Abstract
OBJECTIVES:
The purpose of this study was to determine the paired consequences of food restriction and paradoxical sleep deprivation on lipid profile and spontaneous glucose levels in male rats.
METHOD:
Food restriction began at weaning, with 6 g of food being provided per day, which was subsequently increased by 1 g per week until reaching 15 g per day by the eighth week. At adulthood, both rats subjected to food restriction and those fed ad libitum were exposed to paradoxical sleep deprivation for 96 h or were maintained in their home-cage groups.
RESULTS:
Animals subjected to food restriction exhibited a significant increase in high-density lipoprotein levels compared to animals that were given free access to food. After the paradoxical sleep deprivation period, the food-restricted animals demonstrated reduced concentrations of high-density lipoprotein relative to their respective controls, although the values for the food-restricted animals after sleep deprivation were still higher than those for the ad libitum group. The concentration of low-density lipoproteins was significantly increased in sleep-deprived animals fed the ad libitum diet. The levels of triglycerides, very low-density lipoproteins, and glucose in food-restricted animals were each decreased compared to both ad libitum groups.
CONCLUSION:
These results may help to illustrate the mechanisms underlying the relationship between sleep curtailment and metabolism and may suggest that, regardless of sleep deprivation, dietary restriction can minimize alterations in parameters related to cardiovascular risk.
Keywords: Cardiovascular Diseases, Cholesterol, Food Deprivation, Sleep Deprivation, Triglycerides
INTRODUCTION
Although many studies have demonstrated the importance of sleep duration for overall health, sleep loss is an inevitable consequence of societal pressure. In addition to sleep curtailment, other sleep disorders, such as obstructive sleep apnea (OSA) and insomnia, have become markedly prevalent (1). For instance, sleep apnea has important clinical consequences, including hypertension (2) and increased risks for cardiovascular and endocrine diseases. Patients with OSA experience recurrent sleep disruptions and a significant decrease in rapid eye movement (REM) sleep (3). Moreover, it is well-known that in patients with OSA, most apnea events occur during REM sleep. Therefore, cerebrovascular function may be most affected during REM sleep, due to the high demand for energy by the brain during that sleep stage (4).
In rats, sleep deprivation induced by the platform technique causes numerous awakenings, which predominantly affect the REM stage of sleep. Therefore, we reasoned that this procedure could mimic sleep fragmentation due to repeated awakenings and could therefore be a useful tool for investigating the effects of sleep loss on blood parameters. In a previous study, our group assessed the effects of sleep loss on blood parameters associated with cardiovascular risk in male rats. These data indicated that sleep-deprived rats have significantly increased high-density lipoprotein (HDL) and low-density lipoprotein (LDL) levels compared to controls. Sleep deprivation has also been shown to decrease triglyceride and very-low-density lipoprotein (VLDL) levels significantly (5).
However, one study demonstrated that a reduction in caloric intake is related to lifespan extension because several physiological functions were improved under conditions of food restriction (FR) (6). In rodents, the effects of FR are extensive and include a significant reduction in risk factors associated with the development cardiovascular and neurodegenerative diseases ()()()(7-10). Broderick et al. (11,12) reported enhanced contractile properties of the heart, improved blood-flow regulation and resistance to cerebral ischemia in rats maintained on an 8-month FR diet (45% reduction) compared to ad libitum-fed animals. Similarly, 30-40% of FR rodents had significantly fewer myocardial infarcts over a 10-month period (13). More recently, Venkatachalam et al. (14) reported decreases in inflammation and oxidative damage to heart cells following occlusion of the left anterior descending coronary artery in FR rats compared to similarly treated ad libitum-fed rats.
Although several studies have examined the behavioral alterations in FR rats, no study has addressed the specific effects of FR associated with lack of sleep on lipid profile and spontaneous glucose levels. Thus, the aim of this study was to investigate the consequences of both FR and sleep deprivation on the blood parameters associated with cardiovascular disease risk in male rats.
MATERIALS AND METHODS
Subjects
Thirty-eight male Wistar rats were bred and raised in the animal facility of the Center for Development of Experimental Models for Medicine and Biology (CEDEME) at Universidade Federal de São Paulo. The animals were housed in a colony that was maintained at 22°C on a 12:12-h light-dark cycle (lights on at 07:00 h), and animals were allowed free access to water inside of standard polypropylene cages. The experimental protocol was approved by the Ethical Committee of UNIFESP (CEP N. 05/434) and was conducted under the recommendations of the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes, as well as those of the Brazilian Society of Science in Laboratory Animals.
Food restriction (FR)
From the time of weaning (at 30 days) onward, rats were fed 6 g of chow daily. This amount of food was increased by 1 g per week until it reached 15 g by the eighth week. The animals were kept in separate cages during feeding and remained separated until all food was completely ingested. The protocol that was used for the calculation of chow consumption was in compliance with our previous studies ()()(15-17). The animals were distributed into the following two groups:
those fed an ad libitum diet (AD, n = 19) and allowed to eat and drink ad libitum from the time of weaning throughout the experiment, or
those placed under food restriction (FR, n = 19) and fed according to the dietary restriction protocol described above. For this group, water was offered ad libitum, and the FR rats were fed at approximately 19:00 h. Previously, we demonstrated that there were no statistically significant effects of feeding time on the sleep-wake patterns of diurnal periods (15).
Protocol designs
After eight weeks of FR or AD, the animals were randomly distributed into the following groups:
AD-CTRL (n = 9): AD rats maintained in the home cage.
AD-PSD (n = 10): AD rats subjected to 96 h of PSD.
FR-CTRL (n = 9): FR rats maintained in the home cage.
FR-PSD (n = 10): FR rats subjected to 96 h of PSD.
Paradoxical Sleep Deprivation (PSD)
The rats were distributed into the following two additional groups: the control (CTRL) or the PSD group. The animals in the PSD group were subjected to 96 h of PSD using the modified multiple platform method. We used a duration of 96 h based on the results of previous studies (18,19). Six rats were placed inside of one tiled water tank (143×41×30 cm), which contained 12 circular platforms that were 6.5 cm in diameter, and the water was filled up to 1 cm from the upper surface of the platform. The rats could therefore move around inside of the tank by jumping from one platform to another. When they reached the paradoxical phase of sleep, muscle atonia set in, and the animals fell into the water and woke up. Throughout the study, the experimental room was maintained at a controlled temperature (22±1°C) and light-dark cycle (lights came on at 07:00 h and were turned off at 19:00 h). Food was provided freely to the AD group in the form of chow pellets, and water bottles were made accessible on a grid located on top of the tank. The water in the tank was changed daily throughout the PSD period. The FR rats were fed at approximately 19:00 h in individual cages and were returned to the water tank after they had eaten all of the food pellets. Lastly, cage-control groups (AD and FR) were maintained in separate cages in the same room as the experimental rats during the sleep-deprivation procedure. By housing both groups in the same room, we maintained control over differences in housing conditions between the two groups.
Blood sampling
Following the behavioral tests, the PSD and CTRL rats were brought to an adjacent room and decapitated. Although decapitation is aesthetically questionable, it is an extremely fast and efficient method that does not cause additional pain or distress to the animals. Moreover, decapitation induces an immediate disappearance of the corneal reflex and promotes minimal physiological alterations in the tissue. For the purpose of this study, this method was used to provide the amount of blood required for the assays. Blood samples were immediately collected and stored individually. Blood was collected in glass tubes and centrifuged at 3018.4g for 15 min at room temperature. Cholesterol and triglyceride concentrations were measured using an automated colorimetric method (ADVIA 16/50, BAYER Diagnostics Corporation, NY, USA). Duplicate serum aliquots were also assessed. These assays were performed using the standard automated procedures of our clinical laboratory.
Statistical analyses
The data were analyzed using a one-way ANOVA test followed by a Tukey test for comparisons between groups. Values are expressed as the means ± SEM. The level of significance was set at p<0.05.
RESULTS
Total Cholesterol
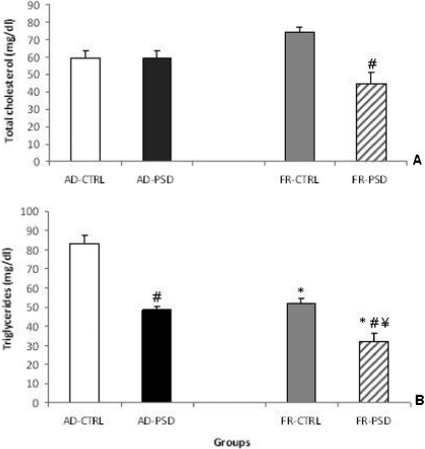
An analysis of the total cholesterol concentrations revealed significant differences between the groups (F(3.33) = 8.38, p<0.0002), as shown in Figure 1A. Following PSD, the FR rats demonstrated significantly lower cholesterol levels did than the respective CTRL group (p<0.01).
Figure 1.
Effects of food restriction (FR) or ad libitum diet (AD), either paired or unpaired with paradoxical sleep deprivation (PSD), on the total cholesterol (panel A) and triglyceride levels (panel B) in male rats. *Significantly different from the AD-CTRL group, ¥ Significantly different from the AD-PSD group and # Significantly different from the respective control group.
Triglycerides
Triglyceride levels were substantially reduced in the AD-PSD and all FR groups compared to the AD-CTRL group (Figure 1B). Indeed, sleep deprivation significantly reduced triglyceride levels in both FR and AD animals compared to the respective non-sleep-deprived controls (p<0.0001).
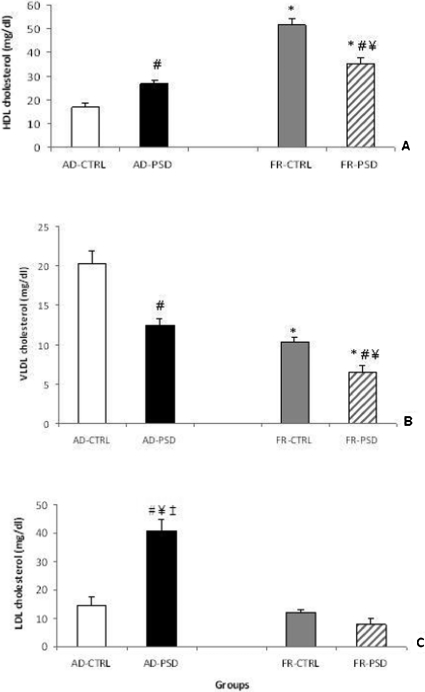
Cholesterol fractions
As shown in Figure 2A, high-density lipoprotein (HDL) levels were higher among animals in the sleep-deprived groups and the FR-CTRL group relative to those in the AD-CTRL group (F(3,33) = 55.19, p<0.0001). In contrast, the very-low-density lipoprotein (VLDL) levels followed an opposite pattern (Figure 2B), as the FR-CTRL group had lower levels than the AD-CTRL group. The sleep-deprived animals exhibited even greater decreases compared to the CTRL groups (p<0.05). With regard to the low-density lipoprotein (LDL) levels, only animals in the AD-PSD group exhibited an increase in comparison to the other groups evaluated (Figure 2C), p<0.0001).
Figure 2.
Concentrations of HDL (panel A), VLDL (panel B) and LDL cholesterol (panel C) in male ad libitum (AD)-fed or food-restricted (FR) rats subjected to normal sleep (CTRL) or paradoxical sleep deprivation (PSD). Data are expressed as the means ± SEM. *Significantly different from the AD-CTRL group, ¥ Significantly different from the AD-PSD group, ‡ Significantly different from the FR-CTRL group and # Significantly different from the respective control group.
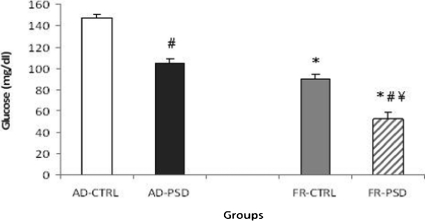
Glucose
As shown in Figure 3, all groups demonstrated decreased glucose levels in comparison to the AD-CTRL group independently of sleep deprivation (p<0.001). Moreover, the FR-PSD animals had reduced glucose levels in comparison to those in the FR-CTRL group (p<0.001).
Figure 3.
Effects of food restriction (FR) or ad libitum diet (AD) on glucose concentrations in home-cage control (CTRL) and paradoxical sleep-deprived (PSD) rats. * Significantly different from the AD-CTRL group, ¥ Significantly different from the AD-PSD group and # Significantly different from the respective control group.
DISCUSSION
These results demonstrate that long-term FR can alter biochemical blood parameters related to cardiovascular risks independently of PSD. The glucose, triglyceride, and VLDL cholesterol concentrations were similarly reduced by FR, and the reductions in these parameters were maintained when PSD was combined with FR. In addition, LDL cholesterol levels were higher only among animals in the AD-PSD group. However, FR was found to increase HDL cholesterol levels. Moreover, these changes were reversed by combining FR with PSD, indicating that selective sleep loss can override the beneficial effects of low caloric intake. However, animals in the FR-PSD group maintained higher levels of the tested parameters than did those in the AD-PSD group.
The beneficial effects of low caloric intake, which include improvements in cardiovascular function, have been widely reported in the literature (7,8,14). However, to the best of our knowledge, this study is the first to focus on whether the positive effects of FR can be maintained when combined with sleep loss. This study showed that for some parameters, such as HDL, triglyceride and glucose levels, FR may reduce the potential damage caused PSD. Of note, HDLs were present at higher concentrations in the FR groups compared to the ad libitum groups, even when FR was combined with PSD.
It has been documented that HDL concentrations are inversely associated with cardiovascular disease risks (20). Pharmacological interventions that increase HDL concentrations typically also improve other lipid parameters. For example, the contribution of increased HDL levels is not easily distinguishable from the contributions of decreased LDL and/or triglycerides levels (21). The low glucose, VLDL and triglyceride levels observed in the FR groups may be protective against an adverse challenge, such as PSD, as a consequence of FR. One example of the beneficial effects of a low caloric intake is the reduction of oxidative stress (22). Moreover, FR leads to alterations in energy balance, such as reduced energy expenditure, as a result of decreased oxygen consumption (23,24) or mitochondrial adaptation (25).
Studies have demonstrated sleep loss affects several blood parameters in rats, and previous studies from our group (5), have isolated the influence of PSD on cardiovascular parameters. In general, these findings indicated increases in total cholesterol and the HDL fraction of cholesterol in male and female rats after sleep loss. In these studies, rats maintained on a normal diet had increased levels of triglycerides, VLDL cholesterol and glucose compared to the other groups. However, during periods of PSD, the levels of HDL and LDL cholesterol increased, whereas those of triglycerides and VLDL cholesterol decreased. It is possible that these results may have been due to reduced food consumption during the sleep deprivation procedure, which was accompanied by weight loss. It has also been reported that sleep deprivation can cause hyperphagia and weight loss (29,30). Therefore, one might hypothesize that the differences observed in the triglyceride and cholesterol levels after PSD were the result of greater food intake. However, during PSD, the impaired capacity to increase food intake in response to increased energy expenditure contributes to an energy deficit in rats (30). Data from animals with normal food intake levels indicate that total cholesterol levels remain unchanged after sleep deprivation, whereas the levels of HDL and LDL increase. The heterogeneity of these responses argues against a simple effect of increased food consumption on these measures.
Recently, there has been growing concern regarding the potential effects of dietary restriction on several health parameters. Most of this attention has been the result of societal interest in longevity, health and welfare. In particular, dietary restriction has been reported as being the only intervention that can promote increased longevity, reduce the incidence of disease and slow processes related to aging (31).
It is important to note that the sleep fragmentation induced by PSD evaluated in the current study did not negatively alter all of the parameters related to cardiovascular risk. In addition, the FR data provide additional evidence regarding the mechanisms underlying an increase in life expectancy. Finally, our data suggest that independently of sleep deprivation, dietary restriction can minimize changes in the parameters related to cardiovascular risk.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the assistance of Waldemarks Leite. This work was supported by grants from the Associação Fundo de Incentivo à Pesquisa (AFIP), the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP-CEPID no. 98/14303-3 to ST.) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) to Andersen ML and Tufik S. Alvarenga TA and Pires GN are recipients of scholarships by FAPESP (no 11/12325-6 and 10/14768-0).
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Tufik S, Santos-Silva R, Taddei JA, Bittencourt LR. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med. 2010;11(5):441–6. doi: 10.1016/j.sleep.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. Bmj. 2000;320(7233):479–82. doi: 10.1136/bmj.320.7233.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goh DY, Galster P, Marcus CL. Sleep architecture and respiratory disturbances in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2000;162(2 Pt 1):682–6. doi: 10.1164/ajrccm.162.2.9908058. [DOI] [PubMed] [Google Scholar]
- 4.Lugaresi E, Partinen M.Prevalence of snoring Saunders NA, Sullivan CE, New York: Marcel Dekker; 1994 [Google Scholar]
- 5.Andersen ML, Martins PJ, D'Almeida V, Santos RF, Bignotto M, Tufik S. Effects of paradoxical sleep deprivation on blood parameters associated with cardiovascular risk in aged rats. Exp Gerontol. 2004;39(5):817–24. doi: 10.1016/j.exger.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 6.McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. 1935. Nutrition. 1989;5(3):155–71; discussion 72.. [PubMed] [Google Scholar]
- 7.Yu ZF, Mattson MP. Dietary restriction and 2-deoxyglucose administration reduce focal ischemic brain damage and improve behavioral outcome: evidence for a preconditioning mechanism. J Neurosci Res. 1999;57(6):830–9. [PubMed] [Google Scholar]
- 8.Mattson MP. Neuroprotective signaling and the aging brain: take away my food and let me run. Brain Res. 2000;886(1-2):47–53. doi: 10.1016/s0006-8993(00)02790-6. [DOI] [PubMed] [Google Scholar]
- 9.Roberge MC, Hotte-Bernard J, Messier C, Plamondon H. Food restriction attenuates ischemia-induced spatial learning and memory deficits despite extensive CA1 ischemic injury. Behav Brain Res. 2008;187(1):123–32. doi: 10.1016/j.bbr.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Rich NJ, Van Landingham JW, Figueiroa S, Seth R, Corniola RS, Levenson CW. Chronic caloric restriction reduces tissue damage and improves spatial memory in a rat model of traumatic brain injury. J Neurosci Res. 2010;88(13):2933–9. doi: 10.1002/jnr.22443. [DOI] [PubMed] [Google Scholar]
- 11.Broderick TL, Belke T, Driedzic WR. Effects of chronic caloric restriction on mitochondrial respiration in the ischemic reperfused rat heart. Mol Cell Biochem. 2002;233(1-2):119–25. doi: 10.1023/a:1015506327849. [DOI] [PubMed] [Google Scholar]
- 12.Broderick TL, Driedzic WR, Gillis M, Jacob J, Belke T. Effects of chronic food restriction and exercise training on the recovery of cardiac function following ischemia. J Gerontol A Biol Sci Med Sci. 2001;56(1):B33–7. doi: 10.1093/gerona/56.1.b33. [DOI] [PubMed] [Google Scholar]
- 13.Crandall DL, Feirer RP, Griffith DR, Beitz DC. Relative role of caloric restriction and exercise training upon susceptibility to isoproterenol-induced myocardial infarction in male rats. Am J Clin Nutr. 1981;34(5):841–7. doi: 10.1093/ajcn/34.5.841. [DOI] [PubMed] [Google Scholar]
- 14.Venkatachalam K, Prabhu SD, Reddy VS, Boylston WH, Valente AJ, Chandrasekar B. Neutralization of interleukin-18 ameliorates ischemia/reperfusion-induced myocardial injury. J Biol Chem. 2009;284(12):7853–65. doi: 10.1074/jbc.M808824200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvarenga TA, Andersen ML, Papale LA, Antunes IB, Tufik S. Influence of long-term food restriction on sleep pattern in male rats. Brain Res. 2005;1057(1-2):49–56. doi: 10.1016/j.brainres.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 16.Alvarenga TA, Andersen ML, Papale LA, Tufik S. Effects of long-term food restriction on genital reflexes in paradoxically sleep-deprived male rats. Brain Res. 2006;1115(1):148–54. doi: 10.1016/j.brainres.2006.07.079. [DOI] [PubMed] [Google Scholar]
- 17.Alvarenga TA, Andersen ML, Velazquez-Moctezuma J, Tufik S. Food restriction or sleep deprivation: which exerts a greater influence on the sexual behaviour of male rats. Behav Brain Res. 2009;202(2):266–71. doi: 10.1016/j.bbr.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Andersen ML, Bignotto M, Tufik S. Influence of paradoxical sleep deprivation and cocaine on development of spontaneous penile reflexes in rats of different ages. Brain Res. 2003;968(1):130–8. doi: 10.1016/s0006-8993(03)02228-5. [DOI] [PubMed] [Google Scholar]
- 19.Andersen ML, Martins PJ, D'Almeida V, Bignotto M, Tufik S. Endocrinological and catecholaminergic alterations during sleep deprivation and recovery in male rats. J Sleep Res. 2005;14(1):83–90. doi: 10.1111/j.1365-2869.2004.00428.x. [DOI] [PubMed] [Google Scholar]
- 20.Miller GJ, Miller NE. Plasma-high-density-lipoprotein concentration and development of ischaemic heart-disease. Lancet. 1975;1(7897):16–9. doi: 10.1016/s0140-6736(75)92376-4. [DOI] [PubMed] [Google Scholar]
- 21.Gotto AM., Jr Low high-density lipoprotein cholesterol as a risk factor in coronary heart disease: a working group report. Circulation. 2001;103(17):2213–8. doi: 10.1161/01.cir.103.17.2213. [DOI] [PubMed] [Google Scholar]
- 22.Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126(9):913–22. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Luz J, Griggio MA, Natrieli RM, Aumond MD. Energy balance of rats subjected to continuous and intermittent food restriction. Braz J Med Biol Res. 1995;28(9):1019–23. [PubMed] [Google Scholar]
- 24.Santos-Pinto FN, Luz J, Griggio MA. Energy expenditure of rats subjected to long-term food restriction. Int J Food Sci Nutr. 2001;52(2):193–200. [PubMed] [Google Scholar]
- 25.Johnson G, Roussel D, Dumas JF, Douay O, Malthiery Y, Simard G, et al. Influence of intensity of food restriction on skeletal muscle mitochondrial energy metabolism in rats. Am J Physiol Endocrinol Metab. 2006;291(3):E460–7. doi: 10.1152/ajpendo.00258.2005. [DOI] [PubMed] [Google Scholar]
- 26.Perry JC, D'Almeida V, Souza FG, Schoorlemmer GH, Colombari E, Tufik S. Consequences of subchronic and chronic exposure to intermittent hypoxia and sleep deprivation on cardiovascular risk factors in rats. Respir Physiol Neurobiol. 2007;156(3):250–8. doi: 10.1016/j.resp.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Antunes IB, Andersen ML, Alvarenga TA, Tufik S. Effects of paradoxical sleep deprivation on blood parameters associated with cardiovascular risk in intact and ovariectomized rats compared with male rats. Behav Brain Res. 2007;176(2):187–92. doi: 10.1016/j.bbr.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 28.Andersen ML, Perry JC, Bignotto M, Tufik S. Differential effects of sleep loss and chronic stressors on lipid metabolism. Sleep Sci. 2009;2(3):135–40. [Google Scholar]
- 29.Kushida CA, Everson CA, Suthipinittharm P, Sloan J, Soltani K, Bartnicke B, et al. Sleep deprivation in the rat: VI. Skin changes. Sleep. 1989;12(1):42–6. doi: 10.1093/sleep/12.1.42. [DOI] [PubMed] [Google Scholar]
- 30.Martins PJ, D'Almeida V, Nobrega JN, Tufik S. A reassessment of the hyperphagia/weight-loss paradox during sleep deprivation. Sleep. 2006;29(9):1233–8. doi: 10.1093/sleep/29.9.1233. [DOI] [PubMed] [Google Scholar]
- 31.Mulas MF, Demuro G, Mulas C, Putzolu M, Cavallini G, Donati A, et al. Dietary restriction counteracts age-related changes in cholesterol metabolism in the rat. Mech Ageing Dev. 2005;126(6-7):648–54. doi: 10.1016/j.mad.2004.11.010. [DOI] [PubMed] [Google Scholar]