Abstract
Background
Sleep disturbances comparable with insomnia occur in up to 80% of people with schizophrenia, but very little is known about the contribution of circadian coordination to these prevalent disruptions.
Aims
A systematic exploration of circadian time patterns in individuals with schizophrenia with recurrent sleep disruption.
Method
We examined the relationship between sleep-wake activity, recorded actigraphically over 6 weeks, along with ambient light exposure and simultaneous circadian clock timing, by collecting weekly 48 h profiles of a urinary metabolite of melatonin in 20 out-patients with schizophrenia and 21 healthy control individuals matched for age, gender and being unemployed.
Results
Significant sleep/circadian disruption occurred in all the participants with schizophrenia. Half these individuals showed severe circadian misalignment ranging from phase-advance/delay to non-24 h periods in sleep-wake and melatonin cycles, and the other half showed patterns from excessive sleep to highly irregular and fragmented sleep epochs but with normally timed melatonin production.
Conclusions
Severe circadian sleep/wake disruptions exist despite stability in mood, mental state and newer antipsychotic treatment. They cannot be explained by the individuals' level of everyday function.
Disturbances of sleep and abnormal sleep-wake cycles are common features of psychiatric disorders.1 In schizophrenia, polysomnographic studies comparing healthy individuals with participants who are drug-naive or drug-treated, when either acutely psychotic or clinically stable, have found delayed sleep onset, impaired sleep continuity and increased time awake.2-5 There are also sleep architectural changes already evident in individuals not on medication at psychosis onset, characterised by shorter rapid eye movement sleep and less slow-wave sleep.6 Slow-wave sleep deficits have been shown to correlate with lower frontal lobe metabolism and ventriculomegaly suggesting a neurodevelopmental trait.7,8 Sleep is only part of the 24 h circadian cycle. The generation and regulation of sleep that arises from multiple brain regions, neurotransmitter systems and modulatory hormones is driven by a complex interaction of wake and sleep mechanisms involving: (a) a wake-dependant homeostatic buildup of sleep pressure that increases with prolonged wakefulness and dissipates during sleep; and (b) a 24 h body clock or circadian system (‘circa diem’ - about a day) that aligns sleep to the dark phase and activity to the light phase of the 24 h day through precisely controlled, cyclic expression of a number of genes, so-called clock genes. It has recently been shown that genetic variability in a number of these genes is associated not only with phenotypic differences in morning v. evening preference, rhythm-related sleep disorders, sleep homeostasis and cognitive performance following sleep loss9 but also with midbrain dopamine regulation and reward processing10 known to be disrupted in schizophrenia.11 These findings suggest that sleep-wake disruption in schizophrenia may have a genetic basis and that the presence of specific sleep-wake patterns may serve as a useful endophenotypic dimension to assess familial pre-disposition.12
To date there have been few studies of circadian rhythms over extended periods of time in people with schizophrenia and these are mainly case reports or small group reports; none has controlled for the possibility that a lack of structured routine, common in schizophrenia, may be a cause of circadian misalignment in sleep-wake activity. The main aim of this study was to provide a comprehensive description of sleep-wake phenotypes in individuals with stable, non-acute schizophrenia who have sleep complaints, and to determine the contribution of probable circadian rhythm dysfunction to self-reported sleep disturbances. We measured day-to-day motor activity and light exposure with unobtrusive ‘watches’ (wrist actigraphy) over 6 weeks, and we established melatonin profiles from weekly urine collections, thereby using melatonin's property as a physiological phase marker of the circadian clock. To control for the non-specific effect that the lack of a daytime routine may have on sleep-wake cycles, we compared the circadian rhythms of participants with schizophrenia with those of unemployed, healthy individuals.
Method
Participants
Twenty people with schizophrenia and on medication were recruited from mental health services in west London (the schizophrenia group). The diagnosis of schizophrenia was confirmed for each patient according to DSM-IV13 criteria by using OPCRIT14 with reference to longitudinal information from the clinical notes. This group was compared with 21 healthy people who were all unemployed and recruited from the same catchment area via the local job centre (the control group). General physical and psychological health was confirmed with our health questionnaire adapted from the Patient Health Questionnaire.15
Exclusion criteria for the control group were: a history of psychiatric illness in themselves or their first-degree relatives and being prescribed medication known to affect sleep. Exclusion criteria for all participants were: a previous head injury, neurological illness or an endocrine disorder affecting brain function, drug or alcohol dependence and visual impairment (assessed using a Snellen chart). The study protocol was approved by the West London Mental Health Trust Local Research Ethics Committee. Participation of one individual was approved by the Lothian Local Research Ethics Committee. All participants gave written informed consent and received an honorarium for their time.
Clinical assessments
Subjective sleep quality was assessed using the self-rated Pittsburgh Sleep Quality Index (PSQI),16 which measures sleep quality over the previous month according to seven subscale domains: sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, use of sleeping medication, and daytime dysfunction. The range of subscale scores is 0-3 and the sum of all seven domains yields a global score of subjective sleep quality (range 0-21) with higher scores representing poorer subjective sleep quality. Studies examining the psychometric properties of the PSQI have found it appropriate for use with healthy volunteers and patients.17,18
Individual differences in diurnal preference of activities were estimated using a self-assessment questionnaire that distinguishes morning, evening and intermediate types.19 This questionnaire has been shown to provide a reliable measure of the so-called ‘chronotype’. For example, the best time of day for performance of an evening type is shifted towards the later hours of the day in comparison to a morning type.
Mood was assessed with the Profile of Mood States (POMS).20 This is a self-report questionnaire assessing emotion over the previous week and comprises 65 items corresponding to six factors: tension-anxiety, depression-dejection, anger-hostility, vigour-activity, fatigue and confusion. This was undertaken by 10 individuals in the schizophrenia group and 11 in the control group at the beginning and end of the 6-week recording period.
The participants in the schizophrenia group were in a clinically stable state according to the referring team and their medication had been unchanged for at least 3 months. They all reported some form of regular social activity during the 6-week observation period. Using self-report and the clinical notes, information concerning activities of daily living and social activity was compiled for participants in the schizophrenia group and rated using the General Assessment of Function (GAF) scale.21 The persistent presence or absence of positive symptoms was also noted.
Sleep-wake monitoring
The sleep-wake cycle of each participant and exposure to light was simultaneously monitored for a period of 6 weeks using wrist-worn actigraphs with an integrated light sensor (Actiwatch-L). Any intrinsic variability among the watches was addressed by ensuring that the same watch was used equally by both groups. Actigraphs contain a miniaturised piezoelectric acceleration sensor that detects the movement-induced force and stores this information in a memory chip. Data were collected in the actigraph's integration mode, which allowed sampling with a frequency of 32 Hz and storage of activity counts for each 2 min epoch. Ambient light of the bedroom was recorded separately by placing a second Actiwatch-L next to the participant's bed because light levels can be missed by the wrist-worn watch if it is covered when the participant is asleep. Each participant was asked to keep a daily record of bedtime, get-up time, naps, daytime activities and medication in a standardised diary for the duration of actigraphic monitoring. Participants were asked to provide very detailed contextual information for 2 consecutive days during their first week, which was used as real-time ‘bio-calibration’ record for the interpretation of individual actigraphic activity levels over the remaining 5 weeks. The consistency and accuracy of diary entries was monitored as part of the weekly home visits and included the engagement of the participant in the onscreen inspection of actigraphic light/activity data and their verification with diary notes.
Melatonin rhythms
To estimate the circadian period and phase of the participants' circadian pacemaker, we established weekly profiles of 6-sulphatoxymelatonin (aMT6s, a metabolite of melatonin) using a protocol suitable for people with schizophrenia.22 Each participant was asked to pass a full volume of urine into a bottle roughly every 4 h (about 8 h when asleep) over a 48 h period and note the date and time of collection. The volume in each bottle was recorded and two 5 ml aliquots of each sample were stored at –20°C. Two participants in the schizophrenia group were instructed to discontinue the collection for 1-2 weeks due to unavoidable circumstances. Three participants in this group collected urine specimens only infrequently and the melatonin sulphate profiles of these three individuals could not be determined with certainty and were discarded. In total 17 people in the schizophrenia group and 21 in the control group completed repeated urine collection that was used to investigate the amount, timing and periodicity of melatonin sulphate.
Data analysis
Activity and light data from Actiwatches were downloaded to a computer and actograms showing the rest-activity and light patterns were generated and analysed with ‘Actiwatch Activity and Sleep Analysis’ software (Actiwatch software version 5, Cambridge Neurotechnology, UK; www.camntech.com/files/The_Actiwatch_User_Manual_V7.2.pdf) on Windows. Diary information was then added manually to the printed actograms to verify the concordance with the actigraphic data, to determine ‘bedtime’ and ‘get-up time’ and to edit gaps when the watch was removed, for example to avoid water when swimming, showering or bathing. Weekly onscreen inspection of activity data, lights on/off and diary entries allowed precise determination of bedtime and get-up time. Sleep start and sleep end was not manually determined but automatically calculated by the ‘SleepWatch’ algorithm using bedtime and get-up time as reference points.
Periodogram analysis and cosinor analysis was carried out across 6 weeks of rest-activity data with the software El Temps (A. Diez-Noguera, University of Barcelona, Spain; www.el-temps.com/contact.htm) to determine period length and peak of the rest-activity cycles respectively. Non-parametric circadian rhythms analysis23 was carried out on complete continuous days and weighted averages were carried out for split periods when days with incomplete data were excluded. We generated ‘average 24 h day’ profiles of activity for each week by overlaying 7 days from midnight to midnight. A 10 h window (M10) and another 5 h window (L5), both moving in 1 h steps across the ‘average day’, were used to determine the periods of the 10 most active hours and the 5 least active hours. The amplitude was calculated from the ratio of the most active 10 h period to the least active 5 h period across the averaged 24 h profile. Its measure ranges from 0 to 1 with higher values indicating a high amplitude hence consolidated low activity during sleep (L5) and consolidated high activity during the day (M10), which is considered healthy in individuals.
The automated algorithm of the Actiwatch software was used to establish ‘sleep onset’ and ‘sleep offset’ for each night and to calculate sleep/wake estimates. Sleep estimates, derived from the actigraphic sleep analysis, include ‘sleep period’ (time between sleep onset and sleep offset, including periods defined as ‘wake time’), ‘total sleep time’ (TST, time between sleep onset and sleep offset, excluding periods defined as ‘wake time’), ‘sleep latency’ (defined as time between ‘bedtime’ and ‘sleep onset’) and ‘sleep efficiency’ (defined as percentage of time spent asleep between ‘sleep onset’ and ‘sleep offset’, thereby separating it from ‘sleep latency’). Sleep/wake estimates were averaged weekly over the entire recording period. Days with missing data of several hours (for example when the participant forgot the Actiwatch or for unexpected circumstances such as admission to hospital for a surgical procedure) were excluded from further analysis.
Actograms were also visually inspected to estimate the daily pattern of light exposure. Our simultaneous recordings of rest-activity and light exposure showed that light exposure remained synchronised with the activity period and dark with the sleep period.
To investigate the diversity in sleep-wake timing using long-term objective recordings, we developed operational criteria to distinguish the following abnormal sleep patterns in accordance with DSM-IV and ICD-1024 diagnostic guidelines: (a) non-shifted sleep/wake phases, defined as sleep onset before 01.00 h and open sleep end; (b) delayed sleep/wake phases, defined as sleep onset after 01.00 h and sleep end after 10.00 h; (c) non-24 h sleep/wake phases, defined as circadian period longer or shorter than 24 h; (d) irregular/fragmented sleep/wake patterns, defined as sleep and wake episodes at irregular times over 24 h; (e) hypersomnia, defined as the majority of consolidated sleep periods >9 h regardless of their sleep onset time. Mixed pattern types can be exhibited.
Melatonin sulphate concentrations were determined for each urine sample by radioimmunoassay.25 To determine rhythms in melatonin sulphate from our unequally spaced collection times, we applied a non-linear regression model to our data using the equation:
where MT represents the aMT6s secretion rate (ng/h) and t represents time. For the parameter estimates c represents mesor (rhythm adjusted daily mean); Amp amplitude; tc phase angle; and T period. The timing of melatonin sulphate peaks were estimated at each 48 h session and these data points were subsequently fitted to a curve. Non-linear regression was performed using SAS software for Windows, Version 8.
To estimate the dose of antipsychotic medication across the various types and applications (for example oral or long-acting injections) of antipsychotics, we converted each participant's dose of antipsychotic medication into a chlorpromazine equivalent dose using chlorpromazine 100 mg/day. Dose equivalency estimates were taken from published tables,26-28 with equivalents being based primarily on dopaminergic blockade and not on a drug receptor profile for cholinergic, serotonergic or histaminergic systems because clinical potency of antipsychotic medication has been shown to correlate closely with affinity for dopamine D2 receptors.
Statistical analysis
Data analysis comparing two groups was based on t-tests for independent variables. Data analysis comparing three groups was based on one-way analysis of variance with post hoc Tukey's test (homogeneous variances) or Dunnett's test (inhomogeneous variances) to explore significant effects. For categorical variables, chi-squared analyses were conducted. For the schizophrenia group, univariate ANCOVAs were used to determine the influence of three factors: (a) presence of positive symptoms, (b) length of illness, and (c) GAF on sleep, rest-activity and melatonin rhythm parameters (dependant variables). The participants with schizophrenia were categorised into two groups, for each of these analyses: with/without positive symptoms, below/above 10 years of illness duration (median split) and below/above a GAF score of 50 (median split). Age was included as a covariate to adjust the scores on dependent variables of the ANCOVA before those scores were related to any independent groups. Main effects of ‘group’ and of ‘age’ as well as the interaction of ‘group×age’ were included in the model. Non-parametric correlation was based on Spearman's rank correlation. Statistical analyses were performed with SPSS 16 for Windows.
Results
Participants
Table 1 shows that there were no differences between the schizophrenia and the control group with respect to: gender; age; chronotype; mood state across the study period; and season when tested. The schizophrenia group reported poor sleep as assessed by the PSQI.
Table 1.
Demographics of schizophrenia and control group
| Test statistics |
||||||
|---|---|---|---|---|---|---|
| Schizophrenia group | Control group | χ2 (d.f.) | t-test (d.f.) | F (d.f.) | P | |
| Gender, n (%) | 0.368 (1) | |||||
| Male | 15 (75) | 13 (61.9) | ||||
| Female | 5 (25) | 8 (38.1) | ||||
| Total | 20 (100) | 21 (100) | ||||
| Employment, n (%) | ||||||
| Yes | 0 | 0 | ||||
| No | 20 (100) | 21 (100) | ||||
| Total | 20 (100) | 21 (100) | ||||
| Chronotype, n (%) | 0.215 (1) | |||||
| Moderate morning | 0 | 1 (5) | ||||
| Neutral | 5 (27.8) | 6 (30) | ||||
| Moderate evening | 12 (66.7) | 8 (40) | ||||
| Definitely evening | 1 (5.6) | 5 (25) | ||||
| Season, n (%) | 1.977 (3) | |||||
| Spring | 6 (30) | 4 (19) | ||||
| Summer | 6 (30) | 9 (42.9) | ||||
| Autumn | 5 (25) | 3 (14.3) | ||||
| Winter | 3 (15) | 5 (23.8) | ||||
| Age, years: mean (s.d.) | 38.8 (8.6) | 37.5 (9.6) | 0.466 (39) | 0.644 | ||
| Sleep quality index, mean (s.d.) | 8.32 (3.77) | 4.83 (2.37) | 3.531 (39) | 0.001 | ||
| Mood profile, mean (s.d.) | ||||||
| Total score, week 1 | 13.3 (10.7) | 12.1 (8.4) | ||||
| Total score, week 6 | 14.3 (10) | 9.5 (9) | ||||
| Between groups | 1.614 | 0.22 | ||||
| Between time points | 0.621 | 0.711 | ||||
| Time and group | 1.278 | 0.332 | ||||
Schizophrenia group
No one in the schizophrenia group was in remunerated employment. Seventeen individuals lived with a family member or independently, two lived in supported accommodation and one was in an in-patient unit, ready for discharge but awaiting accommodation. They all had paranoid subtype schizophrenia. Regarding regularly prescribed psychotropic medication and psychiatric comorbidity, 12 individuals were taking antipsychotic drugs only, and 7 received additional psychotropic medication to control extrapyramidal (antimuscarinics n = 3) and seizure-inducing side-effects of antipsychotics (anticonvulsants n = 4). Eight people had experienced depressive and/or anxiety symptoms at some point during their illness; two of these were currently being treated with a selective serotonin reuptake inhibitor.
Antipsychotic medication ranked by dose using chlorpromazine equivalents is detailed in online Table DS1 along with age, gender and presence/absence of positive symptoms. Length of illness ranged from 2 to 33 years (median 10 years). Sleep, rest-activity and melatonin rhythm parameters were similar for individuals with shorter (below 10 years) and longer (above 10 years) durations of illness (all P>0.1; online Table DS2). Their GAF scores ranged from 34 to 70 (median 50). Sleep, rest-activity and melatonin rhythm parameters were similar for individuals with scores below and above 50 (all P>0.1; online Table DS3). None of the sleep, rest-activity and melatonin rhythm parameters were correlated with antipsychotic drug dose using chlorpromazine equivalents (all P>0.05; online Table DS4).
Persistent positive symptoms were present in eight individuals. Univariate ANCOVAs comparing groups with and without positive symptoms showed no significant differences for sleep parameters, rest-activity and chlorpromazine equivalents of antipsychotic dose (all P>0.1; online Table DS5). Lower melatonin levels (mesor) were found in individuals with positive symptoms (mean 467.6 ng (s.d. = 228.6)) compared with individuals without positive symptoms (mean 1066.3 ng (s.d. = 501)) (group: F(1,15) = 9.207, P= 0.01). However, individuals with positive symptoms and lower melatonin levels were also older (group age interaction: F(1,15) = 5.029, P = 0.045). Melatonin levels were correlated with the age of participants (n = 17, Spearman's rho: –0.513, P = 0.035), with older individuals having lower and younger individuals having higher melatonin levels, indicating that differences in melatonin levels between individuals with and without positive symptoms was an effect of age.
Timing of sleep-wake behaviour
The variability of sleep-wake timing was remarkably greater in the schizophrenia group than in the control group (Fig. 1). Intra-individual difference in sleep onset, sleep offset and sleep mid-point in the schizophrenia group was two- to threefold that of the variability in the control group (Table 2).
Fig. 1.
Variability in sleep timing of schizophrenia group (n = 20) and unemployed, control group (n = 21) for three parameters: sleep onset, sleep offset and sleep mid-point.
Each dot represents a person's average standard deviation (in minutes) derived from 6 weeks of wrist actigraphy. The horizontal bars indicate the median. Those in the schizophrenia group show significantly higher variability than those in the control group, most remarkably in sleep offset.
Table 2.
Sleep timing variability in schizophrenia and control groups
| Group, mean (95% CI) |
Statistical difference |
|||
|---|---|---|---|---|
| Schizophrenia group (n = 20) | Control group (n = 21) | t-test | P | |
| Time at sleep onset, h and min | 01.00 (00.02–01.57) | 01.21 (00.41–02.02) | –0.64 | 0.527 |
| Time at sleep offset, h and min | 11.07 (09.56–12.18) | 08.52 (08.17–09.28) | 3.55 | 0.001 |
| Sleep onset variation, min | 67 (47–87) | 36 (27–46) | 2.92 | 0.007 |
| Sleep offset variation, min | 91 (69–118) | 33 (24–42) | 4.2 | 0.001 |
| Sleep mid-point variation, min | 60 (41–80) | 30 (24–38) | 2.99 | 0.006 |
| Sleep period, h | 9.98 (9.3–10.66) | 7.46 (7.16–7.76) | 7.016 | 0.0001 |
| Total sleep time, h | 8.22 (7.52–8.92) | 6.08 (5.78–6.36) | 5.924 | 0.0001 |
| Sleep latency, min | 34 (26–42) | 19 (15–24) | 3.373 | 0.002 |
| Sleep efficiency, % | 82.4 (78.6–86.3) | 81.7 (78.9–84.6) | 0.308 | 0.76 |
The time between sleep start and sleep end (sleep period), the actual time spent asleep (total sleep time) and sleep latency (time between bedtime and sleep onset) was each significantly longer in the schizophrenia group than the control group (P<0.005; Table 2). Thus, individuals with schizophrenia were inconsistent in their sleep patterns but, in general, took longer to fall asleep, spent a longer time in bed and actually slept longer than those in the control group.
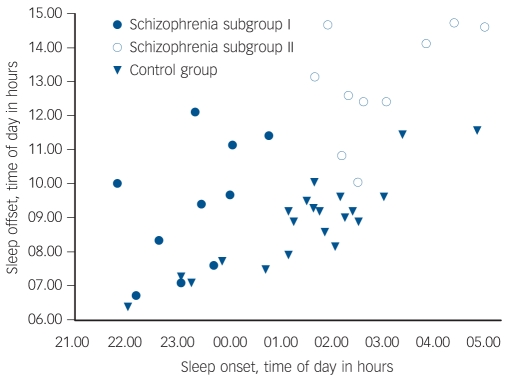
Further inspection of the sleep/wake timing of the schizophrenia group suggested two characteristic profiles. Whereas the majority of individuals in the control group had a sleep onset after 01.00 h and sleep offset before 10.00 h, in half those in the schizophrenia group (n = 10), sleep onset was before 01.00 h and sleep offset before 12.00 h (subgroup I), and in the other half (n = 10) sleep onset was after 01.00 h and sleep offset after 12.00 h (subgroup II) (Fig. 2). An ANOVA of these three groups (controls, schizophrenia subgroup I, schizophrenia subgroup II) confirmed distinct differences in sleep timing and sleep duration (Table 3). Post hoc comparisons revealed that sleep onset was significantly different between all groups (P<0.001), with the control group in the middle and the two schizophrenia subgroups either side (Table 3): schizophrenia subgroup I showed earliest sleep onset and subgroup II showed the latest sleep onset relative to local time. Sleep end was significantly later in subgroup II in comparison with both subgroup I (P<0.001) and the control group (P<0.001) (Table 3).
Fig. 2.
Distribution of sleep timing with sleep onset plotted relative to sleep offset in schizophrenia and control group.
Each dot represents a person's average sleep onset/offset time derived from 6 weeks of wrist actigraphy. Schizophrenia subgroup I: sleep onset before 01.00 h, n = 10; schizophrenia subgroup II: sleep onset after 01.00 h, n = 10; control group n = 21.
Table 3.
Parameters sleep in the schizophrenia subgroups and control group
| Group, mean (s.d.) |
Statistical difference |
||||
|---|---|---|---|---|---|
| Subgroup I (n = 10) | Subgroup II (n = 10) | Control group (n = 21) | F(2,38) | P | |
| Time at sleep onset, h:min (h) | 23:14 (01.86) | 02:46 (01.06) | 01:21 (01.47) | 19.837 | 0.0001a |
| Time at sleep offset, h:min (h) | 09:19 (01.86) | 12:56 (01.63) | 08:52 (01.30) | 24.786 | 0.0001b |
| Sleep period, h | 9.93 (1.65) | 10.03 (1.33) | 7.46 (0.65) | 24.841 | 0.001c |
| Total sleep time, h | 8.08 (1.7) | 8.35 (1.33) | 6.08 (0.62) | 17.978 | 0.0001c |
| Sleep latency, min | 31 (20) | 37 (13) | 19 (9) | 6.468 | 0.004c |
| Sleep efficiency, % | 81.6 (10.7) | 83.3 (5.2) | 81.7 (6.3) | 0.188 | 0.83 |
Indicates significant differences between all groups (Tukey's (homogeneous variances) and Dunnetts (inhomogeneous variances)).
Significant difference distinguishing schizophrenia subgroup II from schizophrenia subgroup I and control group.
Significant difference distinguishing control group from schizophrenia subgroups I and II.
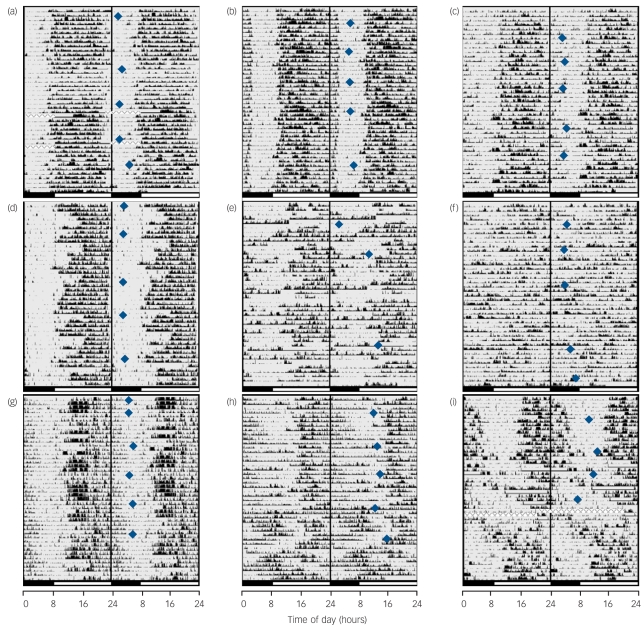
Other sleep/wake characteristics differed in these groups (Fig. 3). All individuals in the control group displayed consistent sleep patterns of either consolidated sleep/activity phases synchronised to the day/night cycle (n = 5, example in Fig. 3(a)), consolidated sleep phases with delayed sleep onsets (n = 11, example in Fig. 3(b)) or less consolidated and fragmented sleep phases (n = 5, example in Fig. 3(c)). In contrast, the schizophrenia group's rest/activity patterns were mostly abnormal (n = 17). Within subgroup I (i.e. those with a sleep onset before 01.00 h, 70% male), five people showed excessive sleep periods (Fig. 3(d)) and five irregular and/or fragmented sleep/activity phases (Fig. 3(e) and (f)). Within subgroup II (i.e. with a sleep onset after 01.00 h and a sleep end after 10.00 h, 80% male), six people showed markedly delayed sleep phases (Fig. 3(g)) and four people exhibited patterns of both delayed and non-24 h (free-running) sleep/activity phases (Fig. 3(h) and (i)).
Fig. 3.
Representative examples of phenotypic variability in rest–activity patterns derived from 6 weeks' wrist activity monitoring and concurrent weekly melatonin profiling carried out in the participants' home environment.
Actograms from individuals in the control group (a-c) show clear entrainment to the day/night cycle with relatively low night-time activity disrupting sleep. Actograms of those in the schizophrenia group without phase shifts (subgroup I) span from (d) robustly entrained with variable sleep periods to (e) disrupted, and (f) highly irregular rest-activity cycles accounting for major complaints about problems with sleep. Actograms of those in the schizophrenia group with circadian misalignment (subgroup II) show (g) highly delayed rest-activity periods with bed times around 04.00 h in the morning and get-up times in the afternoon, (h) reversed rest-activity cycles alternating with free-running periods or (i) delayed rest-activity periods alternating with free-running periods patterns that were never present in the control group. Blue diamonds indicate peaks in melatonin sulphate rhythms, which is a marker for the phase of the circadian clock. All controls and the majority of people in schizophrenia subgroup I had melatonin peak times within the normal range of entrainment whereas individuals in subgroup II had severely delayed or free-running peak times. Actigraphic data are 48 h double-plotted with successive days on vertical axis. Black bars on the bottom indicate night-time, open bars indicate daytime and the midline indicates midnight between day 1 and 2. Edited data are highlighted with ‘___’, and times when watch was removed and information missing are filled with ‘xxxxxxx’.
Thus, in addition to those in the schizophrenia group taking longer to fall asleep and sleeping for longer than those in the control group regardless of sleep onset time, the sleep phase in 50% of individuals in the schizophrenia group was out of synchrony with the environmental night-time.
A similar analysis of the circadian phase of the rest/activity rhythm showed that the shift in sleep/wake timing of schizophrenia subgroup II was also reflected in a later afternoon activity peak compared with earlier peaks in both subgroup I and the control group (P = 0.002) (Table 4). However, those in subgroup II were overall significantly less active during waking hours in comparison with controls (P<0.001). The level of activity of individuals in schizophrenia subgroup I was intermediate between the other two groups (P = 0.678).
Table 4.
Parameters of circadian activity rhythms in the schizophrenia subgroups and control group
| Group |
Statistical difference |
||||
|---|---|---|---|---|---|
| Subgroup I (n = 10) | Subgroup II (n = 10) | Control group (n = 21) | F(2,38) | P | |
| Period length, h: mean (95% CI) | 23.9 (23.7–24.1) | 24.3 (23.8–24.7) | 24.0 (23.99–24.02) | 3.044 | 0.059 |
| Delayed sleep phasea | 24.0 (24.0–24.0) | ns | |||
| Non-24 ha | 24.7 (23.2–26.3) | ns | |||
| Peak of activity cycle, h:min: mean (95% CI) | 15:28 (13:59–16:58) | 18:02 (17:08–18:56) | 16:13 (15:34–16:51) | 7.279 | 0.002b |
| Level of activity (M10 counts), mean (s.d.) | 17381 (11530) | 12935 (4039) | 21286 (5328) | 4.806 | 0.014c |
| Level of inactivity (L5 counts), mean (s.d.) | 1995 (1843) | 2012 (1169) | 1776 (757) | 0.815 | 0.832 |
| Amplitude, ratio: mean (s.d.) | 0.797 (0.139) | 0.776 (0.221) | 0.838 (0.073) | 2.32 | 0.112 |
ns, not significant.
The schizophrenia subgroup II were split into those with a delayed sleep phase (n = 6) and those with non-24 h sleep–wake cycles (n = 4).
Significant difference distinguishing schizophrenia subgroup II from schizophrenia subgroup I and control group.
Significant difference only between schizophrenia subgroup II and control group.
Despite marked differences in sleep onset and sleep end times, most participants had sleep/wake cycles with a near-24 h period (Table 4). Only those individuals in the schizophrenia group with non-24 h sleep/wake cycles had either much longer (n = 3) or shorter (n = 1) periods.
In summary, schizophrenia subgroup II had severe circadian timing abnormalities with desynchronised sleep/wake patterns relative to the environmental day/night cycle, excessive sleep and less activity when awake. Schizophrenia subgroup I showed no underlying circadian abnormality but sleep was excessively prolonged, irregular or fragmented.
The circadian rhythm of melatonin sulphate
To evaluate whether the sleep/wake cycles are synchronised to the body clock phase, we determined weekly 48 h melatonin profiles. Both the control and schizophrenia group showed a clear cyclic waveform in melatonin production. All those in the control group and the majority of those in the schizophrenia group had a melatonin periodicity of near-24 h. The four individuals with non-24 h sleep/wake cycles from the schizophrenia subgroup II also showed non-24 h melatonin cycles (P<0.001; Table 5).
Table 5.
Parameters of melatonin rhythms in the schizophrenia subgroups and control group
| Group, mean (95% CI) |
Statistical difference |
||||
|---|---|---|---|---|---|
| Subgroup I (n = 10) | Subgroup II (n = 10) | Control group (n = 21) | F(2,38) | P | |
| Period length, h | 23.9 (23.8–24.1) | 24.1 (23.98–24.3) | 24.0 (23.97–24.05) | 4.333 | 0.021 |
| Delayed sleep phasea | 23.9 (23.87–24.1) | ns | |||
| Non-24 ha | 24.3 (24.10–24.6) | <0.001 | |||
| Peak of melatonin, h:min | 04:10 (03:21–04:59) | 08:24 (06:28–10:19) | 04:44 (04:01–05:27) | 18.699 | 0.001c |
| Mesor, ngb | 576 (287–864) | 1034 (642–1427) | 992 (805–1179) | 3.257 | 0.05d |
ns, not significant.
The schizophrenia subgroup II were split into those with a delayed sleep phase (n = 5) and those with non-24 h sleep–wake cycles (n = 4).
Mesor: rhythm adjusted mean of the amount of melatonin sulphate over a circadian cycle.
Significant difference distinguishing schizophrenia subgroup II from schizophrenia subgroup I and control group.
Significant difference distinguishing schizophrenia subgroup I from schizophrenia subgroup II and control group.
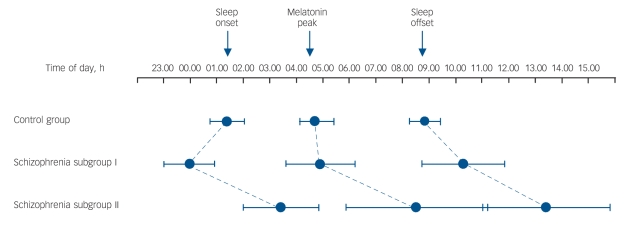
The peak of the melatonin cycle occurred at a similar time of day in the control group and schizophrenia subgroup I but markedly later in the subgroup II (P<0.001) (Fig. 4 and Table 5). Figure 4 illustrates that the melatonin peaks and sleep/activity phases of the control group and subgroup I are in normal temporal order internally and synchronised to the day/night cycle. In subgroup II, sleep/activity phases and melatonin peaks are also in normal temporal order internally but both are shifted to a much later external time, and this shift distinguished them as ‘phase-shifted’ schizophrenia subgroup II from the ‘non-shifted’ subgroup I (χ2(2) = 7.367, P = 0.025). The results indicate that the body clock in this subgroup is not adjusted and internal time does not coincide with day/night phases.
Fig. 4.
Phase relationships between habitual sleep-wake and internal circadian time (derived from melatonin sulphate) and external time of day.
The control group had a somewhat late sleep onset but normal circadian entrainment and sleep offset. Schizophrenia subgroup I show an extended sleep period with normal circadian melatonin entrainment. Schizophrenia subgroup II show a marked delay in sleep-wake cycles and melatonin peak relative to time of day, indicating abnormal circadian entrainment in a subset of the schizophrenia group with sleep complaints. Whiskers indicate 95% confidence intervals of the mean.
The amount of melatonin sulphate (mesor) was borderline different between the three groups (Table 5). Post hoc comparison showed no difference between controls and the phase-shifted schizophrenia subgroup II (P = 0.966) but lower melatonin sulphate levels were found in the non-shifted subgroup I. This was not related to antipsychotic dose because chlorpromazine equivalents of antipsychotic dose was not different between the two schizophrenia subgroups (F = 0.008, P = 0.932, online Table DS6). However, we noted differences with type of antipsychotics. Very low amounts of melatonin were found in those in the schizophrenia group treated with clozapine and amisulpride but normal levels in individuals treated with olanzapine, risperidone and ‘high/medium’ potency antipsychotics (flupenthixol, trifluoperazine, zuclopenthixol) (online Table DS7). The majority of those treated with clozapine and amisulpride belonged to the non-shifted subgroup I, which could explain the lower amount of melatonin in this subgroup.
Discussion
In this study, we recorded profound abnormalities in circadian sleep-wake timing and in the rise and fall of melatonin levels in people with schizophrenia who have long-standing subjective sleep problems.
Circadian rhythm desynchronisation in people with schizophrenia
The sleep-wake cycle and the rise and fall of melatonin levels are subjected to circadian regulation that synchronises these behavioural and physiological rhythms with changes in the environmental 24 h light/dark cycle. The most important difference in the schizophrenia group, in comparison with the control group, was that 50% showed markedly delayed and/or free-running sleep-wake cycles. Our repeated melatonin measurements showed that these sleep-wake shifts were always associated with delayed or free-running circadian rhythms in melatonin, which implies a lack of synchrony between the body's internal rhythm and the external day-night cycle.
Such desynchronisation was not present in our control group but is reminiscent of that observed in people with delayed sleep phase syndrome and non-24 h sleep-wake syndrome,29,30 which can occur in otherwise healthy individuals living in normal environmental conditions.31 There are no clear explanations for the development of this desynchronisation other than when it occurs in people with a genetic predisposition32 or when there is no light input to the circadian clock due to eye loss. All our participants had undergone vision tests and these were found to be normal.
One possible explanation for the abnormal rise and fall in melatonin in some individuals in our schizophrenia group comes from a recent experimental study showing that the human circadian clock, which governs the synthesis of melatonin, can be entrained to a non-24 h cycle in rest-activity through concurrent abnormal light exposure.33 This raises the possibility that the melatonin rhythm in our phase-shifted subgroup became misaligned secondary to delayed and/or non-24 h rest-activity cycles. Another explanation could be that the melatonin rhythm abnormality is primary and that this contributes to sleep and circadian rhythm disruption. In support of this are findings that aetiological mechanisms contributing to circadian misalignment include dysfunction of neurotransmitter systems such as glutamate and dopamine and risk genes that have been associated both with core features of schizophrenia and circadian signalling pathways.12,34
Studies of circadian rhythms over extended periods of time in people with schizophrenia taking medication are scarce and are non-existent for drug-naive individuals. Of the few longitudinal case studies published, sleep-wake behaviour has been reported as free-running sleep-wake cycles for a shortened period in two people with schizophrenia who were released from external time cues,35 as a more irregular and delayed rest-activity pattern in one person,36 whereas more irregular rest-activity patterns and phase-advanced melatonin and core body temperature rhythms were found in another individual.37 In a case series of seven people with schizophrenia, rest-activity recordings over 2-7 weeks showed a variety of irregular circadian rest-activity cycles, but no non-24 h rhythms were observed.38 All of these studies have been limited by small sample sizes and the lack of an adequate control group.
Irregular and fragmented sleep-wake patterns in schizophrenia
The non-phase shifted schizophrenia subgroup (I) also showed melatonin rhythms that were stably synchronised with the 24 h day/night cycle and corresponded well with those of the healthy, unemployed controls but still showed highly irregular periods of sleep-wake episodes. All those in the schizophrenia group took longer to fall asleep and slept consistently longer in comparison with those in the control group. Longer sleep periods are also in accordance with observations by Martin et al,39,40 who analysed rest-activity patterns over 3 days in a cohort of 28 community-dwelling, mostly unemployed, older people with schizophrenia (about 58 years old), which they compared with age- and gender-matched healthy individuals, the majority being employed. Although we studied younger, middle-aged individuals with schizophrenia (about 38 years old) and age- and gender-matched unemployed individuals over 6 weeks (42 days) using actigraphy and melatonin profiles, our results are consistent with some of the findings of Martin et al.39,40 For example, the individuals in our schizophrenia group had similarly low levels of daytime activity, spent about 10 h in bed, slept about 8.25 h and some had severely fragmented sleep and daytime naps despite having regular, synchronised melatonin rhythms.
Mechanism of impairment
One possible explanation of the circadian misalignment and abnormal sleep-wake patterns found in our schizophrenia subgroup II could be a lack of daily routine. We think that this is unlikely because our control group were unemployed and were similar to our schizophrenia group in that they also lacked externally imposed routines. However, although they showed late sleep onset, consistent with lack of daily routine, they displayed none of the abnormalities suggestive of circadian misalignment. In addition there was no association in the schizophrenia group between their circadian parameters and their general level of function as measured by the GAF scale.
Another explanation could be the effect of antipsychotic medication, which may explain increased sleep time, irrespective of group, and decreased daytime activity due to sedation or effects on motivation. However, we found no evidence that medication dose was related to any of the circadian abnormalities. The type of medication was also not specifically associated with any circadian composite profile, although those treated with risperidone showed the least pathological phenotype on all measures taken (rest-activity, sleep, melatonin). Whether this is a consequence of type of medication or other factors such as environmental conditions, social support, clinical status, length of illness or a random effect cannot be established at this time.
Although the possible contribution of these external factors requires further clarification, our data points to the involvement of circadian disruption among the highly diverse sleep-wake phenotypes found in people with schizophrenia.
Consequences of circadian rhythm and sleep-wake disruption
Although we have provided a detailed correlation between schizophrenia and sleep-wake disruption, we have yet to demonstrate a mechanistic link between the two. To establish whether this is genetic, one approach would be to examine the sleep and circadian phenotype of individuals at high familial risk for developing schizophrenia; another would be to examine the sleep phenotype of mice with mutations in genes linked to schizophrenia. Possible drug effects could be examined by studying the longitudinal pattern of sleep in individuals at the time of initial diagnosis when unmedicated and following pharmacological treatment.
Chronically disturbed sleep has a strong impact on social function, mood, cognition and quality of life in people with schizophrenia.41 In a recent study, circadian integrity was strongly associated with outcome measures of cognitive performance but not symptom severity or length of illness.42 Those with disrupted circadian rhythms (daytime sleepiness, more fragmented sleep and delayed melatonin release) performed worse in neuropsychological tasks than those with schizophrenia who had normal circadian rhythms. Thus, whatever the cause of sleep-wake disruption in schizophrenia, actigraphy is a procedure of clinical relevance for establishing the nature of the circadian sleep-wake abnormalities with a view to assessing and tailoring chronotherapeutic interventions.
Limitations of the study
Our results provide the basis for further research. For example, it would be important to clarify the effect of type and dose of antipsychotic and adjunctive medications using a larger number of participants. In addition, although we screened for symptoms of comorbid sleep disorders, we did not utilise objective measurements to detect akathisia, restless leg syndrome, periodic leg movement disorder or obstructive sleep apnoea.
Acknowledgments
We thank the consultants at West London Mental Health NHS Trust for their help with patient referrals.
Footnotes
Declaration of interest
None.
Funding
This work was supported by a Marie Curie Individual Fellowship (HPMF-CT-2002-01795) to K.W. from the European Commission and in part by a grant from the Hammersmith Hospitals Trust Research Committee (20305), the Wellcome Trust (076067/Z/04/Z) to R.G.F., E.M.J. and D.-J.D., the Oxford Biomedical Research Centre (K.W., R.G.F.) and the UCL/UCLH Comprehensive Biomedical Research Centre (E.M.J.).
References
- 1. Wulff K, Gatti S, Wettstein JG, Foster RG. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci 2010; 11: 589-99 [DOI] [PubMed] [Google Scholar]
- 2. Tandon R, Shipley JE, Taylor S, Greden JF, Eiser A, DeQuardo J, et al. Electroencephalographic sleep abnormalities in schizophrenia. Relationship to positive/negative symptoms and prior neuroleptic treatment. Arch Gen Psychiatry 1992; 49: 185-94 [DOI] [PubMed] [Google Scholar]
- 3. Lauer CJ, Schreiber W, Pollmacher T, Holsboer F, Krieg JC. Sleep in schizophrenia: a polysomnographic study on drug-naive patients. Neuropsychopharmacology 1997; 16: 51-60 [DOI] [PubMed] [Google Scholar]
- 4. Nofzinger EA, van Kammen DP, Gilbertson MW, Gurklis JA, Peters JL. Electroencephalographic sleep in clinically stable schizophrenic patients: two-weeks versus six-weeks neuroleptic-free. Biol Psychiatry 1993; 33: 829-35 [DOI] [PubMed] [Google Scholar]
- 5. Chouinard S, Poulin J, Stip E, Godbout R. Sleep in untreated patients with schizophrenia: a meta-analysis. Schizophr Bull 2004; 30: 957-67 [DOI] [PubMed] [Google Scholar]
- 6. Keshavan MS, Reynolds CF, 3rd, Miewald JM, Montrose DM. A longitudinal study of EEG sleep in schizophrenia. Psychiatry Res 1996; 59: 203-11 [DOI] [PubMed] [Google Scholar]
- 7. Keshavan MS, Miewald J, Haas G, Sweeney J, Ganguli R, Reynolds CF. Slow-wave sleep and symptomatology in schizophrenia and related psychotic disorders. J Psychiatr Res 1995; 29: 303-14 [DOI] [PubMed] [Google Scholar]
- 8. van Kammen DP, van Kammen WB, Peters J, Goetz K, Neylan T. Decreased slow-wave sleep and enlarged lateral ventricles in schizophrenia. Neuropsychopharmacology 1988; 1: 265-71 [PubMed] [Google Scholar]
- 9. Dijk DJ, Archer SN. PERIOD3, circadian phenotypes, and sleep homeostasis. Sleep Med Rev 2010; 14: 151-60 [DOI] [PubMed] [Google Scholar]
- 10. Hampp G, Ripperger JA, Houben T, Schmutz I, Blex C, Perreau-Lenz S, et al. Regulation of monoamine oxidase A by circadian-clock components implies clock influence on mood. Curr Biol 2008; 18: 678-83 [DOI] [PubMed] [Google Scholar]
- 11. Fletcher PC, Frith CD. Perceiving is believing: a Bayesian approach to explaining the positive symptoms of schizophrenia. Nat Rev Neurosci 2009; 10: 48-58 [DOI] [PubMed] [Google Scholar]
- 12. Wulff K, Porcheret K, Cussans E, Foster RG. Sleep and circadian rhythm disturbances: multiple genes and multiple phenotypes. Curr Opin Genet Dev 2009; 19: 237-46 [DOI] [PubMed] [Google Scholar]
- 13. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (4th edn) (DSM-IV). APA, 1994. [Google Scholar]
- 14. McGuffin P, Farmer A, Harvey I. A polydiagnostic application of operational criteria in studies of psychotic illness. Development and reliability of the OPCRIT system. Arch Gen Psychiatry 1991; 48: 764-70 [DOI] [PubMed] [Google Scholar]
- 15. Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 1999; 282: 1737-44 [DOI] [PubMed] [Google Scholar]
- 16. Buysse DJ, Reynolds III CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989; 28: 193-213 [DOI] [PubMed] [Google Scholar]
- 17. Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res 2002; 53: 737-40 [DOI] [PubMed] [Google Scholar]
- 18. Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res 1998; 45: 5-13 [DOI] [PubMed] [Google Scholar]
- 19. Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol 1976; 4: 97-110 [PubMed] [Google Scholar]
- 20. McNair DM, Lorr M, Droppleman L. Manual for the Profile of Mood States. Educational and Industrial Testing Service, 1971. [Google Scholar]
- 21. Frances A, Pincus HA, First MB. The Global Assessment of Functioning Scale (GAF). American Psychiatric Association, 1994. [Google Scholar]
- 22. Wulff K, Joyce EM, Middleton B, Dijk DJ, Foster RG. The suitability of actigraphy, diary data, and urinary melatonin profiles for quantitative assessment of sleep disturbances in schizophrenia: a case report. Chronobiol Int 2006; 23: 485-95 [DOI] [PubMed] [Google Scholar]
- 23. Van Someren EJ, Swaab DF, Colenda CC, Cohen W, McCall WV, Rosenquist PB. Bright light therapy: improved sensitivity to its effects on rest-activity rhythms in Alzheimer patients by application of nonparametric methods. Chronobiol Int 1999; 16: 505-18 [DOI] [PubMed] [Google Scholar]
- 24. World Health Organization The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. WHO, 1992. [Google Scholar]
- 25. Aldhous ME, Arendt J. Radioimmunoassay for 6-sulphatoxymelatonin in urine using an iodinated tracer. Ann Clin Biochem 1988; 25: 298-303 [DOI] [PubMed] [Google Scholar]
- 26. Atkins M, Burgess A, Bottomley C, Riccio M. Chlorpromazine equivalents: a consensus of opinion for both clinical and research applications. Psychiatr Bull 1997; 21: 224-6 [Google Scholar]
- 27. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry 2003; 64: 663-7 [DOI] [PubMed] [Google Scholar]
- 28. Bai YM, Ting Chen T, Chen JY, Chang WH, Wu B, Hung CH, et al. Equivalent switching dose from oral risperidone to risperidone long-acting injection: a 48-week randomized, prospective, single-blind pharmacokinetic study. J Clin Psychiatry 2007; 68: 1218-25 [DOI] [PubMed] [Google Scholar]
- 29. Weitzman ED, Czeisler CA, Coleman RM, Spielman AJ, Zimmerman JC, Dement W, et al. Delayed sleep phase syndrome. A chronobiological disorder with sleep-onset insomnia. Arch Gen Psychiatry 1981; 38: 737-46 [DOI] [PubMed] [Google Scholar]
- 30. Hayakawa T, Uchiyama M, Kamei Y, Shibui K, Tagaya H, Asada T, et al. Clinical analyses of sighted patients with non-24-hour sleep-wake syndrome: a study of 57 consecutively diagnosed cases. Sleep 2005; 28: 945-52 [DOI] [PubMed] [Google Scholar]
- 31. Weber AL, Cary MS, Connor N, Keyes P. Human non-24-hour sleep-wake cycles in an everyday environment. Sleep 1980; 2: 347-54 [PubMed] [Google Scholar]
- 32. Toh KL, Jones CR, He Y, Eide EJ, Hinz WA, Virshup DM, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science 2001; 291: 1040-3 [DOI] [PubMed] [Google Scholar]
- 33. Gronfier C, Wright Jr KP, Kronauer RE, Czeisler CA. Entrainment of the human circadian pacemaker to longer-than-24-h days. Proc Natl Acad Sci USA 2007; 104: 9081-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci 2008; 31: 234-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mills JN, Morgan R, Minors DS, Waterhouse JM. The free-running circadian rhythms of two schizophrenics. Chronobiologia 1977; 4: 353-60 [PubMed] [Google Scholar]
- 36. Haug HJ, Wirz-Justice A, Rossler W. Actigraphy to measure day structure as a therapeutic variable in the treatment of schizophrenic patients. Acta Psychiatr Scand Suppl 2000; 102: 91-5 [DOI] [PubMed] [Google Scholar]
- 37. Wirz-Justice A, Cajochen C, Nussbaum P. A schizophrenic patient with an arrhythmic circadian rest-activity cycle. Psychiatry Res 1997; 73: 83-90 [DOI] [PubMed] [Google Scholar]
- 38. Wirz-Justice A, Haug HJ, Cajochen C. Disturbed circadian rest-activity cycles in schizophrenia patients: an effect of drugs? Schizophr Bull 2001; 27: 497-502 [DOI] [PubMed] [Google Scholar]
- 39. Martin J, Jeste DV, Caliguiri MP, Patterson T, Heaton R, Ancoli-Israel S. Actigraphic estimates of circadian rhythms and sleep/wake in older schizophrenia patients. Schizophr Res 2001; 47: 77-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Martin JL, Jeste DV, Ancoli-Israel S. Older schizophrenia patients have more disrupted sleep and circadian rhythms than age-matched comparison subjects. J Psychiatr Res 2005; 39: 251-9 [DOI] [PubMed] [Google Scholar]
- 41. Krystal AD, Thakur M, Roth T. Sleep disturbance in psychiatric disorders: effects on function and quality of life in mood disorders, alcoholism, and schizophrenia. Ann Clin Psychiatry 2008; 20: 39-46 [DOI] [PubMed] [Google Scholar]
- 42. Bromundt V, Köster M, Georgiev-Kill A, Opwis K, Wirz-Justice A, Stoppe G, et al. Sleep-wake cycles and cognitive functioning in schizophrenia. Br J Psychiatry 2011; 198: 269-76 [DOI] [PubMed] [Google Scholar]