabian JW, Calne DB. Multiple receptors for dopamine. Nature. 1979;277(5692):93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- 10.Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379(6566):606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 11.Jones SR, Gainetdinov RR, Mark Wightman R, Caron MG. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. Journal of Neuroscience. 1998;18(6):1979–1986. doi: 10.1523/JNEUROSCI.18-06-01979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willner P. Dopamine and depression: a review of recent evidence. I. Empirical studies. Brain Research. 1983;287(3):211–224. doi: 10.1016/0165-0173(83)90005-x. [DOI] [PubMed] [Google Scholar]
- 13.Maj J. Antidepressants given repeatedly: pharmacological evaluation of their action. Polish Journal of Pharmacology and Pharmacy. 1991;43(3):241–254. [PubMed] [Google Scholar]
- 14.Brown AS, Gershon S. Dopamine and depression. Journal of Neural Transmission. 1993;91(2-3):75–109. doi: 10.1007/BF01245227. [DOI] [PubMed] [Google Scholar]
- 15.D’Aquila PS, Collu M, Gessa GL, Serra G. The role of dopamine in the mechanism of action of antidepressant drugs. European Journal of Pharmacology. 2000;405(1–3):365–373. doi: 10.1016/s0014-2999(00)00566-5. [DOI] [PubMed] [Google Scholar]
- 16.Vetulani J, Nalepa I. Antidepressants: past, present and future. European Journal of Pharmacology. 2000;405(1–3):351–363. doi: 10.1016/s0014-2999(00)00565-3. [DOI] [PubMed] [Google Scholar]
- 17.Willner P. The mesolimbic dopamine system as a target for rapid antidepressant action. International Clinical Psychopharmacology. 1997;12(3):S7–S14. doi: 10.1097/00004850-199707003-00002. [DOI] [PubMed] [Google Scholar]
- 18.Bartoszyk GD. Anxiolytic effects of dopamine receptor ligands: I. Involvement of dopamine autoreceptors. Life Sciences. 1998;62(7):649–663. doi: 10.1016/s0024-3205(97)01160-0. [DOI] [PubMed] [Google Scholar]
- 19.Niskanen P, Tamminen T, Viukari M. Sulpiride vs. amitriptyline in the treatment of depression. Current Therapeutic Research. 1975;17(3):281–284. [PubMed] [Google Scholar]
- 20.Benkert O, Holsboer F. Effect of sulpiride in endogenous depression. Acta Psychiatrica Scandinavica. 1984;69(supplement 311):43–48. doi: 10.1111/j.1600-0447.1984.tb06858.x. [DOI] [PubMed] [Google Scholar]
- 21.Tsukamoto T, Asakura M, Tsuneizumi T, Satoh Y, Shinozuka T, Hasegawa K. Therapeutic effects and side effects in patients with major depression treated with sulpiride once a day. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1994;18(3):615–618. doi: 10.1016/0278-5846(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 22.Brunswick DJ, Amsterdam JD, Mozley PD, Newberg A. Greater availability of brain dopamine transporters in major depression shown by [99mTc]TRODAT-1 SPECT imaging. American Journal of Psychiatry. 2003;160(10):1836–1841. doi: 10.1176/appi.ajp.160.10.1836. [DOI] [PubMed] [Google Scholar]
- 23.Dhir A, Kulkarni SK. Involvement of dopamine (DA)/serotonin (5-HT)/sigma (σ) receptor modulation in mediating the antidepressant action of ropinirole hydrochloride, a D2/D3 dopamine receptor agonist. Brain Research Bulletin. 2007;74(1–3):58–65. doi: 10.1016/j.brainresbull.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Gambarana C, Ghiglieri O, Tagliamonte A, D'Alessandro N, De Montis MG. Crucial role of D1 dopamine receptors in mediating the antidepressant effect of imipramine. Pharmacology Biochemistry and Behavior. 1995;50(2):147–151. doi: 10.1016/0091-3057(94)00274-m. [DOI] [PubMed] [Google Scholar]
- 25.Gambarana C, Ghiglieri O, De Montis MG. Desensitization of the D1 dopamine receptors in rats reproduces a model of escape deficit reverted by imipramine, fluoxetine and clomipramine. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1995;19(5):741–755. doi: 10.1016/0278-5846(95)00128-i. [DOI] [PubMed] [Google Scholar]
- 26.Cyr M, Landry M, Di Paolo T. Modulation by estrogen-receptor directed drugs of 5-hydroxytryptamine-2A receptors in rat brain. Neuropsychopharmacology. 2000;23(1):69–78. doi: 10.1016/S0893-133X(00)00085-3. [DOI] [PubMed] [Google Scholar]
- 27.Cyr M, Calon F, Morissette M, Grandbois M, Callier S, Di Paolo T. Drugs with estrogen-like potency and brain activity: potential therapeutic application for the CNS. Current Pharmaceutical Design. 2000;6(12):1287–1312. doi: 10.2174/1381612003399725. [DOI] [PubMed] [Google Scholar]
- 28.Sherwin BB. Estrogen and cognitive aging in women. Neuroscience. 2006;138(3):1021–1026. doi: 10.1016/j.neuroscience.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 29.Walf AA, Frye CA. Antianxiety and antidepressive behavior produced by physiological estradiol regimen may be modulated by hypothalamic-pituitary-adrenal axis activity. Neuropsychopharmacology. 2005;30(7):1288–1301. doi: 10.1038/sj.npp.1300708. [DOI] [PubMed] [Google Scholar]
- 30.Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31(6):1097–1111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Consoli D, Fedotova J, Micale V, Sapronov NS, Drago F. Stressors affect the response of male and female rats to clomipramine in a model of behavioral despair (forced swim test) European Journal of Pharmacology. 2005;520(1–3):100–107. doi: 10.1016/j.ejphar.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 32.Fedotova J. Effects of mild, moderate and severe stress on depression in female rats: modifications by estrous cycle, ovariectomy and estradiol treatment. Biological Psychiatry and Psychopharmacology. 2006;8:45–47. [Google Scholar]
- 33.Okada M, Hayashi N, Kometani M, Nakao K, Inukai T. Influences of ovariectomy and continuous replacement of 17β-estradiol on the tail skin temperature and behavior in the forced swimming test in rats. Japanese Journal of Pharmacology. 1997;73(1):93–96. doi: 10.1254/jjp.73.93. [DOI] [PubMed] [Google Scholar]
- 34.Pearlstein T, Rosen K, Stone AB. Mood disorders and menopause. Endocrinology and Metabolism Clinics of North America. 1997;26(2):279–294. doi: 10.1016/s0889-8529(05)70247-4. [DOI] [PubMed] [Google Scholar]
- 35.Noble RE. Depression in women. Metabolism. 2005;54(5):49–52. doi: 10.1016/j.metabol.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 36.Halbreich U, Kahn LS. Role of estrogen in the aetiology and treatment of mood disorders. CNS Drugs. 2001;15(10):797–817. doi: 10.2165/00023210-200115100-00005. [DOI] [PubMed] [Google Scholar]
- 37.Wang-Cheng R, Rosenfeld JA. Hormone replacement therapy. British Medical Journal. 2003;326:139–140. [Google Scholar]
- 38.Di Paolo T, Poyet P, Labrie F. Effect of chronic estradiol and haloperidol treatment on striatal dopamine receptors. European Journal of Pharmacology. 1981;73(1):105–106. doi: 10.1016/0014-2999(81)90153-9. [DOI] [PubMed] [Google Scholar]
- 39.Smith CL, Conneely OM, O’Malley BW. Modulation of the ligand-independent activation of the human estrogen receptor by hormone and antihormone. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(13):6120–6124. doi: 10.1073/pnas.90.13.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mani SK, Blaustein JD, O’Malley BW. Progesterone receptor function from a behavioral perspective. Hormones and Behavior. 1997;31(3):244–255. doi: 10.1006/hbeh.1997.1393. [DOI] [PubMed] [Google Scholar]
- 41.Riby JE, Chang GHF, Firestone GL, Bjeldanes LF. Ligand-independent activation of estrogen receptor function by 3,3’-diindolylmethane in human breast cancer cells. Biochemical Pharmacology. 2000;60(2):167–177. doi: 10.1016/s0006-2952(00)00307-5. [DOI] [PubMed] [Google Scholar]
- 42.Porsolt RD, Bertin A, Jalfre M. ’Behavioral despair’ in rats and mice: strain differences and the effects of imipramine. European Journal of Pharmacology. 1978;51(3):291–294. doi: 10.1016/0014-2999(78)90414-4. [DOI] [PubMed] [Google Scholar]
- 43.Leranth C, Roth RH, Elswoth JD, Naftolin F, Horvath TL, Redmond DE. Estrogen is essential for maintaining nigrostriatal dopamine neurons in primates: implications for Parkinson’s disease and memory. Journal of Neuroscience. 2000;20(23):8604–8609. doi: 10.1523/JNEUROSCI.20-23-08604.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walker QD, Cabassa J, Kaplan KA, et al. Sex differences in cocaine-stimulated motor behavior: disparate effects of gonadectomy. Neuropsychopharmacology. 2001;25(1):118–130. doi: 10.1016/S0893-133X(00)00248-7. [DOI] [PubMed] [Google Scholar]
- 45.Ponnusamy R, Nissim HA, Barad M. Systemic blockade of D2-like dopamine receptors facilitates extinction of conditioned fear in mice. Learning and Memory. 2005;12(4):399–406. doi: 10.1101/lm.96605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson ML, Ho CC, Day AE, Walker QD, Francis R, Kuhn CM. Oestrogen receptors enhance dopamine neurone survival in rat midbrain. Journal of Neuroendocrinology. 2010;22(4):226–237. doi: 10.1111/j.1365-2826.2010.01964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Estrada-Camarena E, Fernández-Guasti A, López-Rubalcava C. Antidepressant-like effect of different estrogenic compounds in the forced swimming test. Neuropsychopharmacology. 2003;28(5):830–838. doi: 10.1038/sj.npp.1300097. [DOI] [PubMed] [Google Scholar]
- 48.Estrada-Camarena E, Fernández-Guasti A, López-Rubalcava C. Interaction between estrogens and antidepressants in the forced swimming test in rats. Psychopharmacology. 2004;173(1):139–145. doi: 10.1007/s00213-003-1707-4. [DOI] [PubMed] [Google Scholar]
- 49.Fedotova J, Platonova N. Behavioral response to clomipramine after application of different stressors in ovariectomized female rats. European Neuropsychopharmacology. 2007;17(supplement 4):368 pages. [Google Scholar]
- 50.Takamori K, Yoshida S, Okuyama S. Repeated treatment with imipramine, fluvoxamine and tranylcypromine decreases the number of escape failures by activating dopaminergic systems in a rat learned helplessness test. Life Sciences. 2001;69(16):1919–1926. doi: 10.1016/s0024-3205(01)01279-6. [DOI] [PubMed] [Google Scholar]
- 51.Robertson MM, Trimble MR. Neuroleptics as antidepressants. Neuropharmacology. 1981;20(12 B):1335–1336. [PubMed] [Google Scholar]
- 52.Abbas AI, Hedlund PB, Huang XP, Tran TB, Meltzer HY, Roth BL. Amisulpride is a potent 5-HT7 antagonist: relevance for antidepressant actions in vivo. Psychopharmacology. 2009;205(1):119–128. doi: 10.1007/s00213-009-1521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pawar GR, Agrawal RP, Phadnis P, Paliwal A, Vyas S, Solanki P. Evaluation of antidepressant like property of amisulpride per se and its comparison with fluoxetine and olanzapine using forced swimming test in albino mice. Acta Poloniae Pharmaceutica. 2009;66(3):327–331. [PubMed] [Google Scholar]
- 54.Nikulina EM, Skrinskaya JA, Popova NK. Role of genotype and dopamine receptors in behaviour of inbred mice in a forced swimming test. Psychopharmacology. 1991;105(4):525–529. doi: 10.1007/BF02244374. [DOI] [PubMed] [Google Scholar]
- 55.Silverstone T. Response to bromocriptine distinguishes bipolar from unipolar depression. The Lancet. 1984;1(8382):903–904. doi: 10.1016/s0140-6736(84)91359-x. [DOI] [PubMed] [Google Scholar]
- 56.Borsini F, Lecci A, Mancinelli A, D’Aranno V, Meli A. Stimulation of dopamine D-2 but not D-1 receptors reduces immobility time of rats in the forced swimming test: implication for antidepressant activity. European Journal of Pharmacology. 1988;148(3):301–307. doi: 10.1016/0014-2999(88)90107-0. [DOI] [PubMed] [Google Scholar]
- 57.Tamminga CA, Gerlach J. New neuroleptics and experimental antipsychotics in schizophrenia. In: Meltzer HerbertY., editor. Psychopharmacology. New York, NY, USA: Raven press; 1987. pp. 1129–1140. [Google Scholar]
- 58.Tagliamonte A, De Montis G, Olianas M, Vargiu L, Corsini GU, Gessa GL. Selective increase of brain dopamine synthesis by sulpiride. Journal of Neurochemistry. 1975;24(4):707–710. [PubMed] [Google Scholar]
- 59.Standish Barry HMAS, Bouras N, Bridges PK, Watson JP. A randomized double blind group comparative study of sulpiride and amitriptyline in affective disorder. Psychopharmacology. 1983;81(3):258–260. doi: 10.1007/BF00427274. [DOI] [PubMed] [Google Scholar]
- 60.Mouret JL, Le Moine P, Minuit MP. Marquers polygraphiques Clinique et therapeutique des depressions dopamine-dependantes (DDD) (special issue) Confront Psychiatr. 1989:430–437. [Google Scholar]
- 61.Nelson JC. The use of antipsychotic drugs in the treatment of depression. In: Zohar J, Belmaker RH, editors. Treating Resistant Depression. New York, NY, USA: PMA Corp; 1987. pp. 131–146. [Google Scholar]
- 62.Levant B. The D3 dopamine receptor: neurobiology and potential clinical relevance. Pharmacological Reviews. 1997;49(3):231–252. [PubMed] [Google Scholar]
- 63.Imperato A, Di Chiara G. Dopamine release and metabolism in awake rats after systemic neuroleptics as studied by trans-striatal dialysis. Journal of Neuroscience. 1985;5(2):297–306. doi: 10.1523/JNEUROSCI.05-02-00297.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moghaddam B, Bunney BS. Utilization of microdialysis for assessing the release of mesotelencephalic dopamine following clozapine and other antipsychotic drugs. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1990;14, supplement:S51–S57. doi: 10.1016/0278-5846(90)90086-v. [DOI] [PubMed] [Google Scholar]
- 65.Kawagoe KT, Garris PA, Wiedemann DJ, Wightman RM. Regulation of transient dopamine concentration gradients in the microenvironment surrounding nerve terminals in the rat striatum. Neuroscience. 1992;51(1):55–64. doi: 10.1016/0306-4522(92)90470-m. [DOI] [PubMed] [Google Scholar]


