Abstract
Introduction:
The leptomeningeal collaterals are a subsidiary network of vascular channels that act as anastomotic channels in conditions where cerebral blood flow is pathologically altered. These secondary collateral pathways may be utilized when collateral flow through the circle of Willis is inadequate.
Summary of Review:
The review highlights the importance of leptomeningeal (pial) anastomoses in the brain especially in conditions of hemodynamic impairment such as ischemic stroke. The historical perspective regarding the role of these vessels is discussed. New advancements in the diagnostic and treatment modalities for the evaluation and optimization of these vessels are identified.
Conclusion:
Evaluation and optimization of the leptomeningeal collaterals in ischemic stroke represents an important venue in prevention and treatment of cerebral ischemia.
Keywords: leptomeningeal collaterals, ischemic stroke, cerebral blood flow
Collateral vessels in cerebral circulation are formed during the prenatal period, although secondary changes related to various pathophysiological conditions may occur throughout life.1 In general, collateral blood supply to the brain following vessel occlusion occurs through large vessels (medium sized arteries) that form the circle of Willis.2, 3 Other collateral routes such as the tectal plexus connecting the PCA with superior cerebellar artery and the orbital plexus which links branches of ICA with external carotid artery are important, however, they are less commonly activated in acute ischemic stroke. Leptomeningeal collaterals (LMCs) also known as leptomeningeal anastomoses (LMAs), or pial collaterals, are small arterial connections joining the terminal cortical branches of major (middle, anterior and posterior) cerebral arteries along the surface of the brain. These vessels are dormant under normal conditions when blood flow from all major cerebral arteries is not impeded, but are recruited when one major artery is either chronically or acutely occluded. LMCs of varying magnitude can be seen in approximately 80% of the patients with MCA occlusion4, 5 in angiographic images acquired hours after onset of occlusion in patients with acute ischemic stroke. However, in the absence of pre-occlusion angiographic images and angiographic images concurrent with onset of occlusion, the temporal onset of these collaterals is not known. LMCs in humans demonstrate substantial variation in their distribution, size, number, and compensatory capacity in ischemic stroke.
In the current review, we will explain the background and significance of LMCs and later elaborate on the current advancements and future possibilities for utilizing these vessels in the management of cerebral ischemia.6–8
Historical perspectives of LMCs
The first elaborate description of LMCs was given by Heubner9 in 1874. In the late 1950s and early 60s, Van der Eecken and Adams10 later provided a comprehensive anatomic description of LMCs. They performed several studies on cadaveric brains derived from patients suffering from ischemic stroke secondary to MCA occlusion. They observed that the infarcted area in most patients was smaller than actual distribution supplied by the occluded artery. A variable proportion of the MCA territory was spared as a result of LMC filling from the ACA and/or PCA. In recent years, both anatomic and angiographic studies have confirmed the presence of LMCs in patients’ brains under normal and diseased states.11
The role of various imaging modalities in the evaluation of LMCs
Modern diagnostic imaging techniques, like Xenon enhanced CT, single photon emission CT, PET, CT perfusion, MR perfusion, and TCD, have improved the assessment of cerebral blood flow through collaterals. Relatively subtle findings such as vascular enhancement on CT and MRI scans may also identify collateral blood flow.12 LMCs can be directly visualized by conventional angiography, CTA and MRA. Collateral blood flow as assessed by CT angiography, triphasic perfusion CT, Xenon CT, MR imaging, and SPECT has been shown to correlate with the extent of LMC formation seen on conventional angiograms and with clinical outcome.13, 14 Angiographic scales have attempted to qualitatively classify the extent of collateral blood supply, however, variations in contrast volume and pressure during injection may distort the appearance of distal vessels. Noninvasive techniques including CTA and MRA angiograms have limited resolution, precluding thorough evaluation of LMCs and other secondary collateral pathways. The specific advantages and limitations of each modality must be considered, alongwith the timing of image acquisition as collaterals evolve with time from the incipient ischemic event.
The role of LMCs in acute ischemic stroke patients
Since their description, the role of LMCs in the pathophysiology of cerebral ischemia has been a matter of debate. From the year 2000 onwards, the role of LMCs in ischemic stroke has again gained substantial attention. The PROACT II trial15 investigators semiquantitatively analyzed pial collateral formation on angiography and categorized them as full, partial, or none and found that presence of good collaterals influences NIHSS score at initial presentation and infarct volume on 24-hour CT scan in patients with MCA occlusion. The presence of LMCs has also been associated with better outcomes, reduced infarct size, and faster recanalization. After the introduction of the concept of ischemic penumbra by Astrup et al16 in 1981, Fukuyama et al17 (1983) studied LMCs by nuclear medicine techniques and concluded that cortical infarction occurs in patients with inadequate development of LMCs despite an angiographically normal circle of Willis. Saito et al18 (1987) measured the time interval between opacification of the distal internal carotid and the M2 segment via LMCs on 21 angiograms in patients presenting with acute ischemic stroke within 24 hours of symptom onset. The investigators found that a time interval <5 seconds for opacification resulted in better outcomes with reduced infarct size. In another study8, (1992) visualization of robust LMCs in CT angiograms was associated with rapid recanalization and possible prevention of large infarcts. Presumably, retrograde collateral filling may permit thrombolytic activity in the distal aspects of the clot and dissolution of fragmented thrombi.19, 20 Lee et al21 (2000) correlated triphasic perfusion CT in MCA distribution ischemic stroke with angiographic findings and found a positive correlation between recovery after thrombolytic therapy and the presence of efficient LMCs. Christoforidis et al22 (2005) reviewed 65 patients retrospectively who underwent thrombolysis for acute ischemic stroke and reported that LMC formation before thrombolytic treatment predicted infarct volume and clinical outcome independent of other predictive factors. The benefit of thrombolytic treatment was augmented in patients with greater LMC formation.
Qureshi et al23 (2002) devised a grading scheme for angiographic evaluation of patients before and after intra-arterial thrombolysis. The grading scheme was based on anatomic location of occlusion and presence of LMC pathways in the affected distribution. The scheme showed that presence of good collaterals in patients undergoing thrombolytic therapy for stroke correlated with better recanalization rates, and recovery and lower mortality at 7 days. Prediction of these outcome variables in stroke patients using the abovementioned scheme has been established with high confidence in several studies.4, 24–26 Yamauchi et al27 (2004) studied 42 patients and determined that the presence of ophthalmic or leptomeningeal collaterals in patients with symptomatic ICA occlusion was an independent predictor of increased oxygen extraction suggestive of ischemic stress within the brain tissue but this association was confounded by the presence of ischemic lesions in the brain. Mohammad et al24 (2008) determined the relationship between severity of angiographic occlusion using Qureshi grading scheme and the volume of brain infarction on follow up CT in 55 patients with anterior circulation ischemic stroke who underwent intra-arterial thrombolysis. He found that collateral supply through LMCs was associated with lower volume of brain infarction in patients with proximal MCA occlusion.
Temporal profile of development of LMCs in acute ischemic stroke
LMCs connect arterial watershed terrories (eg: ACA and MCA, MCA and PCA territory) and are activated instantly in the presence of a major proximal arterial occlusion.51 Variable blood flow along these pre-existing connections occurs to fill the territory of the occluded vessel.22
Martin et al28 determined the intracranial hemodynamic response to ICA occlusion following clamping by using TCD measurements. Secondary collaterals were detected in 62 ot the 85 patients. Yamashita et al29 (1996) used Xenon enhanced CT rCBF measurement with acetazolamide challenge in patients with ICA stenosis and demonstrated that LMCs develop to some extent immediately after occlusion and continue to develop for some time. Drake et al30 documented a prominent compensatory potential of LMCs during surgical occlusion of major cerebral arteries for aneurysms based on their findings on conventional angiograms. Enam and Malik31 (1999) observed refilling of arteriovenous malformations as a result of LMCs subsequent to complete occlusion of major feeding vessels documented on cerebral angiography.
The presence of secondary collateral pathways is usually a marker of impaired cerebral hemodynamics. Secondary collateral pathways that require time to develop are presumed to be recruited once primary collaterals at the circle of Willis are inadequate.
LMC adequacy in maintaining rCBF in patients wih major cerebral artery occlusion
Some studies have reported that LMCs are not always adequate in maintaining rCBF32. Derdeyn et al33 (1998) concluded that LMCs are not adequate to maintain normal rCBF in major cerebral artery occlusion based on conventional angiograms and PET scan findings. It is important to note that the effect of concomitant medications on LMC formation is not known although previous studies have not suggested an acute affect of either antiplatelet agents34 or heparin.35
Factors determining functionality and patency of LMCs
From the functional aspect, blood flow can occur in both directions through LMCs depending on the pressure gradient. The functional capacity of LMC vessels is ultimately determined by their lumen caliber36, since it is inversely proportional to hydraulic resistance, ie, the fouth power of the radius. Therefore, size and number of LMCs determine the total capacity to maintain rCBF.11 The activation of these collaterals also depend on less well understood compensatory hemodynamic, metabolic, and neural mechanisms. Angiogenesis may result in collateral growth at the periphery of an ischemic region over time.37
During the incipient development of secondary collaterals rCBF may vary with hemodynamic fluctuations. Similarly, distal fragmentation of a thrombus within the parent vessel may occlude distal branches supplying retrograde collateral flow from cortical arteries. The efficacy of LMCs alo depends upon age, duration of ischemia, and associated co morbidities. Hypertension may impair collateral development in the setting of carotid occlusion and therefore increase stroke risk.38 Chronic hypoperfusion due to arterial flow restrictions such as extracranial carotid or intracranial steno-occlusive disease promotes collateral development, although the relationship of these collaterals with rCBF and clinical symptomatology remains unclear.
The role of animal studies in understanding LMCs
Animal experiments provide the possibility of measuring LMCs in vivo and also manipulating them under various experimental conditions. However, there are certain differences between the cerebrovascular systems of humans and animals, the major one being the presence of single ACA in rats and non-human primates. Furthermore, the P1 segment of the PCA is narrow, lacking, or variable in diameter. Early studies involving LMCs in animals were mostly performed in cats, canines, and non-human primates. They indicated that rCBF after major cerebral arterial occlusion depends on vessel size and collateral circulation and that rCBF via LMCs is established immediately after occlusion followed by chronic adaptation of LMCs by hypertrophy.39, 40 Coyle et al41 (1991) reported that LMCs can increase up to 50% in diameter 3 weeks after MCA occlusion in rats. The luminal width of these LMCs was a major determinant of rCBF in the territory of the occluded artery and of subsequent infarction. Maeda42 and Nallet et al.43 (1999) performed experiments on rats. They found that microvascular perfusion in the penumbra was predominantly preserved by LMCs in MCA occlusion. Recently, a study44 found an increase in the diameter of leptomeningeal anasotomoses after hypoxic preconditioning in rats. This change could be attributable to the upregulation of several angiogenic growth factors such as vascular endothelial growth factor and erythropoietin which have been shown to contribute to vascular remodeling after hypoxic preconditioning.45, 46 Most recently (2008), Todo et al47 observed enhanced LMC growth after giving GM-CSF treatment to mice with chronic unilateral ICA occlusion. After 14 days, the MCA was artificially occluded and a significant reduction in infarct size was observed in the GM-CSF treated mice compared with controls.
Conclusions and future prospects
The current review highlights the important research conducted in the last few decades on the role of LMCs in the cerebral circulation. The presence of robust LMCs is associated with rapid recanalization in acute ischemic stroke and reduction of infarct size. The application of nanotechnology has already begun to show promise in imaging LMCs48, 49. Three dimentional MRA techniques using 5-nm particles of contrast material have produced clear images of blood vessels less than 1 mm in diameter allowing quantitative measurements of angiogenesis and microvascular permeability in both healthy and ischemic tissues.50 Rapid advancements in such diagnostic modalities may allow further elaboration of the role of LMCs under abnormal hemodynamic conditions of the brain. Furthermore, a better understanding of LMCs in acute ischemic stroke might advance knowledge related to mechanism of action and mode of delivery pertaining to thrombolytic drugs.
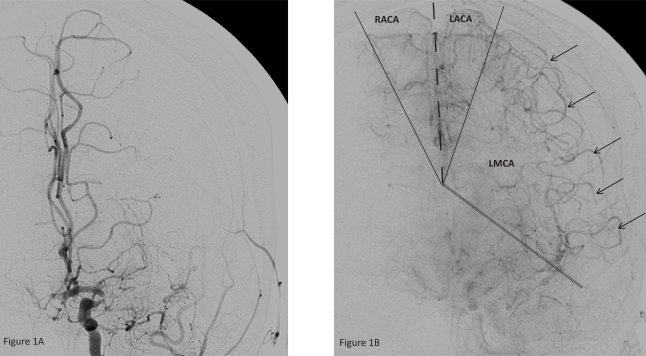
Figure 1, A and B.
Left ICA injection, antero-posterior projection, early (A) and late arterial phase.
1A. The left MCA is occluded. There is no filling of the vascular territory.
1B. Leptomeningeal collaterals (arrows) are now filling the MCA territory. The respective supply territories of the vessels are marked.
Abbreviations:
- DSA
digital subtraction angiography;
- LMCs
leptomeningeal collaterals;
- LMAs
leptomeningeal anastomosis;
- ACA
anterior cerebral artery;
- MCA
middle cerebral artery;
- PCA
posterior cerebral artery;
- ICA
internal carotid artery;
- rCBF
regional cerebral blood flow;
- MRA
magnetic resonance angiography;
- TCD
transcranial Doppler;
- GM-CSF
granulocyte monocyte-colony stimulating factor;
- CT
computed tomography;
- CTA
computed tomographic angiography;
- PET
Positron emission tomography
References
- 1.Liebeskind DS. Collateral circulation. Stroke. 2003;34:2279–2284. doi: 10.1161/01.STR.0000086465.41263.06. [DOI] [PubMed] [Google Scholar]
- 2.Moody DM. Features of the cerebral vascular pattern that predict vulnerability to perfusion or oxygenation deficiency: An anatomic study. American Journal of Neuroradiology. 1990;11:431–439. [PMC free article] [PubMed] [Google Scholar]
- 3.ur Türe U, Ya_argil MG, Krisht AF. The arteries of the corpus callosum: A microsurgical anatomic study. Neurosurgery. 1996;39:1075. doi: 10.1097/00006123-199612000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Mohammad Y, Xavier AR, Christoforidis G, Bourekas E, Slivka A. Qureshi grading scheme for angiographic occlusions strongly correlates with the initial severity and in-hospital outcome of acute ischemic stroke. J Neuroimaging. 2004;14:235–241. doi: 10.1177/1051228404265716. [DOI] [PubMed] [Google Scholar]
- 5.Qureshi AI, Siddiqui AM, Kim SH, Hanel RA, et al. Reocclusion of recanalized arteries during intra-arterial thrombolysis for acute ischemic stroke. AJNR Am J Neuroradiol. 2004;25:322–328. [PMC free article] [PubMed] [Google Scholar]
- 6.Chalela JA, Alsop DC, Gonzalez-Atavales JB, Maldjian JA, Kasner SE, Detre JA. Magnetic resonance perfusion imaging in acute ischemic stroke using continuous arterial spin labeling. Stroke. 2000;31:680–687. doi: 10.1161/01.str.31.3.680. [DOI] [PubMed] [Google Scholar]
- 7.Na DG, Byun HS, Lee KH, et al. Acute occlusion of the middle cerebral artery: Early evaluation with triphasic helical ct--preliminary results. Radiology. 1998;207:113–122. doi: 10.1148/radiology.207.1.9530306. [DOI] [PubMed] [Google Scholar]
- 8.Ringelstein EB, Biniek R, Weiller C, Ammeling B, Nolte PN, Thron A. Type and extent of hemispheric brain infarctions and clinical outcome in early and delayed middle cerebral artery recanalization. Neurology. 1992;42:289–298. doi: 10.1212/wnl.42.2.289. [DOI] [PubMed] [Google Scholar]
- 9.Heubner Die luetischen erkrankungen der hirnarterien. FC Vogel. 1874:170–214. [Google Scholar]
- 10.Van der Eecken HM, Adams RD. The anatomy and functional significance of the meningeal arterial anastomoses of the human brain. J Neuropathol Exp Neurol. 1953;12:132–157. doi: 10.1097/00005072-195304000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Brozici M, van der Zwan A, Hillen B. Anatomy and functionality of leptomeningeal anastomoses: A review. Stroke. 2003;34:2750–2762. doi: 10.1161/01.STR.0000095791.85737.65. [DOI] [PubMed] [Google Scholar]
- 12.Essig M, von Kummer R, Egelhof T, Winter R, Sartor K. Vascular mr contrast enhancement in cerebrovascular disease. AJNR Am J Neuroradiol. 1996;17:887–894. [PMC free article] [PubMed] [Google Scholar]
- 13.P. M. Practical neuroangiography. Baltimore: Williams & Wilkins; 1997. [Google Scholar]
- 14.Wildermuth S, Knauth M, Brandt T, Winter R, Sartor K, Hacke W. Role of ct angiography in patient selection for thrombolytic therapy in acute hemispheric stroke. Stroke. 1998;29:935–938. doi: 10.1161/01.str.29.5.935. [DOI] [PubMed] [Google Scholar]
- 15.Roberts HC, Dillon WP, Furlan AJ, et al. Computed tomographic findings in patients undergoing intra-arterial thrombolysis for acute ischemic stroke due to middle cerebral artery occlusion: Results from the proact ii trial. Stroke. 2002;33:1557–1565. doi: 10.1161/01.str.0000018011.66817.41. [DOI] [PubMed] [Google Scholar]
- 16.Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia - the ischemic penumbra. Stroke. 1981;12:723–725. doi: 10.1161/01.str.12.6.723. [DOI] [PubMed] [Google Scholar]
- 17.Fukuyama H, Akiguchi I, Kameyama M, et al. Krypton-81m single photon emission tomography and the collateral circulation in carotid occlusion: The role of the circle of willis and leptomeningeal anastomosis. J Neurol. 1983;230:7–17. doi: 10.1007/BF00313592. [DOI] [PubMed] [Google Scholar]
- 18.Saito I, Segawa H, Shiokawa Y, Taniguchi M, Tsutsumi K. Middle cerebral artery occlusion: Correlation of computed tomography and angiography with clinical outcome. Stroke. 1987;18:863–868. doi: 10.1161/01.str.18.5.863. [DOI] [PubMed] [Google Scholar]
- 19.Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol. 1998;55:1475–1482. doi: 10.1001/archneur.55.11.1475. [DOI] [PubMed] [Google Scholar]
- 20.Caplan LR, Wong KS, Gao S, Hennerici MG. Is hypoperfusion an important cause of strokes? If so, how? Cerebrovasc Dis. 2006;21:145–153. doi: 10.1159/000090791. [DOI] [PubMed] [Google Scholar]
- 21.Lee KH, Cho SJ, Byun HS, et al. Triphasic perfusion computed tomography in acute middle cerebral artery stroke: A correlation with angiographic findings. Arch Neurol. 2000;57:990–999. doi: 10.1001/archneur.57.7.990. [DOI] [PubMed] [Google Scholar]
- 22.Christoforidis GA, Mohammad Y, Kehagias D, Avutu B, Slivka AP. Angiographic assessment of pial collaterals as a prognostic indicator following intra-arterial thrombolysis for acute ischemic stroke. AJNR Am J Neuroradiol. 2005;26:1789–1797. [PMC free article] [PubMed] [Google Scholar]
- 23.Qureshi AI. New grading system for angiographic evaluation of arterial occlusions and recanalization response to intra-arterial thrombolysis in acute ischemic stroke. Neurosurgery. 2002;50:1405–1414. doi: 10.1097/00006123-200206000-00049. discussion 1414–1405. [DOI] [PubMed] [Google Scholar]
- 24.Mohammad YM, Christoforidis GA, Bourekas EC, Slivka AP. Qureshi grading scheme predicts subsequent volume of brain infarction following intra-arterial thrombolysis in patients with acute anterior circulation ischemic stroke. J Neuroimaging. 2008 doi: 10.1111/j.1552-6569.2007.00233.x. [DOI] [PubMed] [Google Scholar]
- 25.Qureshi AI. A new scheme for grading the quality of scientific reports that evaluate imaging modalities for cerebrovascular diseases. Med Sci Monit. 2007;13:RA181–187. [PubMed] [Google Scholar]
- 26.Shah QA, Georgiadis A, Suri MF, Rodriguez G, Qureshi AI. Preliminary experience with intra-arterial nicardipine in patients with acute ischemic stroke. Neurocrit Care. 2007;7:53–57. doi: 10.1007/s12028-007-0035-7. [DOI] [PubMed] [Google Scholar]
- 27.Yamauchi H, Kudoh T, Sugimoto K, Takahashi M, Kishibe Y, Okazawa H. Pattern of collaterals, type of infarcts, and haemodynamic impairment in carotid artery occlusion. J Neurol Neurosurg Psychiatry. 2004;75:1697–1701. doi: 10.1136/jnnp.2004.040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin PJ, Smith JL, Gaunt ME, Naylor AR. Assessment of intracranial primary collaterals using transcranial color-coded real-time sonography. J Neuroimaging. 1995;5:199–205. doi: 10.1111/jon199554199. [DOI] [PubMed] [Google Scholar]
- 29.Yamashita T, Nakano S, Ishihara H, et al. Surgical modulation of the natural course of collateral circulation in chronic ischemic patients. Acta Neurol Scand Suppl. 1996;166:74–78. doi: 10.1111/j.1600-0404.1996.tb00554.x. [DOI] [PubMed] [Google Scholar]
- 30.Drake CG, Peerless SJ, Ferguson GG. Hunterian proximal arterial occlusion for giant aneurysms of the carotid circulation. J Neurosurg. 1994;81:656–665. doi: 10.3171/jns.1994.81.5.0656. [DOI] [PubMed] [Google Scholar]
- 31.Enam SA, Malik GM. Association of cerebral arteriovenous malformations and spontaneous occlusion of major feeding arteries: Clinical and therapeutic implications. Neurosurgery. 1999;45:1105–1111. doi: 10.1097/00006123-199911000-00018. discussion 1111–1112. [DOI] [PubMed] [Google Scholar]
- 32.Muller M, Schimrigk K. Vasomotor reactivity and pattern of collateral blood flow in severe occlusive carotid artery disease. Stroke. 1996;27:296–299. doi: 10.1161/01.str.27.2.296. [DOI] [PubMed] [Google Scholar]
- 33.Derdeyn CP, Powers WJ, Grubb RL., Jr Hemodynamic effects of middle cerebral artery stenosis and occlusion. AJNR Am J Neuroradiol. 1998;19:1463–1469. [PMC free article] [PubMed] [Google Scholar]
- 34.Ozdemir O, Soylu M, Demir AD, et al. Collaterals that regressed after angioplasty can be recruited to protect the left ventricle in case of an acute occlusion. Angiology. 2005;56:517–523. doi: 10.1177/000331970505600502. [DOI] [PubMed] [Google Scholar]
- 35.Carroll SM, White FC, Roth DM, Bloor CM. Heparin accelerates coronary collateral development in a porcine model of coronary artery occlusion. Circulation. 1993;88:198–207. doi: 10.1161/01.cir.88.1.198. [DOI] [PubMed] [Google Scholar]
- 36.Hoksbergen AW, Fulesdi B, Legemate DA, Csiba L. Collateral configuration of the circle of willis: Transcranial color-coded duplex ultrasonography and comparison with postmortem anatomy. Stroke. 2000;31:1346–1351. doi: 10.1161/01.str.31.6.1346. [DOI] [PubMed] [Google Scholar]
- 37.Wei L, Erinjeri JP, Rovainen CM, Woolsey TA. Collateral growth and angiogenesis around cortical stroke. Stroke. 2001;32:2179–2184. doi: 10.1161/hs0901.094282. [DOI] [PubMed] [Google Scholar]
- 38.Hedera P, Bujdakova J, Traubner P, Pancak J. Stroke risk factors and development of collateral flow in carotid occlusive disease. Acta Neurol Scand. 1998;98:182–186. doi: 10.1111/j.1600-0404.1998.tb07291.x. [DOI] [PubMed] [Google Scholar]
- 39.Meyer JS, Fang HC, Denny-Brown D. Polarographic study of cerebral collateral circulation. AMA Arch Neurol Psychiatry. 1954;72:296–312. doi: 10.1001/archneurpsyc.1954.02330030030003. [DOI] [PubMed] [Google Scholar]
- 40.Symon L. Observations on the leptomeningeal collateral circulation in dogs. J Physiol. 1960;154:1–14. 12. doi: 10.1113/jphysiol.1960.sp006560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coyle P, Heistad DD. Development of collaterals in the cerebral circulation. Blood Vessels. 1991;28:183–189. doi: 10.1159/000158860. [DOI] [PubMed] [Google Scholar]
- 42.Maeda K, Hata R, Bader M, Walther T, Hossmann KA. Larger anastomoses in angiotensinogen-knockout mice attenuate early metabolic disturbances after middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1999;19:1092–1098. doi: 10.1097/00004647-199910000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Nallet H, MacKenzie ET, Roussel S. The nature of penumbral depolarizations following focal cerebral ischemia in the rat. Brain Res. 1999;842:148–158. doi: 10.1016/s0006-8993(99)01859-4. [DOI] [PubMed] [Google Scholar]
- 44.Woitzik J, Hecht N, Schneider UC, Pena-Tapia PG, Vajkoczy P. Increased vessel diameter of leptomeningeal anastomoses after hypoxic preconditioning. Brain Res. 2006;1115:209–212. doi: 10.1016/j.brainres.2006.07.081. [DOI] [PubMed] [Google Scholar]
- 45.Chen WJ, Chen HW, Yu SL, et al. Gene expression profiles in hypoxic preconditioning using cdna microarray analysis: Altered expression of an angiogenic factor, carcinoembryonic antigen-related cell adhesion molecule 1. Shock. 2005;24:124–131. doi: 10.1097/01.shk.0000170352.72694.36. [DOI] [PubMed] [Google Scholar]
- 46.Prass K, Scharff A, Ruscher K, et al. Hypoxia-induced stroke tolerance in the mouse is mediated by erythropoietin. Stroke. 2003;34:1981–1986. doi: 10.1161/01.STR.0000080381.76409.B2. [DOI] [PubMed] [Google Scholar]
- 47.Todo K, Kitagawa K, Sasaki T, et al. Granulocyte-macrophage colony-stimulating factor enhances leptomeningeal collateral growth induced by common carotid artery occlusion. Stroke. 2008;39:1875–1882. doi: 10.1161/STROKEAHA.107.503433. [DOI] [PubMed] [Google Scholar]
- 48.Silva GA. Nanotechnology approaches for the regeneration and neuroprotection of the central nervous system. Surg Neurol. 2005;63:301–306. doi: 10.1016/j.surneu.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 49.Silva GA. Nanotechnology approaches for drug and small molecule delivery across the blood brain barrier. Surg Neurol. 2007;67:113–116. doi: 10.1016/j.surneu.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 50.Hopkins LN, Ecker RD. Cerebral endovascular neurosurgery. Neurosurgery. 2008;62:1483–1501. doi: 10.1227/01.neu.0000333813.95025.a0. discussion 1501–1502. [DOI] [PubMed] [Google Scholar]
- 51.Qureshi AI, El-Gengaihi A, Hussein HM, Suri MFK, Liebeskind DS. Occurance and variability in acute formation of leptomeningeal collaterals in proximal middle cerebral artery occlusion. J Vasc Interv Neurol. 2008;1:70–72. [PMC free article] [PubMed] [Google Scholar]