Abstract
The burden of liver disease in Egypt is exceptionally high due to the highest prevalence of hepatitis C virus (HCV) resulting in rising rates of hepatocellular carcinoma (HCC). The aim of the current study was to determine the isoflavones in soy and to evaluate the protective role of soy against CCl4-induced liver damage in rats. Four experimental groups were treated for 8 weeks and included the control group, soy-supplemented diet (20% w/w) group, the group treated orally with CCl4 (100 mg/kg bw) twice a week, and the group fed soy-supplemented diet and treated with CCl4. Blood and liver tissue samples were collected for biochemical analyses and histological examination. The results indicated that protein content was 45.8% and the total isoflavones recorded 167.3 mg/100 g soy. Treatment with CCl4 resulted in a significant biochemical changes in serum liver tissue accompanied with severe oxidative stress and histological changes. Supplementation with soy succeeded to restore the elevation of liver enzymes activities and improved serum biochemical parameters. Moreover, soy supplementation improved the antioxidant enzymes, decreased lipid peroxidation, and improved the histological picture of the liver tissue. It could be concluded that soy-protein-enriched isoflavones may be a promising agent against liver diseases.
1. Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer in the world with an estimated 473,000 new cases annually [1]. Most patients survive less than 1 year after diagnosis. In Egypt, the annual prevalence of HCC has increased significantly during the past decade [2]. HCC was reported to account for about 4.7% of chronic liver disease (CLD) patients where its epidemiology is characterized by marked demographic and geographic variations [3]. The cancer is more common in men with a male: female ratio exceeding three in high incidence areas.
Liver is subjected to a number of malignant and benign tumors. There are three common tumors of the liver, hemangiomas, adenomas, and focal nodular hyperplasias. Etiology of these benign tumors are either congenital or due to oral contraceptive intake. There is no evidence that any of these benign lesions progress to malignant tumors [4]. Malignant tumors include hepatocellular carcinoma, cholangiocarcinoma, hepatoblastoma, and angiosarcoma. The most common and important primary malignant tumor is hepatocellular carcinoma. This tumor is one of the major malignant diseases in the world today. It occurs most frequently in Asia and sub-Saharan Africa. Cholangiocarcinoma, hepatoblastoma, and angiosarcoma are less common but still important malignant tumors. Metastatic spread of tumors from elsewhere in the body to the liver is frequent [5].
The etiology of HCC seems to be multifactorial, and several events seem to be necessary for malignant transformation to occur. Large geographic differences in the incidence of HCC suggest that environmental factors often contribute to its development. One common factor is the association with chronic liver disease, most frequently cirrhosis. Cirrhosis is seen in about 70–80% of HCC and it has been considered as a preneoplastic condition. The incidence of HCC varies depending on the type and etiology of associated cirrhosis [6].
Soybeans and soy products, which are relatively enriched in isoflavones, are of particular interest due to the fact that they make up a significant dietary protein source in some areas of the world [7]. Moreover, soy is rich in phenols which have been reported to exhibit antioxidant activity [8]. Soy isoflavones have been reported to decrease LDL oxidation both in vitro and in vivo [9]. Dietary flavonoids from a variety of fruits, vegetables, and beverages have also been shown to be potent inducers of this enzyme [10, 11]. Soy phytochemicals can modulate both phase I and II enzymes in the xenobiotic metabolizing system [12–15]. In vivo and in vitro experiments have demonstrated that soy and soy phytochemicals specifically induce QR activity [16–18]. The aim of the current study was to evaluate the protective effect of soy against CCl4-induced liver damage in rats.
2. Materials and Methods
2.1. Chemicals and Kits
Carbon tetrachloride (CCl4) was obtained from Morgan Chemical Co. (Cairo, Egypt). Transaminase (ALT and AST) kits were purchased from Spectrum Diagnostics (Cairo, Egypt). Alpha fetoprotein (AFP) was purchased from Immunospec (CA, USA). Total protein, albumin, Cholesterol, triglycerides, superoxide dismutase, glutathione peroxidase, and lipid peroxidase kits were purchased from Biodiagnostic Co. (Giza, Egypt).
2.2. Plant Materials
Soy bean (Glycine max) was purchased from the Crop Institute, Agricultural Research Center, Giza, Egypt in the season 2010.
2.3. Extraction and Determination of Soy Protein and Isoflavones
Soy samples were ground to a fine powder to pass through a 60-mesh sieve and the soy flour was used for the determination of protein and isoflavones. Crude protein was determined according to the method described by AOAC [19]. The soy flour was extracted by methanol 80% using a magnetic stirrer and the extract was used for the determination of daidzin, genistein, genistin, daidzein by HPLC according to the method described by Duke et al. [20]. The isoflavones were detected using a UV detector (Water 486) and online monitoring was done at 260 nm. The data were integrated and recorded using a Millennium chromatography manger software 2010 (Water Milford MA O1757).
2.4. Experimental Animals
Three-month old Sprague-Dawley male rats (100–120 g, purchased from animal house colony, Giza, Egypt) were maintained on standard lab diet (protein: 160.4; fat: 36.3; fiber: 41 g/kg and metabolizable energy 12.08 MJ) purchased from Meladco Feed Co. (Aubor City, Cairo, Egypt). Animals were housed in a room free from any source of chemical contamination, artificially illuminated and thermally controlled, at the Animal House Lab., National Research Centre, Dokki, Cairo, Egypt. After an acclimatization period of one week, the animals were divided into four groups (10 rats/group) and housed in filter-top polycarbonate cages. All animals received human care in compliance with the guidelines of the Animal Care and Use Committee of the National Research Center, Dokki, Cairo, Egypt.
2.5. Experimental Design
Animals within different treatment groups received their respective treatment for 8 weeks as follows: group (1), untreated control; group (2), fed soy supplemented diet (20% w/w); group (3), treated orally with CCl4 (100 mg/kg bw) twice a week for 8 weeks and group (4), treated orally with CCl4 and fed soy-supplemented diet. At the end of the treatment period (i.e., day 57), all animals were being fasted for 12 hrs and blood samples were collected from the retroorbital venous plexus under diethyl ether anesthesia. Sera were separated using cooling centrifugation and stored at −20°C until analysis. The following serum biochemical analyses were carried out: ALT and AST [21], total protein [22], albumin [23], cholesterol [24], triglycerides [25], and alpha fetoprotein [26]. After blood samples were collected, all animals were killed and sample of liver tissues of each animal was dissected, weighed, and homogenized in phosphate buffer (pH 7.4) to give 20% w/v homogenate [27]. This homogenate was centrifuged at 11500 ×g and 4°C for 10 min and the supernatant was stored at −70°C until analysis. Lipid peroxide (LP) was estimated by measuring the formed malondialdehyde (MDA) using thiobarbituric acid reactive substances method according to the spectrophotometric method of Buege and Aust [28] and Ruiz-Larrea et al. [29]. The level of lipid peroxide was expressed as nmol MDA per gram tissue. Hepatic glutathione peroxidase (GPX) activity was determined by a spectrophotometric method, using reduced glutathione and cumene hydroperoxide as substrate and 20 μL diluted liver homogenate, according to the modified method of Paglia and Valentine [30]. Hepatic superoxide dismutase (SOD) activity was assayed spectrophometrically by the red formazan dye reduction procedure [31] using 50 μL diluted liver homogenate. The specific activity of hepatic glutathione peroxidase and superoxide dismutase was expressed as unit/mg liver protein.
Other samples of the liver from all animals within different treatment groups were excised and fixed in 10% neutral formalin followed by dehydration in ascending grades of alcohol, clearing in xylene and embedding in paraffin wax. Liver sections (5 μm thickness) were stained with hematoxylin and eosin (H&E) for the histological examination [32].
2.6. Statistical Analysis
All data were statistically analyzed by analysis of variance (ANOVA) using the General Linear Model Procedure of the Statistical Analysis System [33]. The significance of the differences among treatment groups was determined by Waller-Duncan k-ratio [34]. All statements of significance were based on probability of P ≤ 0.05.
3. Results
The results of crude protein indicated that crude protein in soy was high and recorded 45.8% based on dry matter contents and the total isoflavones content recorded 167.3 mg/100 g soy (Figure 1).
Figure 1.
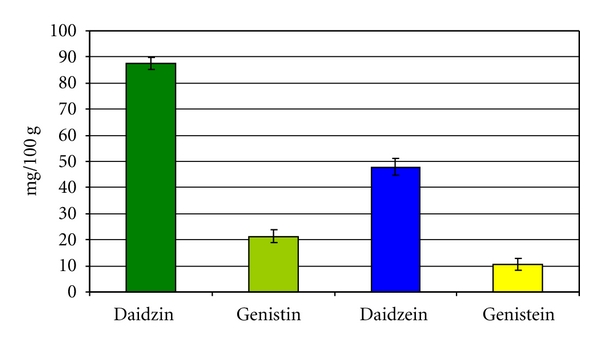
Isoflavones concentration in soy flour extract determined by HPLC (means ± SE).
The results of the current study revealed that treatment with CCl4 resulted in a significant increase in ALT and AST activities, AFP and cholesterol level accompanied with a significant decrease in albumin, total protein, and triglycerides (Table 1). Animals fed soy-supplemented diet showed a significant decrease in AFP, cholesterol, and triglycerides however; ALT and AST activities and albumin and total protein were comparable to the control. ALT, AST, total protein, and cholesterol in animals treated with CCl4 and fed soy-supplemented diet were comparable to the control however; a significant improvement in albumin and triglycerides was observed although this treatment did not normalize them.
Table 1.
The effect of soy supplementation on different treatments on the biochemical serum parameters in rats treated with CCl4.
| Parameters | Groups | |||
|---|---|---|---|---|
| Control | Soy | CCl4 | CCl4 + Soy | |
| ALT (U/mL) | 176.88 ± 3.54a | 173.88 ± 4.88a | 264.33 ± 7.43b | 174.25 ± 6.98a |
| AST (U/mL) | 65.25 ± 3.8a | 63.88 ± 5.04a | 263.63 ± 20.2c | 63.75 ± 3.95a |
| Albumin (g/dL) | 3.64 ± 0.09a | 3.95 ± 0.23a | 1.43 ± 0.12b | 2.98 ± 0.26c |
| Total protein (g/dL) | 9.54 ± 0.66a | 9.32 ± 0.7a | 5.94 ± 0.2b | 8.78 ± 0.58a |
| Cholesterol (mg/dL) | 62.5 ± 4.54a | 54.75 ± 3.03b | 103 ± 4.53c | 67.5 ± 6.07a |
| Triglycerides (mg/dL) | 63.15 ± 4.04a | 56.71 ± 6.23b | 117.83 ± 1.67c | 107 ± 6.51d |
| AFP (IU/mL) | 10.04 ± 2.42a | 6.39 ± 0.73b | 15.71 ± 1.07c | 7.16 ± 0.80d |
Within each row, means superscript with different letters are significantly different (P ≥ 0.05).
The current results revealed that treatment with CCl4 resulted in a severe stress in liver as indicated by the significant increase in MDA and the significant decrease of GPX and SOD in liver tissue (Table 2). Animals fed soy-supplemented diet showed a significant decrease in MDA accompanied with a significant increase in GPX and SOD. However, animals treated with CCl4 and fed soy-supplemented diet showed a significant improvement in the oxidative stress marker as well as the antioxidant enzymes activity although these values were still different significantly than the control group.
Table 2.
The effect of soy supplementation on oxidative stress markers in rat liver treated with CCl4.
| Parameters | Groups | |||
|---|---|---|---|---|
| Control | Soy | CCl4 | CCl4 + Soy | |
| GPX (U/g tissue) | 2512.00 ± 53.59a | 2939.78 ± 521.99b | 949.10 ± 29.39c | 2390.68 ± 561.57d |
| SOD (U/g tissue) | 1289.63 ± 92.3a | 4102.94 ± 191.76b | 834.44 ± 48.56c | 3425.42 ± 205.44d |
| MDA (nmol/g tissue) | 48.48 ± 0.58a | 41.52 ± 5.89b | 139.01 ± 9.43c | 58.27 ± 2.22d |
Within each row, means superscript with different letters are significantly different (P ≥ 0.05).
The biochemical analyses were further confirmed by the histological examination of the liver section. The results indicated that liver tissue in the control group showed normal central vein and hepatocytes (Figure 2(a)). Animal fed soy-supplemented diet also showed normal central vein and hepatocytes (Figure 2(b)). The liver section in the animals treated with CCl4 showed an increase in fibrous tissues and inflammatory cells around the congested blood vessel; moreover, a marked hepatocellular fatty degeneration was also seen (Figure 2(c)). The hepatocytes of same group were observed with large fatty droplets and nuclear pleomorphism (Figure 2(d)). The liver of animals treated with CCl4 plus soy showed marked improvement in most of hepatocytes and prominent decrease in connective tissue (Figure 2(e)) accompanied with a marked improvement in hepatocytes and portal vein area (Figure 2(f)).
Figure 2.
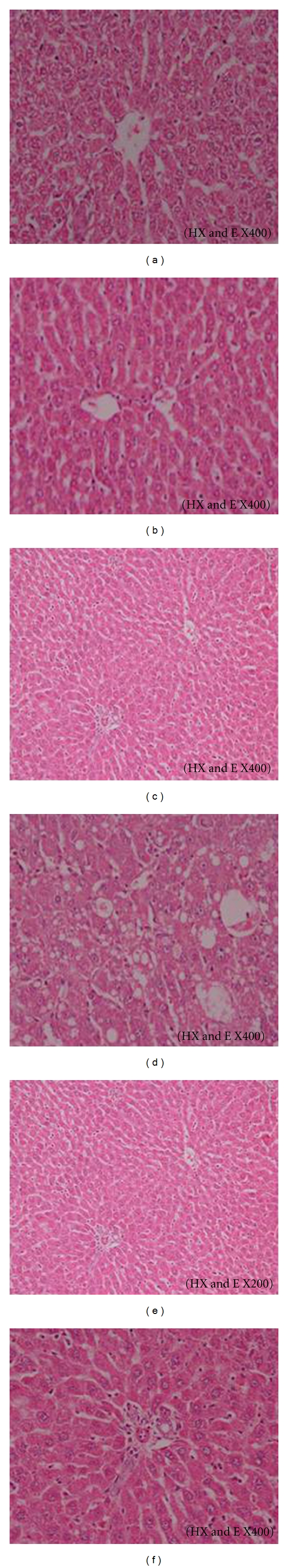
A photomicrograph in liver section of (a) control rat showing the normal central vein and the hepatocytes, (b) rat fed soy-supplemented diet showing the central vein and the hepatocytes, (c) rat treated with CCl4 showing increase in fibrous tissues and inflammatory cells around the congested blood vessel. Marked hepatocellular fatty degeneration also seen, (d) rat treated with CCl4 showing hepatocytes with large fatty droplets and nuclear pleomorphism, (e) rat treated with CCl4 and fed soy supplemented diet showing marked improvement in most of hepatocytes and prominent decrease in connective tissues, and (f) rat treated with CCl4 and fed soy supplemented diet showing marked improvement in hepatocytes and in the portal vein area.
4. Discussion
The current study revealed that soy is enriched in protein and isoflavones which were found at high concentrations. Similar to these results, Simmen et al. [35] reported that soy contained high protein content. Moreover, King and Bignell [36], Hou et al. [37], and Duke et al. [20] reported that soy contained a high content of isoflavones as determined by HPLC. The isoflavones genistin and daidzin constitute 99% of the total isoflavones [38].
In the current study we evaluated the protective role of soy against CCl4-induced liver damage in rats. The selected doses of CCl4 and soy were literature based [39, 40]. It is well known that CCl4 is one of the most extensively studied hepatotoxins. The mechanism by which CCl4 causes hepatotoxicity is well documented in a series of reports. The hepatotoxicity of CCl4 undergoes two phases, the first results from its metabolic conversion to free radical product CCl3− by Cyt P-450 [41]. Once CCl3− has been formed it reacts very rapidly with O2 to produce CCl3OO−, a much more reactive radical than CCl3− [42]. These free radicals attack microsomal lipids leading to its peroxidation and also covalently bind to microsomal lipids and proteins. This results in the generation of reactive oxygen species (ROS), which includes the superoxide anion O2, H2O2 and the hydroxyl radical.
In the current study, CCl4 caused significant changes in serum biochemical parameters typical to those reported in the literature [43, 44]. The liver is considered to be the principal target organ for CCl4 and the activity of transaminases are sensitive indicators of acute hepatic necrosis [45, 46]. The elevated levels of ALT, AST, triglycerides, and cholesterol reported herein indicated severe hepatic parenchymal cells injury [47–49], whereas the decrease in total protein and albumin indicated liver necrosis and/or kidney dysfunction [48]. MDA, an end product of lipid peroxidation, is widely used as a marker of lipid peroxidation. It is well documented that CCl4 enhanced lipid peroxidation [44, 48, 50] that is an indication of free radical mediated toxicity.
Free radicals are known to attack the highly unsaturated fatty acids of the cell membrane and induce lipid peroxidation that is considered a key process in many pathological events induced by oxidative stress [51]. In the present study, MDA was found to be significantly higher in the animals treated with CCl4 suggesting a significant effect on lipid peroxidation and supporting the earlier findings [52, 53].
Alteration in the hepatic antioxidant status may be a manifestation of oxidative stress caused by CCl4 and their metabolites. Both GPX and SOD are considered enzymatic free radical scavengers in cells. In the present study, GPX and SOD were found to decline significantly in rats treated with CCl4. It is well known that SOD plays an important role in the elimination of ROS derived from the peroxidative process in liver tissues [50]. Moreover, SOD removes superoxide by converting it to H2O2, which can be rapidly converted to water by CAT [54]. Taken together, the increased level of MDA and the decreased activity of antioxidant enzymes GPX and SOD may be attributed to free radical formation which initiates chain reactions of direct and indirect bond formation with cellular molecules (nucleic acids, proteins, lipids, and carbohydrates) impairing crucial cellular processes that may ultimately culminate.
In the current study, the elevated serum level of AFP in the animals treated with CCl4 indicated that it is a potent hepatocarcinogen, enhances reactive oxygen species (ROS) formation, and causes oxidative DNA damage, which may play a role in its carcinogenicity [44, 48, 55, 56]. Therefore, the current study affirmed that CCl4 can induce hepatotoxicity and degeneration in liver cells in rats as indicated by the elevation of AFP level in serum. Similarly, Engelhardt et al. [57] reported that AFP was much higher after the exposure to CCl4 for 3-4 days.
The biochemical study was further confirmed by the histopathological examination of the liver tissue. The liver section in CCl4-treated group showed an increase in fibrous tissues and inflammatory cells around the congested blood vessel and a marked hepatocellular fatty degeneration. The hepatocytes of same group were observed with large fatty droplets and nuclear pleomorphism. These histological changes were similar to those reported earlier [44, 58]. Animals fed soy-supplemented diet showed a significant improvement in all the biochemical parameters tested and the histological picture of the liver. Possible explanation for the differential effects of soy on the activities of AST and ALT in plasma is that isoflavones and soy protein may inhibit the liver damage induced by CCl4.
On the other hand, supplementation of the diet with soy protein significantly lowered plasma cholesterol and triglycerides levels caused by CCl4. It is indicated recently that soy protein intake decreased the hepatic lipid depots of triacylglycerols and cholesterol and decreased the concentrations of lipid peroxides [59]. The mechanism by which soy protein decreases serum lipids is not yet fully established. A possible mechanism of this decrease is the enhancement of bile acid excretion, by which soy protein acts as dietary fiber to promote bile acid excretion and increase the rate of cholesterol resynthesis in the liver [60].
Previous report has shown that soy protein may improve hyperlipidemia due to its effects on the transcription factors, called sterol regulatory element binding proteins, which are important in the regulation of enzymes involved in lipid metabolism in vivo [61]. Moreover, Tovar et al. [62] found that soy protein lowered sterol regulatory element binding protein-1 expression in adipocytes, which also helped prevent the development of hepatic lipotoxicity.
AFP was higher in animals treated with CCl4. This is likely related to the progression of these agents in liver cirrhosis [63]. Soy ingestion significantly lowered AFP levels in the serum of rats. One of the mechanisms for lowering AFP level may have been the anti-inflammatory properties of soy. Previous studies have indicated that the isoflavones in soy may regulate the inflammatory response and immune function [64, 65]. Moreover, genistein and secoisolariciresinol have anticancer properties on MCF-7 and BT20 in vitro [66] and have been suggested as the most potent inhibitors of cancer cell growth [67].
In the current study, soy supplementation resulted in a significant decrease in lipid peroxidation measured as MDA concentration in liver. This decrease was accompanied with a significant increase in GPX and SOD activity. Moreover, diet supplemented with soy succeeded to induce a significant improvement in the oxidative stress markers and antioxidant status of the liver tissue in rats treated with CCl4. These results suggested that soy protein may prevent oxidative damage in the liver by lowering plasma-free fatty acids and decreasing CYP2E1 expression. Similar observations were reported recently by Yang et al. [59]. The histopathological study of liver confirmed these findings. The liver section of the animals treated with CCl4 and fed soy-supplemented diet showed marked improvement in hepatocytes and in the portal vein area. According to Lila and Raskin [68], the antioxidative effects of several plant compounds have a potentiating effect of other compounds found in plants. A mixture of compounds rather than a single compound may be the key to full antioxidant potency. In this concern, soy contains other bioactive phytochemicals in addition to isoflavones [69]. Consequently, the antioxidative effects of soy reported in the current study may be due to several compounds present in soy.
5. Conclusion
The current study revealed that CCl4 induced severe hepatotoxicity typical to those reported in the literature. Supplementation with soy succeeded to protect the liver from the toxic effects and the oxidative stress of CCl4. These effects may be due to the antioxidant activity, the anticancer effect, and the enhancement of immune response of soy components since it increased the antioxidant capacity of the body and reduced the oxidative stress and tumor markers. The current results concluded that soy contained a combination of active compounds that may be more efficacious and safer as chemopreventive agents than individual compounds.
Conflict of Interests
The authors declare that there is no conflict of interests.
References
- 1.El-Serag HB. Hepatocellular carcinoma: an epidemiologic view. Journal of Clinical Gastroenterology. 2002;35(5, supplement 2):S72–S78. doi: 10.1097/00004836-200211002-00002. [DOI] [PubMed] [Google Scholar]
- 2.El-Zayadi AR, Badran HM, Barakat EMF, et al. Hepatocellular carcinoma in Egypt: a single center study over a decade. World Journal of Gastroenterology. 2005;11(33):5193–5198. doi: 10.3748/wjg.v11.i33.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Zayadi AR, Abaza H, Shawky S, Mohamed MK, Selim OE, Badran HM. Prevalence and epidemiological features of hepatocellular carcinoma in Egypt—a single center experience. Hepatology Research. 2001;19(2):170–179. doi: 10.1016/s1386-6346(00)00105-4. [DOI] [PubMed] [Google Scholar]
- 4.London WT. Liver Cancer: Etiology and Prevention. 2nd edition. Philadelphia, Pa, USA: Fox Chase Cancer Center; 2003. Encyclopedia of Cancer. [Google Scholar]
- 5.Kew MC. Liver cancer. In: Heggenhougen HK, Quah SR, editors. International Encyclopedia of Public Health. Vol. 4. San Diego, Calif, USA: Acadeomic Press; 2008. pp. 105–114. [Google Scholar]
- 6.Okuda H. Hepatocellular carcinoma development in cirrhosis. Best Practice and Research. 2007;21(1):161–173. doi: 10.1016/j.bpg.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Zhao L, Brinton RD. WHI and WHIMS follow-up and human studies of soy isoflavones on cognition. Expert Review of Neurotherapeutics. 2007;7(11):1549–1564. doi: 10.1586/14737175.7.11.1549. [DOI] [PubMed] [Google Scholar]
- 8.Hammerschmidt PA, Pratt DE. Phenolic antioxidants of dried soybeans. Journal of Food Science. 1978;43(2):556–559. [Google Scholar]
- 9.Kerry N, Abbey M. The isoflavone genistein inhibits copper and peroxyl radical mediated low density lipoprotein oxidation in vitro. Atherosclerosis. 1998;140(2):341–347. doi: 10.1016/s0021-9150(98)00138-5. [DOI] [PubMed] [Google Scholar]
- 10.Uda Y, Price KR, Williamson G, Rhodes MJC. Induction of the anticarcinogenic marker enzyme, quinone reductase, in murine hepatoma cells in vitro by flavonoids. Cancer Letters. 1997;120(2):213–216. doi: 10.1016/s0304-3835(97)00311-x. [DOI] [PubMed] [Google Scholar]
- 11.Prochaska HJ, Santamaria AB, Talalay P. Rapid detection of inducers of enzymes that protect against carcinogens. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(6):2394–2398. doi: 10.1073/pnas.89.6.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ricketts ML, Moore DD, Banz WJ, Mezei O, Shay NF. Molecular mechanisms of action of the soy isoflavones includes activation of promiscuous nuclear receptors. A review. Journal of Nutritional Biochemistry. 2005;16(6):321–330. doi: 10.1016/j.jnutbio.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Moon YJ, Wang X, Morris ME. Dietary flavonoids: effects on xenobiotic and carcinogen metabolism. Toxicology in Vitro. 2006;20(2):187–210. doi: 10.1016/j.tiv.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Schuler MA, Berenbaum MR. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annual Review of Entomology. 2007;52:231–253. doi: 10.1146/annurev.ento.51.110104.151104. [DOI] [PubMed] [Google Scholar]
- 15.Singhal R, Shankar K, Badger TM, Ronis MJ. Estrogenic status modulates aryl hydrocarbon receptor—mediated hepatic gene expression and carcinogenicity. Carcinogenesis. 2008;29(2):227–236. doi: 10.1093/carcin/bgm288. [DOI] [PubMed] [Google Scholar]
- 16.Appelt LC, Reicks MM. Soy feeding induces phase II enzymes in rat tissues. Nutrition and Cancer. 1997;28(3):270–275. doi: 10.1080/01635589709514587. [DOI] [PubMed] [Google Scholar]
- 17.Yannai S, Day AJ, Williamson G, Rhodes MJC. Characterization of flavonoids as monofunctional or bifunctional inducers of quinone reductase in murine hepatoma cell lines. Food and Chemical Toxicology. 1998;36(8):623–630. doi: 10.1016/s0278-6915(98)00022-2. [DOI] [PubMed] [Google Scholar]
- 18.Bolling BW, Parkin KL. Phenolic derivatives from soy flour ethanol extract are potent in Vitro quinone reductase (QR) inducing agents. Journal of Agricultural and Food Chemistry. 2008;56(22):10473–10480. doi: 10.1021/jf801541t. [DOI] [PubMed] [Google Scholar]
- 19.AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists International. 17th edition. Gaithersbuy, Md, USA: 2000. [Google Scholar]
- 20.Duke SO, Rimando AM, Pace PF, Reddy KN, Smeda RJ. Isoflavone, glyphosate, and aminomethylphosphonic acid levels in seeds of glyphosate-treated, glyphosate-resistant soybean. Journal of Agricultural and Food Chemistry. 2003;51(1):340–344. doi: 10.1021/jf025908i. [DOI] [PubMed] [Google Scholar]
- 21.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. American Journal of Clinical Pathology. 1957;28(1):56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 22.Gornal AC, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. Journal of Biological Chemistry. 1949;177:751–761. [PubMed] [Google Scholar]
- 23.Doumas BT, Watson WA, Biggs HG. Albumin standards and the measurement of serum albumin with bromcresol green. Clinica Chimica Acta. 1971;31(1):87–96. doi: 10.1016/0009-8981(71)90365-2. [DOI] [PubMed] [Google Scholar]
- 24.Richmond W. Preparation and properties of a cholesterol oxidase from Nocardia sp. and its application to the enzymatic assay of total cholesterol in serum. Clinical Chemistry. 1973;19(12):1350–1356. [PubMed] [Google Scholar]
- 25.Fassati P, Prencipe L. Triglycerides Enzymetic colorimetric method. Clinical Chemistry. 1982;28:2077–2081. [PubMed] [Google Scholar]
- 26.Ruoslahti E, Pihko H, Seppala M. Alpha fetoprotein: immunochemical purification and chemical properties. Expression in normal state and in malignant and non malignant liver disease. Transplantation Review. 1974;20(1):38–60. doi: 10.1111/j.1600-065x.1974.tb00140.x. [DOI] [PubMed] [Google Scholar]
- 27.Lin CC, Hsu YF, Lin TC, Hsu FL, Hsu HY. Antioxidant and hepatoprotective activity of punicalagin and punicalin on carbon tetrachloride-induced liver damage in rats. Journal of Pharmacy and Pharmacology. 1998;50(7):789–794. doi: 10.1111/j.2042-7158.1998.tb07141.x. [DOI] [PubMed] [Google Scholar]
- 28.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods in Enzymology. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 29.Ruiz-Larrea MB, Leal AM, Liza M, Lacort M, de Groot H. Antioxidant effects of estradiol and 2-hydroxyestradiol on iron-induced lipid peroxidation of rat liver microsomes. Steroids. 1994;59(6):383–388. doi: 10.1016/0039-128x(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 30.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. The Journal of Laboratory and Clinical Medicine. 1967;70(1):158–169. [PubMed] [Google Scholar]
- 31.Suttle NF. Copper deficiency in ruminants; recent developments. Veterinary Record. 1986;119(21):519–522. doi: 10.1136/vr.119.21.519. [DOI] [PubMed] [Google Scholar]
- 32.Drury AR, Wallington EA. Carleton's Histological Techniques. 5th edition. London, UK: Oxford University Press; 1980. [Google Scholar]
- 33.SAS Institute, Inc. SAS User’s Guide: Statistics. Cary, NC, USA: SAS Institute; 1982. [Google Scholar]
- 34.Waller RA, Duncan DB. A Bayes rule for the symmetric multiple comparison problems. Journal of the American Statistical Association. 1969;64:1484–1503. [Google Scholar]
- 35.Simmen FA, Mercado CP, Zavacki AM, et al. Soy protein diet alters expression of hepatic genes regulating fatty acid and thyroid hormone metabolism in the male rat. Journal of Nutritional Biochemistry. 2010;21(11):1106–1113. doi: 10.1016/j.jnutbio.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 36.King RA, Bignell CM. Concentration of isoflavone phytoestrogens and their glucosides in Australian soya beans and soya foods. Australian Journal of Nutrition. 2000;57(2):70–78. [Google Scholar]
- 37.Hou WC, Chen HJ, Lin YH. Antioxidant peptides with angiotensin converting enzyme inhibitory activities and applications for angiotensin converting enzyme purification. Journal of Agricultural and Food Chemistry. 2003;51(6):1706–1709. doi: 10.1021/jf0260242. [DOI] [PubMed] [Google Scholar]
- 38.Fukutake M, Takahashi M, Ishida K, Kawamura H, Sugimura T, Wakabayashi K. Quantification of genistein and genistin in soybeans and soybean products. Food and Chemical Toxicology. 1996;34(5):457–461. doi: 10.1016/0278-6915(96)87355-8. [DOI] [PubMed] [Google Scholar]
- 39.Singab AN, Youssef DT, Noaman E, Kotb S. Hepatoprotective effect of flavonol glycosides rich fraction from Egyptian Vicia calcarata desf. against CCl4-induced liver damage in rats. Archives of Pharmacal Research. 2005;28(7):791–798. doi: 10.1007/BF02977344. [DOI] [PubMed] [Google Scholar]
- 40.DiSilvestro RA, Mattern C, Wood N, Devor ST. Soy protein intake by active young adult men raises plasma antioxidant capacity without altering plasma testosterone. Nutrition Research. 2006;26(2):92–95. [Google Scholar]
- 41.Noguchi T, Fong KL, Lai EK, et al. Specificity of a phenobarbital-induced cytochrome P-450 for metabolism of carbon tetrachloride of the trichloromethyl radical. Biochemical Pharmacology. 1982;31(5):615–624. doi: 10.1016/0006-2952(82)90440-3. [DOI] [PubMed] [Google Scholar]
- 42.Packer JE, Slater TF, Willson RL. Reactions of the carbon tetrachloride-related peroxy free radical (CCl3O2.) with amino acids: pulse radiolysis evidence. Life Sciences. 1978;23(26):2617–2620. doi: 10.1016/0024-3205(78)90378-8. [DOI] [PubMed] [Google Scholar]
- 43.Chandan B, Saxena A, Shukla S, et al. Hepatoprotective potential of aloe barbadensis Mill. against carbon tetrachloride induced hepatotoxicity. Journal of Ethnopharmacology. 2007;111(3):560–566. doi: 10.1016/j.jep.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 44.El Denshary ES, Al-Gahazali MA, Mannaa FA, et al. Dietary honey and ginseng protect against carbon tetrachloride-induced hepatonephrotoxicity in rats. doi: 10.1016/j.etp.2011.01.012. Experimental and Toxicologic Pathology. In press. [DOI] [PubMed] [Google Scholar]
- 45.Kaplan MM. Primary biliary cirrhosis. The New England Journal of Medicine. 1987;316(9):521–528. doi: 10.1056/NEJM198702263160907. [DOI] [PubMed] [Google Scholar]
- 46.Abdel-Wahhab MA, Aly SE. Antioxidant property of Nigella sativa (black cumin) and Syzygium aromaticum (clove) in rats during aflatoxicosis. Journal of Applied Toxicology. 2005;25(3):218–223. doi: 10.1002/jat.1057. [DOI] [PubMed] [Google Scholar]
- 47.Abdel-Wahhab MA, Nada SA, Amra HA. Effect of aluminosilicates and bentonite on aflatoxin-induced developmental toxicity in rat. Journal of Applied Toxicology. 1999;19(3):199–204. doi: 10.1002/(sici)1099-1263(199905/06)19:3<199::aid-jat558>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 48.Abdel-Wahhab MA, Abdel-Galil MM, Hassan AM, et al. Zizyphus spina-christi extract protects against aflatoxin B1-intitiated hepatic carcinogenicity. The African Journal of Traditional. 2007;4(3):248–256. [PMC free article] [PubMed] [Google Scholar]
- 49.Barton CC, Barton EX, Ganey PE, Kunkel SL, Roth RA. Bacterial lipopolysaccharide enhances aflatoxin B1 hepatotoxicity in rats by a mechanism that depends on tumor necrosis factor α. Hepatology. 2001;33(1):66–73. doi: 10.1053/jhep.2001.20643. [DOI] [PubMed] [Google Scholar]
- 50.Abdel-Wahhab MA, Ahmed HH, Hagazi MM. Prevention of aflatoxin B1-initiated hepatotoxicity in rat by marine algae extracts. Journal of Applied Toxicology. 2006;26(3):229–238. doi: 10.1002/jat.1127. [DOI] [PubMed] [Google Scholar]
- 51.Schinella GR, Tournier HA, Prieto JM, Mordujovich DBP, Ríos JL. Antioxidant activity of anti-inflammatory plant extracts. Life Sciences. 2002;18(70):1023–1033. doi: 10.1016/s0024-3205(01)01482-5. [DOI] [PubMed] [Google Scholar]
- 52.Mansour MA. Protective effects of thymoquinone and desferrioxamine against hepatotoxicity of carbon tetrachloride in mice. Life Sciences. 2000;66(26):2583–2591. doi: 10.1016/s0024-3205(00)00592-0. [DOI] [PubMed] [Google Scholar]
- 53.Bhattacharjee R, Sil PC. Protein isolate from the herb, Phyllanthus niruri L. (Euphorbiaceae), plays hepatoprotective role against carbon tetrachloride induced liver damage via its antioxidant properties. Food and Chemical Toxicology. 2007;45(5):817–826. doi: 10.1016/j.fct.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 54.Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? The Lancet. 1994;344(8924):721–724. doi: 10.1016/s0140-6736(94)92211-x. [DOI] [PubMed] [Google Scholar]
- 55.Yang M, Chen K, Shih JC. Yang-Gan-Wan protects mice against experimental liver damage. American Journal of Chinese Medicine. 2000;28(2):155–162. doi: 10.1142/S0192415X00000209. [DOI] [PubMed] [Google Scholar]
- 56.Aziz TA, Aziz MA, Fouad HH, et al. Interferon-alpha gene therapy prevents aflatoxin and carbon tetrachloride promoted hepatic carcinogenesis in rats. International Journal of Molecular Medicine. 2005;15(1):21–26. [PubMed] [Google Scholar]
- 57.Engelhardt NV, Lazareva MN, Abelev GI, et al. Detection of α-fetoprotein in mouse liver differentiated hepatocytes before their progression through S phase. Nature. 1976;263:146–148. doi: 10.1038/263146a0. [DOI] [PubMed] [Google Scholar]
- 58.Song JY, Li L, Ahn JB, et al. Acute liver toxicity by carbon tetrachloride in HSP70 knock out mice. Experimental and Toxicologic Pathology. 2007;59(1):29–34. doi: 10.1016/j.etp.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 59.Yang HY, Tzeng YH, Chai CY, et al. Soy protein retards the progression of non-alcoholic steatohepatitis via improvement of insulin resistance and steatosis. Nutrition. 2011;27(9):943–948. doi: 10.1016/j.nut.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 60.Bakhit RM, Klein BP, Essex-Sorlie D, Ham JO, Erdman JW, Potter SM. Intake of 25 g of soybean protein with or without soybean fiber alters plasma lipids in men with elevated cholesterol concentrations. Journal of Nutrition. 1994;124(2):213–222. doi: 10.1093/jn/124.2.213. [DOI] [PubMed] [Google Scholar]
- 61.Ascencio C, Torres N, Isoard-Acosta F, Gómez-Pérez FJ, Hernández-Pando R, Tovar AR. Soy protein affects serum insulin and hepatic SREBP-1 mRNA and reduces fatty liver in rats. Journal of Nutrition. 2004;134(3):522–529. doi: 10.1093/jn/134.3.522. [DOI] [PubMed] [Google Scholar]
- 62.Tovar AR, Torre-Villalvazo I, Ochoa M, et al. Soy protein reduces hepatic lipotoxicity in hyperinsulinemic obese Zucker fa/fa rats. Journal of Lipid Research. 2005;46(9):1823–1832. doi: 10.1194/jlr.M500067-JLR200. [DOI] [PubMed] [Google Scholar]
- 63.Diehl AM. Nonalcoholic steatohepatitis. Seminars in Liver Disease. 1999;19(2):221–230. doi: 10.1055/s-2007-1007111. [DOI] [PubMed] [Google Scholar]
- 64.Akiyama T, Ishida J, Nakagawa S, et al. Genistein, a specific inhibitor of tyrosine-specific protein kinases. Journal of Biological Chemistry. 1987;262(12):5592–5595. [PubMed] [Google Scholar]
- 65.Chan YC, Wu CC, Chan KC, et al. Nanonized black soybean enhances immune response in senescence-accelerated mice. International Journal of Nanomedicine. 2009;4(1):27–35. doi: 10.2147/ijn.s4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Theil C, Briese V, Gerber B, Richter DU. The effects of different lignans and isoflavones, tested as aglycones and glycosides, on hormone receptor-positive and -negative breast carcinoma cells in vitro. Archives of Gynecology and Obstetrics. 2010;284(2):459–465. doi: 10.1007/s00404-010-1661-4. [DOI] [PubMed] [Google Scholar]
- 67.Onozawa M, Fukuda K, Ohtani M, Akaza H, Sugimura T, Wakabayashi K. Effects of soybean isoflavones on cell growth and apoptosis of the human prostatic cancer cell line LNCaP. Japanese Journal of Clinical Oncology. 1998;28(6):360–363. doi: 10.1093/jjco/28.6.360. [DOI] [PubMed] [Google Scholar]
- 68.Lila MA, Raskin I. Health-related interactions of phytochemicals. Journal of Food Science. 2005;70(1):R20–R27. [Google Scholar]
- 69.Hosny M, Rosazza JPN. Isolation and characterization of novel flavonoid and triterpene glycosides from Novasoy. Jornal of Natural Products. 2002;65:805–813. doi: 10.1021/np010606g. [DOI] [PubMed] [Google Scholar]


