Abstract
Increased protein supply by feeding cow-milk-based infant formula in comparison to lower protein content of human milk is a well-recognized major risk factor of childhood obesity. However, there is yet no conclusive biochemical concept explaining the mechanisms of formula-induced childhood obesity. It is the intention of this article to provide the biochemical link between leucine-mediated signalling of mammalian milk proteins and adipogenesis as well as early adipogenic programming. Leucine has been identified as the predominant signal transducer of mammalian milk, which stimulates the nutrient-sensitive kinase mammalian target of rapamycin complex 1 (mTORC1). Leucine thus functions as a maternal-neonatal relay for mTORC1-dependent neonatal β-cell proliferation and insulin secretion. The mTORC1 target S6K1 plays a pivotal role in stimulation of mesenchymal stem cells to differentiate into adipocytes and to induce insulin resistance. It is of most critical concern that infant formulas provide higher amounts of leucine in comparison to human milk. Exaggerated leucine-mediated mTORC1-S6K1 signalling induced by infant formulas may thus explain increased adipogenesis and generation of lifelong elevated adipocyte numbers. Attenuation of mTORC1 signalling of infant formula by leucine restriction to physiologic lower levels of human milk offers a great chance for the prevention of childhood obesity and obesity-related metabolic diseases.
1. Introduction
Obesity is a serious health problem of Westernized societies with a prevalence of up to 25% with an increasing incidence in children [1]. Obesity is a complex disease that involves interactions between environmental and genetic factors [2]. Approximately 25% of children in the USA are overweight and approximately 11% are obese. From 1976 till 1991, the prevalence rate of overweight children in the USA had increased by approximately 40% [1]. Type 2 diabetes mellitus (T2D) in children and adolescents is an important and increasing public health problem directly related to the epidemic of childhood obesity [3]. High BMI at birth has been identified as an important determinant for overweight later in life [4]. In Western societies, maternal and postnatal nutritions are excessive and may substantially affect developmental programming [5].
2. The Link between Infant Formula Feeding and Childhood Obesity
In 1981, a case-control study of children between 12 to 18 years of age in Montreal presented first evidence that breast feeding protects against later obesity [6]. A cross sectional survey in southern Germany (Bavaria) assessed early feeding, diet, and lifestyle factors of 13 345 children at the time of school entry. The prevalence of obesity in children who had never been breast fed was 4.5% as compared with 2.8% in breast-fed children [7]. A clear dose-response effect on the prevalence of obesity was identified for the duration of breast feeding. Thus, breast feeding is a significant protective factor against the development of obesity and being overweight [7]. During the first 6–8 weeks of life, there is little difference in growth (gain in weight and length) between breast- and formula-fed infants. However, from about 2 months of age to the end of the first year of life, formula-fed infants gain weight and length more rapidly than breast-fed infants [8]. At the end of the first year of life, evidence suggests that breast-fed infants are leaner than formula-fed infants. Formula-fed infants at 4-5 months of age show higher plasma levels of IGF-1, insulin, and certain amino acids than breast-fed infants. Whereas the protein intake of breast-fed infants decreases with age and closely matches the requirements for protein during the early months of life, the protein intake of formula-fed infants exceeds requirements after the first 1-2 months of life consistent with the hypothesis that differences in protein intake are mainly responsible for differences in growth between breast- and formula-fed infants (early protein hypothesis) [8]. A double-blinded, randomized controlled trial presented substantial evidence that infant formulas providing a high protein intake during the first year of life induced excessive weight gain in early childhood [9]. High protein intake in early infancy has thus been confirmed to be an important risk factor for later obesity [9, 10].
Exclusive breast feeding by a healthy mother should be the standard from birth to at least 6 months. During the breast feeding period, the protein intake is low in the human being compared to many other animals. The daily protein intake by breast feeding is approximately 1 g/kg/day. When other foods are introduced during the weaning period, the protein intake increases remarkably to 3-4 g/kg/day in spite of the fact that the protein requirement is decreasing [11]. A systematic review evaluating the ideal quantity of dietary protein for formula-fed low-birth-weight infants <2.5 kg came to the conclusion that higher protein intake (>3.0 g/kg/day but <4.0 g/kg/day) from formula in comparison to lower protein intake (<3 g/kg/day) accelerated weight gain [12]. Furthermore, human milk in comparison to infant formula contains leptin, which has been implicated to play a role in perinatal programming of body weight and the regulation of muscle thermogenesis [13].
3. Postnatal High-Protein Diet Increases Obesity Risk of Rats in Adulthood
Low-protein diets in the rat have probably most extensively been used to try and elucidate mechanisms of metabolic programming [14]. However, only few studies evaluated the effect of high protein diets on developmental programming. A high-protein diet (40% casein wt/wt; 4% leucine) in comparison to a normal diet (20% casein wt/wt, 2% leucine) or to a high-fibre diet (17.3% casein wt/wt; 1.7% leucine) in Wistar rat pups weaned at day 21 was associated with an increased susceptibility for obesity in adulthood [15]. Body weight, fat mass, and glycaemia in adult males and fat mass in females were greater after a high-fat challenge in these rats that consumed a high protein diet from weaning [15, 16]. High-protein diet during early growth exhibited excessive increase in fat mass in response to a high-fat and -sucrose diet in adult rats as well as elevated leptin levels during oral glucose challenge [17]. Maternal diet during pregnancy and lactation as well as early-postnatal feeding are thus critical periods during which intervention strategies could be developed to reduce the prevalence of obesity [18].
4. Mammalian Milk: A Signalling System for Neonatal Growth
Information on the potential endocrine mechanisms of milk protein signalling is very scarce. So far, most attention has been paid to the role of IGF-1 as the driving mechanism of milk-mediated growth [19–26]. IGF-1 has been shown to stimulate the differentiation of preadipocytes to adipocytes [27, 28]. Moreover, the branched-chain amino acids (BCAAs) leucine, isoleucine, and valine have been implicated to be involved in growth promoting effects of milk, because they are physiologic stimuli of insulin secretion [29, 30]. Both insulin and IGF-1 have mitogenic and anabolic effects and stimulate lipogenesis and adipogenesis [31].
It has recently been emphasized that mammalian milk is the most important endocrine species-specific signalling system that promotes neonatal growth by increasing insulin-, IGF-1-, incretin-, and leucine-mediated mammalian target of rapamycin complex 1- (mTORC1-) signalling of pancreatic β-cells [32]. Milk signalling is predominantly based on milk proteins, evolutionarily well-conserved secretory products of the mammalian lactation genome, which are essential for growth and survival of mammalian neonates, and have been established more than 160 million years ago [33]. Remarkably, among all mammalian species, the average protein content of milk in humans is the lowest and has been determined to be 1.21 g protein/100 mL of mature human milk during the first 3 months of lactation and 1.14 g/100 mL at 6 months of lactation, respectively [34]. Recent data of human milk (>15 days postpartum) found protein values of 1.26 g/100 mL during the day period and 1.35 g/100 mL during the night, respectively [35]. In contrast, cow milk contains 3.36 g protein/100 mL.
5. Total Leucine Uptake by Mammalian Milk and Neonatal Growth Rate
Intriguingly, the protein content of mammalian milk of various species is related to the rate of growth of the offspring [36]. Human neonates, who receive the lowest protein content of milk among mammalian species, require 180 days to double their birth weight in comparison to calves who double their birth weight already after 40 days. The highest milk protein concentrations are found in rat or rabbit in the range of 8.7 g/100 mL and 10 g/100 mL, respectively, who double their birth weight already after 4 to 5 days [36]. Thus, there is a correlation between the species-specific protein concentration of mammalian milk and the growth rate of the neonate (Table 1). Remarkably, the amount of leucine per gram milk protein appears to be a mammalian species-independent constant in the range of 100 mg leucine/g milk protein for humans, various primates, and nonprimate species including cow [37] (Table 2). Thus, the total amount of milk protein fed to a mammalian neonate correlates to the total leucine uptake provided by milk of the mammalian species and appears to be associated with leucine-mediated growth. These observations are in accordance with premature infants fed formula containing a higher protein concentration who gained weight faster than those fed formulas with a lower protein concentration closer to that of human milk [38].
Table 1.
Leucine content of mammalian milk and growth rate.
| Species | Protein content of milk (g/100 mL) | Leucine content of milk (mg/100 mL) | Days (n) for doubling birth weight3 |
|---|---|---|---|
| Rat1 | 8.7 | 799 | 4 |
| Cow1 | 3.4 | 333 | 40 |
| Infant formula (HP)2 | 3.2 | 308 | ? |
| Infant formula (LP)2 | 1.6 | 154 | ? |
| Human milk2 | 1.2 | 104 | 180 |
Table 2.
Leucine content per g total milk protein in various species*.
| Species | Total amino acids g/100 mL whole milk | Leucine content mg/g total amino acids |
|---|---|---|
| Human | 0.85 | 104 |
| Chimpanzee | 0.92 | 104 |
| Gorilla | 1.15 | 102 |
| Baboon | 1.15 | 105 |
| Rhesus | 1.16 | 111 |
| Horse | 1.58 | 93 |
| Goat | 2.57 | 96 |
| Llama | 2.96 | 99 |
| Cow | 3.36 | 99 |
| Pig | 3.50 | 89 |
| Elephant | 3.71 | 98 |
| Sheep | 5.41 | 90 |
| Cat | 7.57 | 118 |
| Rat | 8.69 | 92 |
| Range: 0.85–8.69 g/100 mL | Mean: 100 ± 8 mg/g protein |
* Data derived from Davis et al. [37].
6. Milk Proteins: The Richest Animal Protein Source of Leucine
Among all animal proteins, milk proteins contain the highest amount of leucine. Highest leucine levels are found in the water-soluble and easily digestible whey protein fraction in the range of 14% [40]. An important leucine carrier is the whey protein α-lactalbumin. Bovine and human α-lactalbumin contain 10.4% and 11.3% leucine, respectively [41]. Whey proteins and especially α-lactalbumin exert a high insulinaemic response, which is predominantly mediated by the insulinotropic activity of leucine [42].
The bulk of cow milk proteins are contained in the casein fraction (80%). Bovine caseins contain on average 10% leucine. Egg protein (8.5% leucine) and meat protein (8% leucine) contain less leucine than milk proteins, the life starter proteins of mammalian evolution [40]. To understand the role of milk-mediated leucine signalling, the most critical regulatory function of the leucine-dependent kinase mammalian target of rapamycin complex 1 (mTORC1) has to be discussed in more detail.
7. mTORC1: The Central Growth Regulator of Mammalian Cells
Leucine as well as the growth hormones insulin and IGF-1 are important activating stimuli of the nutrient-sensitive kinase mTORC1, a central cell growth regulator conserved from yeast to mammals [43–49]. Amino acids and predominantly leucine are the most critical signals for mTORC1 signalling and are required for mTORC1 activation by growth factors like insulin and IGF-1 [49] (Figure 1). Recent discoveries in the field of molecular biology have established the key role of mTORC1 in the regulation of multiple central cell functions including gene transcription, translation, ribosome biogenesis, protein synthesis, cell growth, cell proliferation, lipid synthesis, mitochondrial activity, and suppression of autophagy [44–47]. The ribosomal S6 kinase (S6K1) and the eukaryote translation initiation factor 4E binding protein (4E-BP1) are the two best characterized mTORC1 substrates. Their phosphorylation by mTORC1 mediates the function of mTORC1 in regulating translation [43, 44]. mTORC1 has been identified as the most important convergence point of nutrient-derived signalling and is thus of pivotal importance for neonatal growth [50].
Figure 1.
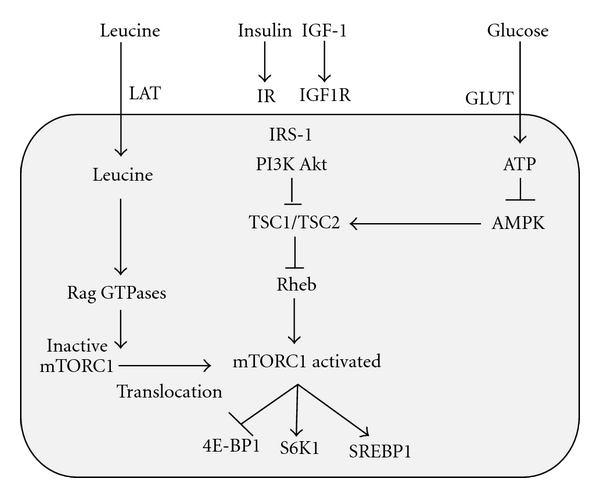
Simplified model of leucine-, insulin/IGF-1-, and glucose-dependent activation of mTORC1; Akt: Akt kinase; AMPK: AMP-activated protein kinase; ATP: adenosine triphosphate; 4E-BP1: eukaryotic initiation factor (eIF) 4E-binding protein 1; GLUT: glucose transporter protein; IGF-1: insulin-like growth factor 1; IGF1R: IGF-1 receptor; IR: insulin receptor; IRS-1: insulin receptor substrate 1; LAT: L-type amino acid transporter; mTORC1: mammalian target of rapamycin complex 1; PI3K: phosphoinositol-3 kinase; Rheb: ras homolog enriched in brain; S6K1: p70 S6 kinase 1; SREBP: sterol regulatory element-binding transcription factor; TSC1: tuberous sclerosis complex 1 (hamartin); TSC2: tuberous sclerosis complex 2 (tuberin).
mTOR is a multidomain protein of approximately 300 kDa exhibiting a protein kinase domain at its C-terminus related to phosphoinositol-3-kinases (PI3Ks). In mammalian cells, two functionally different mTOR complexes exist: mTORC1 and mTORC2, respectively. Among other functional proteins, mTORC1 contains the important partner protein raptor, which interacts with substrates for mTORC1-mediated phosphorylation. mTORC1 controls the G1/S transition and G2/M progression of the cell cycle [51]. In contrast to mTORC2, which contains the partner protein rictor, only mTORC1 plays a special role in sensing cellular nutrients, amino acids, and energy (ATP) levels important for cell growth and proliferation. LKB1 and AMP-activated protein kinase (AMPK) are critical regulators of mTORC1 [52]. Most functions of mTORC1 are inhibited by rapamycin, a triene macrolide antibiotic synthesized by Streptomyces hygroscopicus [44, 47]. Growth factor signals like insulin and IGF-1 are integrated by the tuberous sclerosus proteins TSC1 (hamartin) and TSC2 (tuberin), which form a complex that regulates Rheb (ras homolog enriched in brain), the final activator of mTORC1 [53–57] (Figure 1). Growth factor signalling via TSC2 phosphorylation reduces the inhibitory function of TSC1/TSC2 towards Rheb, which results in activation of Rheb and finally of mTORC1 [53–57]. mTORC1 has to be regarded as a key node in cell signalling, because it integrates many intra- and extracellular signals such as growth factors (insulin, IGF-1), energy-sensing signals (glucose, AMP/ATP ratio regulating AMPK), and most importantly the availability of amino acids, especially leucine for mTORC1 activation [45, 49, 50] (Figure 1).
8. Amino-Acid-Mediated Activation of mTORC1
Two parallel mechanisms of mTORC1 activation have been identified: (1) the upstream activation of the small GTPase Rheb by growth factor signals and high glucose/ATP levels, and (2) the amino-acid-mediated Rag GTPase-dependent translocation of inactive mTORC1 to active Rheb localized at late endosome or lysosome compartments [58–61] (Figure 1). Moreover, mTORC1 activity is regulated by Rab and Arf family small GTPases, which stimulate mTORC1 activation by the regulation of intracellular trafficking in response to amino acids [62]. Raptor has been identified as an interacting partner of the signalling adaptor p62, which is an integral part of mTORC1 and is necessary to mediate amino-acid signalling for the activation of S6K1 and 4E-BP1 [63]. p62 interacts in an amino acid-dependent manner with mTORC1 and raptor and binds the Rag proteins and favours formation of the active Rag heterodimer that is further stabilized by raptor. Interestingly, p62 colocalizes with Rags at the lysosomal compartment and is required for the interaction of mTORC1 with Rag GTPases in vivo and for translocation of mTORC1 to the lysosome, a crucial step for mTORC1 activation [63, 64] (Figure 1). Recent evidence has been provided that mTORC1 also senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H+-ATPase [65].
9. Primacy of Leucine for mTORC1 Activation
Essential BCAAs are important for the regulation of growth, protein biosynthesis, and metabolism [45, 49]. There is substantial evidence that from all amino acids, leucine plays a major role in mTORC1 activation [43, 64]. The system L amino acid transporter 1/2 and the 4F2hc/CD98 glycoprotein are the primary route for cellular entry of neutral amino acids, such as leucine. The accumulation of intracellular amino acids is believed to be achieved by system A transporter, such as SNAT2 (sodium-coupled neutral amino acid transporter 2) [66]. Expression of both system L (LAT1/CD98) and system A (SNAT2) positively correlated with mTORC1 activity [67, 68]. Their functional coupling may explain why glutamine is required for the stimulating effect of leucine on mTORC1 activity [69, 70]. The reciprocal exchange of intracellular glutamine for extracellular leucine is important for activation of mTORC1 and its downstream target S6K1 [71–73]. Since mTORC1 signalling positively stimulates protein synthesis, it makes physiological sense that mTORC1 signalling is tightly regulated by amino acid availability. Withdrawal of leucine in cell culture was nearly as effective in downregulation of mTORC1 signalling as withdrawal of all amino acids [43]. The preeminent effect of leucine withdrawal has been consistently observed in a variety of cell types, thus underlining the primacy of leucine in amino-acid-mediated mTORC1 regulation [45]. Rat plasma levels of leucine were linearly related to the intake of gram protein diet regardless of the dietary source [74]. In humans, the highest postprandial leucine concentrations have been measured after a whey protein meal, followed by a milk meal and a cheese meal, respectively [42]. The strongest correlation between postprandial insulin responses and early increments in plasma amino acids was demonstrated for leucine, valine, lysine, and isoleucine. In comparison to other amino acids, leucine exhibited the highest insulinogenic index [42]. Thus, leucine plays a crucial role for mTORC1 activation, β-cell growth, β-cell proliferation, and insulin secretion [42, 43, 45, 49].
10. Leucine-mTORC1-Dependent β-Cell Proliferation and Insulin Secretion
Insulin is an anabolic and mitogenic hormone important for neonatal growth. Both insulin and IGF-1 are involved in the regulation of adipogenesis [28, 31]. Thus, the regulation of insulin secretion by mammalian milk plays a critical role for infant and adipose tissue growth. Despite a low carbohydrate moiety and a low glycaemic index, both whole cow milk as well as skim milk exhibit a high insulinaemic index (>100), which depends on milk's insulinotropic protein fraction [75, 76]. It is the biological function of milk to promote neonatal growth by stimulating neonatal β-cell mass expansion and insulin secretion. Like in all other peripheral cells, the mTORC1 pathway is highly active in β-cells and plays a central role in leucine-mediated β-cell proliferation and insulin secretion [77]. Leucine activates mTORC1 independent of insulin [29, 30]. The pancreatic β-cells express a variety of growth factor receptors, which stimulate mTORC1 and promote β-cell growth and replication [78]. Insulin and IGFs in concert with leucine, glutamine, and glucose modulate protein translation through mTORC1 in β-cells [29, 30]. Glucose robustly activates mTORC1 in an amino-acid-dependent manner in rodent and human islets [29]. In contrast, the mTORC1 inhibitor rapamycin dose-dependently inhibited DNA synthesis of rat islets exposed to elevated levels of glucose [29]. mTORC1/S6K1/4E-BP1-signalling is known to control cell size and proliferation by increasing mRNA translation and cell cycle progression [44, 51, 78]. Leucine has already been demonstrated to activate the translational regulators, phosphorylated heat- and acid-stable protein regulated by insulin (PHAS-I) and S6K1, in an mTORC1-dependnet manner [79]. Leucine-induced insulin secretion of β-cells involves increased mitochondrial metabolism by oxidative decarboxylation and allosteric activation of glutamate dehydrogenase. Leucine is essential for activation of protein translation through mTORC1 and contributes to enhanced β-cell function by stimulating growth-related protein synthesis and β-cell proliferation [79, 80]. mTORC1 activation in β-cells of TSC2-deficient mice (βTSC2−/−) increased mitochondrial biogenesis and enhanced insulin secretion [81]. In contrast, S6K1-deficient mice displayed hypoinsulinaemia, glucose intolerance, and diminished β-cell size [82]. Thus, there is substantial evidence for the crucial role of leucine in mTORC1-S6K1-mediated activation increasing β-cell proliferation, protein translation, and insulin synthesis [77–82]. The most important function of leucine-transmitted milk signalling is the stimulation of insulin secretion, a fundamental growth-promoting mechanism for insulin-mediated mTORC1 signalling of insulin-sensitive peripheral cells of the body including adipocytes.
11. Excessive Leucine Uptake by Infant Formula Feeding
Cow milk-based infant formulas currently contain almost 50% higher total protein content (2.1 to 2.2 g/100 kcal) than human milk (1.8 g/100 kcal) [83]. Remarkably, the protein intake per kg body weight is 55–80% higher in formula-fed than in breast-fed infants [84]. The most recent randomized clinical trial showed that cow-milk-based infant and followon formulas prepared with lower protein (1.77 and 2.2 g protein/100 kcal) and higher protein (2.9 and 4.4 g protein/100 kcal) had higher leucine content in comparison to human milk [39]. Infant and followon formulas with lower protein content provided 119 and 154 mg leucine/100 mL, whereas infant and followon formula with higher protein content contained 197 and 308 mg leucine/100 mL, respectively. In comparison, human milk only contains 104 mg leucine/100 mL [37, 39] (Table 3). Thus, the leucine amounts provided by these infant formulas were 14.4%, 48.1%, 89.4%, and 196.2% higher compared to physiologic leucine levels provided by human milk. At 6 months of age, serum leucine levels of infants fed higher protein formula were 165 μmol/L, and those of infants fed lower protein formula were 120 μmol/L, respectively, whereas the lowest leucine serum levels were detected in breast-fed infants (106 μmol/L) (Table 3) [39]. Higher leucine serum levels of infants fed higher amounts of leucine by infant formula just reflect the known linear correlation between dietary amino acid intake and amino acid serum levels [74] (Figure 2). The excessive supply of leucine provided by infant formula is thus of most critical concern because the availability of leucine is a crucial determinant for the magnitude of mTORC1 activity [43, 45].
Table 3.
Leucine content of infant diet and serum leucine levels.
| Type of infant diet | Protein content of infant diet (g/100 mL) | Leucine content of infant diet (mg/100 mL) | Serum leucine levels (μmol/L) |
|---|---|---|---|
| Human milk1 whey/casein 3 : 2 | 1.21 | 104 | 106 |
| Low-protein (LP) infant formula2 | 1.25 | 119 | 1104 |
| LP followon formula2 | 1.60 | 154 | 120 |
| High-protein (HP) infant formula2 | 2.05 | 197 | 1334 |
| HP followon formula2 | 3.20 | 308 | 165 |
| Cow milk whey/casein 1 : 5 | 3.40 | 3333 | 1724 |
Figure 2.
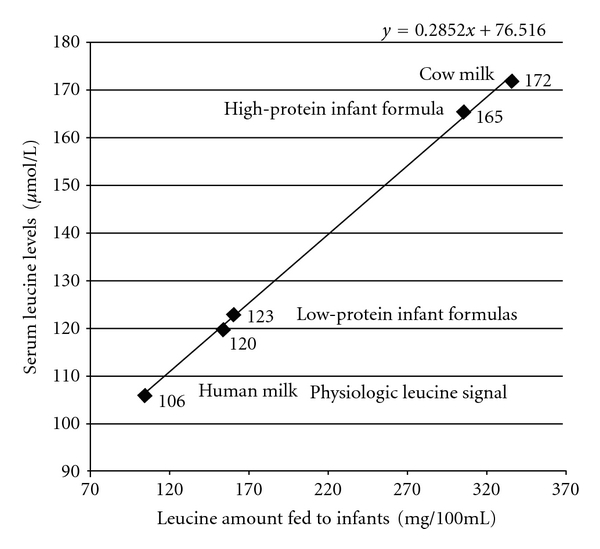
Linear correlation between oral leucine intake by the infant feeding and the infant's serum leucine concentrations.
12. Infant Formula Increases Insulin/IGF-1 Signals for mTORC1 Activation
Infant formula not only increases leucine signals for mTORC1 activation but also insulin and IGF-1 signals, which are all integrated by mTORC1 (Figure 1). It has recently been shown that lower protein content of infant formula was associated with lower total IGF-1 serum levels (34.7 ng/mL) in comparison to high protein formula (48.4 ng/mL) [39]. Remarkably, the lowest total serum IGF-1 levels (14.1 ng/mL) were detected in serum of breast-fed infants [39]. C-peptide serum levels, a measure of insulin secretion, were highest in infants fed the high-protein formula (26.9 ng/mL), lower in infants fed the lower-protein formula (19.5 ng/mL), and again the lowest in breast-fed infants (9.3 ng/mL) [39]. Thus, the controlled feeding study of Socha et al. [39] provided strong evidence that infant formula in comparison to human milk significantly increases serum levels of leucine, insulin, and IGF-1, all most important stimulatory signals for mTORC1 activation [49, 50].
13. mTORC1 Signalling and Milk-Induced Insulin Resistance
The major substrate of mTORC1 is the kinase S6K1 [43]. S6K1 phosphorylates Ser-307 of insulin receptor substrate-1 (IRS-1) and thereby activates an important feedback mechanism, which downregulates insulin signalling by inducing insulin resistance [85–87]. High mTORC1 signalling is thus associated with a higher degree of S6K1-mediated insulin resistance. Remarkably, absence of S6K1 protected S6K1−/− mice against age- and high-fat-diet-induced obesity while enhancing insulin sensitivity, pointing to the crucial role of the mTORC1-S6K1 pathway in the regulation of insulin signalling and the induction of nutrient-induced insulin resistance due to hyperactivated S6K1 [88]. In humans, insulin resistance has been induced by infusions of high concentrations of amino acids, whereas the mTORC1 inhibitor rapamycin improved insulin action [89]. Infusion of an amino acid mixture to healthy men resulted in plasma amino acid elevations, hyperinsulinaemia, and marked activation of S6K1 with increased inhibitory IRS-1 phosphorylation [90]. Infusion of amino acids containing 8.9 g/L leucine impaired insulin-mediated suppression of glucose production and insulin-stimulated glucose disposal in skeletal muscle. Insulin resistance was also observed after incubation of rat skeletal muscle with higher concentrations of both leucine and glucose [91]. In accordance with these findings, are the results of feeding studies of rats with a high-fat diet supplemented with BCAAs exhibiting chronic mTORC1-mediated phosphorylation of IRS-1 at Ser-307, which was reversed by rapamycin treatment [92]. Moreover, the dietary pattern that includes high fat consumption accompanied with high amounts of BCAAs appears to contribute to obesity-associated insulin resistance [92]. Thus, there is substantial evidence that the amount of leucine and other BCAAs, regulate mTORC1-S6K1 signalling, an important pathway leading to insulin resistance.
Remarkably, the daily intake of 53 g cow-milk protein containing 5.7 g leucine in 8-year-old boys resulted in increased insulin secretion and the induction of insulin resistance [22]. Milk-protein-induced insulin resistance can be well explained by leucine-mTORC1-mediated activation of S6K1. Higher leucine/isoleucine serum levels (median 170.0 μM) of obese subjects have been shown to be associated with a higher HOMA of 5.73 in comparison to lower leucine/isoleucine serum levels (149.0 μM) of lean subjects exhibiting a lower HOMA of 2.51 [92].
The magnitude of leucine-mTORC1 signalling for normal growth differs in various mammalian species. The highest leucine intake per volume milk is found in small-fast growing rodents. Calves duplicate their birth weight already after 40 days, whereas human infants are the mammals with the slowest growth rate, duplicating their birth weight after 180 days (Table 1). Thus, each species appears to have its own milk-mediated leucine-mTORC1 signalling axis (Table 2). Remarkably, cow-milk-mediated leucine-mTORC1 signalling greatly exceeds the much lower leucine-mTORC1 signalling maintained by human milk (Figure 2).
Early postnatal restriction or overfeeding of mice with mouse milk resulted in metabolic abnormalities in adult life [93]. Underfed mice exhibited impaired insulin secretion, whereas overfed mice, which received more milk protein and thus more total leucine during the early postnatal period, developed insulin resistance in adult life [93]. The study of Socha et al. [39] implies the presence of higher insulin resistance in infants fed high-protein formula (ratio C-peptide 26.9 ng/mL: fasting glucose 83 mg/dL) in comparison to low-protein formula (C-peptide 19.5 ng/mL: glucose 85 mg/dL). The lowest level for insulin resistance was detected in breast-fed infants (C-peptide 9.3 ng/mL: glucose 86 mg/dL) [39]. Thus, human milk in comparison to infant formula induced the lowest degree of insulin resistance reflecting the lowest magnitude of mTORC1-S6K1-IRS-1 signalling during physiological breast feeding in comparison to artificial formula feeding.
14. Adipogenic Effects of Leucine
Adipose tissue like muscle is a major extrahepatic site of leucine metabolism. Notably, leucine is an important precursor of fatty acid and cholesterol biosynthesis [94]. Most importantly, in adipocytes, leucine has been shown to be the main regulatory amino acid activating mTORC1, S6K1, and 4E-BP1 [95, 96]. mTORC1 plays a crucial role in adipocyte regulation including hypertrophic growth, leptin secretion, protein synthesis, and adipose tissue morphogenesis [96, 97]. The mTORC1 antagonist rapamycin has been shown to block adipocyte differentiation [98]. Rapamycin specifically disrupted the positive transcriptional feedback loop between CCAAT/enhancer-binding protein-α and peroxisome proliferator-activated receptor-γ (PPARγ), two key transcriptional factors in adipogenesis, by directly targeting the transactivation of PPARγ [99]. Remarkably, PPARγ activity was dependent on amino acid sufficiency, linking amino acid status to adipogenesis [99]. In isolated adipocytes, amino acids, and primarily leucine, stimulated phosphorylation of 4E-BP1 and S6K and induced multicellular clustering in adipocytes [100, 101]. mTORC1 signalling alone is sufficient to regulate adipogenesis, because activation of mTORC1 causes a robust increase in mRNA and PPARγ protein expression despite severe insulin resistance and the absence of Akt signalling [102]. Furthermore, it has been demonstrated that the adipogenic effect of insulin is mediated by insulin's stimulatory effect on the Akt-TSC2-mTORC1 pathway [102]. Thus, it is conceivable that leucine-enriched infant formulas in comparison to human milk exert higher leucine-, insulin-, and IGF-1-mediated stimulatory effects on mTORC1 of mesenchymal stem cells and adipocytes. In fact, it has recently been shown in S6K1−/− mice that lack the mTORC1 downstream target S6K1 impaired the generation of de novo adipocytes when these mice were challenged with a high-fat diet, consistent with a reduction in early adipocyte progenitors [103]. Thus, leucine-mediated activation of mTORC1-S6K1 signalling plays a fundamental functional and substrate role in adipogenesis and serves as a pivotal amino acid stimulator of the mTORC1-S6K1 pathway, which drives S6K1-dependent commitment of embryonic stem cells to early adipocyte progenitors, stimulates adipocyte differentiation via crosstalk with PPARγ upregulation and serves as a lipid substrate for adipocyte de novo lipid synthesis [96–103]. The importance of mTORC1-S6K1 signalling in adipogenesis becomes apparent in S6K1-deficient mice, which are protected against age- and diet-induced obesity while enhancing insulin sensitivity [88].
There is another important finding implicating mTORC1 signalling in adipogenesis. mTORC1 promotes the function of sterol (SREBP regulatory element binding transcription factor), a master regulator of lipo- and sterologenic gene transcription. mTORC1 regulates SREBP by controlling the nuclear entry of lipin 1, a phosphatidic acid phosphatase. Dephosphorylated, nuclear, catalytically active lipin-1 promotes nuclear remodelling and mediates the effects of mTORC1 on SREBP target genes, SREBP promoter activity, and nuclear SREBP protein abundance [104]. Hepatic triglycerides and SREBP-1 mRNA concentrations increased significantly in rats fed a 30% casein diet for one month [105]. Thus, mTORC1 is not only a central regulator of protein biosynthesis but also of lipid biosynthesis by regulation of SREBP-1, the key transcription factor of lipid synthesizing enzymes [106].
The recent insights into leucine-mediated mTORC1 signalling allow the conclusion that higher leucine uptake provided by higher milk protein consumption may increase general growth as well as BMI. In fact, higher milk consumption in children has been associated with increased linear growth and BMI [107, 108]. Milk-stimulated acceleration of growth may be well explained by leucine-, insulin-, and IGF-1-mediated mTORC1 activation resulting in increased differentiation of mesenchymal stem cells into adipocytes, osteoblasts, and myocytes, thus promoting adipogenesis, bone growth, as well as myogenesis [109]. In fact, high consumption of whole cow's milk in infancy has unfortunate effects on growth, especially weight acceleration and development of overweight in childhood [19, 110].
15. mTORC1 and Placental Amino Acid Transfer for Fetal Growth
mTORC1 plays a key role for trophoblast growth and differentiation and L-system-mediated amino-acid uptake of trophoblast cells, which is most important for fetal growth [111–113]. The activity of placental amino acid transporters is decreased in intrauterine growth restriction (IUGR). As mTORC1 regulates the activity of the placental L-type amino acid transporter system, the decrease of placental mTORC1 activity in IUGR just explains diminished supply of leucine for mTORC1-dependent fetal growth [114, 115]. Maternal protein restriction in rats inhibited mTORC1 signalling and downregulated placental amino acid transporters [116]. Leucine-rich maternal diet appears to be an important determinant for leucine-stimulated placental-mTORC1-dependent amino acid transfer to the fetus and thus for leucine-mTORC1-dependent fetal growth. Thus, impaired intrauterine growth by protein restriction during pregnancy may be well explained by insufficient leucine-mediated mTORC1 signalling of fetal tissues.
16. mTORC1 and Early Developmental Programming
The periods of fetal growth and postnatal growth are a continuum and are both intimately connected to prenatal and postnatal mTORC1 signalling and nutrient and especially leucine availability. After birth, maternal leucine is no longer provided by the placental leucine transport system but by the secretory leucine-rich family of mammalian milk proteins. The essential role of mTORC1 signalling for early development is illustrated by the early postimplantation lethality after disruption of the mouse mTOR gene [117]. Moreover, mTOR−/− embryos displayed a lesion in inner cell mass proliferation, consistent with the inability to establish embryonic stem cells from mTOR−/− embryos [117]. Inactivation of mTORC1 kinase activity resulted in reduced cell size, diminished cell proliferation, and cell cycle arrest of embryonic stem cells [118]. Transcriptome analysis of fetal baboon kidneys in response to maternal nutrient restriction provided further evidence that mTORC1 is involved in developmental programming of the fetal kidney [119]. Accumulating evidence underlines the important role of mTORC1-S6K1 signalling in mesenchymal stem cell development and mTORC1-dependent regulation of adipogenesis [99–102, 109]. Remarkably, deficient mesenchymal stem cell differentiation into adipocytes has been observed in S6K1−/− mice [103]. Conversely, it can be concluded that increased leucine supply during pregnancy by leucine-rich diets like increased dairy protein consumption during pregnancy may be associated with increased fetal growth and higher birth weight. Indeed, increased dairy protein consumption during pregnancy has been clearly correlated with increased size and birth weight of human neonates [120] (Figure 3).
Figure 3.
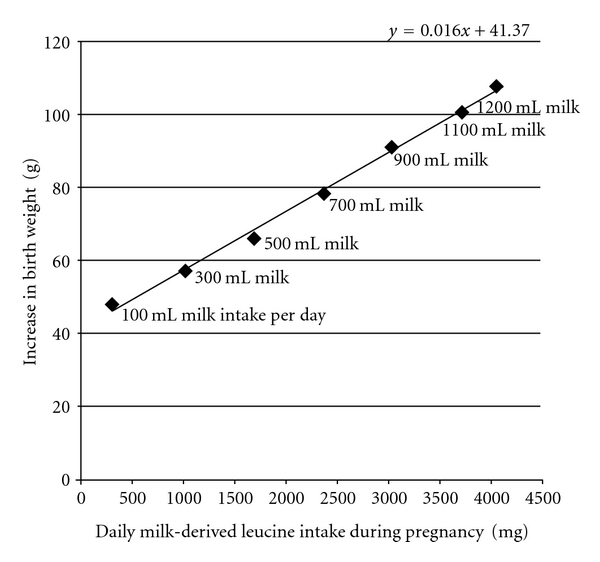
Linear correlation between milk-derived leucine intake of pregnant mothers and increase of the infants' birth weight. Data obtained from Olsen et al. [120].
Continued high leucine supply to the neonate by leucine-rich infant formula may overstimulate the mTORC1-dependent process of mesenchymal stem cell differentiation into adipocytes. Thus, human overnutrition with leucine-rich protein sources provided by Western diet during pregnancy as well as postnatal infant feeding with leucine-rich infant formula may be fundamental factors promoting higher numbers of adipocytes, a long-term adverse effect of early adipogenic programming.
17. Hypothalamic mTORC1 Signalling Controls Food Intake
There is recent concern that formula-mediated regulation of central satiety signalling deviates from natural satiety regulation of breast feeding. The anorexigenic hormone leptin is primarily synthesized by adipose tissue and reduces food intake by central action on the arcuate nucleus and the ventromedial hypothalamus [121]. It has been shown that addition of leucine to isolated rat adipocytes stimulated leptin expression via an mTORC1-dependent pathway [122]. Furthermore, long-term leucine supplementation in old rats promoted hypertrophy and hyperplasia of adipose tissue associated with increased mTORC1/S6K1 activation [123]. Serum leptin levels correlate with adipose tissue levels in both animals and humans and show a stronger correlation with fat mass than total body mass [124]. A dietary pattern that includes high fat consumption accompanied with high amounts of BCAAs appears to contribute to obesity-associated insulin resistance and increased serum leptin levels [92]. Thus, leucine appears to act as a signal nutrient that relays adipocte leptin release, which finally exerts leptin-mediated central regulations of food intake. Intriguingly, recent observations have suggested a potential role for mTORC1 as a cellular fuel sensor in the hypothalamic circuits that regulate energy balance [125, 126]. mTORC1 is critically involved in mediating leptin-induced anorexia [127]. Under physiological conditions, increased hypothalamic availability of leucine stimulates mTORC1 activity and reduces food intake in an mTORC1-dependent manner. Hypothalamic mTORC1 activity is also required for the suppression of feeding by leptin [127]. However, long-term activation of the mTORC1 pathway by deletion of TSC1 with Pomc/Cre (Pomc-Tsc1cKO mice) impairs the function of anorectic Pomc neurons and induced hyperphagic obesity [126]. Recent evidence points to a complex cell-type-dependent mTORC1-mediated regulation in the basomedial hypothalamus by leptin and nutritional status [128]. Remarkably, rats maintained on a high-fat diet had no anorexic response to intracerebroventricular leptin [129]. Under high-fat diet, leptin was unable to modulate hypothalamic mTORC1 signalling [129]. CNS leptin resistance by obesigenic diets is therefore, thought to override CNS regulatory circuits otherwise assuring the maintenance of energy balance. Long-term abnormally high leucine intake provided by infant formula may thus induce a state of chronically imbalanced leptin-mTORC1 signalling promoting “orexigenic metabolic programming” of mTORC1-dependent hypothalamic regulatory networks.
18. Conclusions
There is substantial evidence that breast feeding in comparison to cow-milk-based formulas supplies much lower amounts of leucine to the infant and thus transduces lower leucine-mediated stimulatory effects on mTORC1 signalling of the neonate (Figure 4). Infant formula feeding in comparison to breast feeding results in excessive serum levels of leucine, insulin, and IGF-1, explaining exaggerated mTORC1-dependent early adipogenic programming, the promoting mechanism for early onset of childhood obesity. In comparison to lower levels of mTORC1 signalling mediated by human milk, available cow-milk-based infant formulas transduce increased mTORC1 signalling. Although recent efforts are made to decrease the formulas' total protein content [130, 131], leucine levels even of these low-protein formulas still exceed leucine amounts of human milk [39]. The preparation of whey-predominant infant formula and the addition of increased amounts of bovine α-lactalbumin allow a reduction of total protein content [130, 132–134], however, these procedures do not normalize the elevated leucine content, as whey proteins are carriers of high amounts of leucine. In fact, breast feeding is associated with less weight gain per months (595 g) in comparison to standard formula (730 g) or α-lactalbumin-enriched formula (707 g), respectively [132].
Figure 4.
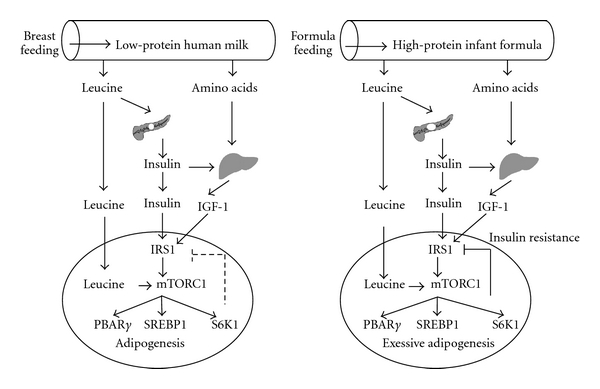
Comparison between physiologic human milk-mediated mTORC1 signalling and exaggerated adipogenic mTORC1 signalling by feeding leucine-rich infant formula (abbreviations see Figure 1).
There are further most serious concerns of the adipogenic impact of formula feeding as mothers often use higher than recommended amounts of formula powder for preparing infant meals. Furthermore, it is not uncommon that parents switch too early from low protein preformula to higher protein followon formulas to keep their infants asleep during the night. Moreover, the total daily volume of formula given to the infant is not naturally restricted in comparison to daily limitations of milk volume by breast feeding. As the postnatal phase is the most critical period for metabolic programming, it is a serious matter of public health to increase the compliance for breast feeding and to provide much more supervision and education for parents feeding formula.
Exaggerated formula-induced mTORC1 signalling appears to be the most critical factor for the early development of childhood obesity and other mTORC1-driven chronic diseases of civilization especially T2D and cancer [135–139]. Future postnatal feeding studies in animals, preferably primates, should clarify the effect of increased versus physiologic leucine intake on adipose tissue development as well as central regulations of leucine- and leptin-mediated food intake. Moreover, future attention should be paid to the regulatory mechanism directing dietary leucine either to adipogenesis or myogenesis, which may be an important aspect in early metabolic programming with special regard to the recently recognized molecular cross-talk between adipose tissue and skeletal muscle [140]. Closely regulated dietary leucine fluxes into various tissues may be the most critical determinant during the neonatal growth period when skeletal muscle activity and myogenesis are less mind controlled. The proper adjustment of the mTORC1-driven signalling axis by leucine restriction of infant formula to physiologic leucine levels of human milk offers a new and most promising chance for the prevention of early adipogenic programming and the epidemic of childhood obesity.
Conflict of Interests
The author has no conflict of interest to declare.
Abbreviations
- Akt:
Akt kinase (=protein kinase B, PKB)
- AMP:
Adenosine monophosphate
- AMPK:
AMP-activated protein kinase
- BCAAs:
Branched-chain essential amino acids
- 4E-BP1:
Eukaryotic initiation factor (eIF) 4E-binding protein 1
- IGF-1:
Insulin-like growth factor 1
- IGF1R:
IGF-1 receptor
- IR:
Insulin receptor
- IRS-1:
Insulin receptor substrate 1
- IUGR:
Intrauterine growth restriction
- LAT1:
L-type amino acid transporter 1
- mTORC1:
Mammalian target of rapamycin complex 1
- PI3K:
Phosphoinositol-3 kinase
- PPARγ:
Peroxisome proliferator-activated receptor gamma
- Raptor:
Regulatory-associated protein of mTOR
- Rictor:
Rapamycin-insensitive companion of mTOR
- Rheb:
Ras homolog enriched in brain
- S6K1:
p70 S6 kinase 1
- SNAT2:
Sodium-coupled neutral amino acid transporter 2
- SREBP:
Sterol regulatory element-binding transcription factor
- TSC1:
Tuberous sclerosis complex 1 (hamartin)
- TSC2:
Tuberous sclerosis complex 2 (tuberin)
References
- 1.Troiano RP, Flegal KM. Overweight children and adolescents: description, epidemiology, and demographics. Pediatrics. 1998;101(3, supplement 2):497–504. [PubMed] [Google Scholar]
- 2.Zhao J, Grant SFA. Genetics of Childhood Obesity. Journal of Obesity. 2011;2011:9 pages. doi: 10.1155/2011/845148. Article ID 845148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yaturu S. Obesity and type 2 diabetes. Journal of Diabetes Mellitus. 2011;1(4):79–95. [Google Scholar]
- 4.Thorn J, Waller M, Johansson M, Marild S. Overweight among four-year-old children in relation to early growth characteristics and socioeconomic factors. Journal of Obesity. 2010;2010:6 pages. doi: 10.1155/2010/580642. Article ID 580642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armitage JA, Taylor PD, Poston L. Experimental models of developmental programming: consequences of exposure to an energy rich diet during development. The Journal of Physiology. 2005;565(1):3–8. doi: 10.1113/jphysiol.2004.079756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kramer MS. Do breast-feeding and delayed introduction of solid foods protect against subsequent obesity? The Journal of Pediatrics. 1981;98(6):883–887. doi: 10.1016/s0022-3476(81)80579-3. [DOI] [PubMed] [Google Scholar]
- 7.Von Kries R, Koletzko B, Sauerwald T, et al. Breast feeding and obesity: cross sectional study. British Medical Journal. 1999;318(7203):147–150. doi: 10.1136/bmj.319.7203.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ziegler EE. Growth of breast-fed and formula-fed infants. Nestlé Nutrition Institute Workshop Series Pediatric Program. 2006;(58):51–59. doi: 10.1159/000095010. [DOI] [PubMed] [Google Scholar]
- 9.Koletzko B, Von Kries R, Closa R, et al. Lower protein in infant formula is associated with lower weight up to age 2 y: a randomized clinical trial. The American Journal of Clinical Nutrition. 2009;89(6):1836–1845. doi: 10.3945/ajcn.2008.27091. [DOI] [PubMed] [Google Scholar]
- 10.Koletzko B, Von Kries R, Closa R, Escribano J. Can infant feeding choices modulate later obesity risk? The American Journal of Clinical Nutrition. 2009;89(5):1502S–1508S. doi: 10.3945/ajcn.2009.27113D. [DOI] [PubMed] [Google Scholar]
- 11.Axelsson I. Effects of high protein intakes. Nestlé Nutrition Institute Workshop Series Pediatric Program. 2006;58:121–129. doi: 10.1159/000095025. [DOI] [PubMed] [Google Scholar]
- 12.Premji SS, Fenton TR, Sauve RS. Higher versus lower protein intake in formula-fed low birth weight infants. Cochrane Database of Systematic Reviews. 2006;(1) doi: 10.1002/14651858.CD003959.pub2. Article ID CD003959. [DOI] [PubMed] [Google Scholar]
- 13.Pico C, Jilkova ZM, Kus V, Palou A, Kopecky J. Perinatal programming of body weight control by leptin: putative roles of AMP kinase and muscle thermogenesis. The American Journal of Clinical Nutrition. 2011;94(6):1830S–1837S. doi: 10.3945/ajcn.110.000752. [DOI] [PubMed] [Google Scholar]
- 14.McArdle HJ, Andersen HS, Jones H, Gambling L. Fetal programming: causes and consequences as revealed by studies of dietary manipulation in rats—a review. Placenta. 2006;27(supplement):56–60. doi: 10.1016/j.placenta.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Maurer AD, Chen Q, McPherson C, Reimer RA. Changes in satiety hormones and expression of genes involved in glucose and lipid metabolism in rats weaned onto diets high in fibre or protein reflect susceptibility to increased fat mass in adulthood. The Journal of Physiology. 2009;587(3):679–691. doi: 10.1113/jphysiol.2008.161844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maurer AD, Reimer RA. Maternal consumption of high-prebiotic fibre or -protein diets during pregnancy and lactation differentially influences satiety hormones and expression of genes involved in glucose and lipid metabolism in offspring in rats. British Journal of Nutrition. 2011;105(3):329–338. doi: 10.1017/S0007114510003533. [DOI] [PubMed] [Google Scholar]
- 17.Maurer AD, Eller LK, Hallam MC, Taylor K, Reimer RA. Consumption of diets high in prebiotic fiber or protein during growth influences the response to a high fat and sucrose diet in adulthood in rats. Nutrition & Metabolism. 2010;7, article 77 doi: 10.1186/1743-7075-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rooney K, Ozanne SE. Maternal over-nutrition and offspring obesity predisposition: targets for preventative interventions. International Journal of Obesity. 2011;35(7):883–890. doi: 10.1038/ijo.2011.96. [DOI] [PubMed] [Google Scholar]
- 19.Larnkjær A, Hoppe C, Mølgaard C, Michaelsen KF. The effects of whole milk and infant formula on growth and IGF-I in late infancy. European Journal of Clinical Nutrition. 2009;63(8):956–963. doi: 10.1038/ejcn.2008.80. [DOI] [PubMed] [Google Scholar]
- 20.Hoppe C, Udam TR, Lauritzen L, Mølgaard C, Juul A, Michaelsen KF. Animal protein intake, serum insulin-like growth factor I, and growth in healthy 2.5-y-old Danish children. The American Journal of Clinical Nutrition. 2004;80(2):447–452. doi: 10.1093/ajcn/80.2.447. [DOI] [PubMed] [Google Scholar]
- 21.Hoppe C, Mølgaard C, Juul A, Michaelsen KF. High intakes of skimmed milk, but not meat, increase serum IGF-I and IGFBP-3 in eight-year-old boys. European Journal of Clinical Nutrition. 2004;58(9):1211–1216. doi: 10.1038/sj.ejcn.1601948. [DOI] [PubMed] [Google Scholar]
- 22.Hoppe C, Mølgaard C, Vaag A, Barkholt V, Michaelsen KF. High intakes of milk, but not meat, increase s-insulin and insulin resistance in 8-year-old boys. European Journal of Clinical Nutrition. 2005;59(3):393–398. doi: 10.1038/sj.ejcn.1602086. [DOI] [PubMed] [Google Scholar]
- 23.Hoppe C, Mølgaard C, Dalum C, Vaag A, Michaelsen KF. Differential effects of casein versus whey on fasting plasma levels of insulin, IGF-1 and IGF-1/IGFBP-3: results from a randomized 7-day supplementation study in prepubertal boys. European Journal of Clinical Nutrition. 2009;63(9):1076–1083. doi: 10.1038/ejcn.2009.34. [DOI] [PubMed] [Google Scholar]
- 24.Rich-Edwards JW, Ganmaa D, Pollak MN, et al. Milk consumption and the prepubertal somatotropic axis. Nutrition Journal. 2007;6, article 28 doi: 10.1186/1475-2891-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crowe FL, Key TJ, Allen NE, et al. The association between diet and serum concentrations of IGF-I, IGFBP-1, IGFBP-2, and IGFBP-3 in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiology Biomarkers and Prevention. 2009;18(5):1333–1340. doi: 10.1158/1055-9965.EPI-08-0781. [DOI] [PubMed] [Google Scholar]
- 26.Mølgaard C, Larnkjær A, Arnberg K, Michaelsen KF. Milk and growth in children: effects of whey and casein. Nestlé Nutrition Institute Workshop Series Pediatric Program. 2011;67:67–78. doi: 10.1159/000325576. [DOI] [PubMed] [Google Scholar]
- 27.Smith PJ, Wise LS, Berkowitz R, Wan C, Rubin CS. Insulin-like growth factor-I is an essential regulator of the differentiation of 3T3-L1 adipocytes. The Journal of Biological Chemistry. 1988;263(19):9402–9408. [PubMed] [Google Scholar]
- 28.Blüher S, Kratzsch J, Kiess W. Insulin-like growth factor I, growth hormone and insulin in white adipose tissue. Best Practice & Research Clinical Endocrinology & Metabolism. 2005;19(4):577–587. doi: 10.1016/j.beem.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 29.Kwon G, Marshall CA, Pappan KL, Remedi MS, McDaniel ML. Signaling elements involved in the metabolic regulation of mTOR by nutrients, incretins, and growth factors in islets. Diabetes. 2004;53(supplement 3):S225–S232. doi: 10.2337/diabetes.53.suppl_3.s225. [DOI] [PubMed] [Google Scholar]
- 30.McDaniel ML, Marshall CA, Pappan KL, Kwon G. Metabolic and autocrine regulation of the mammalian target of rapamycin by pancreatic β-cells. Diabetes. 2002;51(10):2877–2885. doi: 10.2337/diabetes.51.10.2877. [DOI] [PubMed] [Google Scholar]
- 31.Kiess W, Petzold S, Töpfer M, et al. Adipocytes and adipose tissue. Best Practice & Research in Clinical Endocrinology & Metabolism. 2008;22(1):135–153. doi: 10.1016/j.beem.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Melnik BC. Milk signalling in the pathogenesis of type 2 diabetes. Medical Hypotheses. 2011;76(4):553–559. doi: 10.1016/j.mehy.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 33.Lemay DG, Lynn DJ, Martin WF, et al. The bovine lactation genome: insights into the evolution of mammalian milk. Genome Biology. 2009;10(4, article R43) doi: 10.1186/gb-2009-10-4-r43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nommsen LA, Lovelady CA, Heinig MJ, Lonnerdal B, Dewey KG. Determinants of energy, protein, lipid, and lactose concentrations in human milk during the first 12 mo of lactation: the DARLING Study. The American Journal of Clinical Nutrition. 1991;53(2):457–465. doi: 10.1093/ajcn/53.2.457. [DOI] [PubMed] [Google Scholar]
- 35.Sánchez López CL, Hernández A, Rodríguez AB, Rivero M, Barriga C, Cubero J. Nitrogen and protein content analysis of human milk, diurnality vs nocturnality. Nutricion Hospitalaria. 2011;26(3):511–514. doi: 10.1590/S0212-16112011000300012. [DOI] [PubMed] [Google Scholar]
- 36.Bounous G, Kongshavn PAL, Taveroff A, Gold P. Evolutionary traits in human milk proteins. Medical Hypotheses. 1988;27(2):133–140. doi: 10.1016/0306-9877(88)90158-2. [DOI] [PubMed] [Google Scholar]
- 37.Davis TA, Nguyen HV, Garcia-Bravo R, et al. Amino acid composition of human milk is not unique. Journal of Nutrition. 1994;124(7):1126–1132. doi: 10.1093/jn/124.7.1126. [DOI] [PubMed] [Google Scholar]
- 38.Gordon HH, Levin SZ, McNamara H. Feeding of premature infants: a comparison of human and cow’s milk. American Journal of Diseases of Children. 1947;73(4):442–452. doi: 10.1001/archpedi.1947.02020390054002. [DOI] [PubMed] [Google Scholar]
- 39.Socha P, Grote V, Gruszfeld D, et al. Milk protein intake, the metabolic-endocrine response, and growth in infancy: data from a randomized clinical trial. The American Journal of Clinical Nutrition. 2011;94(6):1776S–1784S. doi: 10.3945/ajcn.110.000596. [DOI] [PubMed] [Google Scholar]
- 40.Millward DJ, Layman DK, Tomé D, Schaafsma G. Protein quality assessment: impact of expanding understanding of protein and amino acid needs for optimal health. The American Journal of Clinical Nutrition. 2008;87(5):1576S–1581S. doi: 10.1093/ajcn/87.5.1576S. [DOI] [PubMed] [Google Scholar]
- 41.Lönnerdal B, Lien EL. Nutritional and physiologic significance of α-lactalbumin in infants. Nutrition Reviews. 2003;61(9):295–305. doi: 10.1301/nr.2003.sept.295-305. [DOI] [PubMed] [Google Scholar]
- 42.Nilsson M, Holst JJ, Björck IME. Metabolic effects of amino acid mixtures and whey protein in healthy subjects: studies using glucose-equivalent drinks. The American Journal of Clinical Nutrition. 2007;85(4):996–1004. doi: 10.1093/ajcn/85.4.996. [DOI] [PubMed] [Google Scholar]
- 43.Hara K, Yonezawa K, Weng QP, Kozlowski MT, Belham C, Avruch J. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. The Journal of Biological Chemistry. 1998;273(23):14484–14494. doi: 10.1074/jbc.273.23.14484. [DOI] [PubMed] [Google Scholar]
- 44.Inoki K, Ouyang H, Li Y, Guan KL. Signaling by target of rapamycin proteins in cell growth control. Microbiology and Molecular Biology Reviews. 2005;69(1):79–100. doi: 10.1128/MMBR.69.1.79-100.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Avruch J, Long X, Ortiz-Vega S, Rapley J, Papageorgiou A, Dai N. Amino acid regulation of TOR complex 1. American Journal of Physiology—Endocrinology and Metabolism. 2009;296(4):E592–E602. doi: 10.1152/ajpendo.90645.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suzuki T, Inoki K. Spatial regulation of the mTORC1 system in amino acids sensing pathway. Acta Biochimica et Biophysica Sinica. 2011;43(9):671–679. doi: 10.1093/abbs/gmr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X, Proud CG. mTORC1 signaling: what we still don't know. Journal of Molecular Cell Biology. 2011;3(4):206–220. doi: 10.1093/jmcb/mjq038. [DOI] [PubMed] [Google Scholar]
- 48.Proud CG. A New Link in the Chain from Amino Acids to mTORC1 Activation. Molecular Cell. 2011;44(1):7–8. doi: 10.1016/j.molcel.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 49.Kim J, Guan K-L. Amino acid signaling in TOR activation. Annual Review of Biochemistry. 2011;80:1001–1032. doi: 10.1146/annurev-biochem-062209-094414. [DOI] [PubMed] [Google Scholar]
- 50.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nature Reviews Molecular Cell Biology. 2011;12(1):21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang X, Proud CG. Nutrient control of TORC1, a cell-cycle regulator. Trends in Cell Biology. 2009;19(6):260–267. doi: 10.1016/j.tcb.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 52.Shaw RJ. LKB1 and AMP-activated protein kinase control of mTOR signalling and growth. Acta Physiologica. 2009;196(1):65–80. doi: 10.1111/j.1748-1716.2009.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nature Cell Biology. 2002;4(9):648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 54.Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/Akt pathway. Molecular Cell. 2002;10(1):151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 55.Tee AR, Fingar DC, Manning BD, Kwiatkowski DJ, Cantley LC, Blenis J. Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(21):13571–13576. doi: 10.1073/pnas.202476899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115(5):577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 57.Gwinn DM, Shackelford DB, Egan DF, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Molecular Cell. 2008;30(2):214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sancak Y, Peterson TR, Shaul YD, et al. The rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320(5882):1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan KL. Regulation of TORC1 by Rag GTPases in nutrient response. Nature Cell Biology. 2008;10(8):935–945. doi: 10.1038/ncb1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141(2):290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goberdhan DCI. Intracellular amino acid sensing and mTORC1-regulated growth: new ways to block an old target? Current Opinion in Investigational Drugs. 2010;11(12):1360–1367. [PMC free article] [PubMed] [Google Scholar]
- 62.Li L, Kim E, Yuan H, et al. Regulation of mTORC1 by the Rab and Arf GTPases. The Journal of Biological Chemistry. 2010;285(26):19705–19709. doi: 10.1074/jbc.C110.102483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Proud CG. A New Link in the Chain from Amino Acids to mTORC1 Activation. Molecular Cell. 2011;44(1):7–8. doi: 10.1016/j.molcel.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 64.Duran A, Amanchy R, Linares JF, et al. P62 is a key regulator of nutrient sensing in the mTORC1 pathway. Molecular Cell. 2011;44(1):134–146. doi: 10.1016/j.molcel.2011.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H+-ATPase. Science. 2011;334(6056):678–683. doi: 10.1126/science.1207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mackenzie B, Erickson JD. Sodium-coupled neutral amino acid (System N/A) transporters of the SLC38 gene family. Pflugers Archiv European Journal of Physiology. 2004;447(5):784–795. doi: 10.1007/s00424-003-1117-9. [DOI] [PubMed] [Google Scholar]
- 67.Edinger AL, Thompson CB. An activated mTOR mutant supports growth factor-independent, nutrient-dependent cell survival. Oncogene. 2004;23(33):5654–5663. doi: 10.1038/sj.onc.1207738. [DOI] [PubMed] [Google Scholar]
- 68.Peyrollier K, Hajduch E, Blair AS, Hyde R, Hundal HS. L-leucine availability regulates phosphatidylinositol 3-kinase, p70 s6 kinase and glycogen synthase kinase-3 activity in L6 muscle cells: evidence for the involvement of the mammalian target of rapamycin (mTOR) pathway in the L-leucine-induced up-regulation of System A amino acid transport. Biochemical Journal. 2000;350(2):361–368. [PMC free article] [PubMed] [Google Scholar]
- 69.Xia Y, Wen HY, Young ME, Guthrie PH, Taegtmeyer H, Kellems RE. Mammalian target of rapamycin and protein kinase A signaling mediate the cardiac transcriptional response to glutamine. The Journal of Biological Chemistry. 2003;278(15):13143–13150. doi: 10.1074/jbc.M208500200. [DOI] [PubMed] [Google Scholar]
- 70.Fumarola C, La Monica S, Guidotti GG. Amino acid signaling through the mammalian target of rapamycin (mTOR) pathway: role of glutamine and of cell shrinkage. Journal of Cellular Physiology. 2005;204(1):155–165. doi: 10.1002/jcp.20272. [DOI] [PubMed] [Google Scholar]
- 71.Yanagida O, Kanai Y, Chairoungdua A, et al. Human L-type amino acid transporter 1 (LAT1): characterization of function and expression in tumor cell lines. Biochimica et Biophysica Acta—Biomembranes. 2001;1514(2):291–302. doi: 10.1016/s0005-2736(01)00384-4. [DOI] [PubMed] [Google Scholar]
- 72.Fuchs BC, Bode BP. Amino acid transporters ASCT2 and LAT1 in cancer: partners in crime? Seminars in Cancer Biology. 2005;15(4):254–266. doi: 10.1016/j.semcancer.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 73.Nicklin P, Bergman P, Zhang B, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136(3):521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johnson DJ, Anderson GH. Prediction of plasma amino acid concentration from diet amino acid content. The American Journal of Physiology. 1982;243(1):R99–103. doi: 10.1152/ajpregu.1982.243.1.R99. [DOI] [PubMed] [Google Scholar]
- 75.Östman EM, Liljeberg Elmståhl HGM, Björck IME. Inconsistency between glycemic and insulinemic responses to regular and fermented milk products. The American Journal of Clinical Nutrition. 2001;74(1):96–100. doi: 10.1093/ajcn/74.1.96. [DOI] [PubMed] [Google Scholar]
- 76.Hoyt G, Hickey MS, Cordain L. Dissociation of the glycaemic and insulinaemic responses to whole and skimmed milk. British Journal of Nutrition. 2005;93(2):175–177. doi: 10.1079/bjn20041304. [DOI] [PubMed] [Google Scholar]
- 77.Yang J, Chi Y, Burkhardt BR, Guan Y, Wolf BA. Leucine metabolism in regulation of insulin secretion from pancreatic beta cells. Nutrition Reviews. 2010;68(5):270–279. doi: 10.1111/j.1753-4887.2010.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vasavada RC, Gonzalez-Pertusa JA, Fujinaka Y, Fiaschi-Taesch N, Cozar-Castellano I, Garcia-Ocaña A. Growth factors and beta cell replication. International Journal of Biochemistry & Cell Biology. 2006;38(5-6):931–950. doi: 10.1016/j.biocel.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 79.Xu G, Kwon G, Cruz WS, Marshall CA, McDaniel ML. Metabolic regulation by leucine of translation initiation through the mTOR-signaling pathway by pancreatic β-cells. Diabetes. 2001;50(2):353–360. doi: 10.2337/diabetes.50.2.353. [DOI] [PubMed] [Google Scholar]
- 80.Xu G, Kwon G, Marshall CA, Lin TA, Lawrence JC, McDaniel ML. Branched-chain amino acids are essential in the regulation of PHAS-I and p70 S6 kinase by pancreatic β-cells: a possible role in protein translation and mitogenic signaling. The Journal of Biological Chemistry. 1998;273(43):28178–28184. doi: 10.1074/jbc.273.43.28178. [DOI] [PubMed] [Google Scholar]
- 81.Koyanagi M, Asahara S, Matsuda T, et al. Ablation of TSC2 enhances insulin secretion by increasing the number of mitochondria through activation of mTORC1. PLoS One. 2011;6(8) doi: 10.1371/journal.pone.0023238. Article ID e23238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pende M, Kozma SC, Jaquet M, et al. Hypoinsulinaemia, glucose intolerance and diminished β-cell size in S6K1-deficient mice. Nature. 2000;408(6815):994–997. doi: 10.1038/35050135. [DOI] [PubMed] [Google Scholar]
- 83.Martinez JA, Ballew MP. Infant formulas. Pediatrics in Review. 2011;32(5):179–189. doi: 10.1542/pir.32-5-179. [DOI] [PubMed] [Google Scholar]
- 84.Alexy U, Kersting M, Sichert-Hellert W, Manz F, Schöch G. Macronutrient intake of 3- to 36-month-old German infants and children: results of the DONALD Study. Annals of Nutrition & Metabolism. 1999;43(1):14–22. doi: 10.1159/000012762. [DOI] [PubMed] [Google Scholar]
- 85.Zick Y. Ser/Thr phosphorylation of IRS proteins: a molecular basis for insulin resistance. Science's Signal Transduction Knowledge Environment. 2005;2005(268, pe4) doi: 10.1126/stke.2682005pe4. [DOI] [PubMed] [Google Scholar]
- 86.Boura-Halfon S, Zick Y. Phosphorylation of IRS proteins, insulin action, and insulin resistance. American Journal of Physiology—Endocrinology and Metabolism. 2009;296(4):E581–E591. doi: 10.1152/ajpendo.90437.2008. [DOI] [PubMed] [Google Scholar]
- 87.Um SH, D’Alessio D, Thomas G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metabolism. 2006;3(6):393–402. doi: 10.1016/j.cmet.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 88.Um SH, Frigerio F, Watanabe M, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431(7005):200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 89.Krebs M, Brunmair B, Brehm A, et al. The mammalian target of rapamycin pathway regulates nutrient-sensitive glucose uptake in man. Diabetes. 2007;56(6):1600–1607. doi: 10.2337/db06-1016. [DOI] [PubMed] [Google Scholar]
- 90.Tremblay F, Krebs M, Dombrowski L, et al. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes. 2005;54(9):2674–2684. doi: 10.2337/diabetes.54.9.2674. [DOI] [PubMed] [Google Scholar]
- 91.Saha AK, Xu XJ, Lawson E, et al. Downregulation of AMPK accompanies leucine- and glucose-induced increases in protein synthesis and insulin resistance in rat skeletal muscle. Diabetes. 2010;59(10):2426–2434. doi: 10.2337/db09-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metabolism. 2009;9(4):311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kappeler L, De Magalhaes Filho C, Leneuve P, et al. Early postnatal nutrition determines somatotropic function in mice. Endocrinology. 2009;150(1):314–323. doi: 10.1210/en.2008-0981. [DOI] [PubMed] [Google Scholar]
- 94.Rosenthal J, Angel A, Farkas J. Metabolic fate of leucine: a significant sterol precursor in adipose tissue and muscle. American Journal of Physiology. 1974;226(2):411–418. doi: 10.1152/ajplegacy.1974.226.2.411. [DOI] [PubMed] [Google Scholar]
- 95.Lynch CJ, Fox HL, Vary TC, Jefferson LS, Kimball SR. Regulation of amino acid-sensitive TOR signaling by leucine analogues in adipocytes. Journal of Cellular Biochemistry. 2000;77(2):234–251. doi: 10.1002/(sici)1097-4644(20000501)77:2<234::aid-jcb7>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 96.Lynch CJ. Role of leucine in the regulation of mTOR by amino acids: revelations from structure-activity studies. Journal of Nutrition. 2001;131(3):861S–865S. doi: 10.1093/jn/131.3.861S. [DOI] [PubMed] [Google Scholar]
- 97.Lynch CJ, Gern B, Lloyd C, Hutson SM, Eicher R, Vary TC. Leucine in food mediates some of the postprandial rise in plasma leptin concentrations. American Journal of Physiology—Endocrinology and Metabolism. 2006;291(3):E621–E630. doi: 10.1152/ajpendo.00462.2005. [DOI] [PubMed] [Google Scholar]
- 98.Pham PTT, Heydrick SJ, Fox HL, Kimball SR, Jefferson LS, Lynch CJ. Assessment of cell-signaling pathways in the regulation of mammalian target of rapamycin (mTOR) by amino acids in rat adipocytes. Journal of Cellular Biochemistry. 2000;79(3):427–441. doi: 10.1002/1097-4644(20001201)79:3<427::aid-jcb80>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 99.Kim JE, Chen J. Regulation of peroxisome proliferator-activated receptor-γ activity by mammalian target of rapamycin and amino acids in adipogenesis. Diabetes. 2004;53(11):2748–2756. doi: 10.2337/diabetes.53.11.2748. [DOI] [PubMed] [Google Scholar]
- 100.Fox HL, Kimball SR, Jefferson LS, Lynch CJ. Amino acids stimulate phosphorylation of p70(S6k) and organization of rat adipocytes into multicellular clusters. American Journal of Physiology—Cell Physiology. 1998;274(1):C206–C213. doi: 10.1152/ajpcell.1998.274.1.C206. [DOI] [PubMed] [Google Scholar]
- 101.Fox HL, Pham PT, Kimball SR, Jefferson LS, Lynch CJ. Amino acid effects on translational repressor 4E-BP1 are mediated primarily by L-leucine in isolated adipocytes. American Journal of Physiology—Cell Physiology. 1998;275(5):C1232–C1238. doi: 10.1152/ajpcell.1998.275.5.C1232. [DOI] [PubMed] [Google Scholar]
- 102.Zhang HH, Huang J, Düvel K, et al. Insulin stimulates adipogenesis through the Akt-TSC2-mTORC1 pathway. PLoS One. 2009;4(7) doi: 10.1371/journal.pone.0006189. Article ID e6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Carnevalli LS, Masuda K, Frigerio F, et al. S6K1 plays a critical role in early adipocyte differentiation. Developmental Cell. 2010;18(5):763–774. doi: 10.1016/j.devcel.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Peterson TR, Sengupta SS, Harris TE, et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146(3):408–420. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tovar AR, Torres N. The role of dietary protein on lipotoxicity. Biochimica et Biophysica Acta—Molecular and Cell Biology of Lipids. 2010;1801(3):367–371. doi: 10.1016/j.bbalip.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 106.Porstmann T, Santos CR, Lewis C, Griffiths B, Schulze A. A new player in the orchestra of cell growth: SREBP activity is regulated by mTORC1 and contributes to the regulation of cell and organ size. Biochemical Society Transactions. 2009;37(1):278–283. doi: 10.1042/BST0370278. [DOI] [PubMed] [Google Scholar]
- 107.Hoppe C, Mølgaard C, Michaelsen KF. Cow’s milk and linear growth in industrialized and developing countries. Annual Review of Nutrition. 2006;26:131–173. doi: 10.1146/annurev.nutr.26.010506.103757. [DOI] [PubMed] [Google Scholar]
- 108.Wiley AS. Dairy and milk consumption and child growth: is BMI involved? An analysis of NHANES 1999–2004. American Journal of Human Biology. 2010;22(4):517–525. doi: 10.1002/ajhb.21042. [DOI] [PubMed] [Google Scholar]
- 109.Xiang X, Zhao J, Xu G, Li Y, Zhang W. mTOR and the differentiation of mesenchymal stem cells. Acta Biochimica et Biophysica Sinica. 2011;43(7):501–510. doi: 10.1093/abbs/gmr041. [DOI] [PubMed] [Google Scholar]
- 110.Thorsdottir I, Thorisdottir AV. Whole cow's milk in early life. Nestlé Nutrition Institute Workshop Serries Pediatric Program. 2011;67:29–40. doi: 10.1159/000325573. [DOI] [PubMed] [Google Scholar]
- 111.Wen HY, Abbasi S, Kellems RE, Xia Y. mTOR: a placental growth signaling sensor. Placenta. 2005;26(supplement):S63–S69. doi: 10.1016/j.placenta.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 112.Pollheimer J, Knöfler M. Signalling pathways regulating the invasive differentiation of human trophoblasts: a review. Placenta. 2005;26(supplement):S21–S30. doi: 10.1016/j.placenta.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 113.Roos S, Lagerlöf O, Wennergren M, Powell TL, Jansson T. Regulation of amino acid transporters by glucose and growth factors in cultured primary human trophoblast cells is mediated by mTOR signaling. American Journal of Physiology—Cell Physiology. 2009;297(3):C723–C731. doi: 10.1152/ajpcell.00191.2009. [DOI] [PubMed] [Google Scholar]
- 114.Roos S, Jansson N, Palmberg I, Säljö K, Powell TL, Jansson T. Mammalian target of rapamycin in the human placenta regulates leucine transport and is down-regulated in restricted fetal growth. The Journal of Physiology. 2007;582(1):449–459. doi: 10.1113/jphysiol.2007.129676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Roos S, Powell TL, Jansson T. Placental mTOR links maternal nutrient availability to fetal growth. Biochemical Society Transactions. 2009;37(1):295–298. doi: 10.1042/BST0370295. [DOI] [PubMed] [Google Scholar]
- 116.Rosario FJ, Jansson N, Kanai Y, Prasad PD, Powell TL, Jansson T. Maternal protein restriction in the rat inhibits placental insulin, mTOR, and STAT3 signaling and down-regulates placental amino acid transporters. Endocrinology. 2011;152(3):1119–1129. doi: 10.1210/en.2010-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gangloff YG, Mueller M, Dann SG, et al. Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Molecular and Cellular Biology. 2004;24(21):9508–9516. doi: 10.1128/MCB.24.21.9508-9516.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Murakami M, Ichisaka T, Maeda M, et al. mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells. Molecular and Cellular Biology. 2004;24(15):6710–6718. doi: 10.1128/MCB.24.15.6710-6718.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nijland MJ, Schlabritz-loutsevitch NE, Hubbard GB, Nathanielsz PW, Cox LA. Non-human primate fetal kidney transcriptome analysis indicates mammalian target of rapamycin (mTOR) is a central nutrient-responsive pathway. The Journal of Physiology. 2007;579(3):643–656. doi: 10.1113/jphysiol.2006.122101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Olsen SF, Halldorsson TI, Willett WC, et al. Milk consumption during pregnancy is associated with increased infant size at birth: prospective cohort study. The American Journal of Clinical Nutrition. 2007;86(4):1104–1110. doi: 10.1093/ajcn/86.4.1104. [DOI] [PubMed] [Google Scholar]
- 121.Cripps RL, Martin-Gronert MS, Ozanne SE. Fetal and perinatal programming of appetite. Clinical Science. 2005;109(1):1–11. doi: 10.1042/CS20040367. [DOI] [PubMed] [Google Scholar]
- 122.Roh C, Han J, Tzatsos A, Kandror KV. Nutrient-sensing mTOR-mediated pathway regulates leptin production in isolated rat adipocytes. American Journal of Physiology—Endocrinology and Metabolism. 2003;284(2):E322–E330. doi: 10.1152/ajpendo.00230.2002. [DOI] [PubMed] [Google Scholar]
- 123.Zeanandin G, Balage M, Schneider SM, et al. Differential effect of long-term leucine supplementation on skeletal muscle and adipose tissue in old rats: an insulin signaling pathway approach. Age. 34(2):371–387. doi: 10.1007/s11357-011-9246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liuzzi A, Savia G, Tagliaferri M, et al. Serum leptin concentration in moderate and severe obesity: relationship with clinical, anthropometric and metabolic factors. International Journal of Obesity. 1999;23(10):1066–1073. doi: 10.1038/sj.ijo.0801036. [DOI] [PubMed] [Google Scholar]
- 125.Catania C, Binder E, Cota D. mTORC1 signaling in energy balance and metabolic disease. International Journal of Obesity. 2010 doi: 10.1038/ijo.2010.208. [DOI] [PubMed] [Google Scholar]
- 126.Mori H, Inoki K, Münzberg H, et al. Critical role for hypothalamic mTOR activity in energy balance. Cell Metabolism. 2009;9(4):362–374. doi: 10.1016/j.cmet.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cota D, Proulx K, Blake Smith KA, et al. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312(5775):927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 128.Villanueva EC, Münzberg H, Cota D, et al. Complex regulation of mammalian target of rapamycin complex 1 in the basomedial hypothalamus by leptin and nutritional status. Endocrinology. 2009;150(10):4541–4551. doi: 10.1210/en.2009-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cota D, Matter EK, Woods SC, Seeley RJ. The role of hypothalamic mammalian target of rapamycin complex 1 signaling in diet-induced obesity. Journal of Neuroscience. 2008;28(28):7202–7208. doi: 10.1523/JNEUROSCI.1389-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Räihä NCR, Fazzolari-Nesci A, Cajozzo C, et al. Whey predominant, whey modified infant formula with protein/energy ratio of 1.8 g/100 kcal: adequate and safe for term infants from birth to four months. Journal of Pediatric Gastroenterology and Nutrition. 2002;35(3):275–281. doi: 10.1097/00005176-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 131.Hernell O. Human milk vs. cow's milk and the evolution of infant formulas. Nestlé Nutrition Institute Workshop Series Padiatric Program. 2011;67:17–28. doi: 10.1159/000325572. [DOI] [PubMed] [Google Scholar]
- 132.Sandström O, Lönnerdal B, Graverholt G, Hernell O. Effects of α-lactalbumin-enriched formula containing different concentrations of glycomacropeptide on infant nutrition. The American Journal of Clinical Nutrition. 2008;87(4):921–928. doi: 10.1093/ajcn/87.4.921. [DOI] [PubMed] [Google Scholar]
- 133.Lien EL. Infant formulas with increased concentrations of alpha-lactalbumin. The American Journal of Clinical Nutrition. 2003;77(6) doi: 10.1093/ajcn/77.6.1555S. [DOI] [PubMed] [Google Scholar]
- 134.Heine WE, Klein PD, Reeds PJ. The importance of α-lactalbumin in infant nutrition. Journal of Nutrition. 1991;121(3):277–283. doi: 10.1093/jn/121.3.277. [DOI] [PubMed] [Google Scholar]
- 135.Proud CG. mTOR signalling in health and disease. Biochemical Society Transactions. 2011;39(2):431–436. doi: 10.1042/BST0390431. [DOI] [PubMed] [Google Scholar]
- 136.Mieulet V, Lamb RF. Tuberous sclerosis complex: linking cancer to metabolism. Trends in Molecular Medicine. 2010;16(7):329–335. doi: 10.1016/j.molmed.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 137.Dann SG, Selvaraj A, Thomas G. mTOR Complex1-S6K1 signaling: at the crossroads of obesity, diabetes and cancer. Trends in Molecular Medicine. 2007;13(6):252–259. doi: 10.1016/j.molmed.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 138.Melnik BC. Milk signalling in the pathogenesis of type 2 diabetes. In: Lewis BS, Flugelman MY, Halon DA, editors. In: Proceedings of the 9th International Congress on Coronary Artery Disease; October 2011; Bologna, Italy. pp. 17–22. Medimond International Proceedings. [Google Scholar]
- 139.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441(7092):424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 140.Trayhurn P, Drevon CA, Eckel J. Secreted proteins from adipose tissue and skeletal muscle—Adipokines, myokines and adipose/muscle cross-talk. Archives of Physiology and Biochemistry. 2011;117(2):47–56. doi: 10.3109/13813455.2010.535835. [DOI] [PubMed] [Google Scholar]


