Abstract
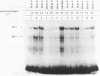
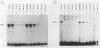
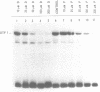
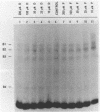
We investigated the effects of the antiviral agent distamycin A and of a distamycin derivative (FCE 24517) which possesses antineoplastic activity on the binding of some regulatory proteins to DNA. Both compounds inhibited the binding to DNA of the ubiquitous octamer binding factor OTF 1 and of the erythroid specific GATAAG protein (NFE 1). This was shown using the electrophoretic mobility shift assay on a DNA fragment of human gamma-globin gene promoter (-156 to -201), on the same fragment with a point mutation (T to C mutation) known to have an increased affinity of binding for NFE 1, on a DNA fragment of human histone H2B promoter and on a DNA fragment of mouse alpha 1 globin promoter. The ability of distamycin or of FCE 24517 to inhibit the binding was specific for AT-rich sequences since neither drug inhibited the binding of nuclear protein factors to the sequence CCACACCC of the human beta globin gene. Binding to DNA was investigated by evaluating the drugs' ability to protect selected sequences from DNase I digestion (DNase footprinting). Distamycins binding was highly preferential for DNA sequences containing stretches of AT. These studies indicate that chemicals which have a high degree of DNA sequence-specific binding can selectively inhibit the binding of regulatory proteins to DNA. These effects might be responsible for modification of the transcription of specific genes and might to some extent account for these drugs' antiviral and antineoplastic activities. This approach offers potential for the investigation of new such drugs.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARCAMONE F., PENCO S., OREZZI P., NICOLELLA V., PIRELLI A. STRUCTURE AND SYNTHESIS OF DISTAMYCIN A. Nature. 1964 Sep 5;203:1064–1065. doi: 10.1038/2031064a0. [DOI] [PubMed] [Google Scholar]
- Carbon P., Murgo S., Ebel J. P., Krol A., Tebb G., Mattaj L. W. A common octamer motif binding protein is involved in the transcription of U6 snRNA by RNA polymerase III and U2 snRNA by RNA polymerase II. Cell. 1987 Oct 9;51(1):71–79. doi: 10.1016/0092-8674(87)90011-0. [DOI] [PubMed] [Google Scholar]
- Casazza A. M., Fioretti A., Ghione M., Soldati M., Verini M. A. Distamycin A, a new antiviral antibiotic. Antimicrob Agents Chemother (Bethesda) 1965;5:593–598. [PubMed] [Google Scholar]
- DIMARCO A., GAETANI M., OREZZI P., SCOTTIT, ARCAMONE F. Experimental studies on distamycin A--a new antibiotic with cytotoxic activity. Cancer Chemother Rep. 1962 May;18:15–19. [PubMed] [Google Scholar]
- DePamphilis M. L. Transcriptional elements as components of eukaryotic origins of DNA replication. Cell. 1988 Mar 11;52(5):635–638. doi: 10.1016/0092-8674(88)90398-4. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopka M. L., Yoon C., Goodsell D., Pjura P., Dickerson R. E. The molecular origin of DNA-drug specificity in netropsin and distamycin. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1376–1380. doi: 10.1073/pnas.82.5.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low C. M., Drew H. R., Waring M. J. Echinomycin and distamycin induce rotation of nucleosome core DNA. Nucleic Acids Res. 1986 Sep 11;14(17):6785–6801. doi: 10.1093/nar/14.17.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
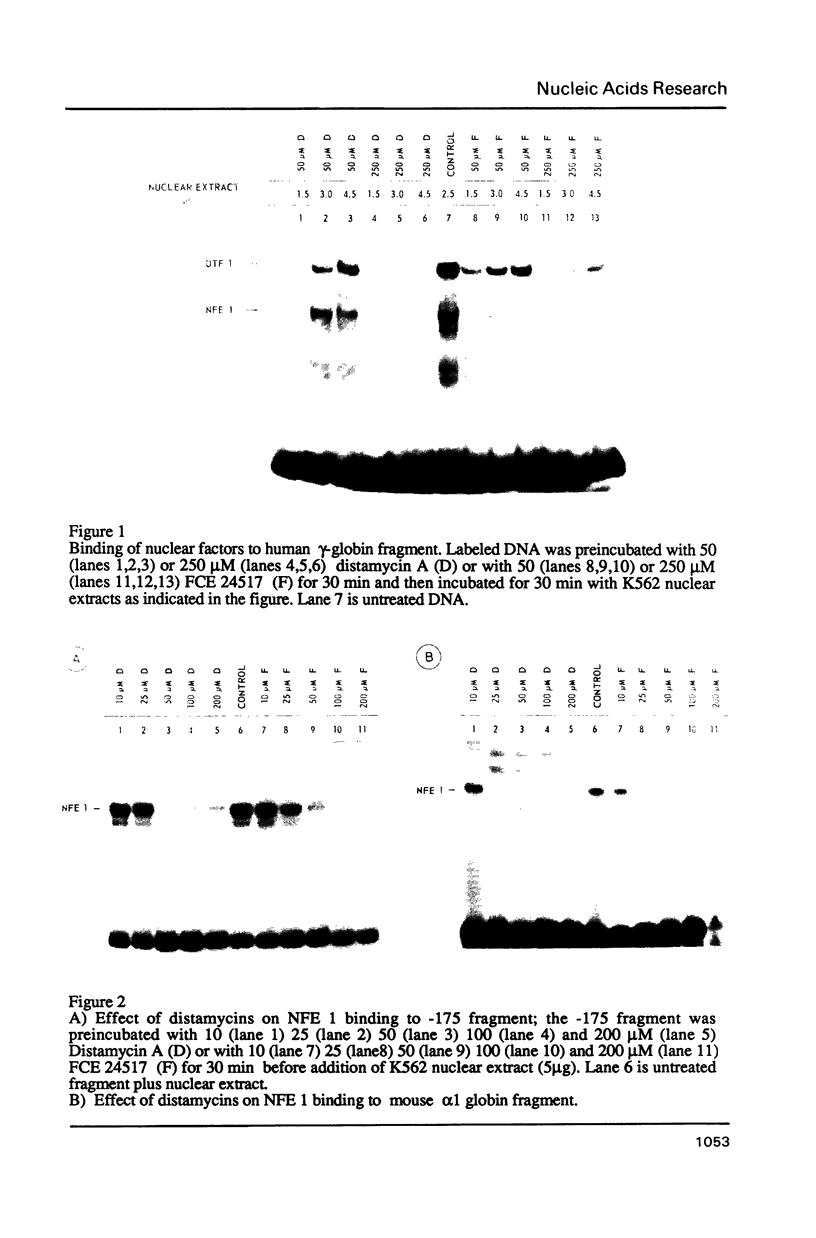
- Mantovani R., Malgaretti N., Giglioni B., Comi P., Cappellini N., Nicolis S., Ottolenghi S. A protein factor binding to an octamer motif in the gamma-globin promoter disappears upon induction of differentiation and hemoglobin synthesis in K562 cells. Nucleic Acids Res. 1987 Nov 25;15(22):9349–9364. doi: 10.1093/nar/15.22.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani R., Malgaretti N., Nicolis S., Giglioni B., Comi P., Cappellini N., Bertero M. T., Caligaris-Cappio F., Ottolenghi S. An erythroid specific nuclear factor binding to the proximal CACCC box of the beta-globin gene promoter. Nucleic Acids Res. 1988 May 25;16(10):4299–4313. doi: 10.1093/nar/16.10.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal J., Waring M. J. Interaction of nucleosome core particles with distamycin and echinomycin: analysis of the effect of DNA sequences. Nucleic Acids Res. 1987 Feb 11;15(3):885–903. doi: 10.1093/nar/15.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
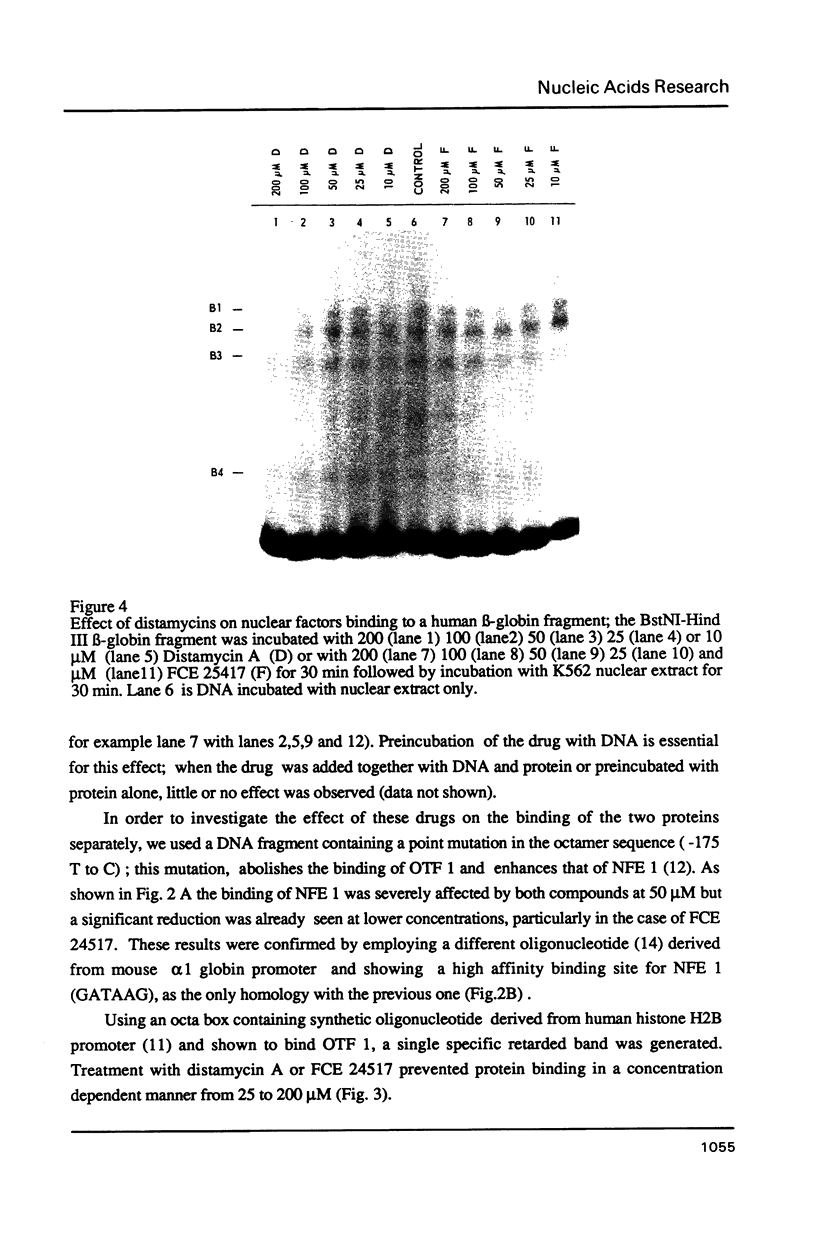
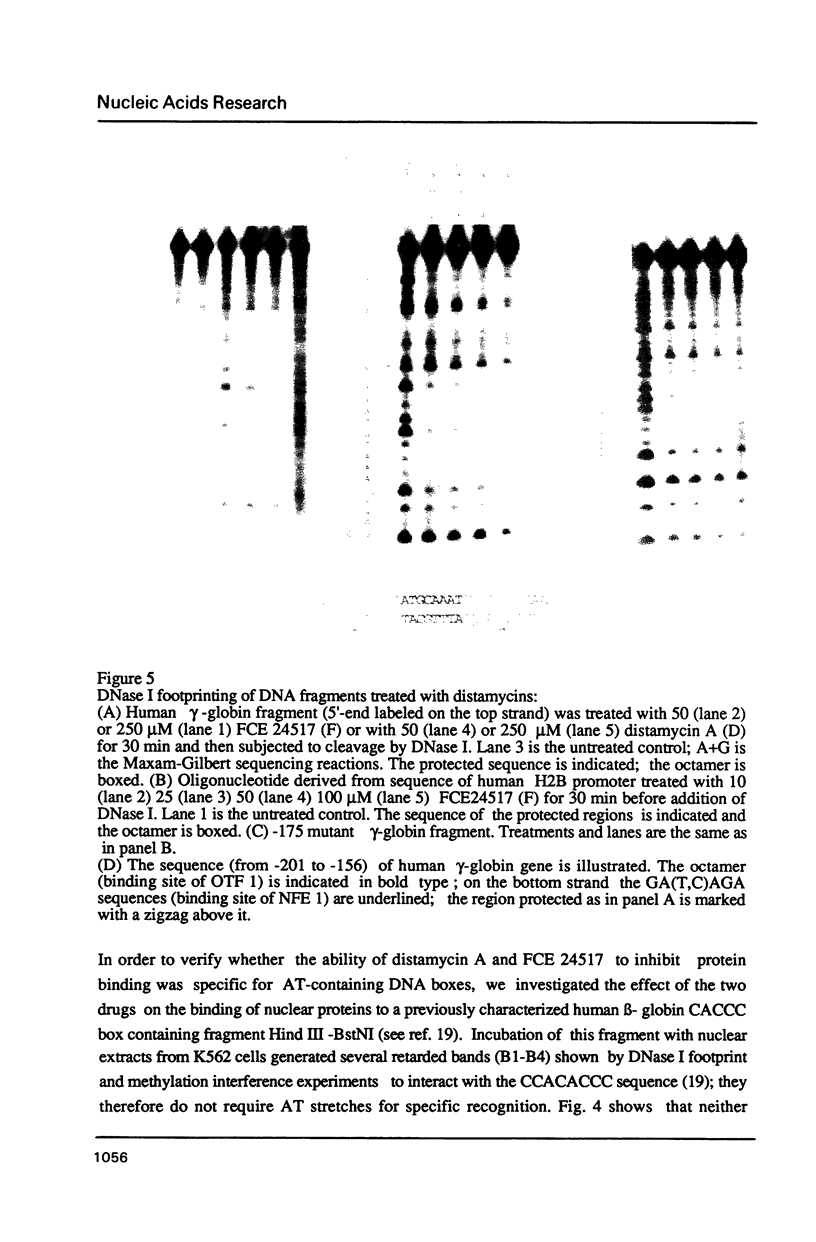
- Pruijn G. J., van Driel W., van Miltenburg R. T., van der Vliet P. C. Promoter and enhancer elements containing a conserved sequence motif are recognized by nuclear factor III, a protein stimulating adenovirus DNA replication. EMBO J. 1987 Dec 1;6(12):3771–3778. doi: 10.1002/j.1460-2075.1987.tb02712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz A., Galas D. J. The interaction of RNA polymerase and lac repressor with the lac control region. Nucleic Acids Res. 1979 Jan;6(1):111–137. doi: 10.1093/nar/6.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., Sen R., Baltimore D., Sharp P. A. A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature. 1986 Jan 9;319(6049):154–158. doi: 10.1038/319154a0. [DOI] [PubMed] [Google Scholar]
- Sive H. L., Roeder R. G. Interaction of a common factor with conserved promoter and enhancer sequences in histone H2B, immunoglobulin, and U2 small nuclear RNA (snRNA) genes. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6382–6386. doi: 10.1073/pnas.83.17.6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt L. M., Singh H., Sen R., Wirth T., Sharp P. A., Baltimore D. A lymphoid-specific protein binding to the octamer motif of immunoglobulin genes. Nature. 1986 Oct 16;323(6089):640–643. doi: 10.1038/323640a0. [DOI] [PubMed] [Google Scholar]
- Wingender E. Compilation of transcription regulating proteins. Nucleic Acids Res. 1988 Mar 25;16(5):1879–1902. doi: 10.1093/nar/16.5.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]