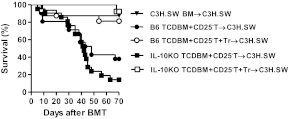
The donor source of IL-10 is critical for regulation of GVHD severity.
Keywords: cytokines, IL-10, BMT, GVHD, Tregs
Abstract
IL-10 is a key immune-regulatory cytokine, and its gene polymorphisms correlate with severity of clinical GVHD. IL-10 is made by a variety of donor and host cells, but the functional relevance of its source and its role in the biology of acute GVHD are not well understood. We used preclinical models to examine the relevance of IL-10−/− in donor and host cellular subsets on the severity of GVHD. IL-10−/− in host tissues or in the donor grafts did not alter donor Teff-mediated severity of GVHD. Furthermore, neither host-derived nor donor Teff-derived IL-10 was required for regulation of GVHD by WT CD4+CD25+ donor Tregs. By contrast, Treg-derived IL-10, although not obligatory, was necessary for optimal reduction of GVHD by mature donor Tregs. Importantly, IL-10 from donor BM grafts was also critical for optimal donor Treg-mediated suppression of GVHD. Together, these data suggest that IL-10 does not contribute to the induction of GVHD severity by the Teffs. However, donor BM graft and Treg-derived IL-10 are important for donor Treg-mediated suppression of GVHD.
Introduction
Allogeneic BMT is a curative, therapeutic option for hematological malignancies [1, 2]. The donor T lymphocytes eliminate host malignancies but also concurrently cause GVHD [3], the major side-effect of allogeneic BMT [1, 4, 5]. A large number of proinflammatory cytokines and alloreactive donor T cells are involved in the pathophysiology of GVHD [5]. Several inflammatory cytokines, such as IL-1, TNF-α, IL-6, and IFN-γ, are elevated after allogeneic BMT and are known to perpetuate GVHD through direct cytotoxic effects on host tissues and also by activation and priming of immune effector cells [4, 6–8]. Whereas the role of proinflammatory cytokines in the biology of GVHD is well appreciated, the role of immune-regulatory cytokines, such as IL-10 and TGF-β, has not been as well studied [9–11].
IL-10 was first identified by Mosmann and colleagues [12, 13] and subsequently named as IL-10 following the cloning of its cDNA. IL-10 can be produced by virtually all T cell subsets, including Th1, Th2, Th17, Th9, Teffs, and CD4+25+Foxp3+ Tregs and induced regulatory Tr1 cells. In addition, tissue epithelial cells can synthesize IL-10 under certain circumstances [14, 15]. IL-10 primarily targets leukocytes, has broad immune-regulatory effects [15–17], and has been implicated directly in the regulation of many experimental and human inflammatory diseases [15, 17, 18].
Several lines of strong, correlative data suggest that IL-10 may be relevant in the induction and severity of clinical GVHD. For example, IL-10 polymorphisms in the recipients of HLA-matched sibling transplants are associated with acute and chronic GVHD, whereas serum levels of IL-10 are elevated in patients with acute GVHD [19–24] and in autologous GVHD [25, 26]. Experimental data demonstrate a dose-dependent modulation of GVHD by exogenous IL-10 [9, 27]. Administration of low doses of IL-10 suppressed GVHD, whereas higher doses exacerbated severity of GVHD in MHC disparate BMT [9]. IL-10 has also been implicated in the suppression of GVHD by Tr1 cells, G-CSF analogues, inverse vaccination, or eosinophil cationic protein [28–32]. However, the role of endogenous sources of IL-10 in modulating GVHD biology and mortality remains poorly understood.
The balance between alloreactive donor Teffs and donor-derived, natural CD4+25+Foxp3+ T cells (Tregs) has been shown to modulate the severity of GVHD [6, 33–36]. Tregs use diverse mechanisms in mediating their regulatory functions [37]. Depending on the context, IL-10 has been implicated in mediating Treg-induced immune regulation [35]. The mechanisms for Treg-mediated suppression of GVHD are largely unknown. Specifically, whether IL-10 plays an important role in Treg-mediated reduction of GVHD severity is not well understood.
We carefully examined the cellular sources and biological role of endogenously derived IL-10 in the induction of acute GVHD by the cytopathic donor Teffs and its regulation by the donor CD4+25+ Tregs. We demonstrate that neither donor- nor host-derived IL-10 modulates GVHD mediated by alloreactive, cytopathic Teffs in the absence of donor CD4+25+Foxp3+ Tregs. By contrast, donor-derived IL-10, either from the mature Tregs or the BM, plays an important role in the suppression of GVHD severity.
MATERIALS AND METHODS
Mice
Female B6 (H-2b, CD45.2+), B6-background, IL-10−/− (B6.129P2-Il10tm1Cgn/J, IL-10KO, H-2b, CD45.2+), and C3H.SW (H-2b, CD45.2+, CD229.1+) mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). B6-Ly5.2/Cr (B6-CD45.1, H-2b, CD45.1+) mice were purchased from NCI-Frederick (Frederick, MD, USA). All animal work must have been conducted according to institutional IACUC guidelines of the University of Michigan (Ann Arbor, MI, USA), Protocol #10006. The IACUC Institutional Review Board Committee specifically approved this study.
Antibodies and flow cytometric analysis
FITC-, PE-, PerCP-Cy5.5 or allophycocyanin-conjugated mAb to mouse CD4 (clone; RM4-4), CD8a (53-6.7), CD25 (PC61.5), CD45.1 (A20), and CD45.2 (104) were purchased from eBioscience (San Diego, CA, USA). Anti-mouse CD229.1 (30C7) was purchased from BD PharMingen (San Diego, CA, USA). Spleen or BM cells were incubated with anti-CD16/CD32 (2.4G2; Fc block, BD PharMingen) mAb for 15 min at 4°C in staining buffer (2% FCS-containing PBS), and then the cells were stained with fluorochrome-conjugated mAb for 15 min at 4°C in the staining buffer. After washing, cells were fixed with FACS lysing solution (BD Biosciences, San Diego, CA, USA) [38]. For intracellular Foxp3 staining, the following procedure was performed after cell-surface staining: fixed cells were washed with permeabilization buffer (eBioscience) and stained with PE-conjugated anti-Foxp3 mAb (FJK-16s; eBioscience) for 30 min at 4°C. The cells were washed with permeabilization buffer and staining buffer (2×) and resuspended with staining buffer for analysis. Cells were analyzed on a C6 flow cytometer (Accuri Cytometers, Ann Arbor, MI, USA).
BM chimeras
Recipient mice (B6-Ly5.2/Cr) were irradiated (137Cs source) with 11 Gy TBI before transplant of syngeneic BM cells [38]. Donor BM cells were harvested from femurs and tibias of donor mice, washed, and counted, and cells (5×106) were infused into recipient mice through the tail vein. Recipient mice were maintained on acidified water (pH <3) for 3 weeks. Donor hematopoietic cell engraftment was confirmed using peripheral blood by flow cytometry, 2–3 months after transplantation. Anti-CD45.1 or -CD45.2 mAb were used for identification of donor-derived white blood cells. All animal studies were performed per the institutional IACUC guidelines of the University of Michigan.
BMT
Recipient mice were irradiated (137Cs source) with 9 to 11 Gy TBI, 1 day before BMT [8, 38]. Donor BM cells were harvested from femurs and tibias, and T cells in the BM were removed magnetically using CD90.2 microbeads (Miltenyi Biotec, Auburn, CA, USA) and a MACS LS column (Miltenyi Biotec). Spleen T cells were magnetically isolated using MACS LS or LD columns (Miltenyi Biotec). Pan T cell isolation kit (Miltenyi Biotec) and biotin anti-CD25 (clone 7D4, BD PharMingen) were used for CD25-depleted T cell isolation. CD8a+ T cell isolation kit and CD4+CD25+ Treg isolation kit (Miltenyi Biotec) were used for CD8+ T cell isolation and CD4+CD25+ T cell isolation, respectively. Purities of isolated T cells were checked by flow cytometry, and T cell numbers were determined based on cell count and purity. Syngeneic or allogeneic BM and T cells were resuspended with L-15 media (Mediatech, Manassas, VA, USA) and infused through the tail vein (250 μl/mouse). Host mice were housed in sterilized microisolator cages and maintained on acidified water (pH <3) for 3 weeks [8]. Survival was monitored daily, and clinical GVHD was assessed weekly [38]. All animal studies were performed per the institutional IACUC guidelines of the University of Michigan.
In vitro suppression assay
CD4+CD25− and CD4+CD25+ T cells were isolated from spleen cells from B6(WT) or IL-10KO animals using the CD4+CD25+ Treg isolation kit (Miltenyi Biotec) [8]. For the anti-CD3e-stimulated suppression assay, CD4+CD25+ T cells were serially diluted from 1 × 104–1250 cells/well and incubated with B6(WT) 1 × 104 CD4+CD25− T cells and 5 × 104 irradiated (30 Gy), syngeneic B6 spleen cells in the presence of 2 μg/ml anti-CD3e mAb (clone 145-2C-11; eBioscience) for 72 h. For the allo-stimulated suppression assay, CD4+CD25+ T cells were serially diluted from 2 × 104–2500 cells/well and incubated with B6(WT) 2 × 104 CD4+CD25− T cells and 1 × 103 allogeneic B6D2F1 or BALB/c BM DCs for 120 h. Incorporation of 3H-thymidine (1 μCi/well) by proliferating cells was measured during the last 6 h of culture [8].
In vivo Treg expansion
C3H.SW recipient mice were irradiated (10 Gy; Day −1) and transplanted B6(WT) 1 × 106 CD25-depleted T cells and B6-CD45.1 0.25 × 106 CD4+CD25+ T cells with 5 × 106 TCD BM from B6(WT) or IL-10KO mice. On day 10, 17, or 24 post-BMT, a phenotype of the spleen cells was collected, counted, and analyzed by flow cytometry. Donor Tregs were defined as CD45.1+CD4+Foxp3+ cells [38].
Histology
Formalin-preserved gut, liver, and skin were embedded in paraffin, cut into 5 μm-thick sections, and stained with H&E for histologic examination. Slides were coded without reference to prior treatment and examined in a blinded manner by a pathologist (C. Liu). A semiquantitative scoring system was used to assess the following abnormalities known to be associated with GVHD.
Statistical analysis
Student's t test was used for the statistical analysis of in vitro data. The log-rank test was used to analyze survival data. P < 0.05 was considered statistically significant.
RESULTS
Host-derived IL-10 is dispensable for induction of GVHD
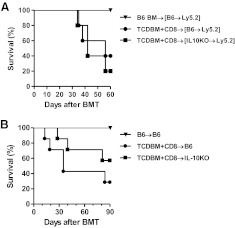
IL-10 can be derived from the host hematopoietic or the nonhematopoietic cells [15]. Host hematopoietic-derived APCs are known to modulate donor T cell responses [5, 39]. As IL-10 secretion by APCs has been suggested to alter T cell responses, we first determined whether IL-10 production by only the host hematopoietic cells plays a critical role in modulating GVHD [40]. To this end, we generated [IL-10−/− B6→B6] (such that only the hematopoietic-derived cells were incapable of producing IL-10) and the control [WTB6→B6] chimeras and 3 months later, used them as recipients in an allogeneic BMT. The chimeras were conditioned and transplanted with TCD BM and donor T cells, which were rigorously depleted of CD25 cells (to eliminate any potential confounding effects of Tregs) from syngeneic B6 or MHC-matched, multiple minor-mismatched C3H.SW allogeneic donors, as in Materials and Methods. As shown in Fig. 1A, the [IL-10−/− B6→B6] and the [WTB6→B6] recipients demonstrated similar mortality from GVHD (P=0.38). The severity of clinical and histopathological GVHD was similar regardless of the ability of hosts to make IL-10 (data not shown).
Figure 1. Effect of host-derived IL-10 on GVHD.
(A) B6(WT) BM chimera recipients [B6→Ly5.2] were irradiated (9 Gy) on Day −1 and transplanted 5 × 106 syngeneic B6 BM (▾; n=6) or allogeneic C3H.SW 5 × 106 TCD BM + 0.2 × 106 CD8 T cells (●; n=5). B6-IL-10KO BM chimera recipients [IL-10KO→Ly5.2] were irradiated (9 Gy) on Day −1 and transplanted allogeneic C3H.SW 5 × 106 TCD BM + 0.2 × 106 CD8 T cells (■; n=5). Survival was monitored daily. Data from one of two similar experiments are shown. ● versus ■, P = 0.6186. (B) B6(WT) recipients were irradiated (10 Gy) on Day −1 and transplanted 5 × 106 syngeneic B6 BM (▾; n=9) or allogeneic C3H.SW 5 × 106 TCD BM + 0.2 × 106 CD8 T cells (●; n=7). B6-IL-10KO recipients were irradiated (10 Gy) on Day −1 and transplanted allogeneic C3H.SW 5 × 106 TCD BM + 0.2 × 106 CD8 T cells (■; n=7). Survival was monitored daily. Data from one of three similar experiments are shown. ● versus ■, P = 0.2516.
We next determined whether IL-10 production from all of the host tissues (hematopoietic and nonhematopoietic cells) altered the severity of GVHD after allogeneic BMT. To this end, we used IL-10KO recipients so that none of the host-derived cells can make any IL-10. Lethally irradiated WT or IL-10−/− B6 animals were transplanted with TCD BM and CD25-depleted T cells from syngeneic B6 or MHC-matched, multiple minor-mismatched C3H.SW allogeneic donors, as in Materials and Methods. As shown in Fig. 1B, there was no significant difference in GVHD-induced mortality in the WT and IL-10−/− hosts (P=0.25). Collectively, these data demonstrate that neither host hematopoietic alone nor the host nonhematopoietic and hematopoietic-derived IL-10 play a critical role in modulating the severity of GVHD caused by alloreactive Teffs.
Impact of donor-derived IL-10 on GVHD severity
Antigen-primed T cells show higher expression of IL-10 than naïve T cells [14]. We therefore tested the hypothesis that lack of expression of IL-10 by alloreactive cytopathic donor Teffs will aggravate GVHD mortality. C3H.SW animals were lethally irradiated and transplanted with TCD BM and T cells from the syngeneic C3H.SW donors. The allogeneic C3H.SW animals received TCD BM from WT B6 animals along with T cells that were rigorously depleted of CD25+ cells (to eliminate any potential confounding effects from Tregs) from WT B6 or IL-10−/− B6 donors, such that only the donor-alloreactive cytopathic T cells were incapable of contributing IL-10. Contrary to our hypothesis, inability of alloreactive donor T cells to make IL-10 did not affect GVHD mortality (Fig. 2A; P=0.41) or clinical severity (score of 5.5±0.8 vs. 6.1±1, 4.3±0.6 vs. 4.1±0.7, 5.2±0.9 vs. 5.1±0.5, 6.3±0.4 vs. 5.9±1.1, on Days 7, 14, 28, and 42, respectively). These results were also reflected in the similar histopathological damage (data not shown). Furthermore, proliferation of WT and IL-10−/− T cells was similar upon in vitro allogeneic stimulation with BALB/c DCs (39,653±948 vs. 42,221±2969; P=0.7), demonstrating that the inability of the T cells to make IL-10 did not affect their intrinsic alloreactivity.
Figure 2. Effect of donor-derived IL-10 on GVHD.
(A) C3H.SW recipients were irradiated (10 Gy) on Day −1 and transplanted 5 × 106 syngeneic C3H.SW BM (▾; n=5), allogeneic B6(WT) 5 × 106 TCD BM + B6(WT) 1 × 106 CD25-depleted T cells (●; n=10), or B6(WT) 5 × 106 TCD BM + IL-10KO 1 × 106 CD25-depleted T cells (■; n=12). Data from one of two similar experiments are shown. ● versus ■, P = 0.4081. (B) C3H.SW recipients were irradiated (10 Gy) on Day −1 and transplanted 5 × 106 syngeneic C3H.SW BM (▾; n=5), allogeneic B6(WT) 5 × 106 TCD BM + B6(WT) 1 × 106 CD25-depleted T cells (●; n=10), or IL-10KO 5 × 106 TCD BM + B6(WT) 1 × 106 CD25-depleted T cells (■; n=10). Survival was monitored daily. Data from one of two similar experiments are shown. ● versus ■, P = 0.0785. (C) Day 24 pathology score of C3H.SW recipients received 1 × 106 allogeneic B6(WT) CD25-depleted T cells along with B6 (WT; n=4, black bar) or IL-10KO (n=4, gray bar) TCD BM. P = not significant. (D) B6D2F1 recipients were irradiated (11 Gy) on Day −1 and transplanted 5 × 106 syngeneic B6D2F1 BM (▾; n=11), allogeneic B6(WT) 5 × 106 TCD BM + B6(WT) 1 × 106 CD25-depleted T cells (●; n=15), or IL-10KO 5 × 106 TCD BM + B6(WT) 1 × 106 CD25-depleted T cells (■; n=15). Survival was monitored daily. Data from three similar experiments were combined and shown. ● versus ■, P = 0.7699.
We next determined whether the expression of IL-10 by the nonalloreactive T cells from the donor modulated severity of GVHD. The C3H.SW animals were conditioned and transplanted as above. The allogeneic recipients were transplanted with WT CD25-depleted B6 T cells, along with TCD BM from the WT B6 or IL-10−/− animals. As shown in Fig. 2B, the lack of expression of IL-10 by donor BM-derived cells did not affect GVHD mortality (P=0.08). Although the animals that received IL-10−/− BM appeared to demonstrate a trend toward greater mortality in this experiment, this was not apparent in repeat experiments, and importantly, the histopathological analyses of the GVHD target organs, the liver, and GI tract demonstrated similar GVHD-specific damage (Fig. 2C). Furthermore, the allogeneic recipient animals in a second [MHC disparate, B6→F1] BMT model, which were transplanted with IL-10−/− BM, also demonstrated similar GVHD mortality (Fig. 2D). Together, these data demonstrate that absence of IL-10 secretion by cytopathic alloreactive, mature donor T cells or the BM-derived cells does not modulate GVHD.
IL-10 secretion by donor Tregs is required for their optimal regulation of GVHD
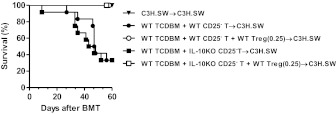
The severity of GVHD is determined by the balance between the mature, alloreactive, cytopathic T cells and Tregs from the donors [5, 6]. IL-10 has been implicated as one of the mechanisms for Treg-mediated suppression [37]. We therefore determined whether IL-10 expression by donor Tregs is critical for regulation of GVHD. The C3H.SW animals were irradiated and transplanted with TCD BM and CD25-depleted T cells from WT B6 animals. There was no intrinsic difference in the expression of CD25+ and Foxp3 between the WT and IL-10−/− animals at baseline. Hence, donor-derived CD25+CD4+ natural Tregs (98–99% of these cells were Foxp3) from WT B6 or IL-10−/− B6 animals were transplanted at 1:4 and 1:16 ratios with CD25− alloreactive T cells. As expected, allogeneic animals that received a WT Treg:Teff ratio of 1:4 demonstrated significant protection from GVHD mortality when compared with animals that did not receive any Tregs (20% vs. 80%; P=0.0010). As shown in Fig. 3A, infusion of lower ratios of Tregs:Teffs (1:16) did not offer any survival benefit (P=0.25), demonstrating that the ability of Tregs to suppress GVHD is a function of the balance between Tregs and Teffs. By contrast, infusion of IL-10−/− Tregs at 1:16 and 1:4 ratios with WT Teffs induced similar GVHD mortality when compared with control animals that did not receive any Tregs, thus demonstrating a failure of protection from GVHD by the IL-10−/− Tregs (Fig. 3B; P=0.4). However, further increasing the ratio of IL-10−/− Tregs: WT Teffs (to 1:2) modestly mitigated GVHD mortality (50% vs. 90%; P<0.03) when compared with animals that did not receive any Tregs. This GVHD-protective effect of IL-10−/− Tregs at a 1:2 ratio was nonetheless still inferior to that afforded by WT Tregs at a lower ratio (1:4 50% vs. 20% mortality, respectively; Figs. 3A and B). By contrast, Tregs suppressed T cell responses, regardless of the allogeneic stimulus (Supplemental Fig. 1). Furthermore, consistent with previous data demonstrating lack of requirement of IL-10 secretion by Tregs in suppressing in vitro alloresponses [35, 41, 42], IL-10−/− Tregs demonstrated equivalent suppression of nonspecific T cell proliferation in vitro when compared with WT Tregs (Fig. 3C). Furthermore, the WT and IL-10−/− Tregs demonstrated no significant differences in the surface expression of CTLA4 and glucocorticoid-induced TNFR (data not shown). These data indicate that the reduced ability of IL-10−/− Tregs to suppress GVHD is likely, not because of a Treg-intrinsic defect.
Figure 3. Effect of donor Treg-derived IL-10 on GVHD.
(A) C3H.SW recipients were irradiated (10 Gy) on Day −1 and transplanted 5 × 106 syngeneic C3H.SW BM (▾; n=5), allogeneic B6(WT) 5 × 106 TCD BM + B6(WT) 1 × 106 CD25-depleted T cells (●; n=10), B6(WT) 5 × 106 TCD BM + B6(WT) 1 × 106 CD25-depleted T cells + B6(WT) 0.25 × 106 CD4+CD25+ T cells (○; n=14), or B6(WT) 5 × 106 TCD BM + B6(WT) 1 × 106 CD25-depleted T cells + B6(WT) 0.0625 × 106 CD4+CD25+ T cells (▴; n=6). Survival was monitored daily. Data from two combined similar experiments are shown. ● versus ○, P = 0.0010; ● versus ▴, P = 0.2544. (B) C3H.SW recipients were irradiated (10 Gy) on Day −1 and transplanted 5 × 106 syngeneic C3H.SW BM (▾; n=5), allogeneic B6(WT) 5 × 106 TCD BM + B6(WT) 1 × 106 CD25-depleted T cells (●; n=10), B6(WT) 5 × 106 TCD BM + B6(WT) 1 × 106 CD25-depleted T cells + IL-10KO 0.5 × 106 CD4+CD25+ T cells (♦; n=4), B6(WT) 5 × 106 TCD BM + B6(WT) 1 × 106 CD25-depleted T cells + IL-10KO 0.25 × 106 CD4+CD25+ T cells (□; n=5), or B6(WT) 5 × 106 TCD BM + B6(WT) 1 × 106 CD25-depleted T cells + IL-10KO 0.0625 × 106 CD4+CD25+ T cells (▴; n=4). Survival was monitored daily. Data from one of two similar experiments are shown. ● versus ♦, P = 0.4115. (C) Suppressive activity of B6(WT) and IL-10KO Tregs. Serially diluted (1×104–1250/well) B6(WT) or IL-10KO CD4+CD25+ T cells were cocultured with 1 × 104 B6(WT) CD4+CD25− T cells in the presence of irradiated syngeneic 5 × 104 B6(WT) spleen cells and 2 μg/ml anti-CD3e mAb for 72 h. Incorporation of 3H-thymidine (1 μCi/well) by proliferating cells was measured during the last 6 h of culture.
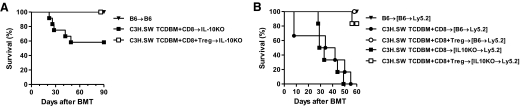
Donor Tregs mitigate GVHD induced by IL-10−/− Teffs
IL-10−/− alloreactive Teffs caused similar GVHD as the WT T cells. We next examined whether this GVHD was amenable to suppression by WT Tregs. The C3H.SW animals were lethally irradiated and transplanted with IL-10−/− CD25− T cells and TCD BM from WT B6 animals, along with or without CD25+CD4+ Tregs from WT donors. As shown in Fig. 4, a vast majority of the animals that received only the CD25− Teffs from IL-10−/− donors died from GVHD, whereas nearly all of the allogeneic animals that received WT donor CD25+ Tregs along with the CD25− Teffs survived (mortality rate of 70% vs. 0%; P=0.0080). These data demonstrate that donor Tregs that express IL-10 can effectively mitigate GVHD, even when the cytopathic Teffs do not express IL-10.
Figure 4. Donor Teff-derived IL-10 is not required for Treg-mediated suppression of GVHD.
C3H.SW recipients were irradiated (10 Gy) on Day −1 and transplanted 5 × 106 syngeneic C3H.SW BM (▾; n=6), allogeneic B6(WT) 5 × 106 TCD BM + B6(WT) 1 × 106 CD25-depleted T cells (●; n=12), B6(WT) 5 × 106 TCD BM + B6(WT) 1 × 106 CD25-depleted T cells + B6(WT) 0.25 × 106 CD4+CD25+ T cells (○; n=7), B6(WT) 5 × 106 TCD BM + IL-10KO 1 × 106 CD25-depleted T cells (■; n=12), or B6(WT) 5 × 106 TCD BM + IL-10KO 1 × 106 CD25-depleted T cells + B6(WT) 0.25 × 106 CD4+CD25+ T cells (□; n=7). Survival was monitored daily. Data from two combined similar experiments are shown. ● versus ○, P = 0.0080; ■ versus □, P = 0.0080.
IL-10 by host is not required for Treg-mediated GVHD suppression
Our data demonstrated that IL-10 expression by host hematopoietic or nonhematopoietic tissues is dispensable for induction of GVHD by Treg-depleted alloreactive Teffs. However, IL-10 expression by APCs has been suggested to be important for Treg-mediated suppression [40, 43]. Therefore, we next analyzed whether expression of IL-10, only by host hematopoietic-derived APCs or by all hematopoietic and nonhematopoietic cells, is critical for Treg-mediated mitigation of GVHD. The [IL-10−/− B6→WT B6] and the [WTB6→WTB6] chimeras were transplanted with TCD BM and alloreactive CD25−CD8+, along with, or without, CD25hiCD4+ T cells from allogeneic C3H.SW animals. Donor CD25hiCD4+ Tregs significantly suppressed GVHD in the [IL-10−/− B6→WT B6] and [WTB6→WTB6] chimeras, regardless of the ability of the host hematopoietic-derived cells to make IL-10 (Fig. 5A; P=0.0005). Furthermore, as shown in Fig. 5B, donor CD25hiCD4+ Tregs also robustly suppressed GVHD mortality in the IL-10−/− and WT B6 animals (P=0.01), demonstrating that host-derived IL-10 is dispensable for Treg-mediated regulation of GVHD.
Figure 5. Effect of host-derived IL-10 on Treg-mediated suppression of GVHD.
(A) B6(WT) recipients were irradiated (10 Gy) on Day −1 and transplanted 5 × 106 syngeneic B6 BM (▾; n=9), B6-IL-10KO recipients were irradiated (10 Gy) on Day −1 and transplanted allogeneic C3H.SW 5 × 106 TCD BM + 0.2 × 106 CD8 T cells (■; n=12), or C3H.SW 5 × 106 TCD BM + 0.2 × 106 CD8 T cells + 0.1 × 106 CD4+CD25+ T cells (□; n=12). Survival was monitored daily. Data from two similar experiments are combined and shown. ■ versus □, P = 0.0136. (B) B6(WT) BM chimera recipients [B6→Ly5.2] were irradiated (9 Gy) on Day −1 and transplanted 5 × 106 syngeneic B6 BM (▾; n=5), allogeneic C3H.SW 5 × 106 TCD BM + 0.2 × 106 CD8 T cells (●; n=6), or C3H.SW 5 × 106 TCD BM + 0.2 × 106 CD8 T cells + 0.1 × 106 CD4+CD25+ T cells (○; n=5). B6-IL-10KO BM chimera recipients [IL-10KO→Ly5.2] were irradiated (9 Gy) on Day −1 and transplanted allogeneic C3H.SW 5 × 106 TCD BM + 0.2 × 106 CD8 T cells (■; n=6) or C3H.SW 5 × 106 TCD BM + 0.2 × 106 CD8 T cells + 0.1 × 106 CD4+CD25+ T cells (□; n=6). Survival was monitored daily. Data from one of two similar experiments are shown. ● versus ○, P = 0.0013; ■ versus □, P = 0.0005.
Donor BM-derived IL-10 is required for optimal Treg-mediated reduction in GVHD
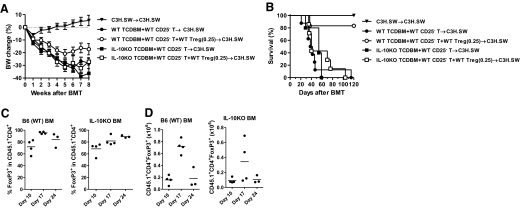
In light of data demonstrating a role for APC-derived IL-10 in Treg-mediated suppression [15, 40, 43] and given the lack of impact of host-derived APCs in Treg-mediated regulation of GVHD [38], we next examined whether donor BM-derived IL-10 is critical for Treg-mediated suppression of GVHD. The C3H.SW animals were lethally irradiated and transplanted with CD25-depleted B6 WT Teffs, along with, or without, WT CD25hiCD4+ Tregs and TCD BM from IL-10−/− B6 animals. The clinical severity of GVHD (Fig. 6A) and the mortality (Fig. 6B; P=0.9) and histopathological damage in the GI tract (Supplemental Fig. 2) were similar, regardless of whether the recipient animals received WT donor Tregs. These data demonstrate that Tregs failed to suppress the clinical severity (Fig. 6A) or reduce GVHD mortality (Fig. 6B) in the animals that received BM from IL-10−/− donors.
Figure 6. Effect of donor BM-derived IL-10 on Treg-mediated suppression of GVHD.
(A) C3H.SW recipients were irradiated (10 Gy) on Day −1 and transplanted 5 × 106 syngeneic C3H.SW BM (▾; n=10), allogeneic B6(WT) 5 × 106 TCD BM + B6(WT) 1 × 106 CD25-depleted T cells (●; n=20), B6(WT) 5 × 106 TCD BM + B6(WT) 1 × 106 CD25-depleted T cells + B6(WT) 0.25 × 106 CD4+CD25+ T cells (○; n=14), IL-10KO 5 × 106 TCD BM + B6(WT) 1 × 106 CD25-depleted T cells (■; n=15), or IL-10KO 5 × 106 TCD BM + B6(WT) 1 × 106 CD25-depleted T cells + B6(WT) 0.25 × 106 CD4+CD25+ T cells (□; n=14). Body weight (BW) of recipients was monitored weekly. Data from four similar experiments were combined and shown. (B) C3H.SW recipients were irradiated (10 Gy) on Day −1 and transplanted 5 × 106 syngeneic C3H.SW BM (▾; n=4), allogeneic B6(WT) 5 × 106 TCD BM + B6(WT) 1 × 106 CD25-depleted T cells (●; n=8), B6(WT) 5 × 106 TCD BM + B6(WT) 1 × 106 CD25-depleted T cells + B6(WT) 0.25 × 106 CD4+CD25+ T cells (○; n=6), IL-10KO 5 × 106 TCD BM + B6(WT) 1 × 106 CD25-depleted T cells (■; n=5), or IL-10KO 5 × 106 TCD BM + B6(WT) 1 × 106 CD25-depleted T cells + B6(WT) 0.25 × 106 CD4+CD25+ T cells (□; n=7). Survival was monitored daily. Data from one of two similar experiments were shown. ● versus ○, P = 0.0037; ■ versus □, P = 0.4752; ● versus ■, P = 0.2806. (C and D) C3H.SW recipients were irradiated (10 Gy) on Day −1 and transplanted allogeneic B6(WT) 5 × 106 TCD BM + B6(WT) 1 × 106 CD25-depleted T cells + B6-CD45.1 0.25 × 106 CD4+CD25+ T cells or IL-10KO 5 × 106 TCD BM + B6(WT) 1 × 106 CD25-depleted T cells + B6-CD45.1 0.25 × 106 CD4+CD25+ T cells. Spleen cells were harvested, counted, and analyzed by flow cytometry on Days 10, 17, and 24. Percentage of Foxp3+ cells in donor (CD45.1+) CD4+ T cells (C) and calculated donor Treg (CD45.1+CD4+Foxp3+) numbers (D) was shown. Data from one of two experiments are shown.
We next explored the mechanisms for the requirement of donor BM-derived IL-10 in Treg-mediated suppression of GVHD. The stability of the expression of Foxp3 in Tregs is critical for their regulatory function [44, 45]. Therefore, we first analyzed whether the Foxp3 expression in donor Tregs was stable in the absence of IL-10 production by donor BM cells. The expression of Foxp3 was stable in the infused, mature donor Tregs, regardless of whether the donor BM made IL-10 (Fig. 6C). We next hypothesized that IL-10 secretion by donor BM is critical for the maintenance of donor Treg numbers after BMT. Contrary to the hypothesis, as shown in Fig. 6C, we found that equivalent numbers of donor Tregs were recovered from the spleens of the recipients early after BMT (Day 10), and the total numbers of donor Tregs were reduced significantly at later time-points (Day 17) after BMT in the animals that received IL-10−/− donor BM when compared with the animals that received WT BM (7.5±1.1×105 vs.3.8±2.3×105; P=0.041). However, the numbers of Foxp3+ Tregs were reduced in both groups and were once again similar (P=0.7) at later time-points (on Day +24) between the groups, suggesting that donor BM-derived IL-10 does not significantly contribute to stable expression of Foxp3. We therefore next determined the relevance of donor BM-derived IL-10 in the magnitude of donor Treg-mediated suppression of GVHD by increasing the Treg:Teff ratio (1:2). As shown in Fig. 7, in contrast to lack of protection at lower Treg ratios, enhancing the ratio of Tregs suppressed GVHD mortality. Together, these data suggest that IL-10, from engrafting donor BM cells, is required for an optimal GVHD protection mediated by the infusion of mature donor Tregs.
Figure 7. Donor BM-derived IL-10 is necessary for optimal Treg-mediated suppression of GVHD.
C3H.SW recipients were irradiated (10 Gy) on Day −1 and transplanted 5 × 106 syngeneic B6D2F1 BM (▾; n=16), allogeneic B6(WT) 5 × 106 TCD BM + B6(WT) 0.4 × 106 CD25-depleted T cells (●; n=21), B6(WT) 5 × 106 TCD BM + B6(WT) 0.4 × 106 CD25-depleted T cells + B6(WT) 0.2 × 106 CD4+CD25+ T cells (○; n=16), IL-10KO 5 × 106 TCD BM + B6(WT) 0.4 × 106 CD25-depleted T cells (■; n=21), or IL-10KO 5 × 106 TCD BM + B6(WT) 0.4 × 106 CD25-depleted T cells + B6(WT) 0.2 × 106 CD4+CD25+ T cells (□; n=13). Survival was monitored daily. Data from three similar experiments were combined and shown. ● versus ○, P = 0.0124; ■ versus □, P = 0.0001; ● versus ■, P = 0.1792; ○ versus □, P = 0.3703.
DISCUSSION
IL-10 is a key immune-regulatory cytokine that has been implicated in the function of Tregs and in suppressing several immune responses [15]. However, its source and role modulating in vivo allogeneic responses, such as acute GVHD, are not well understood. We demonstrate an important role for donor- but not host-derived IL-10 in the severity of acute GVHD and its regulation by donor Tregs. With the use of clinically relevant murine models of GVHD, we show that (1) host-derived IL-10 does not play a critical role in the severity of GVHD, or (2) in its regulation by donor Tregs, (3) donor Teff-derived and donor BM-derived IL-10 does not contribute to the Teff-mediated GVHD severity; (4) by contrast, Treg-intrinsic expression of IL-10 and (5) donor BM-derived IL-10 are critical for optimal suppression of GVHD by the donor CD4+25+ Tregs.
The induction of acute GVHD is a direct consequence of donor T cell responses to host alloantigens and the deregulation of several cytokines [5]. The role of individual cytokines in the biology and clinical severity of GVHD is increasingly understood. A clinically relevant role for IL-10 in GVHD has been suggested by several recent clinical observations. The IL-10 gene polymorphisms in host and donors have implicated a key role for IL-10 pathways in the severity of clinical acute GVHD after matched-related and URD BMT [19, 20]. However, whether the identified polymorphisms result in quantitative and/ or qualitative alterations in IL-10 from donor or host is unclear. We now demonstrate that complete absence of IL-10 from donor Treg or BM cells can modulate GVHD when the donor graft contains mature CD4+25+ Tregs and CD25– Teffs. Thus, our data add clarity and provide insight into the clinical observations about the role of IL-10 and GVHD. Furthermore, our data, demonstrating an important role for donor BM-derived IL-10, may also provide a potential explanation for a seminal, clinical observation by Blazar and colleagues [46] on the impact of high spontaneous production of IL-10 by the URD BM graft in causing reduced mortality after clinical BMT.
Experimental studies with murine models have demonstrated seemingly conflicting results about the role of IL-10 in GVHD. Whereas infusion of exogenous IL-10 has demonstrated protection at very low doses, higher doses aggravated GVHD mortality in MHC-mismatched BMT [9, 27], These experiments, nonetheless, did not evaluate the role of endogenous IL-10. By contrast, infusion of IL-10−/− donor T cells without BM support caused enhanced mortality in sublethally irradiated, MHC-disparate models of allogeneic BMT [9]. Our data show that infusion of IL-10−/− Teffs without Tregs did not alter GVHD, whereas infusion of Tregs, which are IL-10−/−, caused greater GVHD than WT Tregs. The distinctions in the models aside, in the earlier study with IL-10−/− donors, pan T cells without CD25 depletion (Teffs and Tregs) were transplanted [9]. Therefore the severe GVHD might be a consequence of infusion of IL-10−/− Tregs, along with Teffs. Our results thus further expand the earlier observations.
Naturally occurring CD4+CD25+Foxp3+ T cells (Tregs) are a distinct CD4+ T cell subset that causes potent suppression-immune responses across multiple experimental models [34]. Tregs also cause potent suppression of GVHD across multiple experimental models [34]. Clinical data indicate that donor Tregs correlated with GVHD [47]. Furthermore, clinical trials are currently under way to examine the ability of freshly isolated or ex vivo-expanded Tregs to prevent GVHD [34, 48], whereas a recent clinical study has demonstrated the clinical feasibility of this approach after allogeneic BMT [49].
Tregs appear to use diverse mechanisms for mediating immune regulation [37]. Among the diverse pathways, the critical pathway for Treg-mediated suppression of GVHD is not clear. An earlier study suggested a key role for IL-10 in Treg-mediated suppression of GVHD [35]. We now extend those observations and demonstrate that the inferior ability of the IL-10−/− Tregs could be overcome by enhancing their numbers in the donor inoculum. Thus, our data show that IL-10 expression by the donor Tregs is necessary only for optimal suppression of GVHD. Our data did not specifically explore the role of IL-10 in other Treg subset-mediated protection of GVHD but when taken together, suggest that IL-10 is important for Treg- and Tr1-mediated reduction in GVHD [28]. Furthermore, it remains to be determined whether the Treg-mediated suppression of GVHD is also dependent on other suppressor cytokines, such as TGF-β, and/or predominantly through targeting Teffs (by IL-2 consumption or cytolysis) or those that primarily target APCs (such as decreased costimulation or antigen presentation) [37].
We also demonstrate a novel role for donor BM-derived IL-10 in mediating a Treg-induced reduction of GVHD. Our data suggest that donor BM-derived IL-10 is required by the donor Tregs to be more efficacious at smaller ratios of Treg:Teffs after BMT. Future studies will determine which non-T cellular subsets from the donor graft are the key producers of IL-10. However, consistent with a previous report [38], host-derived IL-10 was not important for Treg-mediated suppression of GVHD. These results, when taken together with previous reports, indicate that although interaction of Tregs with the host BM-derived APCs [38] is important for induction of Treg-mediated reduction of GVHD, the ability of these host BM-derived APCs to make IL-10 is, however, not critical. The dependence of donor BM-derived IL-10 is consistent with other reports, demonstrating a role for IL-10 from hematopoietic-derived APCs in mediating Treg function in the GI tract [43, 50]. Future studies will determine whether this requirement is critical for the donor Treg function and migration to the GI tract in reducing GVHD severity after allo-BMT.
In summary, we provide a novel perspective on the role of immune-regulatory cytokine IL-10 in modulating GVHD. We demonstrate that in the absence of donor Tregs, host- or donor-derived IL-10 does not play an important role in the modulation of GVHD. However, IL-10 from donor Tregs or donor BM is important for optimal Treg-mediated suppression of GVHD. These observations might be relevant for an effective use of Tregs in treating clinical GVHD.
Supplementary Material
ACKNOWLEDGMENTS
Support for this work was provided by NIH grants AI-075284 and HL-090775 (to P.R.). P.R. is a recipient of the Scholar in Clinical Research from the Leukemia Lymphoma Society and the Basic Science Investigator Award from the American Society of Transplantation.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- B6
- C57BL/6
- BM
- bone marrow
- BMT
- bone marrow transplantation
- Foxp3
- forkhead box p3
- GI
- gastrointestinal
- GVHD
- graft-versus-host disease
- IACUC
- Institutional Animal Care and Use Committee
- IL-10−/−
- IL-10-deficient
- KO
- knockout
- TBI
- total body irradiation
- TCD
- T cell depletion
- Teff
- T effector cell
- Treg
- regulatory T cell
- URD
- unrelated donor
AUTHORSHIP
I.T. designed and performed research, analyzed the data, and wrote the paper; Y.S., T.T., E.N., R.E., M.A., N.M., and H.T. performed research; C.L. performed pathological analyses; and P.R. designed research, analyzed the data, and wrote the paper.
DISCLOSURES
There are no conflicts of interest.
REFERENCES
- 1. Appelbaum F. R. (2007) Hematopoietic-cell transplantation at 50. N. Engl. J. Med. 357, 1472–1475 [DOI] [PubMed] [Google Scholar]
- 2. Appelbaum F. R. (2001) Haematopoietic cell transplantation as immunotherapy. Nature 411, 385–389 [DOI] [PubMed] [Google Scholar]
- 3. Fowler D. H. (2006) Shared biology of GVHD and GVT effects: potential methods of separation. Crit. Rev. Oncol. Hematol. 57, 225–244 [DOI] [PubMed] [Google Scholar]
- 4. Sun Y., Tawara I., Toubai T., Reddy P. (2007) Pathophysiology of acute graft-versus-host disease: recent advances. Transl. Res. Oct. 150, 197–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Paczesny S., Hanauer D., Sun Y., Reddy P. (2010) New perspectives on the biology of acute GVHD. Bone Marrow Transplant. 45, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Welniak L. A., Blazar B. R., Murphy W. J. (2007) Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu. Rev. Immunol. 25, 139–170 [DOI] [PubMed] [Google Scholar]
- 7. Chen X., Das R., Komorowski R., Beres A., Hessner M. J., Mihara M., Drobyski W. R. (2009) Blockade of interleukin-6 signaling augments regulatory T-cell reconstitution and attenuates the severity of graft-versus-host disease. Blood 114, 891–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tawara I., Koyama M., Liu C., Toubai T., Thomas D., Evers R., Chockley P., Nieves E., Sun Y., Lowler K. P., Malter C., Nishimoto N., Hill G. R., Reddy P. (2011) Interleukin-6 modulates graft-versus-host responses after experimental allogeneic bone marrow transplantation. Clin. Cancer Res. 17, 77–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blazar B. R., Taylor P. A., Panoskaltsis-Mortari A., Narula S. K., Smith S. R., Roncarolo M. G., Vallera D. A. (1998) Interleukin-10 dose-dependent regulation of CD4+ and CD8+ T cell-mediated graft-versus-host disease. Transplantation 66, 1220–1229 [DOI] [PubMed] [Google Scholar]
- 10. Rowe V., Banovic T., MacDonald K. P., Kuns R., Don A. L., Morris E. S., Burman A. C., Bofinger H. M., Clouston A. D., Hill G. R. (2006) Host B cells produce IL-10 following TBI and attenuate acute GVHD after allogeneic bone marrow transplantation. Blood 108, 2485–2492 [DOI] [PubMed] [Google Scholar]
- 11. Banovic T., MacDonald K. P., Morris E. S., Rowe V., Kuns R., Don A., Kelly J., Ledbetter S., Clouston A. D., Hill G. R. (2005) TGF-β in allogeneic stem cell transplantation: friend or foe? Blood Sep. 106, 2206–2214 [DOI] [PubMed] [Google Scholar]
- 12. Fiorentino D. F., Bond M. W., Mosmann T. R. (1989) Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th 1 clones. J. Exp. Med. 170, 2081–2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fiorentino D. F., Zlotnik A., Mosmann T. R., Howard M., O'Garra A. (1991) IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 147, 3815–3822 [PubMed] [Google Scholar]
- 14. Sabat R., Grutz G., Warszawska K., Kirsch S., Witte E., Wolk K., Geginat J. (2010) Biology of interleukin-10. Cytokine Growth Factor Rev. 21, 331–344 [DOI] [PubMed] [Google Scholar]
- 15. Ouyang W., Rutz S., Crellin N. K., Valdez P. A., Hymowitz S. G. (2011) Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu. Rev. Immunol. 29, 71–109 [DOI] [PubMed] [Google Scholar]
- 16. Fiorentino D. F., Zlotnik A., Vieira P., Mosmann T. R., Howard M., Moore K. W., O'Garra A. (1991) IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J. Immunol. 146, 3444–3451 [PubMed] [Google Scholar]
- 17. O'Garra A., Barrat F. J., Castro A. G., Vicari A., Hawrylowicz C. (2008) Strategies for use of IL-10 or its antagonists in human disease. Immunol. Rev. 223, 114–131 [DOI] [PubMed] [Google Scholar]
- 18. Hawrylowicz C. M., O'Garra A. (2005) Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat. Rev. Immunol. 5, 271–283 [DOI] [PubMed] [Google Scholar]
- 19. Lin M. T., Storer B., Martin P. J., Tseng L. H., Gooley T., Chen P. J., Hansen J. A. (2003) Relation of an interleukin-10 promoter polymorphism to graft-versus-host disease and survival after hematopoietic-cell transplantation. N. Engl. J. Med. 349, 2201–2210 [DOI] [PubMed] [Google Scholar]
- 20. Lin M. T., Storer B., Martin P. J., Tseng L. H., Grogan B., Chen P. J., Zhao L. P., Hansen J. A. (2005) Genetic variation in the IL-10 pathway modulates severity of acute graft-versus-host disease following hematopoietic cell transplantation: synergism between IL-10 genotype of patient and IL-10 receptor β genotype of donor. Blood 106, 3995–4001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cavet J., Middleton P. G., Segall M., Noreen H., Davies S. M., Dickinson A. M. (1999) Recipient tumor necrosis factor-α and interleukin-10 gene polymorphisms associate with early mortality and acute graft-versus-host disease severity in HLA-matched sibling bone marrow transplants. Blood 94, 3941–3946 [PubMed] [Google Scholar]
- 22. Kim D. H., Lee N. Y., Sohn S. K., Baek J. H., Kim J. G., Suh J. S., Lee K. B., Shin I. H. (2005) IL-10 promoter gene polymorphism associated with the occurrence of chronic GVHD and its clinical course during systemic immunosuppressive treatment for chronic GVHD after allogeneic peripheral blood stem cell transplantation. Transplantation 79, 1615–1622 [DOI] [PubMed] [Google Scholar]
- 23. Hempel L., Korholz D., Nussbaum P., Bonig H., Burdach S., Zintl F. (1997) High interleukin-10 serum levels are associated with fatal outcome in patients after bone marrow transplantation. Bone Marrow Transplant. 20, 365–368 [DOI] [PubMed] [Google Scholar]
- 24. Korholz D., Kunst D., Hempel L., Söhngen D., Heyll A., Bönig H., Göbel U., Zintl F., Burdach S. (1997) Decreased interleukin 10 and increased interferon-γ production in patients with chronic graft-versus-host disease after allogeneic bone marrow transplantation. Bone Marrow Transplant. 19, 691–695 [DOI] [PubMed] [Google Scholar]
- 25. Miura Y., Thoburn C. J., Bright E. C., Chen W., Nakao S., Hess A. D. (2002) Cytokine and chemokine profiles in autologous graft-versus-host disease (GVHD): interleukin 10 and interferon γ may be critical mediators for the development of autologous GVHD. Blood 100, 2650–2658 [DOI] [PubMed] [Google Scholar]
- 26. Wu J. M., Bensen-Kennedy D., Miura Y., Thoburn C. J., Armstrong D., Vogelsang G. B., Hess A. D. (2005) The effects of interleukin 10 and interferon γ cytokine gene polymorphisms on survival after autologous bone marrow transplantation for patients with breast cancer. Biol. Blood Marrow Transplant. 11, 455–464 [DOI] [PubMed] [Google Scholar]
- 27. Blazar B. R., Taylor P. A., Smith S., Vallera D. A. (1995) Interleukin-10 administration decreases survival in murine recipients of major histocompatibility complex disparate donor bone marrow grafts. Blood 85, 842–851 [PubMed] [Google Scholar]
- 28. Roncarolo M. G., Gregori S., Lucarelli B., Ciceri F., Bacchetta R. (2011) Clinical tolerance in allogeneic hematopoietic stem cell transplantation. Immunol. Rev. 241, 145–163 [DOI] [PubMed] [Google Scholar]
- 29. MacDonald K. P., Rowe V., Filippich C., Thomas R., Clouston A. D., Welply J. K., Hart D. N., Ferrara J. L., Hill G. R. (2003) Donor pretreatment with progenipoietin-1 is superior to granulocyte colony-stimulating factor in preventing graft-versus-host disease after allogeneic stem cell transplantation. Blood 101, 2033–2042 [DOI] [PubMed] [Google Scholar]
- 30. Toubai T., Malter C., Tawara I., Liu C., Nieves E., Lowler K. P., Sun Y., Reddy P. (2010) Immunization with host-type CD8{α}+ dendritic cells reduces experimental acute GVHD in an IL-10-dependent manner. Blood 115, 724–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Capitini C. M., Davis J. P., Larabee S. M., Herby S., Nasholm N. M., Fry T. J. (2011) Extracorporeal photopheresis attenuates murine graft-versus-host disease via bone marrow-derived interleukin-10 and preserves responses to dendritic cell vaccination. Biol. Blood Marrow Transplant. 17, 790–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Foley J. E., Mariotti J., Ryan K., Eckhaus M., Fowler D. H. (2008) Th2 cell therapy of established acute graft-versus-host disease requires IL-4 and IL-10 and is abrogated by IL-2 or host-type antigen-presenting cells. Biol. Blood Marrow Transplant. 14, 959–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Taylor P. A., Noelle R. J., Blazar B. R. (2001) CD4(+)CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J. Exp. Med. 193, 1311–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Riley J. L., June C. H., Blazar B. R. (2009) Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity 30, 656–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hoffmann P., Ermann J., Edinger M., Fathman C. G., Strober S. (2002) Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J. Exp. Med. 196, 389–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Edinger M., Hoffmann P., Ermann J., Drago K., Fathman C. G., Strober S., Negrin R. S. (2003) CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat. Med. 9, 1144–1150 [DOI] [PubMed] [Google Scholar]
- 37. Shevach E. M. (2009) Mechanisms of Foxp3+ T regulatory cell-mediated suppression. Immunity 30, 636–645 [DOI] [PubMed] [Google Scholar]
- 38. Tawara I., Shlomchik W. D., Jones A., Zou W., Nieves E., Liu C., Toubai T., Duran-Struuck R., Sun Y., Clouthier S. G., Evers R., Lowler K. P., Levy R. B., Reddy P. (2010) A crucial role for host APCs in the induction of donor CD4+CD25+ regulatory T cell-mediated suppression of experimental graft-versus-host disease. J. Immunol. 185, 3866–3872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Toubai T., Sun Y., Tawara I., Friedman A., Liu C., Evers R., Nieves E., Malter C., Chockley P., Maillard I., Winandy S., Reddy P. (2011) Ikaros-Notch axis in host hematopoietic cells regulates experimental graft-versus-host disease. Blood 118, 192–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kryczek I., Wei S., Zou L., Zhu G., Mottram P., Xu H., Chen L., Zou W . (2006) Cutting edge: induction of B7–H4 on APCs through IL-10: novel suppressive mode for regulatory T cells. J. Immunol. 177, 40–44 [DOI] [PubMed] [Google Scholar]
- 41. O'Garra A., Vieira P. L., Vieira P., Goldfeld A. E. (2004) IL-10-producing and naturally occurring CD4+ Tregs: limiting collateral damage. J. Clin. Invest. 114, 1372–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vieira P. L., Christensen J. R., Minaee S., O'Neill E. J., Barrat F. J., Boonstra A., Barthlott T., Stockinger B., Wraith D. C., O'Garra A. (2004) IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J. Immunol. 172, 5986–5993 [DOI] [PubMed] [Google Scholar]
- 43. Hadis U., Wahl B., Schulz O., Hardtke-Wolenski M., Schippers A., Wagner N., Müller W., Sparwasser T., Förster R., Pabst O. (2011) Intestinal tolerance requires gut homing and expansion of Foxp3+ regulatory T cells in the lamina propria. Immunity. 34, 237–246 [DOI] [PubMed] [Google Scholar]
- 44. Beres A., Komorowski R., Mihara M., Drobyski W. R. (2011) Instability of Foxp3 expression limits the ability of induced regulatory T cells to mitigate graft versus host disease. Clin. Cancer Res. 17, 3969–3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rubtsov Y. P., Niec R. E., Josefowicz S., Li L., Darce J., Mathis D., Benoist C., Rudensky A. Y. (2010) Stability of the regulatory T cell lineage in vivo. Science 329, 1667–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Baker K. S., Roncarolo M. G., Peters C., Bigler M., DeFor T., Blazar B. R. (1999) High spontaneous IL-10 production in unrelated bone marrow transplant recipients is associated with fewer transplant-related complications and early deaths. Bone Marrow Transplant. 23, 1123–1129 [DOI] [PubMed] [Google Scholar]
- 47. Zorn E., Kim H. T., Lee S. J., Floyd B. H., Litsa D., Arumugarajah S., Bellucci R., Alyea E. P., Antin J. H., Soiffer R. J., Ritz J. (2005) Reduced frequency of FOXP3+ CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood 106, 2903–2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hippen K. L., Merkel S. C., Schirm D. K., Sieben C. M., Sumstad D., Kadidlo D. M., McKenna D. H., Bromberg J. S., Levine B. L., Riley J. L., June C. H., Scheinberg P., Douek D. C., Miller J. S., Wagner J. E., Blazar B. R. (2011) Massive ex vivo expansion of human natural regulatory T cells (Tregs) with minimal loss of in vivo functional activity. Sci. Transl. Med. 3, 83ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brunstein C. G., Miller J. S., Cao Q., McKenna D. H., Hippen K. L., Curtsinger J., Defor T., Levine B. L., June C. H., Rubinstein P., McGlave P. B., Blazar B. R., Wagner J. E. (2011) Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood 117, 1061–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vieira P., O'Garra A. (2007) Regula“ten” the gut. Nat. Immunol. 8, 905–907 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.