Abstract
The prevalence of gestational diabetes mellitus (GDM) in the developed world has increased at an alarming rate over the last few decades. GDM has been shown to be associated with postpartum diabetes, insulin resistance, hypertension, and dyslipidemia. A history of previous GDM (pGDM), associated or not with any of these metabolic abnormalities, can increase the risk of developing not only type 2 diabetes mellitus but also cardiovascular disease (CVD) independent of a diagnosis of type 2 diabetes later in life. In this paper we discuss the relationship among inflammatory markers, metabolic abnormalities, and vascular dysfunction in women with pGDM. We also review the current knowledge on metabolic modifications occurring in normal pregnancy and the link between alterations of a normal metabolic state with the long-term maternal complications that may result in increased CVD risk. Our review of studies on pGDM prompts us to recommend that these women be considered a population at risk for later CVD events, which however could be avoided via the use of specially designed follow-up programs in the future.
1. Introduction
Gestational diabetes mellitus (GDM) is any degree of glucose intolerance with onset or first recognition during pregnancy [1, 2]. In early gestation fasting blood glucose is lower and insulin sensitivity decreases slightly. This is followed by progressively increasing insulin resistance in the second and third trimesters with a borderline increase of insulin production or hyperinsulinemia. Furthermore, insulin resistance occurs as a result of placental hormones that antagonize insulin, estrogen, progesterone, human placental lactogen (HPL), human placental growth hormone, cortisol, prolactin, and tumor necrosis factor-alpha (TNF-α) [3]. The above different pathophysiologic mechanisms accompanying pregnancy result in metabolic changes that allow for higher postprandial maternal glucose. Pregnancy is a hyperinsulinemic state which may develop into impaired glucose tolerance if insulin secretion is unable to compensate for pregnancy-associated insulin resistance [3–5].
The condition of GDM is a state of chronic low-grade subclinical inflammation characterized by abnormal production of cytokine and mediators and activation of a network of inflammatory signaling pathways. Although the characteristic of GDM is insulin resistance, the exact mechanism involved in this process is still unknown. The increased insulin resistance during pregnancy has been, as just described, attributed to cortisol and gestational hormones, but more recent data have shown that cytokines may also be involved in this process [6]. The most significant maternal risk is that of development of metabolic syndrome characterized by central obesity, dyslipidemia, and insulin resistance, which predispose to increased risk for coronary artery disease, stroke, and type 2 diabetes later in life [7–11].
The incidence of type 2 diabetes in women with previous GDM (pGDM) who were examined six weeks to 28 years postpartum was estimated to range from 2.6% to 70% [12, 13]. Other researchers found that women with pGDM have a 18–50% risk of developing type 2 diabetes mellitus within 5 years following pregnancy [14–17], and diabetes is an established risk factor for CVD [18, 19]. In addition, women with a history of GDM are at increased risk of other cardiovascular risk factors, such as obesity, hypertension, dyslipidemia, and subclinical atherosclerosis [20–22]. It is unclear whether women with a history of GDM who do not subsequently develop type 2 diabetes mellitus are also at an increased CVD risk in the future. The metabolic abnormalities which accompany GDM preceding type 2 diabetes and which remain in effect during the natural course of the disease place women at high risk for CVD [23].
In this paper we review the interrelationship among inflammatory markers, metabolic abnormalities, and endothelium dysfunction in pGDM and discuss whether these women could be considered at risk for cardiovascular disease later in life. Based on the small amount of existing literature, we discuss the inflammatory and metabolic abnormalities underlying the status of pGDM and the potential that endothelial dysfunction is a marker of future CVD risk. To our knowledge, this is the first paper presented in the literature dealing with markers of CVD risk in women with a history of gestational diabetes.
2. Surrogate Markers of Increased Cardiovascular Risk
Although the majority of women with GDM return to normal glucose tolerance after delivery, they remain, as a group, at substantially increased risk of developing type 2 diabetes in later life, a known condition that leads to an increased risk for CVD [24].
Inflammation may contribute to atherosclerosis by a variety of mechanisms depending on the stage of the disease. Circulating markers of systemic inflammation have been shown to predict future CVD [25]. These markers include C-reactive protein (CRP), proinflammatory cytokines such as interleukin-6 (IL-6), and soluble adhesion molecules. Most attention has been focused on CRP which, along with IL-6, has been revealed in large prospective studies to be a consistent predictor of future cardiovascular events [26, 27].
Epidemiological and experimental studies have established the association of markers of subclinical inflammation with CVD, type 2 diabetes, and metabolic syndrome (Figure 1). Pregnancy is a hyperinsulinemic state in which the increased insulin resistance during pregnancy may be attributed not only to gestational hormones but also possibly to cytokines, which, as mentioned in Section 1, may play a role [28–30]. Increased levels of inflammatory markers such as CRP, plasminogen activator inhibitor-1 (PAI-1), and IL-6 are predictors of future establishment of type 2 diabetes and CVD [31–34]. Adiponectin, a peptide with anti-inflammatory properties, has in some studies been associated with a decreased risk of type 2 diabetes and CVD [35, 36].
Figure 1.
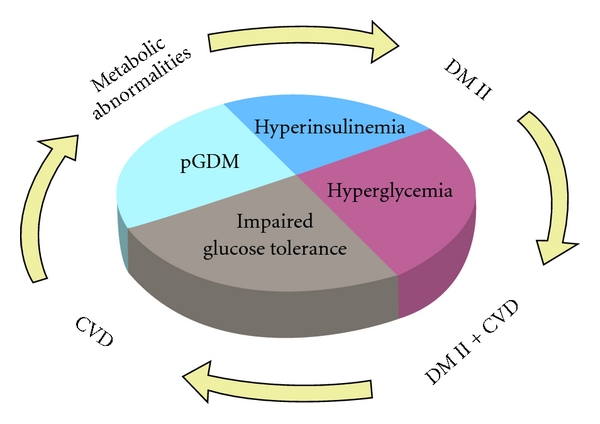
Metabolic status during pregnancy and pGDM may result in outcomes later in life like metabolic abnormalities, DMII, CVD, and DMII + CVD as it is shown following the arrows.
Markers of endothelial dysfunction, like circulating levels of E-selectin, vascular adhesion molecule-1 (VCAM-1), intercellular adhesion molecule 1 (ICAM-1), as well as inflammatory parameters like CRP and IL-6, have been reported to be associated with CVD in several studies [37–42]. Furthermore, the adhesion molecules E-selectin, VCAM-1, and ICAM-1 are thought to play a major role in the pathogenesis of vascular disease [6]. These molecules are markers of endothelial dysfunction and are expressed on the endothelial wall in response to inflammatory mediators. They contribute to the formation of atherosclerotic plaques and can be detected in soluble form in the circulation [43]. More reliable, and thus of great interest, are the measurements of carotid intima media thickness (IMT) and flow-mediated vasodilatation (FMD). Both indexes have been used in epidemiological studies as surrogate markers of early atherosclerosis. Carotid IMT increases with age, is correlated with cardiovascular risk factors, and identifies subjects at increased risk of severe coronary artery disease and cardiovascular morbidity [37]. Intima media thickness (IMT) is an ultrasound marker of CVD risk. Heiss et al. in their study found positive relation of this marker to cardiovascular risk factors and CVD risk [44].
Mediators of inflammation may exert pathologic action by inducing vascular dysfunction, thus leading to many of the diverse effects of the insulin resistance condition, like hypertension, dyslipidemia, and impaired fibrinolysis [62]. Insulin resistance has been associated with impaired endothelial function, which interacts with coagulation and hypofibrinolysis [63, 64], while hypofibrinolysis and procoagulant activity are linked with increased risk for cardiovascular events (Table 1). It is of note that raised levels of circulating inhibitors of the fibrinolytic system have been observed in patients with insulin resistance [64]. Plasminogen activator inhibitor-1 (PAI-1) is elevated in a variety of clinical situations that are associated with insulin resistance and cardiovascular disorders [65].
Table 1.
Markers of increased CVD risk in normoglycemic women with GDM or pGDM.
| Authors | Number of subjects | Months/years postpartum | Markers of CVD risk |
|---|---|---|---|
| Kousta et al., 2003 [45] | 78 | 3 years | ↑ Insulin resistance ↑ lipidemia |
| Anastasiou et al., 1998 [46] | 68 | 3–6 months | ↓ FMD |
| Hu et al., 1998 [47] | 37 | 2–4 years | ↓ Acetylcholine induced vasodilatation |
| Knock et al., 1997 [48] | 32 | During cesarean section | ↑ Vascular pathology, vessel myography |
| Paradisi et al., 2002 [49] | 38 | During GDM pregnancy | ↓ FMD |
| Heitritter et al., 2005 [50] | 48 | 1 year | ↓ Adiponectin |
| Winzer et al., 2004 [51] | 108 | 3 months | ↓ Adiponectin |
| Bo et al., 2007 [52] | 195 | 6-7 years | ↑ E-selectin ↑ ICAM-1 ↑ IMT |
| Thomaseth et al., 1997 [53] | 10 | 1 year | ↑ E-selectin ↑ VCAM-1 |
| Lawrence et al., 2002 [54] | 265 | recent GDM | ≈ E-selectin |
| Kautzky-Willer et al., 1997 [55] | 41 | 3 months | ↑ E-selectin ↑ VCAM-1 |
| Shah et al., 2008 [56] | 89.500 | p GDM | ↑ CVD events |
| Akinci et al., 2008 [57] | 76 | p GDM | ↑ OPG ↑ IMT |
| Akinci et al., 2011 [58] | 195 | 3 years | ↑ OPG |
| Banerjee et al., 2011 [59] | 29 | 2 years | ↑ Vascular pathology, vessel myography |
| Farhan et al., 2006 [60] | 70 | recent GDM | ↑ PAI-1 |
| Madarász et al., 2009 [61] | 107 | 4 years | ↑ CVD risk factors, disturbed carbohydrate metabolism |
Osteoprotegerin (OPG) is a glycoprotein, a soluble member of the tumor necrosis factor (TNF) receptor superfamily, which inhibits receptor activator of nuclear-factor-κB-ligand (RANKL-) mediated osteoclastic bone resorption [66]. It has been reported to be expressed in the arterial wall [67]. Elevated serum OPG levels have been found to be associated with atherosclerosis [68].
Vessel stiffness measured by arterial tonometry is associated with endothelial dysfunction and increased CVD risk [69, 70].
3. Studies in pGDM Women for Identification of the Risk of Cardiovascular Complications
The study of women in the pGDM state serves as a model for the detection of early metabolic abnormalities. Normoglycemic women with pGDM have increased insulin resistance and decreased endothelium-dependent vasodilatation when compared with women who had uncomplicated pregnancies [45, 46]. During the first 3–6 months postpartum, women with pGDM had impaired endothelial function assessed by FMD, this tending to confirm the assumption that glucose metabolism derangement is closely related to vascular dysfunction [46]. A cross-sectional study showed that 2–4 years after the postpartum period, pGDM had impaired acetylcholine-induced skin vasodilatation in hand and foot, as assessed by laser Doppler flow, when compared with normal controls [47]. Two cohort studies have reported signs of vascular endothelial dysfunction in vitro and in vivo during pregnancies complicated by GDM. The first study evaluated vascular endothelial function in small subcutaneous arteries dissected from biopsies obtained at cesarean section using vessel myograph and the second during pregnancy with impaired glucose tolerance and gestational diabetes mellitus assessing brachial artery FMD [48, 49].
Heitritter et al. compared biochemical and hemodynamic surrogate markers of CVD in nondiabetic women with and without a history of GDM who were at least one year postpartum and concluded that nondiabetic women with pGDM have evidence of subclinical inflammation, hypoadiponectinemia, and early vascular dysfunction and may be at increased risk of developing CVD [50]. Lower plasma adiponectin concentrations characterize women with pGDM by contrast to controls, independently of the prevailing insulin sensitivity or the degree of obesity and are associated with subclinical inflammation and atherogenic parameters [51]. Bo et al. showed in their study that pGDM women had higher values of markers of endothelial dysfunction and IMT than controls and an increased future CVD risk; however, few data are available concerning the association between pGDM and inflammation markers of endothelial dysfunction [52]. E-selectin and VCAM-1 concentrations were found to be elevated in a cohort study of women with pGDM one year after delivery [53]. A larger study many months postpartum failed to display the same results [54]. Kautzky-Willer et al. demonstrated that pGDM was characterized by persistently raised levels of E-selectin and VCAM-1 12 weeks after delivery [55]. In a large population-based study, women who had GDM in pregnancy compared with controls were at higher risk of CVD events [56].
Akinci et al. observed that OPG serum levels tended to be elevated in pGDM and moreover reported an association with carotid IMT, thus showing that osteoprotegerin may play a role in the pathogenesis of endothelial dysfunction in these women [57]. Furthermore, a very recent study conducted by the same group concluded that OPG was related to CVD risk factors and metabolic syndrome and may be involved in the development of CVD disorders in pGDM [58]. Farhan et al. recorded elevated PAI-1 levels in pGDM [60].
Another study examined the relationship between glycemia during pregnancy and small artery function 2 years postpartum. In this study subcutaneous arteries from gluteal fat biopsy were examined as to structure, stiffness, and vasoconstrictor response using myography. The results showed that vascular pathology is detectable very early in women at risk of type 2 diabetes [59]. Studying the prevalence of abnormal glucose tolerance and metabolic syndrome in a cohort of pGDM, the results demonstrated disturbed carbohydrate metabolism and a clustering of CVD factors in these women [61, 71].
Surrogate markers of increased cardiovascular risk in population-based studies are commonly used in routine practice. However, though in studies of pGDM markers are used that link this condition with future CVD risk [72], the evidence is as yet inadequate for the markers to be applied in the routine followup of these women. Nevertheless, the aforementioned studies are promising, as several of these biochemical and hemodynamic markers may in future prove to be of great value in follow-up programs, contributing to reducing the risk in pGDM for cardiovascular morbidities later in life.
4. Conclusions
It has been shown that women with pGDM are more insulin resistant than women with normal carbohydrate tolerance during their pregnancies. Diabetic complications may be in progress during the phase of insulin resistance in pregnancy even in the absence of hyperglycemia, while furthermore there is evidence that pGDM is associated with postpartum diabetes, insulin resistance, hypertension, and dyslipidemia. A history of pGDM can increase the risk of developing not only type 2 diabetes mellitus, which is a major risk factor for the development of cardiovascular disorders, but also CVD independent of the presence of type 2 diabetes. Also mentioned in this paper is the fact that a number of studies have reported pGDM to be additionally associated with the increased prevalence of metabolic syndrome, an important factor of cardiovascular disorders.
Having reviewed the current literature concerning the relationship between inflammatory markers, metabolic abnormalities, and vascular dysfunction in pGDM, we proceeded to evaluate, for the first time to our knowledge, the sum total of this information for the purpose of seeking to identify women at future risk. We additionally reviewed the current knowledge on normal metabolic modifications that occur in pregnancy and the link between these normal modifications and the ensuing long-term complications in this group of women. Based on the evidence related to pGDM, we suggest that these women be considered at an increased risk for subsequent cardiovascular morbidity. Identifying women at increased risk for developing cardiovascular morbidities and, at a later date, placing them in follow-up programs that will include the use of established selected markers, has the potential to substantially hold back their CVD risk in terms of both, lower incidence and reduced severity of cardiovascular events later in life.
References
- 1.Jovanovic L, Knopp RH, Kim H, et al. Elevated pregnancy losses at high and low extremes of maternal glucose in early normal and diabetic pregnancy: evidence for a protective adaptation in diabetes. Diabetes Care. 2005;28(5):1113–1117. doi: 10.2337/diacare.28.5.1113. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman L. Gestational diabetes mellitus (GDM) The Medical journal of Australia. 1998;168(3):p. 140. [PubMed] [Google Scholar]
- 3.Richardson AC, Carpenter MW. Inflammatory mediators in gestational diabetes mellitus. Obstetrics and Gynecology Clinics of North America. 2007;34(2):213–224. doi: 10.1016/j.ogc.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Ryan EA, Imes S, Liu D, et al. Defects in insulin secretion and action in women with a history of gestational diabetes. Diabetes. 1995;44(5):506–512. doi: 10.2337/diab.44.5.506. [DOI] [PubMed] [Google Scholar]
- 5.Lain KY, Catalano PM. Metabolic changes in pregnancy. Clinical Obstetrics and Gynecology. 2007;50(4):938–948. doi: 10.1097/GRF.0b013e31815a5494. [DOI] [PubMed] [Google Scholar]
- 6.Petry CJ. Gestational diabetes: risk factors and recent advances in its genetics and treatment. British Journal of Nutrition. 2010;104(6):775–787. doi: 10.1017/S0007114510001741. [DOI] [PubMed] [Google Scholar]
- 7.Hollander MH, Paarlberg KM, Huisjes AJM. Gestational diabetes: a review of the current literature and guidelines. Obstetrical and Gynecological Survey. 2007;62(2):125–136. doi: 10.1097/01.ogx.0000253303.92229.59. [DOI] [PubMed] [Google Scholar]
- 8.Vrachnis N, Iliodromiti S, Samoli E, Iliodromiti Z, Dendrinos S, Creatsas G. Maternal mortality in Greece, 1996-2006. International Journal of Gynecology and Obstetrics. 2011;115(1):16–19. doi: 10.1016/j.ijgo.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Vitoratos N, Vrachnis N, Valsamakis G, Panoulis K, Creatsas G. Perinatal mortality in diabetic pregnancy. Annals of the New York Academy of Sciences. 2010;1205:94–98. doi: 10.1111/j.1749-6632.2010.05670.x. [DOI] [PubMed] [Google Scholar]
- 10.Pérez-Ferre N, Galindo M, Fernández MD, et al. The outcomes of gestational diabetes mellitus after a telecare approach are not inferior to traditional outpatient clinic visits. International Journal of Endocrinology. 2010;2010:6 pages. doi: 10.1155/2010/386941. Article ID 386941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vohr BR, Boney CM. Gestational diabetes: the forerunner for the development of maternal and childhood obesity and metabolic syndrome? Journal of Maternal-Fetal and Neonatal Medicine. 2008;21(3):149–157. doi: 10.1080/14767050801929430. [DOI] [PubMed] [Google Scholar]
- 12.Getahun D, Nath C, Ananth CV, Chavez MR, Smulian JC. Gestational diabetes in the United States: temporal trends 1989 through 2004. American Journal of Obstetrics and Gynecology. 2008;198(5):p. 525. doi: 10.1016/j.ajog.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 13.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25(10):1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 14.Lambrinoudaki I, Vlachou SA, Creatsas G. Genetics in gestational diabetes mellitus: association with incidence, severity, pregnancy outcome and response to treatment. Current diabetes reviews. 2010;6(6):393–399. doi: 10.2174/157339910793499155. [DOI] [PubMed] [Google Scholar]
- 15.Fox KM, Rodbard HW, Green AJ, Grandy S. Trends in method of diagnosis of type 2 diabetes mellitus: results from SHIELD. International Journal of Endocrinology. 2009;2009:6 pages. doi: 10.1155/2009/796206. Article ID 796206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metzger BE, Cho NH, Roston SM, Radvany R. Prepregnancy weight and antepartum insulin secretion predict glucose tolerance five years after gestational diabetes mellitus. Diabetes Care. 1993;16(12):1598–1605. doi: 10.2337/diacare.16.12.1598. [DOI] [PubMed] [Google Scholar]
- 17.Kaufmann RC, Schleyhahn FT, Huffman DG, Amankwah KS. Gestational diabetes diagnostic criteria: long-term maternal follow-up. American Journal of Obstetrics and Gynecology. 1995;172(2 I):621–625. doi: 10.1016/0002-9378(95)90582-0. [DOI] [PubMed] [Google Scholar]
- 18.Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Annals of Internal Medicine. 2004;141(6):413–420. doi: 10.7326/0003-4819-141-6-200409210-00006. [DOI] [PubMed] [Google Scholar]
- 19.Daviglus ML, Liu K, Yan LL, et al. Relation of body mass index in young adulthood and middle age to medicare expenditures in older age. Journal of the American Medical Association. 2004;292(22):2743–2749. doi: 10.1001/jama.292.22.2743. [DOI] [PubMed] [Google Scholar]
- 20.Lauenborg J, Mathiesen E, Hansen T, et al. The prevalence of the metabolic syndrome in a Danish population of women with previous gestational diabetes mellitus is three-fold higher than in the general population. Journal of Clinical Endocrinology and Metabolism. 2005;90(7):4004–4010. doi: 10.1210/jc.2004-1713. [DOI] [PubMed] [Google Scholar]
- 21.Carr DB, Utzschneider KM, Hull RL, et al. Gestational diabetes mellitus increases the risk of cardiovascular disease in women with a family history of type 2 diabetes. Diabetes Care. 2006;29(9):2078–2083. doi: 10.2337/dc05-2482. [DOI] [PubMed] [Google Scholar]
- 22.Tarim E, Yigit F, Kilicdag E, et al. Early onset of subclinical atherosclerosis in women with gestational diabetes mellitus. Ultrasound in Obstetrics and Gynecology. 2006;27(2):177–182. doi: 10.1002/uog.2687. [DOI] [PubMed] [Google Scholar]
- 23.Fonseca VA. Management of diabetes mellitus and insulin resistance in patients with cardiovascular disease. The American journal of cardiology. 2003;92(4 A):50J–60J. doi: 10.1016/s0002-9149(03)00616-7. [DOI] [PubMed] [Google Scholar]
- 24.O'Sullivan JB. Diabetes mellitus after GDM. Diabetes. 1991;40(2):131–135. doi: 10.2337/diab.40.2.s131. [DOI] [PubMed] [Google Scholar]
- 25.Ross R. Atherosclerosis—an inflammatory disease. The New England Journal of Medicine. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 26.Danesh J, Wheeler JG, Hirschfield GM, et al. C-Reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. New England Journal of Medicine. 2004;350(14):1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 27.Lowe GDO, Rumley A, McMahon AD, Ford I, O’Reilly DSJ, Packard CJ. Interleukin-6, fibrin D-dimer, and coagulation factors VII and XIIa in prediction of coronary heart disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24(8):1529–1534. doi: 10.1161/01.ATV.0000135995.39488.6c. [DOI] [PubMed] [Google Scholar]
- 28.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. New England Journal of Medicine. 2000;342(12):836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 29.Freeman DJ, Norrie J, Caslake MJ, et al. C-reactive protein is an independent predictor of risk for the development of diabetes in the west of Scotland coronary prevention study. Diabetes. 2002;51(5):1596–1600. doi: 10.2337/diabetes.51.5.1596. [DOI] [PubMed] [Google Scholar]
- 30.Festa A, D’Agostino R, Howard G, Mykkänen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the insulin resistance atherosclerosis study (IRAS) Circulation. 2000;102(1):42–47. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 31.Koenig W, Löwel H, Baumert J, Meisinger C. C-reactive protein modulates risk prediction based on the framingham score—implications for future risk assessment: results from a large cohort study in Southern Germany. Circulation. 2004;109(11):1349–1353. doi: 10.1161/01.CIR.0000120707.98922.E3. [DOI] [PubMed] [Google Scholar]
- 32.Hamsten A, Walldius G, Szamosi A. Plasminogen activator inhibitor in plasma: risk factor for recurrent myocardial infarction. Lancet. 1987;2(8549):3–9. doi: 10.1016/s0140-6736(87)93050-9. [DOI] [PubMed] [Google Scholar]
- 33.Jenny NS, Tracy RP, Ogg MS, et al. In the elderly, interleukin-6 plasma levels and the -174G>C polymorphism are associated with the development of cardiovascular disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 2002;22(12):2066–2071. doi: 10.1161/01.atv.0000040224.49362.60. [DOI] [PubMed] [Google Scholar]
- 34.Sanchez PL, Morinigo JL, Pabon P, et al. Prognostic relations between inflammatory markers and mortality in diabetic patients with non-ST elevation acute coronary syndrome. Heart. 2004;90(3):264–269. doi: 10.1136/hrt.2002.007443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi KM, Lee J, Lee KW, et al. Serum adiponectin concentrations predict the developments of type 2 diabetes and the metabolic syndrome in elderly Koreans. Clinical Endocrinology. 2004;61(1):75–80. doi: 10.1111/j.1365-2265.2004.02063.x. [DOI] [PubMed] [Google Scholar]
- 36.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. Journal of the American Medical Association. 2004;291(14):1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 37.Rohde LE, Richard TL, Rivero J, et al. Circulating cell adhesion molecules are correlated with ultrasound-based assessment of carotid atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 1998;18(11):1765–1770. doi: 10.1161/01.atv.18.11.1765. [DOI] [PubMed] [Google Scholar]
- 38.Van Der Meer IM, De Maat MPM, Bots ML, et al. Inflammatory mediators and cell adhesion molecules as indicators of severity of atherosclerosis: the Rotterdam study. Arteriosclerosis, Thrombosis, and Vascular Biology. 2002;22(5):838–842. doi: 10.1161/01.atv.0000016249.96529.b8. [DOI] [PubMed] [Google Scholar]
- 39.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 40.Pradhan AD, Rifai N, Ridker PM. Soluble intercellular adhesion molecule-1, soluble vascular adhesion molecule-1, and the development of symptomatic peripheral arterial disease in men. Circulation. 2002;106(7):820–825. doi: 10.1161/01.cir.0000025636.03561.ee. [DOI] [PubMed] [Google Scholar]
- 41.Kondo K, Kitagawa K, Nagai Y, et al. Associations of soluble intercellular adhesion molecule-1 with carotid atherosclerosis progression. Atherosclerosis. 2005;179(1):155–160. doi: 10.1016/j.atherosclerosis.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 42.Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GDO, Fowkes FGR. C-reactive protein, interleukin-6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population: Edinburgh artery study. Circulation. 2005;112(7):976–983. doi: 10.1161/CIRCULATIONAHA.104.513085. [DOI] [PubMed] [Google Scholar]
- 43.Gearing AJH, Hemingway I, Pigott R, Hughes J, Rees AJ, Cashman SJ. Soluble forms of vascular adhesion molecules, E-selectin, ICAM-1, and VCAM-1: pathological significance. Annals of the New York Academy of Sciences. 1992;667:324–331. doi: 10.1111/j.1749-6632.1992.tb51633.x. [DOI] [PubMed] [Google Scholar]
- 44.Heiss G, Sharrett AR, Barnes R, Chambless LE, Szklo M, Alzola C. Carotid atherosclerosis measured by B-mode ultrasound in populations: associations with cardiovascular risk factors in the ARIC study. American Journal of Epidemiology. 1991;134(3):250–256. doi: 10.1093/oxfordjournals.aje.a116078. [DOI] [PubMed] [Google Scholar]
- 45.Kousta E, Lawrence NJ, Godsland IF, et al. Insulin resistance and β-cell dysfunction in normoglycaemic European women with a history of gestational diabetes. Clinical Endocrinology. 2003;59(3):289–297. doi: 10.1046/j.1365-2265.2003.01820.x. [DOI] [PubMed] [Google Scholar]
- 46.Anastasiou E, Lekakis JP, Alevizaki M, et al. Impaired endothelium-dependent vasodilatation in women with previous gestational diabetes. Diabetes Care. 1998;21(12):2111–2115. doi: 10.2337/diacare.21.12.2111. [DOI] [PubMed] [Google Scholar]
- 47.Hu J, Norman M, Wallensteen M, Gennser G. Increased large arterial stiffness and impaired acetylcholine induced skin vasodilatation in women with previous gestational diabetes mellitus. British Journal of Obstetrics and Gynaecology. 1998;105(12):1279–1287. doi: 10.1111/j.1471-0528.1998.tb10006.x. [DOI] [PubMed] [Google Scholar]
- 48.Knock GA, McCarthy AL, Lowy C, Poston L. Association of gestational diabetes with abnormal maternal vascular endothelial function. British Journal of Obstetrics and Gynaecology. 1997;104(2):229–234. doi: 10.1111/j.1471-0528.1997.tb11051.x. [DOI] [PubMed] [Google Scholar]
- 49.Paradisi G, Biaggi A, Ferrazzani S, De Carolis S, Caruso A. Abnormal carbohydrate metabolism during pregnancy: association with endothelial dysfunction. Diabetes Care. 2002;25(3):560–564. doi: 10.2337/diacare.25.3.560. [DOI] [PubMed] [Google Scholar]
- 50.Heitritter SM, Solomon CG, Mitchell GF, Skali-Ounis N, Seely EW. Subclinical inflammation and vascular dysfunction in women with previous gestational diabetes mellitus. Journal of Clinical Endocrinology and Metabolism. 2005;90(7):3983–3988. doi: 10.1210/jc.2004-2494. [DOI] [PubMed] [Google Scholar]
- 51.Winzer C, Wagner O, Festa A, et al. Plasma adiponectin, insulin sensitivity, and subclinical inflammation in women with prior gestational diabetes mellitus. Diabetes Care. 2004;27(7):1721–1727. doi: 10.2337/diacare.27.7.1721. [DOI] [PubMed] [Google Scholar]
- 52.Bo S, Valpreda S, Menato G, et al. Should we consider gestational diabetes a vascular risk factor? Atherosclerosis. 2007;194(2):e72–e79. doi: 10.1016/j.atherosclerosis.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 53.Thomaseth K, Pacini G, Clodi M, et al. Amylin release during oral glucose tolerance test. Diabetic Medicine. 1997;14(2):S29–S34. doi: 10.1002/(sici)1096-9136(199706)14:2+<s29::aid-dia401>3.3.co;2-s. [DOI] [PubMed] [Google Scholar]
- 54.Lawrence NJ, Kousta E, Penny A, et al. Elevation of soluble E-selectin levels following gestational diabetes is restricted to women with persistent abnormalities of glucose regulation. Clinical Endocrinology. 2002;56(3):335–340. doi: 10.1046/j.1365-2265.2002.01473.x. [DOI] [PubMed] [Google Scholar]
- 55.Kautzky-Willer A, Fasching P, Jilma B, Waldhäusl W, Wagner OF. Persistent elevation and metabolic dependence of circulating E-selectin after delivery in women with gestational diabetes mellitus. Journal of Clinical Endocrinology and Metabolism. 1997;82(12):4117–4121. doi: 10.1210/jcem.82.12.4419. [DOI] [PubMed] [Google Scholar]
- 56.Shah BR, Retnakaran R, Booth GL. Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care. 2008;31(8):1668–1669. doi: 10.2337/dc08-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Akinci B, Demir T, Celtik A, et al. Serum osteoprotegerin is associated with carotid intima media thickness in women with previous gestational diabetes. Diabetes Research and Clinical Practice. 2008;82(2):172–178. doi: 10.1016/j.diabres.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 58.Akinci B, Celtik A, Yuksel F, et al. Increased osteoprotegerin levels in women with previous gestational diabetes developing metabolic syndrome. Diabetes Research and Clinical Practice. 2011;91(1):26–31. doi: 10.1016/j.diabres.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 59.Banerjee M, Anderson SG, Malik RA, Austin CE, Cruickshank JK. Small artery function 2 years postpartum in women with altered glycaemic distributions in their preceding pregnancy. Clinical Science. 2012;122(2):53–61. doi: 10.1042/CS20110033. [DOI] [PubMed] [Google Scholar]
- 60.Farhan S, Winzer C, Tura A, et al. Fibrinolytic dysfunction in insulin-resistant women with previous gestational diabetes. European Journal of Clinical Investigation. 2006;36(5):345–352. doi: 10.1111/j.1365-2362.2006.01630.x. [DOI] [PubMed] [Google Scholar]
- 61.Madarász E, Tamás G, Tabák AGy, Kerényi Z. Carbohydrate metabolism and cardiovascular risk factors 4 years after a pregnancy complicated by gestational diabetes. Diabetes Research and Clinical Practice. 2009;85(2):197–202. doi: 10.1016/j.diabres.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 62.Meigs JB, Hu FB, Rifai N, Manson JE. Biomarkers of Endothelial Dysfunction and Risk of Type 2 Diabetes Mellitus. Journal of the American Medical Association. 2004;291(16):1978–1986. doi: 10.1001/jama.291.16.1978. [DOI] [PubMed] [Google Scholar]
- 63.Yki-Järvinen H. Insulin resistance and endothelial dysfunction. Best Practice and Research: Clinical Endocrinology and Metabolism. 2003;17(3):411–430. doi: 10.1016/s1521-690x(03)00033-2. [DOI] [PubMed] [Google Scholar]
- 64.Grant PJ. Diabetes mellitus as a prothrombotic condition. Journal of Internal Medicine. 2007;262(2):157–172. doi: 10.1111/j.1365-2796.2007.01824.x. [DOI] [PubMed] [Google Scholar]
- 65.Juhan-Vague I, Alessi MC. PAI-1, obesity, insulin resistance and risk of cardiovascular events. Thrombosis and Haemostasis. 1997;78(1):656–660. [PubMed] [Google Scholar]
- 66.Min H, Morony S, Sarosi I, et al. Osteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesis. Journal of Experimental Medicine. 2000;192(4):463–474. doi: 10.1084/jem.192.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dhore CR, Cleutjens JPM, Lutgens E, et al. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arteriosclerosis, Thrombosis, and Vascular Biology. 2001;21(12):1998–2003. doi: 10.1161/hq1201.100229. [DOI] [PubMed] [Google Scholar]
- 68.Abedin M, Omland T, Ueland T, et al. Relation of Osteoprotegerin to Coronary Calcium and Aortic Plaque (from the Dallas Heart Study) American Journal of Cardiology. 2007;99(4):513–518. doi: 10.1016/j.amjcard.2006.08.064. [DOI] [PubMed] [Google Scholar]
- 69.Nigam A, Mitchell GF, Lambert J, Tardif JC. Relation between conduit vessel stiffness (assessed by tonometry) and endothelial function (assessed by flow-mediated dilatation) in patients with and without coronary heart disease. American Journal of Cardiology. 2003;92(4):395–399. doi: 10.1016/s0002-9149(03)00656-8. [DOI] [PubMed] [Google Scholar]
- 70.Weber T, Auer J, O’Rourke MF, et al. Arterial Stiffness, Wave Reflections, and the Risk of Coronary Artery Disease. Circulation. 2004;109(2):184–189. doi: 10.1161/01.CIR.0000105767.94169.E3. [DOI] [PubMed] [Google Scholar]
- 71.Di Cianni G, Ghio A, Resi V, Volpe L. Gestational diabetes mellitus: an opportunity to prevent type 2 diabetes and cardiovascular disease in young women. Women’s Health. 2010;6(1):97–105. doi: 10.2217/whe.09.76. [DOI] [PubMed] [Google Scholar]
- 72.Bentley-Lewis R. Late cardiovascular consequences of gestational diabetes mellitus. Seminars in Reproductive Medicine. 2009;27(4):322–329. doi: 10.1055/s-0029-1225260. [DOI] [PMC free article] [PubMed] [Google Scholar]


