Abstract
Background:
Pure arm monoparesis is an uncommon presentation of stroke. Localization of the lesions is variable, including cortical, subcortical or deep brain infarcts. No particular risk factors or unifying mechanisms have been clearly identified.
Methods:
Seven patients (5 women, 2 men) presented with isolated arm weakness and brain magnetic resonance imaging (MRI) documented an infarct in the precentral gyrus. All were evaluated for stroke risk factors, had telemetry monitoring, transthoracic echocardiogram (TTE) and magnetic resonance angiography (MRA) of the head and neck. Transesophageal echocardiogram (TEE) was performed in three cases. Hyper-coagulable work-up was performed in one case. Trans-cranial Doppler was performed in one case.
Results:
Mean age was 73 years (range 55–88 years). Arm weakness in all patients was ranging from mild (−5/5) to moderate (2/5) and was predominantly distal (without plegia). None of the patients complained of limb pain or sensory deficit. Infarcts affected one gyrus (5/7) or, less often, 2 adjacent gyri (2/7), along the most distal aspect of the middle cerebral artery (MCA) territory. Risk factors included hypertension (6/7), diabetes (2/7), hyper-lipidemia (7/7), smoking (1/7) and prior TIA/stroke (3/7). The mechanisms of ischemic stroke were determined to be large artery atherosclerosis (2/5), cardioembolic (2/5), other determined etiology [hypoperfusion (1/5)] and undetermined etiology (2/5).
Conclusions:
Our series of patients with small cortical infarcts and pure motor arm weakness show heterogeneous etiologies of stroke mechanisms and related long term outcomes. The risk factors appear to distribute as in most stroke populations, without a pattern specific to this unusual clinical presentation.
Keywords: mechanisms of stroke, isolated monoparesis, hand, precentral gyrus
Introduction
Pure arm monoparesis is an uncommon presentation of stroke with a reported frequency of less than 1% of all ischemic strokes.1,2 In the majority of cases it results from small ischemic cortical infarcts in the MCA territory due to occlusion of superficial branches of its anterior (superior) and, less frequently its posterior (inferior) division.3,4 Several cases of subcortical infarcts have also been reported.5
Most of the available information relates to cases presenting with isolated weakness of the hand due to small cortical infarcts. In these, weakness of the extensor muscles of the hand has predominated, while others have simulated a pattern suggestive of median or ulnar neuropathy.4 The use of magnetic resonance imaging (MRI), has helped visualize lesions that were missed on CT.6,7 The use of transcranial magnetic stimulation (TMS) and functional MRI (fMRI) has helped to precisely identify the anatomical area referred to as the “hand knob” (extending from the precentral gyrus into the central sulcus and described in the shape of an inverted omega or a horizontal epsilon in the axial plane),8,9 which is the area affected in patients presenting with isolated hand paresis.
Data from large series of patients have failed to identify specific risk factors or mechanisms of infarction that would suggest a standard plan of investigation in these patients.10 An embolic etiology of either cardiac or large artery origin has been often suggested, but small vessel disease and hemorrhage have been implicated as well.5,11,12 In a number of instances the stroke mechanism remains undetermined.13 We describe a group of patients with isolated arm paresis as a result of a small cortical infarct. We review their diagnostic evaluation, clinical and imaging characteristics, and discuss potential mechanisms of stroke and clinical course during follow-up.
Methods and patients
We report seven consecutive patients who were admitted to our Stroke Unit between January 2007 and March 2009. We included patients who presented with a) pure motor upper limb weakness and b) evidence of acute infarction on diffusion weighted imaging (DWI) obtained within 24 hours from symptom onset. All patients were examined by at least two neurologists and were evaluated for traditional stroke risk factors and underwent thorough stroke work-up (Table 1). Neurologists with expertise in vascular neurology (A.P., J.R.R, and C.S.K.) assigned the stroke subtype according to the TOAST criteria14 as: large-artery atherosclerosis, small-vessel occlusion, cardioembolic, other determined etiology, and undetermined etiology. Stroke subtypes were categorized based on the data available from clinical presentations, imaging studies, laboratory studies, noninvasive vascular studies, and cardiac evaluations for a source of embolus. Medical management of stroke risk factors was initiated in all patients during hospitalization. The mean follow-up period was 14 months; one patient was lost to follow-up. This study was approved by the Institutional Review Board at Boston Medical Center.
Table 1.
Demographic, Clinical and Radiological Characteristics.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | |
|---|---|---|---|---|---|---|---|
| Age/gender | 78/F | 73/F | 88/F | 74/M | 63/F | 55/M | 83/F |
| Clinical Presentation | L hand weakness | R hand decreased dexterity | L arm weakness L pronator drift | R hand decreased dexterity (after cardiac catheterization) | R hand decreased dexterity R pronator drift | L hand weakness L pronator drift | L hand weakness |
| Risk Factors | HTN, DM, HLD, ex-smoker | HTN, HLD, prior TIA | HTN, DM, HLD, atrial fibrillation, prior stroke | HLD, CAD, smoker | HTN, HLD, ex-smoker, old L ICA occlusion with prior stroke | HTN, HLD, ex-smoker | HTN, HLD, ex-smoker |
| Workup | TTE: normal | TTE: normal | INR < 1.8 TTE: normal | TTE: LVEF 40%, inferolateral wall hypokinesis | TTE/TEE: aortic valve papillary fibroelastoma | EKG, TTE,TEE: normal findings, negative hypercoagulable work up | EKG, TTE, TEE: normal findings |
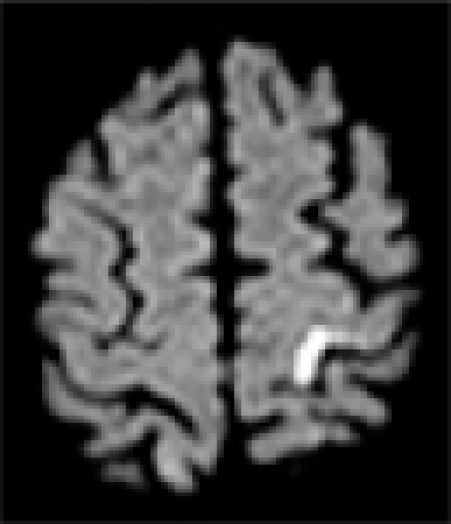
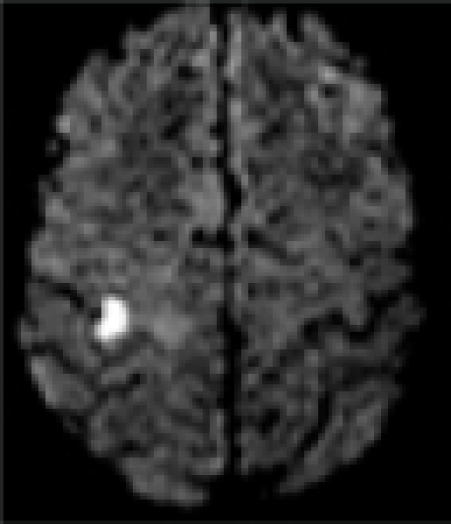
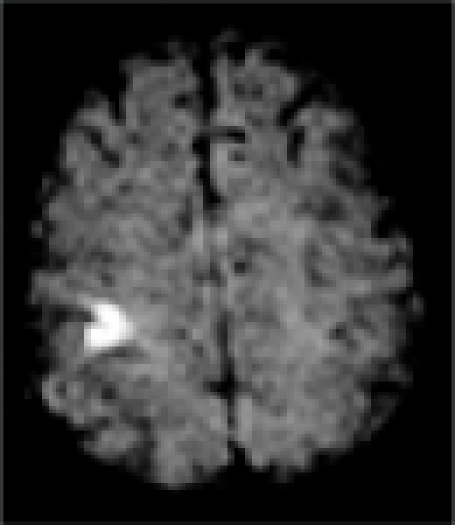
| MRI/ MRA | R precentral gyrus infarct, R ICA stenosis | L precentral gyrus infarct, L ICA stenosis | R pre/post-central gyrus infarct, normal vessels | L pre/post-central gyrus infarct, normal vessels | L precentral gyrus infarct, L- ICA occlusion, TCD: preserved vasomotor reactivity | R precentral gyrus infarct, normal vessels | R precentral gyrus infarct, normal vessels |
| DWI | 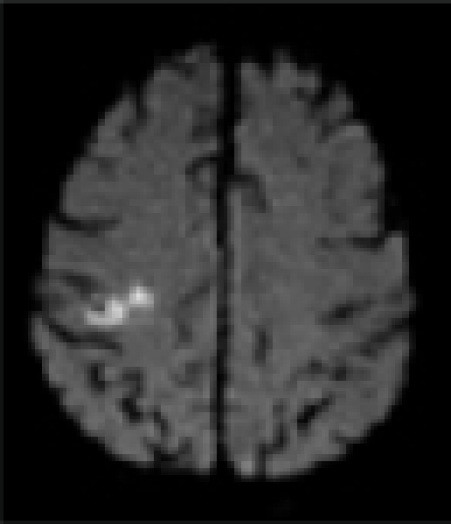 |
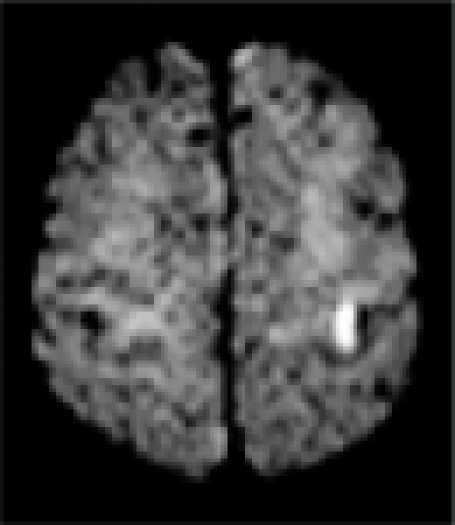 |
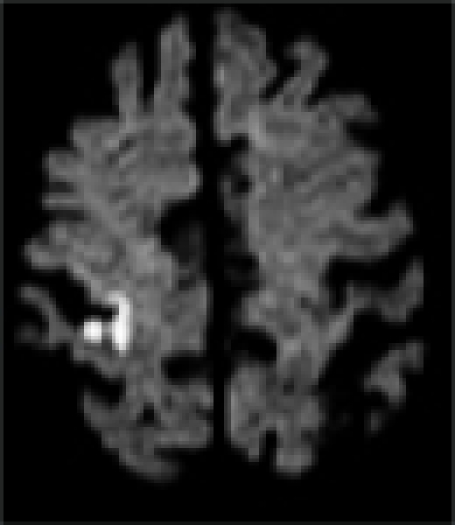 |
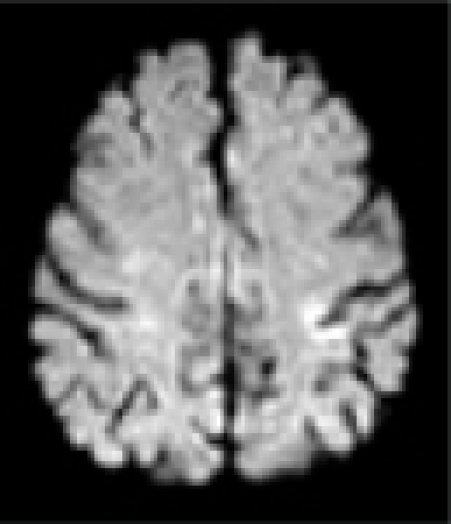 |
 |
 |
 |
| Classification | Large artery atherothrombosis | Large artery atherothrombosis | Cardioembolic | Cardioembolic | Determined (Hypoperfusion) | Undetermined | Undetermined |
F= female, M= male, HTN= hypertension, DM= diabetes mellitus, HLD= hyperlipidemia, MCA= middle cerebral artery, TIA= transient ischemic attack, CAD= coronary artery disease, ICA= internal carotid artery, EKG= electrocardiogram, US= ultrasound, TTE= transthoracic echocardiogram, TEE= transesophageal echocardiogram, LVEF= left ventricular ejection fraction, INR= international normalized ratio, MRI= magnetic resonance imaging, MRA= magnetic resonance angiography, TCD= transcranial Doppler, DWI= Diffusion weighted imaging,
Results
The mean age in our series was 73 years (range 55–88 years). Table 1 shows the demographic, clinical and radiological features in our seven patients. All patients presented with isolated mild (−5/5) to moderate (2/5) arm weakness, predominantly affecting distal muscle groups; clinically presenting with mild pronator drift and/or slow fine finger movements to moderate weakness of the finger extensor muscles.
In all patients, brain MRI/DWI documented small infarcts affecting one gyrus or, less often, 2 adjacent gyri (in 2/7), along the most distal aspect of the MCA territory, adjacent to the MCA/ACA border zone. In three patients MRA demonstrated atherosclerotic changes of the ipsilateral ICA. Risk factors included hypertension (6/7), diabetes mellitus (2/7), hyper-lipidemia (7/7), smoking (1/7) and prior TIA/stroke (3/7). One patient was in atrial fibrillation. TEE was performed in 3/7 and failed to demonstrate a cardiac source of embolism. In one patient an aortic valve papillary fibroelastoma was detected; the same patient also had complete occlusion of the ipsilateral ICA (Case 5). Hyper-coagulable work-up failed to reveal the stroke mechanism in a 55 year old patient (Case 6), the only case where it was performed.
Upon completion of the work-up, the mechanism of infarction was determined to be large artery athero-thrombosis in two cases, cardioembolic in two, secondary to hypoperfusion in one, and of undetermined mechanism in the remaining two.
During a mean follow-up of 14 months (range 5 to 31 months), one patient died from complications of traumatic abdominal injury while on warfarin (Case 3), and two had recurrent ischemic events: one had multiple ipsilateral recurrent ischemic strokes in the setting of progressing ICA stenosis (Case 1), and the other underwent surgical removal of a papillary fibroelastoma, but later sustained recurrent stroke in the distribution of the occluded ipsilateral ICA, in the setting of poor medication compliance and hypoperfusion (Case 5). All others had a favorable outcome without stroke recurrence and excellent recovery from the initial deficits.
Discussion
Isolated arm monoparesis due to cortical infarction accounts for less than 1% of strokes.1,2 As in previous studies,6,12 we found that lesions within the precentral gyrus present with mild or moderate weakness of distal muscles and the infarcts are mostly confined to a single gyrus.
In our study, 6/7 patients had hypertension and all patients had hyper-lipidemia, but other major vascular and stroke risk factors (smoking, atrial fibrillation, diabetes) were also present. A study by Maeder et al., examining all types of monoparesis (face, arm or leg), documented a high prevalence of hypertension, although not significantly different from other groups of patients with stroke.10
Isolated hand weakness has been previously reported as a result of embolic stroke involving the hand knob area,1,12 large-artery atherosclerotic infarct of vascular border zones,15 and small subcortical lacunar infarct.16 It has been suggested that “fractional weakness of the hand” is a strong predictor of atherosclerotic infarction affecting vascular border zones.15 In our study the majority of cases (4/7) were found to be embolic in nature, but hypoperfusion in the presence of ipsilateral ICA occlusion was also identified.
In the Lausanne Stroke Registry, isolated motor arm involvement was seen usually with cortical infarction in the anterior (superior) division of the MCA. However, posterior (inferior) division infarction was also implicated in cases of monoparesis. Two of our seven patients had involvement of both the precentral and postcentral gyrus, without evidence of any sensory deficit.
Prior reports observed a favorable prognosis in most patients with ischemic stroke presenting with isolated arm weakness. However, two of our seven cases demonstrate that the actual mechanism of stroke is a more likely determinant of long term outcome.
In summary, our series of patients with small cortical infarcts and pure motor arm weakness showed heterogeneous etiologies of stroke mechanisms and related long term outcomes. The risk factors appear to distribute as in most stroke populations, without a pattern specific to this unusual clinical presentation.
The main limitations of our study are its descriptive design and a small sample size. Further prospective studies are required to confirm if a comprehensive investigation in patients with small cortical strokes and pure motor arm weakness may help elucidate a particular etiology of stroke mechanism in this unusual stroke entity.
References:
- 1.Celebisoy M, Ozdemirkiran T, Tokucoglu F, Kaplangi DN, Arici S. Isolated hand palsy due to cortical infarction: localization of the motor hand area. Neurologist. 2007;13:376–379. doi: 10.1097/NRL.0b013e31814db093. [DOI] [PubMed] [Google Scholar]
- 2.Peters N, Muller-Schunk S, Freilinger T, et al. Ischemic stroke of the cortical “hand knob” area: stroke mechanisms and prognosis. J Neurol. 2009;256:1146–1151. doi: 10.1007/s00415-009-5104-8. [DOI] [PubMed] [Google Scholar]
- 3.Bogousslavsky J, Van Melle G, Regli F. Middle cerebral artery pial territory infarcts: a study of the Lausanne Stroke Registry. Ann Neurol. 1989;25:555–560. doi: 10.1002/ana.410250605. [DOI] [PubMed] [Google Scholar]
- 4.Chen P-L, Hsu H-Y, Wang P-Y. Isolated hand weakness in cortical infarctions. J Formos Med Assoc. 2006;105:861–865. doi: 10.1016/S0929-6646(09)60276-X. [DOI] [PubMed] [Google Scholar]
- 5.Paciaroni M, Caso V, Milia P, et al. Isolated monoparesis following stroke. J Neurol Neurosurg Psychiatry. 2005;76:805–807. doi: 10.1136/jnnp.2004.047779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tei H. Monoparesis of the right hand following a localised infarct in the left “precentral knob”. Neuroradiology. 1999;41:269–270. doi: 10.1007/s002340050745. [DOI] [PubMed] [Google Scholar]
- 7.Hopkins WD, Pilcher DL. Neuroanatomical localization of the motor hand area with magnetic resonance imaging: the left hemisphere is larger in great apes. Behav Neurosci. 2001;115:1159–1164. [PMC free article] [PubMed] [Google Scholar]
- 8.Boroojerdi B, Foltys H, Krings T, et al. Localization of the motor hand area using transcranial magnetic stimulation and functional magnetic resonance imaging. Clin Neurophysiol. 1999;110:699–704. doi: 10.1016/s1388-2457(98)00027-3. [DOI] [PubMed] [Google Scholar]
- 9.Yousry TA, Schmid UD, Alkadhi H, et al. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997;120(Pt 1):141–157. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]
- 10.Maeder-Ingvar M, van Melle G, Bogousslavsky J. Pure monoparesis: a particular stroke subgroup? Arch Neurol. 2005;62:1221–1224. doi: 10.1001/archneur.62.8.1221. [DOI] [PubMed] [Google Scholar]
- 11.Yoneda Y, Mori E, Tabuchi M, Yamadori A. Pure motor monoparesis due to intracerebral hemorrhage. Stroke. 1993;24:142–143. doi: 10.1161/01.str.24.1.142. [DOI] [PubMed] [Google Scholar]
- 12.Gass A, Szabo K, Behrens S, Rossmanith C, Hennerici M. A diffusion-weighted MRI study of acute ischemic distal arm paresis. Neurology. 2001;57:1589–1594. doi: 10.1212/wnl.57.9.1589. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi N, Kawamura M, Araki S. Isolated hand palsy due to cortical infarction: localization of the motor hand area. Neurology. 2002;58:1412–1414. doi: 10.1212/wnl.58.9.1412. [DOI] [PubMed] [Google Scholar]
- 14.Adams HPJ, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 15.Timsit S, Logak M, Manai R, Rancurel G. Evolving isolated hand palsy: a parietal lobe syndrome associated with carotid artery disease. Brain. 1997;120(Pt 12):2251–2257. doi: 10.1093/brain/120.12.2251. [DOI] [PubMed] [Google Scholar]
- 16.Lampl Y, Gilad R, Eshel Y, Sarova-Pinhas I. Strokes mimicking peripheral nerve lesions. Clin Neurol Neurosurg. 1995;97:203–207. doi: 10.1016/0303-8467(95)00037-k. [DOI] [PubMed] [Google Scholar]


