Abstract
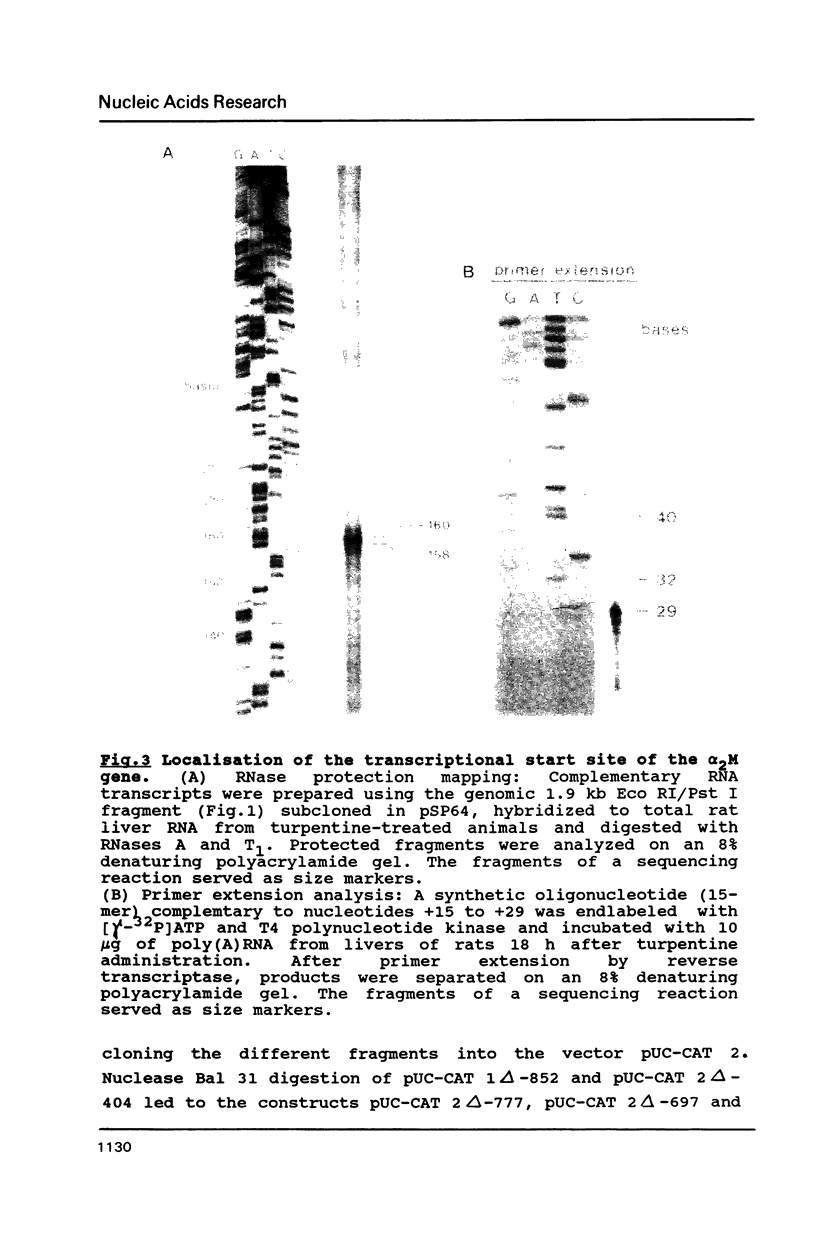
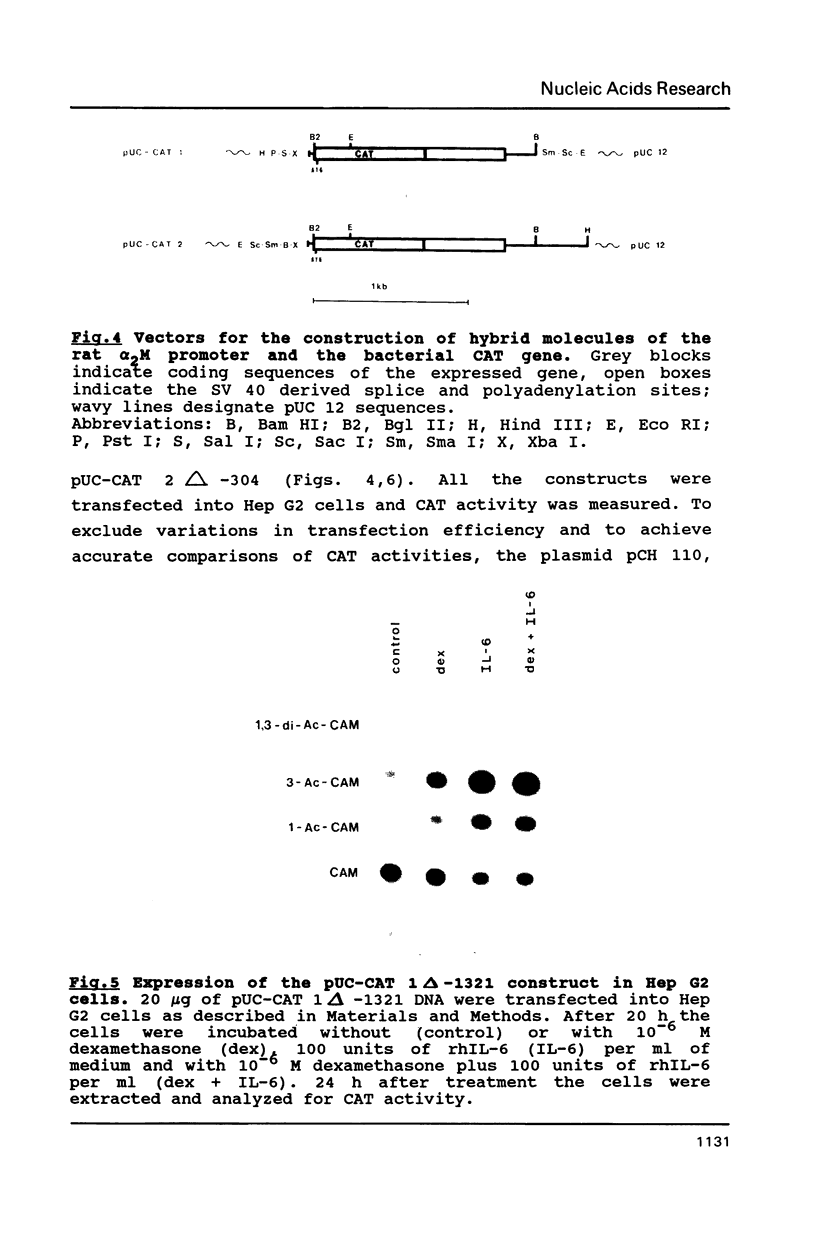
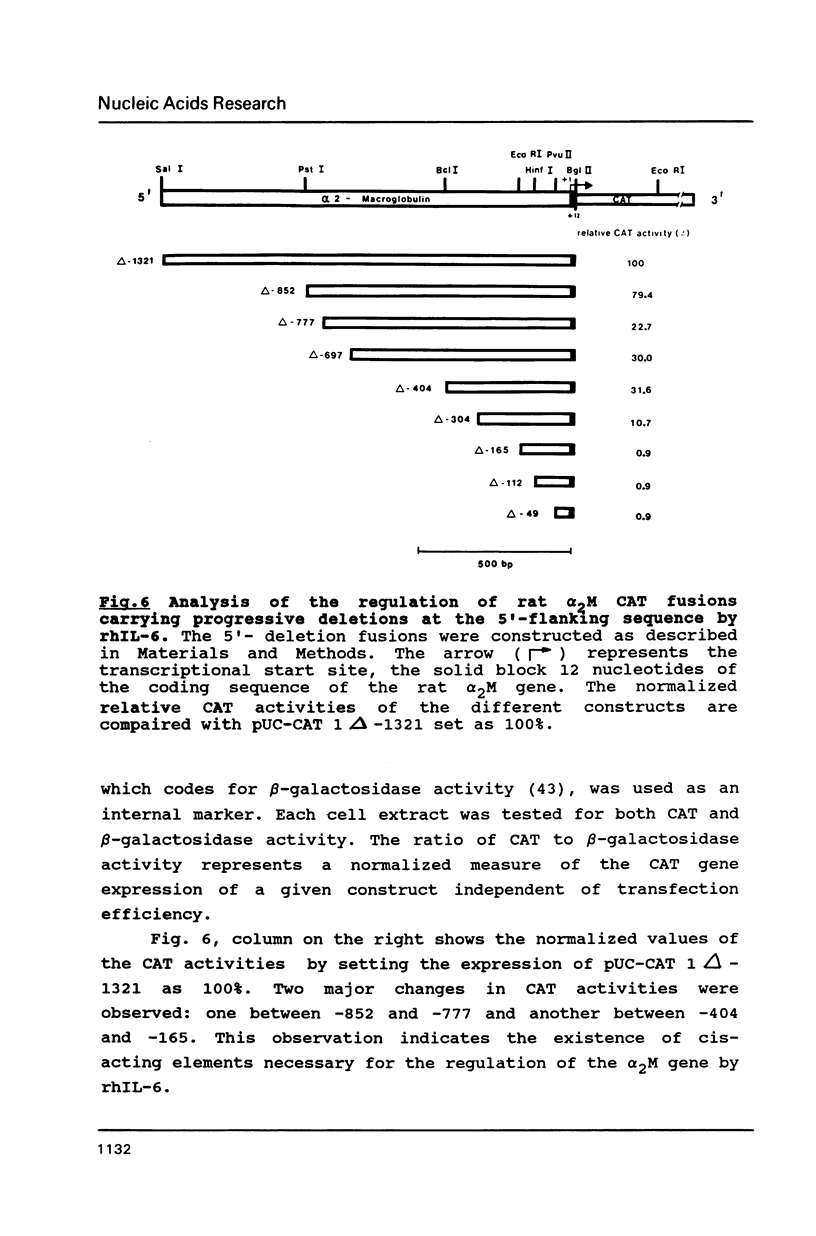
The 5'-region of the rat alpha 2-macroglobulin gene has been characterized. A 5.6 kb Sal I - Xba I fragment containing the first 4 exons of the alpha 2-macroglobulin gene and 1.3 kb of its 5'-flanking region was sequenced. The putative transcriptional start site was determined by RNase protection and primer extension analysis. TATA- and CAAT-box equivalent sequences were found. A potential glucocorticoid receptor binding site was located on the antisense strand. DNA sequences containing the 5'-flanking region of the rat alpha 2-macroglobulin gene were linked to the gene coding for the bacterial chloramphenicol acetyltransferase and introduced into Hep G2 cells. In these transfected Hep G2 cells CAT activity could be induced by recombinant human interleukin-6. Deletion analyses have shown that the sequences between -852 and -777 as well as between -404 and -165 relative to the cap site, contain regulatory elements involved in the interleukin-6 dependent induction of the alpha 2-macroglobulin gene.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian G. S., Korinek B. W., Bowman B. H., Yang F. The human transferrin gene: 5' region contains conserved sequences which match the control elements regulated by heavy metals, glucocorticoids and acute phase reaction. Gene. 1986;49(2):167–175. doi: 10.1016/0378-1119(86)90277-5. [DOI] [PubMed] [Google Scholar]
- Andus T., Geiger T., Hirano T., Kishimoto T., Tran-Thi T. A., Decker K., Heinrich P. C. Regulation of synthesis and secretion of major rat acute-phase proteins by recombinant human interleukin-6 (BSF-2/IL-6) in hepatocyte primary cultures. Eur J Biochem. 1988 Apr 15;173(2):287–293. doi: 10.1111/j.1432-1033.1988.tb13997.x. [DOI] [PubMed] [Google Scholar]
- Andus T., Geiger T., Hirano T., Northoff H., Ganter U., Bauer J., Kishimoto T., Heinrich P. C. Recombinant human B cell stimulatory factor 2 (BSF-2/IFN-beta 2) regulates beta-fibrinogen and albumin mRNA levels in Fao-9 cells. FEBS Lett. 1987 Aug 31;221(1):18–22. doi: 10.1016/0014-5793(87)80344-7. [DOI] [PubMed] [Google Scholar]
- Bauer J., Birmelin M., Northoff G. H., Northemann W., Tran-Thi T. A., Ueberberg H., Decker K., Heinrich P. C. Induction of rat alpha 2-macroglobulin in vivo and in hepatocyte primary cultures: synergistic action of glucocorticoids and a Kupffer cell-derived factor. FEBS Lett. 1984 Nov 5;177(1):89–94. doi: 10.1016/0014-5793(84)80987-4. [DOI] [PubMed] [Google Scholar]
- Bauer J., Tran-Thi T. A., Northoff H., Hirsch F., Schlayer H. J., Gerok W., Heinrich P. C. The acute-phase induction of alpha 2-macroglobulin in rat hepatocyte primary cultures: action of a hepatocyte-stimulating factor, triiodothyronine and dexamethasone. Eur J Cell Biol. 1986 Mar;40(1):86–93. [PubMed] [Google Scholar]
- Bauer J., Weber W., Tran-Thi T. A., Northoff G. H., Decker K., Gerok W., Heinrich P. C. Murine interleukin 1 stimulates alpha 2-macroglobulin synthesis in rat hepatocyte primary cultures. FEBS Lett. 1985 Oct 14;190(2):271–274. doi: 10.1016/0014-5793(85)81298-9. [DOI] [PubMed] [Google Scholar]
- Baumann H., Jahreis G. P., Sauder D. N., Koj A. Human keratinocytes and monocytes release factors which regulate the synthesis of major acute phase plasma proteins in hepatic cells from man, rat, and mouse. J Biol Chem. 1984 Jun 10;259(11):7331–7342. [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Burch J. B. Identification and sequence analysis of the 5' end of the major chicken vitellogenin gene. Nucleic Acids Res. 1984 Jan 25;12(2):1117–1135. doi: 10.1093/nar/12.2.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cato A. C., Geisse S., Wenz M., Westphal H. M., Beato M. The nucleotide sequences recognized by the glucocorticoid receptor in the rabbit uteroglobin gene region are located far upstream from the initiation of transcription. EMBO J. 1984 Dec 1;3(12):2771–2778. doi: 10.1002/j.1460-2075.1984.tb02208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciliberto G., Dente L., Cortese R. Cell-specific expression of a transfected human alpha 1-antitrypsin gene. Cell. 1985 Jun;41(2):531–540. doi: 10.1016/s0092-8674(85)80026-x. [DOI] [PubMed] [Google Scholar]
- Darlington G. J., Wilson D. R., Lachman L. B. Monocyte-conditioned medium, interleukin-1, and tumor necrosis factor stimulate the acute phase response in human hepatoma cells in vitro. J Cell Biol. 1986 Sep;103(3):787–793. doi: 10.1083/jcb.103.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simone V., Ciliberto G., Hardon E., Paonessa G., Palla F., Lundberg L., Cortese R. Cis- and trans-acting elements responsible for the cell-specific expression of the human alpha 1-antitrypsin gene. EMBO J. 1987 Sep;6(9):2759–2766. doi: 10.1002/j.1460-2075.1987.tb02570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowlkes D. M., Mullis N. T., Comeau C. M., Crabtree G. R. Potential basis for regulation of the coordinately expressed fibrinogen genes: homology in the 5' flanking regions. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2313–2316. doi: 10.1073/pnas.81.8.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung W. P., Schreiber G. Structure and expression of the genes for major acute phase alpha 1-protein (thiostatin) and kininogen in the rat. J Biol Chem. 1987 Jul 5;262(19):9298–9308. [PubMed] [Google Scholar]
- Fung W. P., Thomas T., Dickson P. W., Aldred A. R., Milland J., Dziadek M., Power B., Hudson P., Schreiber G. Structure and expression of the rat transthyretin (prealbumin) gene. J Biol Chem. 1988 Jan 5;263(1):480–488. [PubMed] [Google Scholar]
- Gauldie J., Richards C., Harnish D., Lansdorp P., Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring M. R., Shiels B. R., Northemann W., de Bruijn M. H., Kan C. C., Chain A. C., Noonan D. J., Fey G. H. Sequence of rat liver alpha 2-macroglobulin and acute phase control of its messenger RNA. J Biol Chem. 1987 Jan 5;262(1):446–454. [PubMed] [Google Scholar]
- Geiger T., Andus T., Klapproth J., Hirano T., Kishimoto T., Heinrich P. C. Induction of rat acute-phase proteins by interleukin 6 in vivo. Eur J Immunol. 1988 May;18(5):717–721. doi: 10.1002/eji.1830180510. [DOI] [PubMed] [Google Scholar]
- Geiser M., Mattaj I. W., Wilks A. F., Seldran M., Jost J. P. Structure and sequence of the promoter area and of a 5' upstream demethylation site of the estrogen-regulated chicken vitellogenin ii gene. J Biol Chem. 1983 Jul 25;258(14):9024–9030. [PubMed] [Google Scholar]
- Gordon A. H. The alpha macroglobulins of rat serum. Biochem J. 1976 Dec 1;159(3):643–650. doi: 10.1042/bj1590643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Groner B., Kennedy N., Skroch P., Hynes N. E., Ponta H. DNA sequences involved in the regulation of gene expression by glucocorticoid hormones. Biochim Biophys Acta. 1984 Feb 24;781(1-2):1–6. doi: 10.1016/0167-4781(84)90116-7. [DOI] [PubMed] [Google Scholar]
- Grégori C., Besmond C., Kahn A., Dreyfus J. C. Characterization of messenger RNA for aldolase B in adult and fetal human liver. Biochem Biophys Res Commun. 1982 Jan 29;104(2):369–375. doi: 10.1016/0006-291x(82)90646-5. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hall C. V., Jacob P. E., Ringold G. M., Lee F. Expression and regulation of Escherichia coli lacZ gene fusions in mammalian cells. J Mol Appl Genet. 1983;2(1):101–109. [PubMed] [Google Scholar]
- Hayashida K., Okubo H., Noguchi M., Yoshida H., Kangawa K., Matsuo H., Sakaki Y. Molecular cloning of DNA complementary to rat alpha 2-macroglobulin mRNA. J Biol Chem. 1985 Nov 15;260(26):14224–14229. [PubMed] [Google Scholar]
- Herbomel P., Bourachot B., Yaniv M. Two distinct enhancers with different cell specificities coexist in the regulatory region of polyoma. Cell. 1984 Dec;39(3 Pt 2):653–662. doi: 10.1016/0092-8674(84)90472-0. [DOI] [PubMed] [Google Scholar]
- Jantzen H. M., Strähle U., Gloss B., Stewart F., Schmid W., Boshart M., Miksicek R., Schütz G. Cooperativity of glucocorticoid response elements located far upstream of the tyrosine aminotransferase gene. Cell. 1987 Apr 10;49(1):29–38. doi: 10.1016/0092-8674(87)90752-5. [DOI] [PubMed] [Google Scholar]
- Jost J. P., Seldran M., Geiser M. Preferential binding of estrogen-receptor complex to a region containing the estrogen-dependent hypomethylation site preceding the chicken vitellogenin II gene. Proc Natl Acad Sci U S A. 1984 Jan;81(2):429–433. doi: 10.1073/pnas.81.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Haslinger A., Holtgreve H., Richards R. I., Krauter P., Westphal H. M., Beato M. Characterization of DNA sequences through which cadmium and glucocorticoid hormones induce human metallothionein-IIA gene. Nature. 1984 Apr 5;308(5959):513–519. doi: 10.1038/308513a0. [DOI] [PubMed] [Google Scholar]
- Koj A., Gauldie J., Regoeczi E., Sauder D. N., Sweeney G. D. The acute-phase response of cultured rat hepatocytes. System characterization and the effect of human cytokines. Biochem J. 1984 Dec 1;224(2):505–514. doi: 10.1042/bj2240505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner I. The phenomenon of the acute phase response. Ann N Y Acad Sci. 1982;389:39–48. doi: 10.1111/j.1749-6632.1982.tb22124.x. [DOI] [PubMed] [Google Scholar]
- Lei K. J., Liu T., Zon G., Soravia E., Liu T. Y., Goldman N. D. Genomic DNA sequence for human C-reactive protein. J Biol Chem. 1985 Oct 25;260(24):13377–13383. [PubMed] [Google Scholar]
- Lowell C. A., Potter D. A., Stearman R. S., Morrow J. F. Structure of the murine serum amyloid A gene family. Gene conversion. J Biol Chem. 1986 Jun 25;261(18):8442–8452. [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northemann W., Andus T., Gross V., Heinrich P. C. Cell-free synthesis of rat alpha 2-macroglobulin and induction of its mRNA during experimental inflammation. Eur J Biochem. 1983 Dec 1;137(1-2):257–262. doi: 10.1111/j.1432-1033.1983.tb07823.x. [DOI] [PubMed] [Google Scholar]
- Northemann W., Andus T., Gross V., Nagashima M., Schreiber G., Heinrich P. C. Messenger RNA activities of four acute phase proteins during inflammation. FEBS Lett. 1983 Sep 19;161(2):319–322. doi: 10.1016/0014-5793(83)81033-3. [DOI] [PubMed] [Google Scholar]
- Northemann W., Heisig M., Kunz D., Heinrich P. C. Molecular cloning of cDNA sequences for rat alpha 2-macroglobulin and measurement of its transcription during experimental inflammation. J Biol Chem. 1985 May 25;260(10):6200–6205. [PubMed] [Google Scholar]
- Okubo H., Miyanaga O., Nagano M., Ishibashi H., Kudo J., Ikuta T., Shibata K. Purification and immunological determination of alpha 2-macroglobulin in serum from injured rats. Biochim Biophys Acta. 1981 Apr 28;668(2):257–267. doi: 10.1016/0005-2795(81)90033-7. [DOI] [PubMed] [Google Scholar]
- Oliviero S., Morrone G., Cortese R. The human haptoglobin gene: transcriptional regulation during development and acute phase induction. EMBO J. 1987 Jul;6(7):1905–1912. doi: 10.1002/j.1460-2075.1987.tb02450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter D. H., Dinarello C. A., Punsal P. I., Colten H. R. Cachectin/tumor necrosis factor regulates hepatic acute-phase gene expression. J Clin Invest. 1986 Nov;78(5):1349–1354. doi: 10.1172/JCI112721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter D. H., Goldberger G., Dinarello C. A., Mizel S. B., Colten H. R. Regulation of class III major histocompatibility complex gene products by interleukin-1. Science. 1986 May 16;232(4752):850–852. doi: 10.1126/science.3010455. [DOI] [PubMed] [Google Scholar]
- Prowse K. R., Baumann H. Hepatocyte-stimulating factor, beta 2 interferon, and interleukin-1 enhance expression of the rat alpha 1-acid glycoprotein gene via a distal upstream regulatory region. Mol Cell Biol. 1988 Jan;8(1):42–51. doi: 10.1128/mcb.8.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G., Sipe J. D., Dinarello C. A., Mizel S. B., Colten H. R. Pretranslational modulation of acute phase hepatic protein synthesis by murine recombinant interleukin 1 (IL-1) and purified human IL-1. J Exp Med. 1985 Sep 1;162(3):930–942. doi: 10.1084/jem.162.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke R., Feigelson P. Rat alpha 1-acid glycoprotein. Gene sequence and regulation by glucocorticoids in transfected L-cells. J Biol Chem. 1985 Apr 10;260(7):4397–4403. [PubMed] [Google Scholar]
- Renkawitz R., Schütz G., von der Ahe D., Beato M. Sequences in the promoter region of the chicken lysozyme gene required for steroid regulation and receptor binding. Cell. 1984 Jun;37(2):503–510. doi: 10.1016/0092-8674(84)90380-5. [DOI] [PubMed] [Google Scholar]
- Ritchie D. G., Fuller G. M. Hepatocyte-stimulating factor: a monocyte-derived acute-phase regulatory protein. Ann N Y Acad Sci. 1983 Jun 27;408:490–502. doi: 10.1111/j.1749-6632.1983.tb23268.x. [DOI] [PubMed] [Google Scholar]
- Saluz H. P., Jiricny J., Jost J. P. Genomic sequencing reveals a positive correlation between the kinetics of strand-specific DNA demethylation of the overlapping estradiol/glucocorticoid-receptor binding sites and the rate of avian vitellogenin mRNA synthesis. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7167–7171. doi: 10.1073/pnas.83.19.7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent T. D., Wu J. R., Sala-Trepat J. M., Wallace R. B., Reyes A. A., Bonner J. The rat serum albumin gene: analysis of cloned sequences. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3256–3260. doi: 10.1073/pnas.76.7.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent T. D., Wu J. R., Sala-Trepat J. M., Wallace R. B., Reyes A. A., Bonner J. The rat serum albumin gene: analysis of cloned sequences. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3256–3260. doi: 10.1073/pnas.76.7.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidereit C., Beato M. Contacts between hormone receptor and DNA double helix within a glucocorticoid regulatory element of mouse mammary tumor virus. Proc Natl Acad Sci U S A. 1984 May;81(10):3029–3033. doi: 10.1073/pnas.81.10.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidereit C., Geisse S., Westphal H. M., Beato M. The glucocorticoid receptor binds to defined nucleotide sequences near the promoter of mouse mammary tumour virus. Nature. 1983 Aug 25;304(5928):749–752. doi: 10.1038/304749a0. [DOI] [PubMed] [Google Scholar]
- Schmid W., Scherer G., Danesch U., Zentgraf H., Matthias P., Strange C. M., Röwekamp W., Schütz G. Isolation and characterization of the rat tryptophan oxygenase gene. EMBO J. 1982;1(10):1287–1293. doi: 10.1002/j.1460-2075.1982.tb00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya Y., Hattori M., Hayashida K., Ishibashi H., Okubo H., Sakaki Y. Sequence analysis of the putative regulatory region of rat alpha 2-macroglobulin gene. Gene. 1987;57(1):73–80. doi: 10.1016/0378-1119(87)90178-8. [DOI] [PubMed] [Google Scholar]
- Woo P., Korenberg J. R., Whitehead A. S. Characterization of genomic and complementary DNA sequence of human C-reactive protein, and comparison with the complementary DNA sequence of serum amyloid P component. J Biol Chem. 1985 Oct 25;260(24):13384–13388. [PubMed] [Google Scholar]





