Abstract
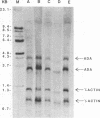
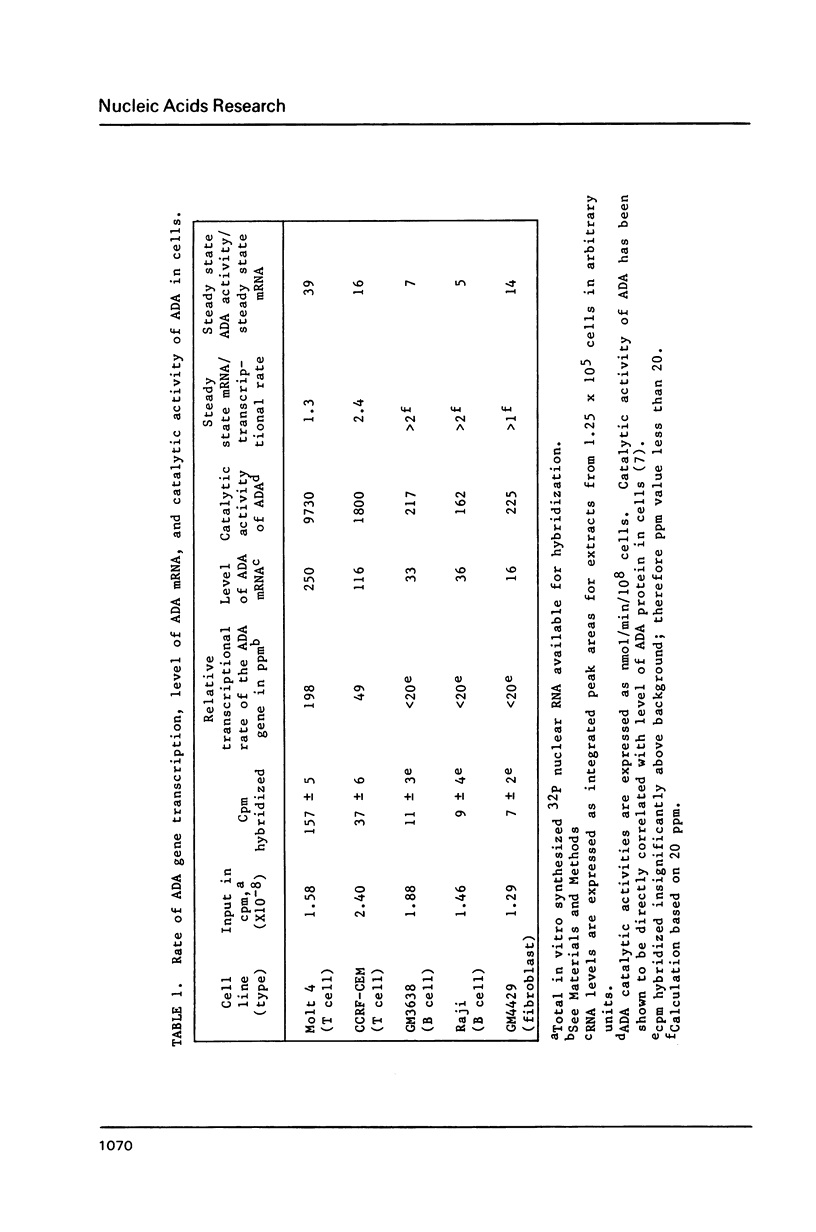
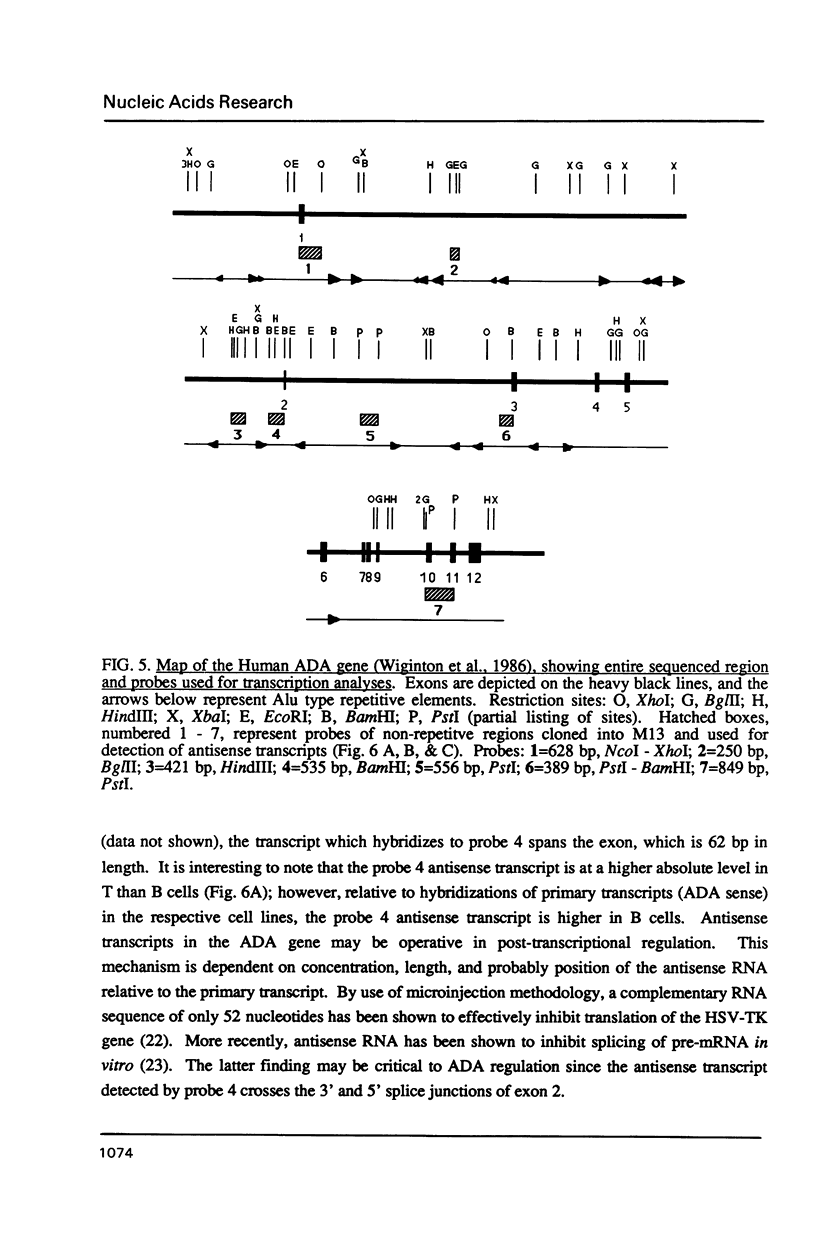
The relative rates of transcription of the human adenosine deaminase (ADA) gene were determined in isolated nuclei from T and B lymphoblasts and skin fibroblasts. ADA gene transcription occurs at higher rates in T cells than in B cells and fibroblasts. Relative steady state ADA mRNA levels were also determined for each cell line, and these values were found to correlate with relative rates of transcription of the gene. Therefore, the primary mechanism for control of expression of this ubiquitous enzyme is at the level of transcription. The ratios of ADA enzymatic activity to specific mRNA content were also compared between cell lines. The B lymphoblasts exhibited lower ratios than did the T lymphoblasts, suggesting that rates of protein degradation were several fold greater in B than in T lymphoblast cell lines. This finding is consistent with previous direct measurements of ADA protein turnover. Differential rates of protein turnover in B as compared to T cells provide a secondary mechanism for the regulation of ADA expression. In addition to transcription initiation being the major control mechanism of steady state ADA mRNA in all cell lines, first intron elongation pausing occurs in fibroblasts, and discrete regions of RNA polymerase II and RNA polymerase III antisense transcripts are observed in all cell lines studied.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian G. S., Hutton J. J. Adenosine deaminase messenger RNAs in lymphoblast cell lines derived from leukemic patients and patients with hereditary adenosine deaminase deficiency. J Clin Invest. 1983 Jun;71(6):1649–1660. doi: 10.1172/JCI110920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian G. S., Wiginton D. A., Hutton J. J. Characterization of normal and mutant adenosine deaminase messenger RNAs by translation and hybridization to a cDNA probe. Hum Genet. 1984;68(2):169–172. doi: 10.1007/BF00279309. [DOI] [PubMed] [Google Scholar]
- Adrian G. S., Wiginton D. A., Hutton J. J. Structure of adenosine deaminase mRNAs from normal and adenosine deaminase-deficient human cell lines. Mol Cell Biol. 1984 Sep;4(9):1712–1717. doi: 10.1128/mcb.4.9.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender T. P., Thompson C. B., Kuehl W. M. Differential expression of c-myb mRNA in murine B lymphomas by a block to transcription elongation. Science. 1987 Sep 18;237(4821):1473–1476. doi: 10.1126/science.3498214. [DOI] [PubMed] [Google Scholar]
- Berger F. G., Loose D., Meisner H., Watson G. Androgen induction of messenger RNA concentrations in mouse kidney is posttranscriptional. Biochemistry. 1986 Mar 11;25(5):1170–1175. doi: 10.1021/bi00353a034. [DOI] [PubMed] [Google Scholar]
- Berkvens T. M., Schoute F., van Ormondt H., Meera Khan P., van der Eb A. J. Adenosine deaminase gene expression is regulated posttranscriptionally in the nucleus. Nucleic Acids Res. 1988 Apr 25;16(8):3255–3268. doi: 10.1093/nar/16.8.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chechik B. E., Schrader W. P., Minowada J. An immunomorphologic study of adenosine deaminase distribution in human thymus tissue, normal lymphocytes, and hematopoietic cell lines. J Immunol. 1981 Mar;126(3):1003–1007. [PubMed] [Google Scholar]
- Coleman M. S., Hutton J. J. Micromethod for quantitation of adenosine deaminase activity in cells from human peripheral blood. Biochem Med. 1975 May;13(1):46–55. doi: 10.1016/0006-2944(75)90139-8. [DOI] [PubMed] [Google Scholar]
- Daddona P. E. Human adenosine deaminase. Properties and turnover in cultured T and B lymphoblasts. J Biol Chem. 1981 Dec 10;256(23):12496–12501. [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Giblett E. R., Anderson J. E., Cohen F., Pollara B., Meuwissen H. J. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972 Nov 18;2(7786):1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- Green P. J., Pines O., Inouye M. The role of antisense RNA in gene regulation. Annu Rev Biochem. 1986;55:569–597. doi: 10.1146/annurev.bi.55.070186.003033. [DOI] [PubMed] [Google Scholar]
- Lis J. T., Neckameyer W., Dubensky R., Costlow N. Cloning and characterization of nine heat-shock-induced mRNAs of Drosophila melanogaster. Gene. 1981 Oct;15(1):67–80. doi: 10.1016/0378-1119(81)90105-0. [DOI] [PubMed] [Google Scholar]
- Martin D. W., Jr, Gelfand E. W. Biochemistry of diseases of immunodevelopment. Annu Rev Biochem. 1981;50:845–877. doi: 10.1146/annurev.bi.50.070181.004213. [DOI] [PubMed] [Google Scholar]
- Marzluff W. F., Jr, Murphy E. C., Jr, Huang R. C. Transcription of ribonucleic acid in isolated mouse myeloma nuclei. Biochemistry. 1973 Aug 28;12(18):3440–3446. doi: 10.1021/bi00742a013. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- Mory Y. Y., Gefter M. L. Synthesis of messenger RNA-like molecules in isolated myeloma nuclei. Nucleic Acids Res. 1977 Jun;4(6):1739–1757. doi: 10.1093/nar/4.6.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munroe S. H. Antisense RNA inhibits splicing of pre-mRNA in vitro. EMBO J. 1988 Aug;7(8):2523–2532. doi: 10.1002/j.1460-2075.1988.tb03100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepveu A., Marcu K. B. Intragenic pausing and anti-sense transcription within the murine c-myc locus. EMBO J. 1986 Nov;5(11):2859–2865. doi: 10.1002/j.1460-2075.1986.tb04580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanstrom R., Shank P. R. X-Ray Intensifying Screens Greatly Enhance the Detection by Autoradiography of the Radioactive Isotopes 32P and 125I. Anal Biochem. 1978 May;86(1):184–192. doi: 10.1016/0003-2697(78)90333-0. [DOI] [PubMed] [Google Scholar]
- Wiginton D. A., Adrian G. S., Friedman R. L., Suttle D. P., Hutton J. J. Cloning of cDNA sequences of human adenosine deaminase. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7481–7485. doi: 10.1073/pnas.80.24.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiginton D. A., Adrian G. S., Hutton J. J. Sequence of human adenosine deaminase cDNA including the coding region and a small intron. Nucleic Acids Res. 1984 Mar 12;12(5):2439–2446. doi: 10.1093/nar/12.5.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiginton D. A., Kaplan D. J., States J. C., Akeson A. L., Perme C. M., Bilyk I. J., Vaughn A. J., Lattier D. L., Hutton J. J. Complete sequence and structure of the gene for human adenosine deaminase. Biochemistry. 1986 Dec 16;25(25):8234–8244. doi: 10.1021/bi00373a017. [DOI] [PubMed] [Google Scholar]