Abstract
OBJECTIVE:
The aim of this study was to measure dynamic lung hyperinflation and its influence on dyspnea perception in moderate and severe chronic obstructive pulmonary disease patients after performing activities of daily living.
METHODS:
We measured inspiratory capacity, sensation of dyspnea, peripheral oxygen saturation, heart rate and respiratory rate in 19 chronic obstructive pulmonary disease patients. These measurements were taken at rest and after performing activities of daily living (e.g., going up and down a set of stairs, going up and down a ramp and sweeping and mopping a room).
RESULT:
The inspiratory capacity of patients at rest was significantly decreased compared to the capacity of patients after performing activities. The change in inspiratory capacity was -0.67 L after going up and down a ramp, -0.46 L after sweeping and mopping a room, and -0.55 L after climbing up and down a set of stairs. Dyspnea perception increased significantly between rest, sweeping and mopping, and going up and down a set of stairs. Dyspnea perception correlated positively with inspiratory capacity variation (r = 0.85) and respiratory rate (r = 0.37) and negatively with peripheral oxygen saturation (r = -0.28).
CONCLUSION:
Chronic obstructive pulmonary disease patients exhibited reductions in inspiratory capacity and increases in dyspnea perception during commonly performed activities of daily living, which may limit physical performance in these patients.
Keywords: COPD, Activities of daily living, Pulmonary hyperinflation, Exercise
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality worldwide. In COPD patients, structural changes occur in the lung parenchyma, including remodeling, airway obstruction, destruction of the alveolar septa and cellular communication, increases in air space volume distal to the terminal bronchioles, and decreases in pulmonary elastic recoil. These changes interfere with the structures that allow airways to remain open during expiration (1-3). These disease-related changes cause air trapping and difficulty in air removal, leading to airflow limitation and pulmonary hyperinflation, which increases functional residual capacity/total lung capacity and residual volume/total lung capacity ratios (4,5).
Dyspnea is the main symptom in COPD patients and the first motivation for individuals to seek medical help. As the disease progresses, the intensity of dyspnea increases and leads to an anxiety state and a deterioration in quality of life. Many COPD patients experience dyspnea even during activities of daily living, such as walking and dressing. There are several mechanisms responsible for dyspnea during exercise in COPD. Among them, the most significant includes the ineffectiveness of respiratory muscle function, which leads to increased ventilation requirements and dynamic pulmonary hyperinflation (1,3,6). It is well known that increased ventilatory demand results in dynamic lung hyperinflation, thus increasing the sensation of dyspnea during exercise and significantly contributing to reductions in physical performance (4). Dynamic hyperinflation occurs in COPD patients and can be measured by a simple method that does not require sophisticated equipment and that should be part of routine evaluations of COPD patients.
It is well described that dynamic pulmonary hyperinflation occurs in COPD patients during incremental exercise tests (5,7). It has also been shown that COPD patients develop lung hyperinflation during simple activities, such as lifting pots and storing them in overhead bins (8). However, whether this phenomenon occurs during the activities of daily living that COPD patients can currently accomplish has not yet been addressed. We hypothesize that some specific activities of daily living induce dynamic pulmonary hyperinflation in COPD patients. Thus, the objective of this study was to measure dynamic pulmonary hyperinflation and its influence on the sensation of dyspnea in COPD patients with moderate to very severe airway obstruction during the performance of activities of daily living.
MATERIALS AND METHODS
This was a cross-sectional study that evaluated moderate to very severe COPD patients according to GOLD 2010 (9). The study was approved by the Ethics Committee of UNIFESP (CEP No. 0353/03). We included 19 COPD patients (13 males) with moderate to very severe degrees of airway obstruction, with a mean post-bronchodilator forced expiratory volume in the first second (FEV1) of 48.7±7.1% predicted and a mean age of 64.1±5.3 years. Demographic and pulmonary function data are displayed in Table 1.
Table 1.
Anthropometric, pulmonary function, hemodynamic and respiratory baseline characteristics of the COPD patients in this study.
| Variables | Pre-bronchodilator | Post-bronchodilator |
| Age (years) | 64.1±5.3 | - |
| BMI (kg/m2) | 28.0±3.0 | - |
| BDI | 8.0±2.0 | - |
| BODE | 2.0±1.0 | - |
| Smoking history (pack-years) | 37.7±13.1 | - |
| FEV1 (%) | 46.4±9.1 | 48.7±7.1 |
| FVC (%) | 71.2±13.0 | 81.4±11.2 |
| FEV1/FVC (%) | 65.5±8.2 | 62.3±6.8 |
| IC (L) | 2.4± 0.1 | 2.5±0.1 |
| IC (%) | 70.8±1.6 | 72.8±1.3 |
| Heart rate (bpm) | 88.1±10.3 | - |
| Blood pressure (mmHg) | 125.8±13.5 | - |
| Respiratory rate (rpm) | 27.6±3.9 | - |
| SpO2 (%) | 93.1±4.2 | - |
Data are presented as the means±standard deviations.
BMI = Body mass index; BDI = Baseline dyspnea index; BODE = Body mass index, airway obstruction, dyspnea and exercise index; FEV1 = Forced expiratory volume in 1 second; FVC = Forced vital capacity, FEV1/FVC = forced expiratory volume in 1 second/forced vital capacity ratio; IC = Inspiratory capacity; SpO2 = arterial oxyhemoglobin saturation.
The inclusion criteria were: COPD patients diagnosed with moderate to very severe airway obstruction according to the GOLD classification (9) and clinical stability with no clinical exacerbation during the 30 days prior to the beginning of the study. Furthermore, all participants were required to sign an informed consent form. The exclusion criteria were: inability to perform the slow vital capacity maneuver, excessive perceived dyspnea during the test, arterial oxyhemoglobin saturation (SpO2)≤80% (7) during activities and/or the need for oxygen supplementation. All recruited patients participated in the study, and no dropouts occurred.
Protocol
All COPD patients enrolled in the study were part of the cardiopulmonary rehabilitation program of the São Paulo Adventist University Center Policlinic. All COPD patients were taught in a simple manner to perform the proposed activities of daily living (namely, walking up and down two sets of stairs for one minute, walking twice up and down a ramp 40 m in length with an 11% incline and sweeping and mopping in two rooms that were 12 m2 each).
Heart rate (HR) was monitored with Polar® heart rate monitors (model FS1, Sao Paulo, Brazil), respiratory frequency (f) was measured by observing the elevation and depression of the chest for a period of 60 seconds, and blood pressure was monitored with the patients in the sitting position with an aneroid sphygmomanometer (Glicomed Aneróide Premium®, São Paulo, Brazil) and a stethoscope (Littmann Classic II®, São Paulo, Brazil) placed directly over the brachial artery, thereby allowing the examiner to hear the Korotkoff sounds as the cuff deflated. SpO2 was monitored using a Nonin Onyx® pulse oximeter (model Onyx 9500, Minnesota, USA) placed on the finger of the patient, and the Borg scale (10) was used to verify dyspnea and fatigue in the patients' legs (0 represented the slightest sensation of dyspnea and fatigue, and 10 represented the maximum sensation). Patients were first taught about the Borg scale, and they were then asked to enumerate their dyspnea and lower-limb fatigue scores during the study.
Spirometry was performed using an EASY ONE® spirometer (model frontline, Boston, USA) in the forced (FVC) and slow (SVC) vital capacity modalities. After calibration of the equipment, data such as name, age, sex, race, weight, height, smoking habits, and diagnosis of asthma were recorded. Spirometry was accomplished according to the ATS criteria (11). The forced vital capacity (FVC) modality was used to obtain the diagnosis and the severity of disease for each patient. With the patient in the sitting position, he/she was asked to place the mouthpiece inside his/her mouth and to put on a nose clip to prevent any air outflow leakage. Through a verbal stimulus, each patient was asked to inhale deeply to his/her limit and then to make a maximum expiration as quickly as possible. The slow vital capacity (SVC) modality was used to obtain the inspiratory capacity (IC). For this measurement, patients were first asked to breathe normally into the mouthpiece; then, when requested, a maximal inspiration followed by a slow maximal expiration was performed.
The activities of daily living (walking up and down two sets of stairs for one minute, walking twice up and down a ramp 40 m in length with an incline of 11% and sweeping and mopping two rooms that each had an area of 12 m2) were performed in a random sequence. COPD patients were instructed not to use any bronchodilators before performing the activities (four hours for short-term medications and 12 hours for long-term medications).
At the end of each activity and with the patient seated, heart rate, respiratory rate, blood pressure, and oxyhemoglobin saturation were measured. Additionally, patients were asked to score the sensations of dyspnea and fatigue in their lower limbs according to the Borg scale; afterward, a slow vital capacity maneuver was performed to obtain their inspiratory capacities. The activities could either be performed on the same day or on a different day. If performed on the same day, an interval period between each activity was set as at least 40 minutes or until the HR, f, IC, dyspnea, and fatigue parameters had returned to baseline values.
For the IC measurements, each patient used a nasal clip and breathed through a mouthpiece connected to the circuit of the KoKo® equipment (Koko Spirometer, PDS Instrumentation, Lousville, Kentucky, USA⦆), which numerically and graphically recorded tidal volume values. When stability of the end-expiratory volume was reached, the patient was asked to inspire up to his/her total pulmonary capacity. A minimum of three maneuvers up to a maximum of eight were performed; to be considered reproducible, two curves could not present a variation greater than 5% or 0.15 L (12,13). Inspiratory capacity was registered as the value measured between the line of the end-expiratory volume and total pulmonary capacity. The higher value of two reproducible curves was considered for analysis. Measurement of inspiratory capacity was performed within two minutes after spirometry using the slow vital capacity maneuver.
Pulmonary hyperinflation was considered when, after the spirometry, a reduction in inspiratory capacity of 10% and/or 0.15 L compared to the basal value was observed (14).
Up and down stairs (UDS)
COPD patients were instructed to walk up and down two sets of stairs totaling 20 steps (each step was 0.18 m high and 0.33 m deep) at a comfortable speed over a period of one minute. This time was chosen because we believe that this is the time required for a healthy individual to perform this activity in his/her day-to-day life. If they wished, they could stop climbing the stairs; however, the timer was not stopped. When the patient decided to continue with the activity until the end of the test period, the time at which the patient stopped and returned to complete the activity was noted.
Up and down a ramp (UDR)
The patient was instructed to go up and down a ramp 40 m in length with an average slope of 11% twice at a comfortable pace (totaling 160 m in ascent and descent). The baseline time for task completion was not pre-determined.
Sweeping and mopping in two rooms with areas of 12 m2 each (SM)
Prior to this activity, we spread an equivalent of 0.5 kg of wet sand in each room. The patient was asked to sweep the room floors with a broom and then mop them with a wet cloth in a squeegee. The activity was terminated when the patient perceived that the rooms were clean. We recommended that the activity should be performed at a comfortable pace.
During each activity, each patient was accompanied by one investigator who asked him/her how he/she felt and if he/she was able to continue testing. The patient was informed that he/she could stop the test at any time.
The activities could be performed either on the same day or on a different day; it was stipulated that either an interval of 40 minutes of rest or enough time for the IC values, dyspnea and fatigue to return to baseline should pass before activities recommenced. The time interval between activities was chosen according to previous publications from our group (15,16).
Statistical analysis
The data are expressed as the means and standard deviations. To analyze the normality of the data, we used the Kolmogorov-Smirnov test. We used analysis of variance with repeated measurements (ANOVA with the Bonferroni post hoc test) to analyze IC, dyspnea, fatigue and SpO2 in the three different activities (walking up and down the ramp, sweeping and mopping and walking up and down the stairs). Pearson's correlation test was used to establish correlations between IC variability and dyspnea. We considered p<0.05 to be statistically significant. The sample size was calculated based on the outcome variable (IC) from the equation, namely the expected effect/standard deviation (E/S) ratio. In this study, the IC expected effect chosen was 0.15 L (minimal clinical difference to diagnose pulmonary hyperinflation), and the sample standard deviation considered was the IC variability after performance of the activities (e.g., 0.15 L). Therefore, for an α = 0.05 and a statistical power of β = 0.2, seventeen COPD patients were needed to achieve outcomes significance.
RESULTS
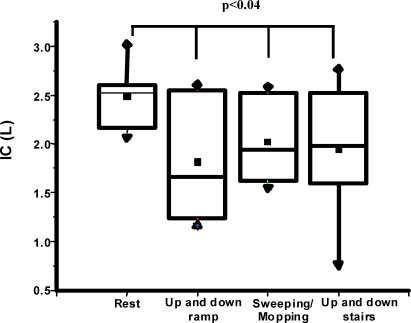
There was a significant reduction in IC after the performance of activities compared to resting IC values (p = 0.04). The changes in IC were 0.67±0.15 L/- 19.1±1.0 % (p = 0.003) after walking up and down the ramp, -0.46±0.15 L/- 13.1±0.9 % (p = 0.05) after sweeping and mopping and -0.55±0.14 L/-15.7±1.1 (p = 0.002) after walking up and down the stairs. Six, one, and six COPD patients had the greatest degree of pulmonary hyperinflation after walking up and down the ramp, mopping and walking up and down the stairs, respectively. Six patients showed no loss of IC greater than 0.15 L (Figure 1).
Figure 1.
Boxplot of resting and post-activity (walking up and down a ramp, sweeping/mopping and walking up and down stairs) inspiratory capacities in COPD patients. Data are presented as medians±maximum/minimum values.
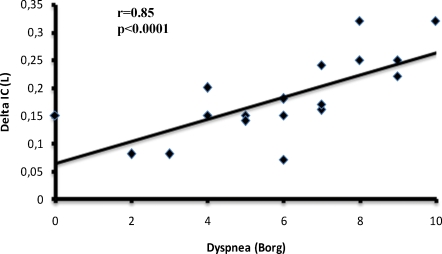
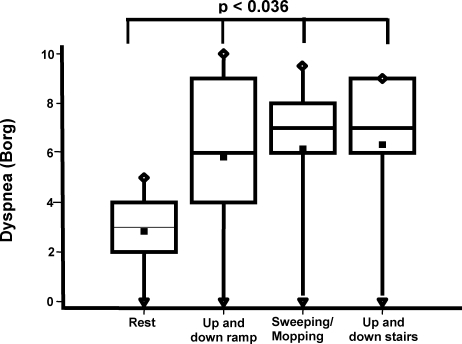
Dyspnea perception correlated positively with the variation in inspiratory capacity (r = 0.85, p = 0.0001) (Figure 2) and respiratory rate (r = 0.37, p = 0.04) and negatively with peripheral oxygen saturation (r = -0.28, p = 0.04). On average, the value of perceived dyspnea increased two-fold after the patients had walked to the end of the ramp, swept and mopped the floors and completed the UDS test compared to the resting values (p = 0.0036) (Figure 3). Likewise, the average value of lower-limb fatigue perception in the COPD patients increased more than two-fold for the same activities between when they commenced and ended (p<0.005) (Figure 4).
Figure 2.
Scatterplot of the association between the sensation of dyspnea and delta (post-pre) inspiratory capacity. Data are presented as individual inspiratory capacity values.
Figure 3.
Boxplot of resting and post-activity (walking up and down ramp, sweeping/mopping and up and down stairs) sensations of dyspnea in COPD patients. Data are presented as medians±maximum/minimum values.
Figure 4.
Boxplot of resting and post-activity (walking up and down a ramp, sweeping/mopping and walking up and down stairs) lower-limb fatigue levels in COPD patients. Data are presented as medians±maximum/minimum values.
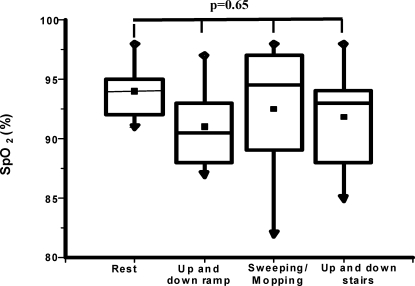
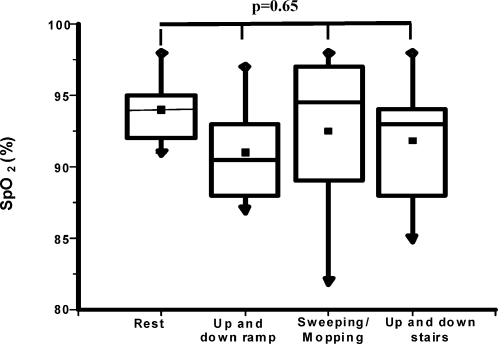
SpO2 values at rest and after each activity revealed no significant decreases in any patient, and there was no difference in SpO2 between the resting and post-activity states (p = 0.65) (Figure 5). Likewise, blood pressure, heart and respiratory rates were not significantly increased between the resting and post-activity periods (p = 0.33, p = 0.51 and p = 0.27, respectively).
Figure 5.
Boxplot of resting and post-activity (walking up and down a ramp, sweeping/mopping and walking up and down stairs) arterial oxyhemoglobin saturations in COPD patients. Data are presented as medians±maximum/minimum values.
DISCUSSION
The importance of this study resides in the fact that it investigated the dynamic pulmonary hyperinflation resulting from activities of daily living in COPD patients. The novel finding is that activities that were never tested before (however commonly performed by COPD patients) were shown to induce dynamic pulmonary hyperinflation. There are several studies in the literature comparing the increases in metabolic demand for activities with a high energy cost, such as the 6-minute walking test (60 to 80% of oxygen consumption) (10) and the endurance test (90% of oxygen consumption) (13), and activities with decreased metabolic demand, such as upper-limb exercises (e.g., the proprioceptive neuromuscular facilitation [PNF] technique) (16) and activities of daily living (e.g., erasing black boards, lifting pots and replacing lamps) (17). Nevertheless, when comparing these studies to our data, either the methods of activity assessment (e.g., accelerometer) (18) or the activities themselves (19) were different. We believe that this is an extremely important issue to be studied, as there are several activities (over 30) that our patients perform in their day-to-day lives that did not predispose the patients to any deleterious effects that we are aware of.
For more than 30 years, IC has been measured to determine lung volume at end-expiration, usually after performing a physical activity. Researchers use it with a high level of confidence by assuming that the total lung capacity does not change from rest to peak exercise and stating that any IC reduction is associated with the increase in functional residual capacity (FRC) (4). The slow vital capacity (SVC) is a reproducible maneuver both at rest and at peak exercise (14). It has also been demonstrated to be a reliable maneuver that provides accurate data, such as the decrease in inspiratory reserve volume, which is associated with increases in FRC (14). Additionally, measuring IC is a low-cost, noninvasive method.
Dynamic pulmonary hyperinflation is one factor that contributes to the reduction of the physical capacity of patients and correlates positively with oxygen consumption at peak exercise (peak VO2) (4). It is already known that a small lung capacity or ventilatory reserve can lower the blood oxygen supply, thereby reducing the aerobic capacity of the patient (4).
We analyzed IC behavior after each of the activities performed in this study. We believe that most of the pulmonary hyperinflation occurred in patients after walking up and down the ramp and the stairs. This was due to a higher demand for physical effort and the use of larger muscle groups than those required in the sweeping and mopping activity. In their study, O'Donnell et al. (20) identified a strong correlation between IC and the distance walked during the 6-minute walking test. The same authors argued that the reduction of IC leads to an increase in ventilation required by the large muscle groups recruited in a symptom-limit cycle ergometer test. In our study, we did not measure minute ventilation and oxygen consumption; however, the activities performed by our patients also involved large muscle groups, and it is possible that they required increased energy and oxygen consumption. This was confirmed by the increased respiratory rate and maximum oxygen consumption. This suggests that the dynamic pulmonary hyperinflation that took place in these activities occurred for the same reason. Many authors have demonstrated the association of increased pulmonary ventilation and the development of dynamic pulmonary hyperinflation during upper-limb exercises (10,14,20) in COPD patients.
Despite the fact that the sweeping and mopping activities are not considered as intense as the other activities mentioned above, they also resulted in a significant IC reduction. Most of the muscles required for the completion of this activity are located in the upper limbs, which are typically composed of muscle groups with less muscle mass and lower maximal oxygen consumption compared to the lower-limb muscles. This proportionality has been demonstrated by Celli et al. (21), who showed that, after exercise with a cycle ergometer, oxygen consumption was lower in the upper limbs (9.3±1.1 ml/kg) than in the lower limbs (30.8± 3.2 ml/kg). However, both the range of motion and the degree of freedom of the shoulder girdle joint are significantly larger than those of the lower limbs. This allows the arms to perform movements that depart from the midline of the body. Therefore, proportionally, the force and torque required for the execution of each arm movement increases significantly, resulting in greater oxygen consumption (22). Porto et al. (15) were the first to demonstrate the development of dynamic lung hyperinflation in COPD patients during exercises with diagonal movements (PNF technique) that resemble the activities of daily living that involve the arms. The authors also showed that the proportion of IC reduction in the upper-limb exercises was greater than in the lower limbs in patients with COPD for the same metabolic demand (16,22). In our study, we did not intend to compare the relative degrees of IC reduction between the upper and lower limbs; however, we demonstrated the occurrence of dynamic pulmonary hyperinflation during activities of daily living with the arms. Furthermore, the movements resemble many unsupported upper-limb exercises that are commonly used during pulmonary rehabilitation sessions for COPD patients.
Our study revealed a strong correlation between both IC and Borg dyspnea and peak exercise. Similarly, Marin et al. (10) demonstrated that pulmonary hyperinflation leads to a greater degree of dyspnea, fatigue, and physical capacity limitations. The sensation of dyspnea has been widely reported in COPD patients during activities of daily living (23), physical exercises (24-26) and even breathing exercises (27). However, none of these authors demonstrated an association between dyspnea and dynamic pulmonary hyperinflation in COPD patients when performing daily activities.
Dynamic hyperinflation occurred due to an acute lung adaptation to hyperpnea and enhanced metabolic requirements. Once this overwhelmed stimulus was interrupted (i.e., during post-activity rest), the respiratory pattern returned to baseline, and the inspiratory capacity increased. Many studies in the literature exist regarding the reversibility of dynamic hyperinflation with the use of bronchodilators (15,21,28). However, no data have yet been reported regarding the spontaneous reversibility of dynamic hyperinflation. Nevertheless, the tests that we have conducted in our laboratory confirm that dynamic hyperinflation is spontaneously reversible and that it may require up to 10 minutes for the inspiratory capacity to increase to its baseline value after the end of the exercise period (15,16).
The Borg scale was also used to measure lower-limb fatigue during these activities. For all activities, the perception of fatigue was greater than the sensation of dyspnea. We did not assess muscle alterations in our patients, as this was not our goal; however, due to the systemic characteristics of COPD, we can assume that there is a reason for this difference that is associated with skeletal muscle characteristics (29,30). It is likely that the skeletal muscle alterations triggered early metabolic acidosis, which may have led to greater perceptions of fatigue reported by our patients. COPD is a systemic disease that also affects the musculoskeletal system, decreasing muscle mass due to disuse and leading to the development of many bioenergetic abnormalities (9). Maltais et al. (29) reported a reduction in thigh muscle mass in COPD patients and found that this group of patients had higher lower-limb fatigue than sensations of dyspnea during physical activity, possibly due to physical deconditioning. Many authors have reported changes in muscle mass (31,32) and bioenergetics, such as that related to severe myopathy (33), favoring early skeletal muscle fatigue in COPD patients.
Our patients were not hypoxemic at rest; after performing all of the activities in this study, SpO2 remained above 80%. Other studies (31,33) were conducted with patients who were not hypoxemic at rest, and they remained within normal SpO2 levels during exercise. Ramírez-Venegas et al. (34) did not identify any strong associations between either SpO2 or Borg dyspnea and the 12-minute walking test (r = -0.45, p<0.05). In our study, we found only a weak association between these variables (r = -0.28, p = 0.04), and there were no clinical differences between either SpO2 or Borg dyspnea and the activities of daily living performed. Despite the fact that the exercise type is strictly different between the two parameters, SpO2 and Borg dyspnea behaved similarly in both studies. This suggests that the sensation of dyspnea is multifactorial and has a low association with hemoglobin oxygen uptake. It is likely that vascular outflow and pulmonary perfusion are crucial for the development of these changes (35,36).
A possible limitation of our study is the fact that we did not measure the static lung volume of our patients as residual volume and total lung capacity. This might have revealed additional data regarding lung function status for our patients. However, our focus was to evaluate dynamic changes in lung volume, and the method used was appropriate to address the problem. As this is one of the first studies to compare dynamic lung hyperinflation in patients performing activities of daily living, it is difficult to compare our results with those from other studies.
However, these findings have an important impact on the pulmonary rehabilitation of COPD patients in clinical practice. Based on our results, we can infer that the activities of daily living should be assessed in these patients, and patients should be guided by a comprehensive multidisciplinary team to provide them with better guidance.
We conclude that during activities of daily living, COPD patients develop dynamic pulmonary hyperinflation, increased dyspnea and lower-limb fatigue, which are the main causes of physical limitations.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Lindberg A, Jonsson AC, Ronmark E, Lundger R, Larsson L-G, Lundbäck B. Prevalence of chronic obstructive pulmonary disease according to BTS, ERS, GOLD and ATS criteria in relation to doctor's diagnosis, symptoms, age, gender, and smoking habits. Respiration. 2005;72(5):471–9. doi: 10.1159/000087670. [DOI] [PubMed] [Google Scholar]
- 2.Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–46. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 3.Celli B, Criner G, Rassulo J. Ventilatory muscle recruitment during unsupported arm exercise in normal subjects. J. Applied Physiol. 1988;64(5):1936–41. doi: 10.1152/jappl.1988.64.5.1936. [DOI] [PubMed] [Google Scholar]
- 4.Chinarro JB. Enfermedad pulmonar obstructiva crónica. Jano. 2001;(60):54–65. [Google Scholar]
- 5.Walker PP, Burnett A, Flavahan PW, Calverley PMA. Lower limb activity and its determinants in COPD. Thorax. 2008;63(8):683–9. doi: 10.1136/thx.2007.087130. [DOI] [PubMed] [Google Scholar]
- 6.Diaz O, Villafranca C, Ghezzo H, Borzone G, Leiva A, Milic-Emil J, et al. Role of inspiratory capacity on exercise tolerance in COPD patients with and without tidal expiratory flow limitation at rest. Eur Resp J. 2000;16(2):269–75. doi: 10.1034/j.1399-3003.2000.16b14.x. [DOI] [PubMed] [Google Scholar]
- 7.ATS statement: pulmonary rehabilitation: American Thoracic Society. Am J Respir Crit Care Med. 1999;159(5 Pt 1):1666–82. doi: 10.1164/ajrccm.159.5.ats2-99. [DOI] [PubMed] [Google Scholar]
- 8.Sclauser IMB, Parreira VF, Lorenzo VAP, Reis MAS, Costa D. Analysis of dynamic pulmonary hyperinflation (DH) following activities of daily living in patients with chronic obstructive pulmonary disease. Rev Bras Fisioter. 2007;6(11):469–74. [Google Scholar]
- 9.Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2010. Available from: http://www.goldcopd.org.
- 10.Marin JM, Carrizo SJ, Gascon M, Sanchez A, Gallego B, Celli BR. Inspiratory capacity, dynamic hyperinflation, breathlessness, and exercise performance during the 6-minute walk test in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163(6):1395–9. doi: 10.1164/ajrccm.163.6.2003172. [DOI] [PubMed] [Google Scholar]
- 11.American thoracic society. Standards for the diagnosis and care of patients with chronic pulmonary obstructive disease. Am J Respir Crit Care Med. 1995;52:S77–S120. [PubMed] [Google Scholar]
- 12.Neder JÁ, Adreonni S, Lerario MC, Nery LE. Reference values for lung function test. II. Maximal respiratory pressure and voluntary ventilation. Braz J Med Biol Res. 1999;32(6):719–27. doi: 10.1590/s0100-879x1999000600007. [DOI] [PubMed] [Google Scholar]
- 13.O'Donnell DE, Revil SM Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(5):770–7. doi: 10.1164/ajrccm.164.5.2012122. [DOI] [PubMed] [Google Scholar]
- 14.Yan S, Kaminski D, Sliwinski P. Reliability of inspiratory capacity for estimating end-expiratory lung volume changes during exercise in patients with chronic obstruction pulmonary disease. Am J Respir Crit Care Med. 1997;156(1):55–9. doi: 10.1164/ajrccm.156.1.9608113. [DOI] [PubMed] [Google Scholar]
- 15.Porto EF, Castro AM, Nascimento O, Oliveira RC, Cardoso F, Jardim JR. Modulation of operational lung volumes with the use of salbutamol in COPD patients accomplishing upper limbs exercise tests. Respiratory Medicine. 2009;103(2):251–7. doi: 10.1016/j.rmed.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 16.Porto EF, Castro AM, Velloso M, Nascimento O, Dal Maso F, Jardim JR. Exercises using the upper limbs hyperinflate COPD patients more than exercises using the lower limbs at the same metabolic demand. Monaldi Arch Chest Dis. 2009;71(1):21–6. doi: 10.4081/monaldi.2009.372. [DOI] [PubMed] [Google Scholar]
- 17.Velloso M, Stella SG, Cendon S, Silva AC, Jardim JR. Metabolic and ventilatory parameters of four activities of daily living accomplished with arms in COPD patients. Chest. 2003;123(4):1047–53. doi: 10.1378/chest.123.4.1047. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Rio F, Lores V, Mediano O, Rojo B, Hernanz A, Lopez-Collazo E, et al. Daily physical activity in patients with chronic obstructive pulmonary disease is mainly associated with dynamic hyperinflation. Am J Respir Crit Care Med. 2009;180(6):506–12. doi: 10.1164/rccm.200812-1873OC. [DOI] [PubMed] [Google Scholar]
- 19.Velloso M, Jardim JR. Study of energy expenditure during activities of daily living using and not using body position recommended by energy conservation techniques in patients with COPD. Chest. 2006;130(1):126–32. doi: 10.1378/chest.130.1.126. [DOI] [PubMed] [Google Scholar]
- 20.O'Donnell DE, Webb KA. Exertional breathlessness in patients with chronic airflow limitation – the role of lung hyperinflation. Am J Rev Respir Dis. 1993;148(5):1351–7. doi: 10.1164/ajrccm/148.5.1351. [DOI] [PubMed] [Google Scholar]
- 21.Celli B, ZuWallack R, Wang S, Kesten S. Improvement in resting inspiratory capacity and hyperinflation with tiotropium in COPD patients with increased static lung volumes. Chest. 2003;124(5):1743–8. doi: 10.1378/chest.124.5.1743. [DOI] [PubMed] [Google Scholar]
- 22.Gigliotti F, Coli C, Bianchi R, Grazzini M, Stendardi L, Castellani C, et al. Arm exercise and hyperinflation in patients with COPD: effect of arm training. Chest. 2005;128(3):1225–32. doi: 10.1378/chest.128.3.1225. [DOI] [PubMed] [Google Scholar]
- 23.Langer D, Pitta F, Troosters T, Burtin C, Decramer M, Gosselink R. Quantifying physical activity in COPD: different measures for different purposes. Thorax. 2009;64(5):458–9. [PubMed] [Google Scholar]
- 24.O'Donnell DE, Bertley JC, Chau LK, Webb KA. Qualitative aspects of exertional breathlessness in chronic airflow limitation. Am J Respir Crit Care Med. 1997;155(1):109–15. doi: 10.1164/ajrccm.155.1.9001298. [DOI] [PubMed] [Google Scholar]
- 25.Bauldoff GS, Hoffman LA, Sciurba F, Zullo TG. Home-based, upper arm exercise training for patients with chronic obstructive pulmonary disease. Heart & Lung. 1996;25(4):288–94. doi: 10.1016/s0147-9563(96)80064-1. [DOI] [PubMed] [Google Scholar]
- 26.Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–46. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 27.Gosselink R. Breathing techniques in patients with chronic obstructive pulmonary disease (COPD). Chron Respir Dis. 2004;1(3):163–72. doi: 10.1191/1479972304cd020rs. [DOI] [PubMed] [Google Scholar]
- 28.Soriano JB, Vestbo J, Pride NB, Soriano JB, Kiri V, Maden C, et al. Survival in COPD patients after regular use of fluticasone propionate and salmeterol in general practice. Eur Resp J. 2002;20(4):819–25. doi: 10.1183/09031936.02.00301302. [DOI] [PubMed] [Google Scholar]
- 29.Bernard S, Leblanc P, Whitton F, Carrier G, Jobin J, Belleau R, et al. Peripheral muscle weakness in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158(2):629–34. doi: 10.1164/ajrccm.158.2.9711023. [DOI] [PubMed] [Google Scholar]
- 30.Man WD, Hopkinson NS, Harraf F, Nikoletou D, Polkey MI, Moxham J. Abdominal muscle and quadriceps strength in chronic obstructive pulmonary disease. Thorax. 2005;60(9):718–22. doi: 10.1136/thx.2005.040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacIntyre NR. Mechanisms of functional loss in patients with chronic lung disease. Respir Care. 2008;53(9):1177–84. [PubMed] [Google Scholar]
- 32.Vogiatzis I, Stratakos G, Simoes DC, Terzis G, Georgiadou O, Roussos C, et al. Effects of rehabilitative exercise on peripheral muscle TNF alpha, IL-6, IGF-I and MyoD expression in patients with COPD. Thorax. 2007;62(11):950–6. doi: 10.1136/thx.2006.069310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagner PD. Skeletal muscles in chronic obstructive pulmonary disease: deconditioning, or myopathy. Respirology. 2006;11(6):681–6. doi: 10.1111/j.1440-1843.2006.00939.x. [DOI] [PubMed] [Google Scholar]
- 34.Ramírez-Venegas A, Sánchez C, Regalado J, Sansores RH. Severity of dyspnea during exercise: similarities and differences between patients with COPD or pulmonary fibrosis. Arch Bronconeumol. 2001;37(5):221–6. doi: 10.1016/s0300-2896(01)75058-2. [DOI] [PubMed] [Google Scholar]
- 35.Mispelaere D, Glerant JC, Audebert M, Remond A, Sevestre-Pietri MA, Jounieaux V. Pulmonary embolism and sibilant types of chronic obstructive pulmonary disease decompensations. Rev Mal Respir. 2002;19(4):415–23. [PubMed] [Google Scholar]
- 36.Vassaux C, Torre-Bouscoulet L, Zeineldine S, Cortopassi F, Paz-Díaz H, Celli BR, et al. Effects of hyperinflation on the oxygen pulse as a marker of cardiac performance in COPD. Eur Respir J. 2008;32(5):1275–82. doi: 10.1183/09031936.00151707. [DOI] [PubMed] [Google Scholar]