Abstract
We examined the independent association between moderate-to-vigorous physical activity (MVPA) and insulin resistance (IR) among obese Latino children (N = 113; 7–15 years) who were enrolled in a community-based obesity intervention. Baseline information on physical activity was gathered by self-report. Clinical assessments of body composition, resting energy expenditure (REE), as well as glucose and insulin responses to an oral glucose tolerance test (OGTT) were performed after an overnight fast. Insulin resistance was defined as a 2 h insulin concentration >57 μU·mL−1. We observed that those obese children who met the 2008 Guidelines for MVPA (≥60 min/day) experienced a significantly lower odds of IR compared with those not meeting the Guidelines (OR = 0.29; 95% CI: (0.10–0.92)) and these findings were independent of age, sex, pubertal stage, acculturation, fasting insulin, and 2 h glucose concentrations. Efforts to promote 60 min or more of daily MVPA among children from ethnic minority and high-risk communities should assume primary public health importance.
1. Introduction
Childhood obesity has reached epidemic proportions in the United States, with 32 percent of children and adolescents defined as either overweight or obese [1]. Among Latino American children and adolescents, the prevalence and complications of obesity are even higher compared with children of other racial and ethnic identity [1]. This issue has substantial public health relevance as Hispanics are the largest and fastest growing ethnic minority in the United States, and data suggest that 85% of overweight youth will remain overweight as adults [2]. The reason behind this observed racial/ethnic disparity in obesity is not clear. Potential explanations include: (1) biological differences in the susceptibility or resistance to weight gain; (2) differences in metabolic/mechanical efficiency; (3) sociocultural differences in lifestyle factors (such as dietary composition or participation in physical activities); or (4) environmental factors such as access to preventive or treatment programs [3–6].
Obesity is commonly accompanied by insulin resistance, which precedes and plays a major role in the etiology of the metabolic syndrome, type 2 diabetes mellitus and subsequent cardiovascular disease [7–11]. Among US adults and adolescents, Latinos have the highest prevalence of the metabolic syndrome [12], and more recent reports indicate an increasing incidence of type 2 diabetes among obese Latino American, African American, and Native American adolescents [13].
Sedentary behavior is associated with both the risk of obesity and of insulin resistance [14–16], and there are data to suggest that the prevalence of sedentary behaviors is higher among children from less-represented minority groups [3, 4, 17–19]. In an effort to reduce physical inactivity in children, the United States Public Health Service (USPHS) 2008 Physical Activity Guidelines for Americans recommends a minimum of 60 min·day−1 of moderate-to-vigorous physical activity (MVPA: e.g., bicycle riding, skate boarding, soccer, jump roping). Unfortunately, the majority of children and adolescents do not meet these recommendations [20]. Furthermore, there are now data in adults confirming the distinct contributions of physical activity and sedentary behavior to the development of obesity and metabolic impairments [21]; whether this remains so among children, however, is not clear.
Few studies have examined the associations among physical activity, sedentary behavior, body composition, and metabolic markers in defined samples of Latino children. Moreover, determining the interrelatedness of these factors is complicated by the fact that obesity lies in the biologic pathway between MVPA or sedentary behavior and metabolic risk, thereby minimizing the true magnitude of contribution from these lifestyle behaviors. We studied cross-sectionally a sample of obese Latino children in order to determine the independent contributions of MVPA and sedentary behaviors on the odds of insulin resistance (IR) over and above that of obesity. We hypothesized that obese children reporting more MVPA will have a lower odds of IR compared with their less active counterparts; however, the magnitude of this association would be mediated by obesity and other risk factors in the biological pathway between obesity and insulin resistance (e.g., sedentary behavior, glucose, and fasting insulin concentrations).
2. Methods
2.1. Subjects
Participants were 113 Latino youth (58 boys and 55 girls), ages of 7–15 years enrolled in a comprehensive community-based obesity intervention program. Participants were recruited through advertisements placed at community facilities such as clinics, schools, and churches in Washington, DC, USA. To avoid selection biases from large families, as well as correlated reporting biases within families, only one child from each family was permitted to participate in the study. Eligibility requirements included Latino ethnicity (as determined by the parent's identifying themselves, their spouses, and both sets of grandparents within the Hispanic or Latino cultural group), a body mass index (BMI) ≥ 95th percentile for age and sex, and otherwise good health. Children who were chronically ill and/or with known medical conditions such as type 2 diabetes, pervasive development delay, cerebral palsy, severe asthma, Cushing syndrome, obesity-associated genetic syndrome (e.g., Prader-Willi syndrome), or untreated hypothyroidism were excluded from study. All study procedures were approved by the Institutional Review Board of Children's National Medical Center (CNMC), and parents provided informed written consent, while the children provided signed assent prior to their participation. All data were collected at one baseline visit prior to participation in the comprehensive treatment program. On the day of testing, participants were admitted to the General Clinical Research Center (GCRC) at CNMC at 07:00 following a 12 hr overnight fast.
2.2. Anthropometry
Body weight was measured on a digital scale (Healthometer, Bridgeview, IL, USA) with the participant in underwear and a hospital gown, while height was measured using a wall-mounted stadiometer (SECA 216, Hanover, MD, USA). The body mass index (BMI) was then calculated as weight (kg)·height (m)−2. Waist circumference was measured in triplicate at the umbilicus using a non-elastic tape measure to the nearest 0.1 centimeter. Total body fat mass (kg; %) and fat-free mass (kg) were determined by air displacement plethysmography (Life Measurement Inc., Concord, CA, USA) according to methods previously described [22]. Pubertal stage was determined by a pediatrician separately for boys and girls, and children were classified as prepubertal (Tanner stage 1); mid prepubertal (Tanner stage 2); or late prepubertal (Tanner stage 3).
2.3. Indirect Calorimetry
Resting energy expenditure (REE) was measured using an open-circuit indirect computerized calorimeter equipped with a mask (Sensor Medics, Yorba Linda, CA, and Ultima CardiO2 system, Medical Graphics Corporation, St. Paul, MN, USA). All calibration procedures were performed to manufacturer specifications prior to testing each morning, including manual flow calibrations using a 3 L syringe and gas calibration with gases of known concentration. The REE measurement was performed in a quiet, thermoneutral room. All measurements were done after a 20-minute rest period. Participants were measured at rest in a supine position for a total of 30 minutes including a 5-minute acclimation period. Data collected during the initial five minutes of testing were excluded from the total 30-minute test period to allow for an adjustment period to the test conditions. Measurements were recorded at 1 minute intervals and then averaged over the remainder of the test period to obtain a measure of REE that was adjusted for body weight (kg). The respiratory exchange ratio (RER = vCO2/vO2) was also determined at 1-min intervals and averaged over the test period. The RER under fasting conditions provides an indirect measure of metabolic flexibility. Values of the RER closer to 0.70 indicate a greater reliance on fat (relative to glucose) oxidation, while values closer to 1.00 indicate greater glucose oxidation.
2.4. Oral Glucose Tolerance Test
A 2-hour oral glucose tolerance test was performed using the protocol described by the World Health Organization [23]. Fasting blood samples were collected from an antecubital vein for the determination of basal glucose, insulin, and free fatty acids (FFAs) concentrations. Participants were then given oral glucose drink (1.75 gm·kg−1 (body weight) of glucose with a maximum of 75 gm) to consume within 2-3 min. At 120 min after challenge, the blood sampling was repeated. Blood samples were placed in prechilled tubes and then were centrifuged at 4°C. Plasma was stored at −70°C until analyzed in the Core Laboratory of CNMC. Serum insulin concentrations were determined by a solid-phase, 2-site chemiluminescent immunometric assay (Immulite 2000 Analyzer, Diagnostic Products, Los Angeles, CA, USA). Plasma glucose levels were determined using the hexokinase-glucose-6-phosphate dehydrogenase method (Dade Behring Inc, Deerfield, IL, USA). Plasma concentrations of FFF were determined using standard microflourimetric procedures (Sigma; St. Louis, MO, USA).
Insulin sensitivity was calculated using the whole-body insulin sensitivity index (WBISI = 10,000/√ ((fasting glucose (mg·dL−1) × fasting insulin (μU·mL−1)) × (average glucose × average insulin))), which has been validated in children and adolescents [24]. The WBISI has demonstrated excellent correlation with clamp-derived insulin sensitivity estimates in children and adolescents and with intramyocellular lipid content, a tissue marker for insulin resistance [24]. We also considered the 2 h postchallenge insulin concentration as an indicator of insulin action. Postchallenge insulin concentrations correlated strongly with the WBISI (r = −0.81; P < 0.0001) and is easier to interpret compared with the WBISI. There currently exist no evidence-based cut-points for defining insulin resistance in children. Therefore, in order to determine the odds of insulin resistance (IR) associated with physical activity in these obese children, those with a 2 hr postchallenge insulin concentration > 57 μU·mL−1 were characterized as IR; otherwise they were considered not insulin resistant. This postchallenge insulin concentration corresponds to a Homeostatic Model Assessment (HOMA-IR = (fasting glucose × 0.056) × (fasting insulin/22.1)) [25] value of >2.5, which is a clinical cut-point often used to characterize insulin resistance in children [26].
2.5. Physical Activity and Sedentary Behavior
Children completed the Activity and Sedentary Behavior Questionnaire (ASBQ), which comprised questions from the validated 2001 Youth Risk Behavior Survey and the World Health Organization Study of Health Behavior of School Children Survey [5]. The ASBQ queried both the frequency and duration of moderate-to-vigorous physical activity acquired through organized sport, informal recreational pursuits, and physical education class. In addition, the ASBQ contained questions about time per day watching television and at the computer. All questions contained categorical responses, which were scored on an ordinal scale. Two indices were then calculated by multiplying the frequency score by the duration score for these activities: (1) moderate-vigorous physical activity (MVPA) Index, with scores that ranged from 0 to 150; (2) sedentary index (SI)—which was derived from questions pertaining to TV viewing and computer time—with scores ranging from 0 to 150. Physical education (PE) volume was derived by multiplying reported frequency by duration of weekly PE class resulting in scores ranging from 0 to 300 min·wk−1. To address the aims of the 2008 Physical Activity Guidelines, the MVPA index was then dichotomized based on a volume of MVPA that did or did not meet those Guidelines for moderate-to-vigorous physical activity (every day for 60 min or more).
2.6. Acculturation
Level of acculturation is an important confounder of the associations among physical activity, obesity and diabetes risk [27]. To assess this important study variable, mothers answered questions (administered in Spanish by a fluently bilingual trained research assistant) derived from the Bicultural Involvement Questionnaire [28]. Acculturation was measured via 17 items assessing constructs of linguistic fluency and comfort level in Spanish and in English; language use at home, and with work and friends; preferences in music, books, magazines and visual media. Each of the items was scored on a scale with a maximum score of 4 for 14 items, of 3 for two items, and of 5 for one item. Individual scores were then summed over all items to create a total score. Total scale scores ranged from 17 to 67, with higher scores reflecting greater acculturation to American culture. In the current study, the acculturation scale demonstrated adequate internal consistency (α = .77).
2.7. Statistical Analyses
Descriptive statistics (mean ± SD; frequencies (%)) were generated on all study variables according to those who were and who were not IR. Between-group differences in mean levels of the study variables were tested using t-tests for independent samples, while the chi-square statistic tested associations between categorical variable frequencies and IR status. Study variables demonstrating a statistically significant association with IR status in the bivariable analysis were then entered into a multivariable logistic regression model in order to determine their independent contribution to the odds of insulin resistance. The variables age, sex, pubertal status, and acculturation were forced into this model. The regression model was then run backward, with those variables demonstrating an alpha level of 0.10 or greater eliminated from the model.
Parameter estimates, as well as adjusted odds ratios (ORs) and 95% confidence intervals (CI) are reported for the final regression model. Because our primary study variables (MVPA and sedentary behavior) were ordinal indices, the OR represents the increase (or decrease) in the odds of IR for each unit increase of the given index. The odds of IR among children meeting the 2008 MVPA Guidelines were compared with the odds of children who did not (the referent group).
3. Results
Study characteristics according to IR status are presented in Table 1. As indicated, subjects with and without IR were similar with regard to age, BMI, fat mass, and percent body fat and Tanner score. Girls tended to have a slightly higher prevalence of IR (0.56) compared with boys (0.44) (P = 0.13), and those with IR also tended to have a higher Z-score for waist circumference (0.33). Fasting RER was comparable between the groups; however, it is worth noting that it was high in both groups, suggesting some degree of metabolic inflexibility among these obese children. Weight-adjusted REE was significantly lower (P < 0.01) and acculturation score was significantly higher (P < 0.05) in those children with IR, compared with those without IR.
Table 1.
Subject characteristics.
| Insulin sensitive (n = 53) | Insulin resistant* (n = 61) | |
|---|---|---|
| Age (y) | 11 ± 2 | 12 ± 2 |
| Sex (% male) | 58 | 44 |
| BMI (kg/m2) | 29.3 ± 5.45 | 31.0 ± 4.9 |
| Fat mass (kg) | 30.0 ± 11.6 | 32.2 ± 10.0 |
| Body fat (%) | 42.2 ± 5.7 | 43.3 ± 5.5 |
| Waist circ. (Z score) | 1.46 ± 0.65 | 1.58 ± 0.64 |
| Tanner score | 2 | 2 |
| REE (kcal/kg) | 35.7 ± 16.4 | 29.2 ± 11.7a |
| Fasting RER | 0.86 ± 0.07 | 0.88 ± 0.07 |
| Acculturation score | 32 ± 7 | 35 ± 7b |
*Based on a 2 h postchallenge insulin concentration greater than 64 μU/mL (median value); aP < 0.01; bP < 0.05. BMI: body mass index; body fat determined by DXA; A Tanner score of 2: mid prepubertal; REE: resting energy expenditure; RER: respiratory exchange ratio.
Fasting glucose and FFA concentrations were no different between study groups; however, fasting insulin and 2 h glucose levels were significantly greater (P < 0.01) in the children with IR compared with those without it (Table 2). As expected, insulin sensitivity, based on the WBISI, was markedly lower (P < 0.001) and 2 h insulin concentrations substantially higher (P < 0.001) in those children who were defined as IR compared with those who were not. Of note is the observation that both groups of children were able to maintain normal glucose tolerance based on a fasting glucose concentration criterion of <100 mg·dL−1.
Table 2.
Metabolic response characteristics to the OGTT.
| Insulin sensitive (n = 53) | Insulin resistant (n = 61) | |
|---|---|---|
| Fasting glucose (mg·dL−1) | 83.5 ± 5.8 | 85.7 ± 12.1 |
| 2 h glucose (mg·dL−1) | 102.1 ± 19.7 | 118.4 ± 30.2* |
| Fasting insulin (μU·mL−1) | 12.0 ± 8.9 | 21.5 ± 13.5** |
| Fasting FFA (μmol·L−1) | 0.72 ± 0.28 | 0.67 ± 0.28 |
| WBISI | 10.6 ± 8.2 | 3.7 ± 2.2** |
| 2 h insulin (μU·mL−1) | 29.8 ± 16.1 | 133.4 ± 83.8** |
OGTT: oral glucose tolerance test; *P < 0.01; **P < 0.001; WBISI: whole-body insulin sensitivity index.
Reported sedentary behavior was similar between groups (SI = 10.5 ± 6.2 versus 10.9 ± 6.5 for those with and without IR, resp.; P = 0.75; Figure 1). In contrast, the reported MVPA index was significantly lower among those characterized as insulin resistant compared with who were not (MVPA index = 14.8 ± 18.4 versus 24.9 ± 32.1, resp.; P < 0.05). Participation in physical education tended to be lower among those children with IR compared with those without it (PE volume = 73.7 ± 74.3 versus 80.0 ± 79.6 min·wk−1, resp.; P = 0.06). Only 18% of the participants met the USPHA guidelines for 60 min or more of MVPA on most days of the week. Those who met the guidelines had an MVPA index score of 58.6 ± 31.7, compared with a score of 8.3 ± 6.9 among those who did not (P < 0.001). We observed a strong unadjusted association between meeting those guidelines and protection from insulin resistance (OR = 0.28; P < 0.01).
Figure 1.
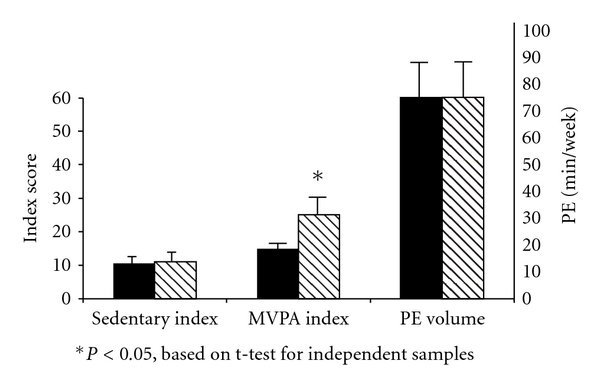
Mean (±SEM) values for the sedentary and MVPA indices and PE volume between obese children with (solid bars) and without (striped bars) insulin resistance.
Study variables that were statistically significant in the bivariable analysis were then entered in a stepwise multivariable logistic regression model to determine their independent contribution to the odds of IR. These variables were MVPA, as well as fasting insulin, 2 h glucose concentrations, REE, and acculturation score. The variables age, sex, pubertal status, and waist circumference were also included due to their reported association with IR in the literature. The MVPA index maintained marginal statistical significance in the multivariable model. Each unit increases in the MVPA index lowered the odds of IR by 3% (OR = 0.97; 95% (CI: (0.95–1.00)). We then repeated the analysis and substituted the dichotomous MVPA variable for the MVPA index. Parameter estimates from the final logistic regression model are presented in Table 3. In the presence of two very powerful correlates of insulin resistance: 2 hr postchallenge glucose (P < 0.01) and fasting insulin (P < 0.001) concentrations, MVPA maintained a significant association with IR as those obese children who reported enough MVPA to meet the 2008 Guidelines experienced a substantially lower odds of IR, compared with the children who did not ((OR = 0.29; 95% CI: (0.10–0.92))). Age, sex, puberty status, REE, and acculturation score did not remain statistically significant in the multivariable model.
Table 3.
Parameter estimates* from the final logistic regression modeling (N = 113).
| Estimate | P value | OR | 95% CI | |
|---|---|---|---|---|
| MVPA | −1.247 | 0.035 | 0.29 | (0.10–0.92) |
| Fasting insulin (μU·mL−1) | 0.086 | 0.001 | 1.09 | (1.04–1.15) |
| 2 hr glucose (mg·dL−1) | 0.037 | 0.004 | 1.04 | (1.01–1.06) |
*Estimates represent the decrease in the log odds of IR in those children meeting the 2008 Guidelines for MVPA (≥60 min/day) compared with those who did not, and the increase in the log odds of IR with each unit increase in fasting insulin or in 2 hr glucose concentrations. Model was adjusted for age, sex, pubertal stage, and acculturation.
4. Discussion
Since obesity lies along the biological pathway between MVPA and insulin resistance, it would seem as though the contribution of MVPA to IR would be diminished in the presence of obesity. Similarly, the association among the weight-adjusted REE, the RER, the waist circumference, and IR appeared blunted in these obese children. That we observed MVPA to be associated with the odds of IR (independent of fasting insulin and 2 h postchallenge glucose levels) underscores the distinct relation of muscle contraction to insulin action (via improvements in mitochondrial function, insulin signaling, and glucose transport) from that of obesity, as well as the continued importance of MVPA in mitigating the risk of metabolic impairments with obesity.
Other research has described the benefits of vigorous activity or MVPA to IR among diverse samples of children, and these reported benefits were also independent of total or central adiposity [29–31]. Importantly, these studies relied on both self-report and objective measures of physical activity. Pubertal status is often implicated as an effect modifier in the pathway toward pediatric diabetes. Previous studies consistently report that puberty is associated with about a 25–30% reduction in insulin sensitivity, with peak reduction occurring at Tanner stage 3 [32]; whether this functional impairment operates independent of accelerated fat deposition during puberty is not clear [7]. An ample body of evidence also supports the complicated relation that acculturation has with physical activity, obesity, and diabetes risk [27]. In general, a high level of acculturation is associated with greater amounts of reported physical activity and exercise; however, acculturation also increases the risk of obesity and diabetes risk, presumably through dietary changes. We did not observe an association between acculturation and MVPA, but those with IR had a significantly higher acculturation score compared with those without IR. This later association did not maintain statistical significance in the presence of fasting insulin and 2 hr glucose concentrations, however.
Adolescents watching greater than two hours of television per day engage in significantly less vigorous physical activity than those who spend less time in front of the screen [33]; however, very few studies report a strong correlation between television viewing and total physical activity, indicating that time engaging in one behavior does not necessarily displace time in the other. Moreover, the small positive correlation often reported between television viewing per se and obesity in children has been attributed to higher caloric intake with television viewing, rather than to lower energy expenditure [33, 34]. We observed that reported sedentary behavior (TV viewing and computer use time) was similar between those with and without IR in our obese pediatric sample, and similar to other studies [33–35], our measure of sedentary behavior demonstrated no association with the MVPA index (r = −0.02; P = 0.83), to BMI (r = 0.13; P = 0.16), or to body fat% (r = 0.17; P = 0.11). This assumes that sedentary behavior is not sufficiently biologically relevant to IR in the face of pediatric obesity, or that sedentary behavior was not measured accurately. Studies that used objective measures of sedentary time in children [30, 35, 36] report associations with metabolic outcomes that are distinct from those using measures of MVPA—thereby supporting the notion that sedentary time and MVPA are two separate behavioral and physiologic constructs.
Among the strengths of this study was the reliance on several clinical measures of metabolic control under both fasting and postprandial conditions. In addition, we believe the 2-h insulin response to an actual physiologic challenge (the OGTT) to be a more valid indicator of insulin resistance than a simple fasting value or the HOMA. We note, however, the limitations inherent to a cross-sectional analysis in that the temporal sequencing between physical activity, attained obesity, and insulin resistance could not be established. Indeed, there is now evidence that children with the greatest impairments in insulin sensitivity have the greatest susceptibility to weight gain over time [16, 37, 38] and the greatest resistance to weight loss interventions [39, 40]. Second, we relied on a valid and widely used self-reported measure of physical activity and sedentary behavior; yet the information bias associated with any self-reported measure is well known. We assume, however, that the tendency to over- or underreport MVPA was nondifferential between those with and without IR, since participants were not aware of their 2 hr postchallenge insulin values when answering the physical activity questions. Therefore, we are confident that no systematic reporting bias occurred which would have falsely inflated the independent contribution of MVPA to IR risk.
These findings support the notion that even in the presence of frank pediatric obesity, moderate-to-vigorous physical activity conveys important benefits to insulin sensitivity—although further longitudinal evidence is necessary to infer a causal relation with confidence. Nonetheless, home-, school-, and community-based programs for promoting MVPA through physical education, sports, and/or culturally appropriate physical activities should be of primary public health importance in order to protect the health of children at risk for obesity and insulin resistance.
Acknowledgments
The authors thank the study participants, their research assistants Fernanda Porto Carreiro, Caroline Collins, and Ana Jaramillo, and the GCRC staff. This research was supported by NIH Grants K23-RR022227 (to N. Mirza), MO1-RR-020359 awarded by the National Center for Research Resources (NCRR, Bethesda, MD, USA) to GCRC at Children's National Medical Center, and the following foundations and organizations: Consumer Health Foundation; The Jessie Ball DuPont Foundation; United Way of the National Capital Area.
References
- 1.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007-2008. JAMA. 2010;303(3):242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 2.Dietz WH, Gortmaker SL. Preventing obesity in children and adolescents. Annual Review of Public Health. 2001;22:337–353. doi: 10.1146/annurev.publhealth.22.1.337. [DOI] [PubMed] [Google Scholar]
- 3.Fontvieille AM, Dwyer J, Ravussin E. Resting metabolic rate and body composition of Pima Indian and Caucasian children. International Journal of Obesity. 1992;16(8):535–542. [PubMed] [Google Scholar]
- 4.Treuth MS, Butte NF, Wong WW. Effects of familial predisposition to obesity on energy expenditure in multiethnic prepubertal girls. American Journal of Clinical Nutrition. 2000;71(4):893–900. doi: 10.1093/ajcn/71.4.893. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Research Unit in Health and Behavioural Change. Edinburgh, UK: Department of Community Health Sciences. University of Edinburgh Medical School; 1998. Health behavior in school-aged children: a WHO cross- national survey (HBSC). Research protocol for the 1997-98 study; pp. 1–101. [Google Scholar]
- 6.Yanovski SZ. Resting metabolic rate in African-American and caucasian girls. Obesity Research. 1997;5(4):321–325. doi: 10.1002/j.1550-8528.1997.tb00558.x. [DOI] [PubMed] [Google Scholar]
- 7.Goran MI, Ball GDC, Cruz ML. Cardiovascular endocrinology 2: obesity and risk of type 2 diabetes and cardiovascular disease in children and adolescents. Journal of Clinical Endocrinology and Metabolism. 2003;88(4):1417–1427. doi: 10.1210/jc.2002-021442. [DOI] [PubMed] [Google Scholar]
- 8.Goran MI, Lane C, Toledo-Corral C, Weigensberg MJ. Persistence of pre-diabetes in overweight and obese hispanic children; Association with progressive insulin resistance, Poor β-cell function, and increasing visceral fat. Diabetes. 2008;57(11):3007–3012. doi: 10.2337/db08-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrison JA, Friedman LA, Wang P, Glueck CJ. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. Journal of Pediatrics. 2008;152(2):201–206. doi: 10.1016/j.jpeds.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Treuth MS, Butte NF, Ellis KJ, Martin LJ, Comuzzie AG. Familial resemblance of body composition in prepubertal girls and their biological parents. American Journal of Clinical Nutrition. 2001;74(4):529–533. doi: 10.1093/ajcn/74.4.529. [DOI] [PubMed] [Google Scholar]
- 11.Ventura EE, Lane CJ, Weigensberg MJ, Toledo-Corral CM, Davis JN, Goran MI. Persistence of the metabolic syndrome over 3 annual visits in overweight hispanic children: association with progressive risk for type 2 diabetes. Journal of Pediatrics. 2009;155(4):535–e1. doi: 10.1016/j.jpeds.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the Third National Health and Nutrition Examination Survey, 1988–1994. Archives of Pediatrics and Adolescent Medicine. 2003;157(8):821–827. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association. Type 2 diabetes in children and adolescents. Pediatrics. 2000;105:671–680. doi: 10.1542/peds.105.3.671. [DOI] [PubMed] [Google Scholar]
- 14.Berkey CS, Rockett HRH, Gillman MW, Colditz GA. One-year changes in activity and in inactivity among 10- to 15-year-old boys and girls: relationship to change in body mass index. Pediatrics. 2003;111(4):836–843. doi: 10.1542/peds.111.4.836. [DOI] [PubMed] [Google Scholar]
- 15.McGavock JM, Anderson TJ, Lewanczuk RZ. Sedentary lifestyle and antecedents of cardiovascular disease in young adults. American Journal of Hypertension. 2006;19(7):701–707. doi: 10.1016/j.amjhyper.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Robinson TN. Reducing children’s television viewing to prevent obesity: a randomized controlled trial. JAMA. 1999;282(16):1561–1567. doi: 10.1001/jama.282.16.1561. [DOI] [PubMed] [Google Scholar]
- 17.Butte NF, Cai G, Cole SA, et al. Metabolic and behavioral predictors of weight gain in Hispanic children: the Viva la Familia Study. American Journal of Clinical Nutrition. 2007;85(6):1478–1485. doi: 10.1093/ajcn/85.6.1478. [DOI] [PubMed] [Google Scholar]
- 18.Dugas LR, Ebersole K, Schoeller D, et al. Very low levels of energy expenditure among pre-adolescent Mexican-American girls. International Journal of Pediatric Obesity. 2008;3(2):123–126. doi: 10.1080/17477160801902248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon-Larsen P, Adair LS, Nelson MC, Popkin BM. Five-year obesity incidence in the transition period between adolescence and adulthood: the National Longitudinal Study of Adolescent Health. American Journal of Clinical Nutrition. 2004;80(3):569–575. doi: 10.1093/ajcn/80.3.569. [DOI] [PubMed] [Google Scholar]
- 20. Youth Risk Behavior Survey. National Center for Chronic Diseases Prevention and Health Promotion. Adolescent and School Health, 2001, http://www.cdc.gov/nccdphp/dash/yrbs.
- 21.Healy GN, Dunstan DW, Salmon J, et al. Breaks in sedentary time: beneficial associations with metabolic risk. Diabetes Care. 2008;31(4):661–666. doi: 10.2337/dc07-2046. [DOI] [PubMed] [Google Scholar]
- 22.Nicholson JC, McDuffie JR, Bonat SH, et al. Estimation of body fatness by air displacement plethysmography in African American and white children. Pediatric Research. 2001;50(4):467–473. doi: 10.1203/00006450-200110000-00008. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and Its complications. Geneva, Switzerland: Department of Non-communicable Disease Surveillance. World Health Organization; 1999. [Google Scholar]
- 24.Yeckel CW, Weiss R, Dziura J, et al. Validation of insulin sensitivity indices from oral glucose tolerance test parameters in obese children and adolescents. Journal of Clinical Endocrinology and Metabolism. 2004;89(3):1096–1101. doi: 10.1210/jc.2003-031503. [DOI] [PubMed] [Google Scholar]
- 25.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21(12):2191–2192. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 26.Da Silva RCQ, Miranda WL, Chacra AR, Dib SA. Metabolic syndrome and insulin resistance in normal glucose tolerant Brazilian adolescents with family history of type 2 diabetes. Diabetes Care. 2005;28(3):716–718. doi: 10.2337/diacare.28.3.716. [DOI] [PubMed] [Google Scholar]
- 27.Pérez-Escamilla R, Putnik P. The role of acculturation in nutrition, lifestyle, and incidence of type 2 diabetes among Latinos. Journal of Nutrition. 2007;137(4):860–870. doi: 10.1093/jn/137.4.860. [DOI] [PubMed] [Google Scholar]
- 28.Szapocznik J, Kurtines WM, Fernandez T. Bicultural involvement and adjustment in Hispanic-American youths. International Journal of Intercultural Relations. 1980;4(3-4):353–365. [Google Scholar]
- 29.Ku CY, Gower BA, Hunter GR, Goran MI. Racial differences in insulin secretion and sensitivity in prepubertal children: role of physical fitness and physical activity. Obesity Research. 2000;8(7):506–515. doi: 10.1038/oby.2000.63. [DOI] [PubMed] [Google Scholar]
- 30.Saudinha LB, Andersen LBO, Anderssen SA, et al. Objectively measured time spent sedentary is associated with insulin resistance independent of overall and central body fat in 9- To 10-year-old Portuguese children. Diabetes Care. 2008;31(3):569–575. doi: 10.2337/dc07-1286. [DOI] [PubMed] [Google Scholar]
- 31.Platat C, Wagner A, Klumpp T, Schweitzer B, Simon C. Relationships of physical activity with metabolic syndrome features and low-grade inflammation in adolescents. Diabetologia. 2006;49(9):2078–2085. doi: 10.1007/s00125-006-0320-6. [DOI] [PubMed] [Google Scholar]
- 32.Moran A, Jacobs DR, Steinberger J, et al. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes. 1999;48(10):2039–2044. doi: 10.2337/diabetes.48.10.2039. [DOI] [PubMed] [Google Scholar]
- 33.Marshall SJ, Biddle SJH, Gorely T, Cameron N, Murdey I. Relationships between media use, body fatness and physical activity in children and youth: a meta-analysis. International Journal of Obesity. 2004;28(10):1238–1246. doi: 10.1038/sj.ijo.0802706. [DOI] [PubMed] [Google Scholar]
- 34.Ekelund U, Brage S, Froberg K, et al. TV viewing and physical activity are independently associated with metabolic risk in children: the European youth heart study. PLoS Medicine. 2006;3(12, article e488) doi: 10.1371/journal.pmed.0030488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Helmerhorst HJF, Wijndaele K, Brage S, Wareham NJ, Ekelund U. Objectively measured sedentary time may predict insulin resistance independent of moderate- and vigorous-intensity physical activity. Diabetes. 2009;58(8):1776–1779. doi: 10.2337/db08-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janz KF, Burns TL, Levy SM. Tracking of activity and sedentary behaviors in childhood: the Iowa bone development study. American Journal of Preventive Medicine. 2005;29(3):171–178. doi: 10.1016/j.amepre.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Johnson MS, Figueroa-Colon R, Huang TTK, Dwyer JH, Goran MI. Longitudinal changes in body fat in African American and Caucasian children: influence of fasting insulin and insulin sensitivity. Journal of Clinical Endocrinology and Metabolism. 2001;86(7):3182–3187. doi: 10.1210/jcem.86.7.7665. [DOI] [PubMed] [Google Scholar]
- 38.Odeleye OE, De Courten M, Pettitt DJ, Ravussin E. Fasting hyperinsulinemia is a predictor of increased body weight gain and obesity in Pima Indian children. Diabetes. 1997;46(8):1341–1345. doi: 10.2337/diab.46.8.1341. [DOI] [PubMed] [Google Scholar]
- 39.Pinhas-Hamiel O, Lerner-Geva L, Copperman N, Jacobson MS. Insulin resistance and parental obesity as predictors to response to therapeutic life style change in obese children and adolescents 10–18 years old. Journal of Adolescent Health. 2008;43(5):437–443. doi: 10.1016/j.jadohealth.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Reinehr T, Kiess W, Kapellen T, Andler W. Insulin sensitivity among obese children and adolescents, according to degree of weight loss. Pediatrics. 2004;114(6):1569–1573. doi: 10.1542/peds.2003-0649-F. [DOI] [PubMed] [Google Scholar]


