Abstract
Background:
Patients with chronic kidney disease (CKD) are at higher risk for stroke because of higher prevalence of traditional and non-traditional cardiovascular risk factors.
Methods:
We performed an extensive literature review with pre-defined keywords. We summarized the results of the studies evaluating for risk factors predisposing to stroke in CKD patients.
Results:
The incidence of stroke and stroke-related mortality is higher in CKD patients compared with the general population. Presence of anemia, hypoalbuminemia, malnutrition, uremia, and hyperhomocysteinemia in patients with CKD is associated with higher incidence of stroke. Hemodialysis and renal transplant patients are at higher risk of developing stroke compared with those who do not require renal replacement therapy.
Conclusion:
The early recognition of risk factors associated with stroke in CKD population is imperative. Early interventions may potentially decrease the incidence and associated mortality of stroke in CKD patients.
Keywords: stroke, chronic kidney disease, risk factors, dialysis, renal transplant, ischemic stroke
Almost 20 million people in United States have chronic kidney disease (CKD).1 CKD is defined as glomerular filteration rate (GFR) less than 60ml/min/1.73 m2. 1,2 Patients with CKD have markedly increased incidence of vascular disease when compared to the general population.3 Traditional risk factors for stroke such as diabetes mellitus, hypertension, cardiac disease, hyperlipidemia and cigarette smoking are quite common in this population2 but little is known about the risk factors which are different from the general population. In this review article, we will emphasize on important non-traditional risk factors unique to the CKD population.
Incidence of stroke
The incidence of stroke is much higher in CKD patients than in the general population.4 The United States Renal Data System (USRDS) and National Hospital Discharge Survey (NHDS) datasets show that the incident dialysis population suffers a five to ten fold higher risk of hospitalized stroke in comparison with non-End Stage Renal Disease (ESRD) population.5 (relative risk; RR of 6.1 in ESRD population). Stroke risk is elevated for both ischemic and hemorrhagic stroke; however there is a higher risk for ischemic stroke than for hemorrhagic stroke, especially among women (ischemic stroke RR ranges from 4.3 to 10.1; hemorrhagic stroke, RR ranges from 4.1 to 6.7).6
In a study of 6685 hospitalized patients, Koren-Moreg et al 7 found that an eGFR less than 60 ml/min had a hazard ratio (HR) of 1.5 for increased rate of incident stroke or transient ischemic attack in patients with CKD and pre-existing cardiovascular diseases. The risk increased with decreasing eGFR values. Preliminary results in the Kidney Early Evaluation Program (KEEP) 8 found that eGFR less than 60/min/1.73m2 or urine albumin to creatinine ratio >30mg/g was associated with higher risk of either myocardial infarction or stroke.
Stroke related mortality
The short and long term mortality associated with stroke appears to be higher in CKD patients than in the general population. In the Okinawa Dialysis Study (OKIDS) 9, 30 day stroke mortality rate was higher in CKD patients compared with the rate observed in general population in Okinawa, Japan. The size of the lesion in hemorrhagic strokes was larger in the CKD patients than in the general population. McWalter et al 10 also found that renal dysfunction was a predictor of an increased mortality in the 7 year follow up study of acute stroke patients. A summary of risk factors for stroke unique to the CKD population are presented in Table 1:
Table 1:
Risk factors of stroke in the general population in comparison to the CKD population.
| General population | CKD population |
|---|---|
| Risk factors | Exclusive risk factors |
| Diabetes mellitus | Female gender |
| Hypertension | Caucasian race |
| Cigeratte smoking | Anemia |
| Hyperlipidemia | Malnutrition |
| Age | Uremia |
| Family history | Hemodialysis |
| Prior stroke | Low systolic blood pressure |
| Black race | Renal transplant |
| Male gender | Hyperhomocysteinemia |
| Metabolic syndrome | Bleeding diathesis |
1). Age, Ethnicity and Gender:
Seliger et al 5 found in their population based study that the age adjusted RR of stroke among dialysis patients was 6.1 [95% Confidence Interval (95% CI) 5.1, 7.1] compared with the general population for Caucasian men, 4.4 (95% CI 3.3, 5.5) for African American men, 9.7 (95%CI 8.2, 11.2) for Caucasians women and 6.2 (95%CI 4.8, 7.6) for African American women. Overall, the RR for stroke was higher among women than in men. In the USRDS and NHDS datasets, African Americans were at lower risk for stroke than Caucasians (HR for strokes; 0.7) among patients with cardiac disease, but opposite effect was observed among individuals without cardiac disease (HR for strokes among African Americans vs. Caucasians; HR; 1.2). Although age is an independent risk factor of stroke in the general population, this relationship was not observed among chronic dialysis patients6. The lack of relationship can be explained either by the overall high death rate in elderly dialysis patients or by the relatively early onset of severe hypertension in CKD patients.
2). Anemia:
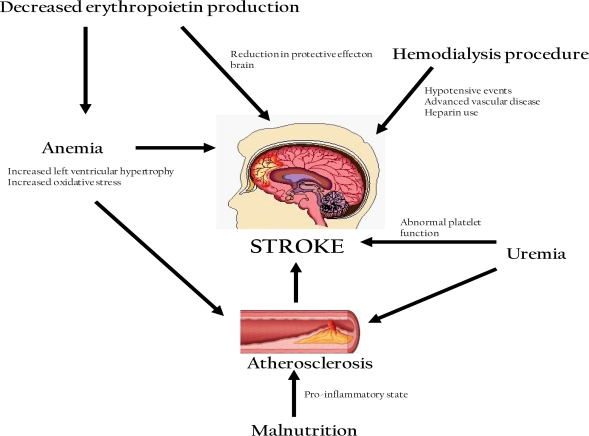
Atherosclerosis Risk in Communities Study (ARIC)11 demonstrated the association between anemia and stroke in CKD patients. In this study, 13,716 patients with both eGFR less than 60ml/min and anemia (defined as hemoglobin <13g/dl for men and <12g/dl for women) were followed for 9 years. They found that the overall stroke risk was increased in this CKD population: HR 1.81, (95% CI 1.3 to 2). This risk was higher in patients with anemia (HR 5.4; 95% CI 2.04 to 14.4) compared with those without anemia (HR 1.4; 95% CI 0.93 to 2.1). This finding is in contrast to the general population where high rather than low hematocrit is associated with an increased risk of stroke. However, since severe anemia is uncommon in the non-ESRD population, these studies may have insignificant power to detect such an association in the general population. The exact reasons for this substantially higher risk of stroke among patients with both CKD and anemia are still not entirely clear. Further studies are needed to clarify this concept. There are several proposed mechanisms for this association between anemia and stroke in CKD patients. Pathophysiological mechanisms of stroke in CKD are shown in Figure 1.
Figure 1:
Pathophysiological mechanisms predisposing to stroke in patients with CKD
Cardiovascular disease (CVD) is prevalent in ESRD.12 Framingham Offspring Based Community Study prospectively examined 6233 patients for 15 years and found that mild renal insufficiency (creatinine of 1.3 –2.9 mg/dl) carries almost two fold risk of CVD diseases compared with the general population. CKD may induce oxidative stress 13 which is known to promote atherosclerosis. 14 Furthermore, 75 % of patients who require dialysis, have pre-existing left ventricular hypertrophy. 15 Studies have shown that LVH is a strong predictor of stroke risk.16 Therefore anemia may increase the risk of stroke by inducing atherosclerosis and LVH in CKD patients.
Studies using animal models demonstrate the neuro-protective effect of erythropoietin for ischemia-induced stroke. 17–20 However, when renal function is reduced, the erythropoietin production from kidneys is impaired, which may limit erythropoietin-induced neuronal protection against anemia-associated strokes. In this way, the combination of anemia and reduced kidney erythropoietin production may interact to increase the probability of stroke.
3). Hypoalbuminemia and malnutrition:
An elevated serum albumin concentration has been associated with a reduced risk of coronary heart disease, 21–23 death from cardiovascular disease and death from all causes in Caucasian men and women and African American men and women. In the National Health Epidemiologic Followup Study (NHEFS) dataset,23 a decreased serum albumin concentration or albumin/globulin ratio was found to be more frequent in stroke cases than in controls. However, data regarding macroalbuminuria as a risk factor for stoke is not entirely clear. In a Japanese study,24 analyzing first symptomatic stroke events, reduced kidney function was associated with increased relative HR (HR, 3.1 in creatinine clearance < 40 ml/min, 1.9 in creatinine clearance 40–70 ml/min). The presence of macroalbuminuria tended to increase HR but was not statistical significant (HR, 1.4). Another report from Japan25 also supported this finding revealing that macroalbuminuria is not a significant risk factor for death due to stroke, although it is a significant risk factor for all-cause and CVD mortality. Many authors24 speculate that the patients with macroalbuminuria are likely to have systemic vasculopathy and therefore the death events due to all-causes may be more apparent than those seen in the First Symptomatic Stroke Events Study.26 Malnutrition is a well recognized risk factor for all27–30 and cardiovascular-specific31 mortality in the dialysis population. Significant protein malnutrition has been ascribed to a higher incidence of intra-cerebral hemorrhage in both the general population and dialysis patients.23 Seliger et al5 found that three markers of malnutrition specifically low serum albumin (per 1 g/dl decrease, HR = 1.4), low height-adjusted body weight (per 25% decrease, HR = 1.2), and a subjective assessment of undernourishment (HR = 1.3), were associated with a higher risk of incident stroke. This is in contrast to the general population, in which obesity, rather than malnutrition, confers a higher stroke risk.27 Several authors have suggested the following pathophysiological mechanisms:
Malnutrition reflects not merely poor nutrient intake but also the effects of a chronic micro-inflammatory state in CKD patients. 32,33 Studies have shown that elevated inflammatory markers are associated with higher rates of stroke. 34, 35 Chronic inflammation may explain the observed association between malnutrition and stroke.
Low serum albumin may be an indicator of some other factor influencing the atherosclerotic process. 21, 23, 36 The effect of albumin concentration on platelet function, blood viscosity, free fatty acid transport, and antioxidant levels have also been considered. 21, 23
4). Uremia induced accelerated atherosclerosis:
An additional factor responsible for higher stroke rates in CKD population is accelerated atherosclerotic vascular disease caused in part by uremia itself. 37,38 This is supported by the reports using noninvasive imaging techniques, demonstrating a greater degree of carotid artery atherosclerosis among the dialysis patients compared to controls, even after adjusting for traditional cardiovascular risk factors. In one study,38 investigators found that vessel wall elasticity of the carotid artery is decreased in younger hemodialysis patients compared with age-matched healthy subjects. The enhanced stiffness of the arteries may contribute to the higher incidence of stroke in hemodialysis patients.
5). Hemodialysis procedure:
Toyoda et al 39 found that the occurrence of ischemic stroke was more common during or shortly after the dialysis procedure (34%) compared with the timing of hemorrhagic stroke. Given the relatively short exposure time during hemodialysis (3 times/wk for 5 hours) the frequency of 34% is meaningful. This result is unexpected because patients usually are administered anticoagulants during hemodialysis which would increase the tendency for hemorrhagic than the ischemic events. The potential pathophysiological mechanism may be related to drastic decrease in intravascular blood volume secondary to hemodialysis and diminished vascular responses secondary to diabetic autonomic neuropathy and advanced arteriosclerosis, These factors result in an abrupt decrease in blood pressure and may induce brain ischemia.40
6). Blood pressure:
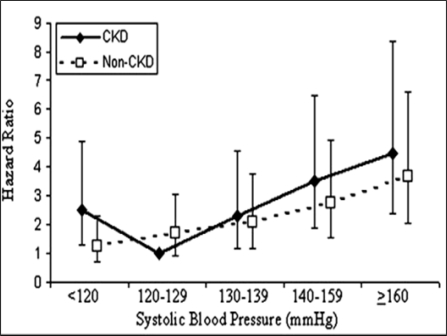
Hypertension, notably elevated systolic blood pressure (SBP), is a risk factor for stroke in the general population. Stroke risk doubles for almost every 20/10 mm Hg increase in blood pressure over 115/75 mm Hg41. In addition, hypertension is a major component of CKD, both as a cause and a result of impaired kidney function. Weiner et al 42 found that the individuals with CKD had a J-shaped relationship with stroke events such that those with SBP <120 mmHg were at significantly increased risk for stroke compared with the individuals with CKD and SBP 120 to 129 mmHg (HR 2.51; 95% CI 1.30 to 4.87); risk increased for SBP >130 mmHg in CKD patients. This J-shaped relationship was not seen in the individuals without CKD. Seliger et al 5 did not find this J shaped effect of BP on stroke. This J shaped effect was also not seen in Perindopril Protection against Recurrent Stroke trial (PROGRESS).43 The association of incident stroke with systolic BP and CKD is depicted in figure2.
Figure 2:
The hazard of incident stroke associated with systolic BP (SBP) and chronic kidney disease (CKD) using an unadjusted model adjusted for age, race, gender, history of diabetes, history of coronary disease, left ventricular hypertrophy, use of antihypertensive medication, education status, smoking status, serum albumin, non- HDL cholesterol, hemoglobin, and study of origin). Reference group is individuals with CKD and SBP 120 to 129 mmHg. Reprinted with permission from journal of American Society of Nephrology.42
7). Anticoagulation during dialysis and uremic bleeding diathesis:
Routine anticoagulation during hemodialysis, combined with a uremic bleeding diathesis, may theoretically lead to an increased risk of hemorrhagic stroke. Studies showing the definitive relationship are lacking.
8). Renal transplant:
Cardiovascular and cerebrovascular diseases are the main causes of death in patients with end-stage renal disease (ESRD) including those on dialysis and those who have received renal transplant. Oliveras et al44 found that the prevalence of stroke in transplant recipients was 8% at 10 yrs. Mean time elapsed between renal transplantation and stroke was 49 months. The rate of intra-cerebral hemorrhage was found to be high in this population. The outcome of all strokes was also extremely poor. They identified three predictors of stroke in these patients: diabetic nephropathy (OR= 4.8; p < 0.01), peripheral vascular disease (OR = 8.2; p < 0.001) and age > 40 yr (OR = 3.3; p < 0.02). Other analyzed risk factors including gender, renal function, cytomegalovirus infection, hyperlipidemia, hyperuricemia, erythrocytosis, or hypertensive donor failed to show any significant predictive value for stroke in these patients. This is very similar to the data from other studies evaluating such a relationship among renal transplant and hemodialysis patients. Another study 45 primarily of 1633 Caucasian patients submitted to renal transplant found a low stroke rate (3.9%) over four years. The pre-transplantation risk factors identified were atrial fibrillation (p = 0.001), and diabetes mellitus (p=0 .037). More common risk factors like prior transient ischemic attack, stroke, hypertension, hyperlipidemia, the primary cause of kidney disease, duration of renal replacement therapy, smoking, and gender were not associated with stroke.
In a recent prospective European study,46 a total of 184 (8.8%, 95% confidence interval 4.6–12.9) of 2102 patients experienced stroke during follow-up of 6.7 years, corresponding to an incidence of 1.3% stroke events per year. For ischemic stroke, diabetes mellitus (HR of 3.5; 95% CI 2.4–5.2), previous cerebrovascular event (HR 3.5; 95% CI 2.2–5.6), age (HR of 1.1; 95% CI 0.1–1.1), and serum creatinine (HR of 1; 95% CI 1.0–1.01) were identified as risk factors. The risk of a hemorrhagic stroke was increased by diabetes mellitus (HR of 4.9; 95% CI 2.1–11.6), polycystic kidney disease (HR of 4.2; 95% CI 1.4–11.2), left ventricular hypertrophy (HR of 3.0; 95% CI 1.2–7.2), and SBP (HR of 1.0; 95% CI 1.00–1.04).
9). Hyperhomocysteinemia:
Wilcken et al47 found homocysteine levels to be 2 fold higher in CKD patients than the controls. Several clinical studies have supported that hyperhomocysteinemia is an important risk factor for atherosclerosis.48, 49 Mild hyperhomocysteinemia has been found in 30 % of the patients having premature cerebral, vascular and coronary artery disease. Bachmann et al50 found that high serum homocysteine was significantly associated with occlusive arterial disease (RR = 0.2). Clark et al51 also found a significant association between hyperhomocysteinemia and premature atherosclerosis; even after the adjustment of other atherogenic factors. Homocysteine enhances the auto oxidation of LDL cholesterol and induces a strong increase in binding of lipoprotein to fibrin. Abnormalities of lipid metabolism may explain the proatherognic and prothrombogenic effects of homocysteine.
Future directions:
Risk factors which predispose CKD population to stroke are still being analyzed. The potential importance of macroalbuminuria, hyperuricemia, hyperhomocysteinemia, and low SBP for stroke events warrants further studies. The data regarding stroke in renal transplant patients also demands further investigation.
Acknowledgments
The authors thank these people for their generous support.
Janet A. Jokela MD, Robert Healy MD, James Kumar MD, Rana Zaman MD, Jahangir Ali Randhawa MD, Prof. Saleh Saeed, Farzana Saeed. Dewan Ali and Ahmad Unisa.
References:
- 1.K/doqi clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 2.Khella S. New insights into stroke in chronic kidney disease. Adv Chronic Kidney Dis. 2008;15:338–346. doi: 10.1053/j.ackd.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Longenecker J, Coresh J, Powe N, Levey A, Fink N, Martin A, Klag M. Traditional cardiovascular disease risk factors in dialysis patients compared with the general population: The choice study. J Am Soc Nephrol. 2002;13:1918–1927. doi: 10.1097/01.asn.0000019641.41496.1e. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy R, Case C, Fathi R, Johnson D, Isbel N, Marwick T. Does renal failure cause an atherosclerotic milieu in patients with end-stage renal disease? Am J Med. 2001;110:198–204. doi: 10.1016/s0002-9343(00)00695-1. [DOI] [PubMed] [Google Scholar]
- 5.Seliger S, Gillen D, Tirschwell D, Wasse H, Kestenbaum B, Stehman-Breen C. Risk factors for incident stroke among patients with end-stage renal disease. J Am Soc Nephrol. 2003;14:2623–2631. doi: 10.1097/01.asn.0000088722.56342.a8. [DOI] [PubMed] [Google Scholar]
- 6.Seliger SL, Gillen DL, Longstreth WT, Jr, Kestenbaum B, Stehman-Breen CO. Elevated risk of stroke among patients with end-stage renal disease. Kidney Int. 2003;64:603–609. doi: 10.1046/j.1523-1755.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- 7.Koren-Morag N, Goldbourt U, Tanne D. Renal dysfunction and risk of ischemic stroke or tia in patients with cardiovascular disease. Neurology. 2006;67:224–228. doi: 10.1212/01.wnl.0000229099.62706.a3. [DOI] [PubMed] [Google Scholar]
- 8.McCullough P, Li S, Jurkovitz C, Stevens L, Wang C, Collins A, Chen S, Norris K, McFarlane S, Johnson B, Shlipak M, Obialo C, Brown W, Vassalotti J, Whaley-Connell A. Ckd and cardiovascular disease in screened high-risk volunteer and general populations: The kidney early evaluation program (keep) and national health and nutrition examination survey (nhanes) 1999–2004. Am J Kidney Dis. 2008;51:S38–45. doi: 10.1053/j.ajkd.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 9.Iseki K, Tozawa M, Iseki C, Takishita S, Ogawa Y. Demographic trends in the okinawa dialysis study (okids) registry (1971–2000) Kidney Int. 2002;61:668–675. doi: 10.1046/j.1523-1755.2002.00147.x. [DOI] [PubMed] [Google Scholar]
- 10.MacWalter R, Wong S, Wong K, Stewart G, Fraser C, Fraser H, Ersoy Y, Ogston S, Chen R. Does renal dysfunction predict mortality after acute stroke? A 7-year follow-up study. Stroke. 2002;33:1630–1635. doi: 10.1161/01.str.0000016344.49819.f7. [DOI] [PubMed] [Google Scholar]
- 11.Abramson J, Jurkovitz C, Vaccarino V, Weintraub W, McClellan W. Chronic kidney disease, anemia, and incident stroke in a middle-aged, community-based population: The aric study. Kidney Int. 2003;64:610–615. doi: 10.1046/j.1523-1755.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 12.Culleton B, Larson M, Wilson P, Evans J, Parfrey P, Levy D. Cardiovascular disease and mortality in a community-based cohort with mild renal insufficiency. Kidney Int. 1999;56:2214–2219. doi: 10.1046/j.1523-1755.1999.00773.x. [DOI] [PubMed] [Google Scholar]
- 13.Himmelfarb J, Stenvinkel P, Ikizler T, Hakim R. The elephant in uremia: Oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62:1524–1538. doi: 10.1046/j.1523-1755.2002.00600.x. [DOI] [PubMed] [Google Scholar]
- 14.Martinet W, Knaapen M, De Meyer G, Herman A, Kockx M. Elevated levels of oxidative DNA damage and DNA repair enzymes in human atherosclerotic plaques. Circulation. 2002;106:927–932. doi: 10.1161/01.cir.0000026393.47805.21. [DOI] [PubMed] [Google Scholar]
- 15.Levin A. Prevalence of cardiovascular damage in early renal disease. Nephrol Dial Transplant. 2001;16(Suppl 2):7–11. doi: 10.1093/ndt/16.suppl_2.7. [DOI] [PubMed] [Google Scholar]
- 16.Bots M, Nikitin Y, Salonen J, Elwood P, Malyutina S, Freire de Concalves A, Sivenius J, Di Carlo A, Lagiou P, Tuomilehto J, Koudstaal P, Grobbee D. Left ventricular hypertrophy and risk of fatal and non-fatal stroke. Eurostroke: A collaborative study among research centres in europe. J Epidemiol Community Health. 2002;56(Suppl 1):i8–13. doi: 10.1136/jech.56.suppl_1.i8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sirén A, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, Keenan S, Gleiter C, Pasquali C, Capobianco A, Mennini T, Heumann R, Cerami A, Ehrenreich H, Ghezzi P. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci U S A. 2001;98:4044–4049. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakanaka M, Wen T, Matsuda S, Masuda S, Morishita E, Nagao M, Sasaki R. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci U S A. 1998;95:4635–4640. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadamoto Y, Igase K, Sakanaka M, Sato K, Otsuka H, Sakaki S, Masuda S, Sasaki R. Erythropoietin prevents place navigation disability and cortical infarction in rats with permanent occlusion of the middle cerebral artery. Biochem Biophys Res Commun. 1998;253:26–32. doi: 10.1006/bbrc.1998.9748. [DOI] [PubMed] [Google Scholar]
- 20.Brines M, Ghezzi P, Keenan S, Agnello D, de Lanerolle N, Cerami C, Itri L, Cerami A. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuller L, Eichner J, Orchard T, Grandits G, McCallum L, Tracy R. The relation between serum albumin levels and risk of coronary heart disease in the multiple risk factor intervention trial. Am J Epidemiol. 1991;134:1266–1277. doi: 10.1093/oxfordjournals.aje.a116030. [DOI] [PubMed] [Google Scholar]
- 22.Phillips A, Shaper A, Whincup P. Association between serum albumin and mortality from cardiovascular disease, cancer, and other causes. Lancet. 1989;2:1434–1436. doi: 10.1016/s0140-6736(89)92042-4. [DOI] [PubMed] [Google Scholar]
- 23.Gillum R, Makuc D. Serum albumin, coronary heart disease, and death. Am Heart J. 1992;123:507–513. doi: 10.1016/0002-8703(92)90667-k. [DOI] [PubMed] [Google Scholar]
- 24.Nakayama M, Metoki H, Terawaki H, Ohkubo T, Kikuya M, Sato T, Nakayama K, Asayama K, Inoue R, Hashimoto J, Totsune K, Hoshi H, Ito S, Imai Y. Kidney dysfunction as a risk factor for first symptomatic stroke events in a general japanese population--the ohasama study. Nephrol Dial Transplant. 2007;22:1910–1915. doi: 10.1093/ndt/gfm051. [DOI] [PubMed] [Google Scholar]
- 25.Ninomiya T, Kiyohara Y, Kubo M, Tanizaki Y, Doi Y, Okubo K, Wakugawa Y, Hata J, Oishi Y, Shikata K, Yonemoto K, Hirakata H, Iida M. Chronic kidney disease and cardiovascular disease in a general japanese population: The hisayama study. Kidney Int. 2005;68:228–236. doi: 10.1111/j.1523-1755.2005.00397.x. [DOI] [PubMed] [Google Scholar]
- 26.Nakayama M, Metoki H, Terawaki H, Ohkubo T, Kikuya M, Sato T, Nakayama K, Asayama K, Inoue R, Hashimoto J, Totsune K, Hoshi H, Ito S, Imai Y. Kidney dysfunction as a risk factor for first symptomatic stroke events in a general japanese population--the ohasama study. Nephrol Dial Transplant. 2007;22:1910–1915. doi: 10.1093/ndt/gfm051. [DOI] [PubMed] [Google Scholar]
- 27.Avram M, Sreedhara R, Fein P, Oo K, Chattopadhyay J, Mittman N. Survival on hemodialysis and peritoneal dialysis over 12 years with emphasis on nutritional parameters. Am J Kidney Dis. 2001;37:S77–80. doi: 10.1053/ajkd.2001.20754. [DOI] [PubMed] [Google Scholar]
- 28.Combe C, Chauveau P, Laville M, Fouque D, Azar R, Cano N, Canaud B, Roth H, Leverve X, Aparicio M. Influence of nutritional factors and hemodialysis adequacy on the survival of 1,610 french patients. Am J Kidney Dis. 2001;37:S81–88. doi: 10.1053/ajkd.2001.20756. [DOI] [PubMed] [Google Scholar]
- 29.Lowrie E, Lew N. Death risk in hemodialysis patients: The predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis. 1990;15:458–482. doi: 10.1016/s0272-6386(12)70364-5. [DOI] [PubMed] [Google Scholar]
- 30.Pifer T, McCullough K, Port F, Goodkin D, Maroni B, Held P, Young E. Mortality risk in hemodialysis patients and changes in nutritional indicators: Dopps. Kidney Int. 2002;62:2238–2245. doi: 10.1046/j.1523-1755.2002.00658.x. [DOI] [PubMed] [Google Scholar]
- 31.Fung F, Sherrard D, Gillen D, Wong C, Kestenbaum B, Seliger S, Ball A, Stehman-Breen C. Increased risk for cardiovascular mortality among malnourished end-stage renal disease patients. Am J Kidney Dis. 2002;40:307–314. doi: 10.1053/ajkd.2002.34509. [DOI] [PubMed] [Google Scholar]
- 32.Stenvinkel P. Malnutrition and chronic inflammation as risk factors for cardiovascular disease in chronic renal failure. Blood Purif. 2001;19:143–151. doi: 10.1159/000046932. [DOI] [PubMed] [Google Scholar]
- 33.Kalantar-Zadeh K, Kopple J. Relative contributions of nutrition and inflammation to clinical outcome in dialysis patients. Am J Kidney Dis. 2001;38:1343–1350. doi: 10.1053/ajkd.2001.29250. [DOI] [PubMed] [Google Scholar]
- 34.Ridker P, Buring J, Shih J, Matias M, Hennekens C. Prospective study of c-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98:731–733. doi: 10.1161/01.cir.98.8.731. [DOI] [PubMed] [Google Scholar]
- 35.Rost N, Wolf P, Kase C, Kelly-Hayes M, Silbershatz H, Massaro J, D’Agostino R, Franzblau C, Wilson P. Plasma concentration of c-reactive protein and risk of ischemic stroke and transient ischemic attack: The framingham study. Stroke. 2001;32:2575–2579. doi: 10.1161/hs1101.098151. [DOI] [PubMed] [Google Scholar]
- 36.Hopkins P, Williams R. Identification and relative weight of cardiovascular risk factors. Cardiol Clin. 1986;4:3–31. [PubMed] [Google Scholar]
- 37.Amann K, Neusüss R, Ritz E, Irzyniec T, Wiest G, Mall G. Changes of vascular architecture independent of blood pressure in experimental uremia. Am J Hypertens. 1995;8:409–417. doi: 10.1016/0895-7061(94)00248-a. [DOI] [PubMed] [Google Scholar]
- 38.Zoungas S, Ristevski S, Lightfoot P, Liang Y, Branley P, Shiel L, Kerr P, Atkins R, McNeil J, McGrath B. Carotid artery intima-medial thickness is increased in chronic renal failure. Clin Exp Pharmacol Physiol. 2000;27:639–641. doi: 10.1046/j.1440-1681.2000.03301.x. [DOI] [PubMed] [Google Scholar]
- 39.Toyoda K, Fujii K, Fujimi S, Kumai Y, Tsuchimochi H, Ibayashi S, Iida M. Stroke in patients on maintenance hemodialysis: A 22-year single-center study. Am J Kidney Dis. 2005;45:1058–1066. doi: 10.1053/j.ajkd.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 40.Balci K, Utku U, Asil T, Unlu E. Simultaneous onset of hemorrhagic and ischemic strokes. Neurologist. 2007;13:148–149. doi: 10.1097/01.nrl.0000256434.19734.77. [DOI] [PubMed] [Google Scholar]
- 41.Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The antihypertensive and lipid-lowering treatment to prevent heart attack trial (allhat) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 42.Weiner D, Tighiouart H, Levey A, Elsayed E, Griffith J, Salem D, Sarnak M. Lowest systolic blood pressure is associated with stroke in stages 3 to 4 chronic kidney disease. J Am Soc Nephrol. 2007;18:960–966. doi: 10.1681/ASN.2006080858. [DOI] [PubMed] [Google Scholar]
- 43.Ninomiya T, Perkovic V, Gallagher M, Jardine M, Cass A, Arima H, Anderson C, Neal B, Woodward M, Omae T, MacMahon S, Chalmers J. Lower blood pressure and risk of recurrent stroke in patients with chronic kidney disease: Progress trial. Kidney Int. 2008;73:963–970. doi: 10.1038/ki.2008.5. [DOI] [PubMed] [Google Scholar]
- 44.Oliveras A, Roquer J, Puig J, Rodríguez A, Mir M, Orfila M, Masramon J, Lloveras J. Stroke in renal transplant recipients: Epidemiology, predictive risk factors and outcome. Clin Transplant. 2003;17:1–8. doi: 10.1034/j.1399-0012.2003.02042.x. [DOI] [PubMed] [Google Scholar]
- 45.Aull-Watschinger S, Konstantin H, Demetriou D, Schillinger M, Habicht A, Hörl W, Watschinger B. Pre-transplant predictors of cerebrovascular events after kidney transplantation. Nephrol Dial Transplant. 2008;23:1429–1435. doi: 10.1093/ndt/gfm766. [DOI] [PubMed] [Google Scholar]
- 46.Abedini S, Holme I, Fellström B, Jardine A, Cole E, Maes B, Holdaas H. Cerebrovascular events in renal transplant recipients. Transplantation. 2009;87:112–117. doi: 10.1097/TP.0b013e31818bfce8. [DOI] [PubMed] [Google Scholar]
- 47.Wilcken D, Wilcken B. The natural history of vascular disease in homocystinuria and the effects of treatment. J Inherit Metab Dis. 1997;20:295–300. doi: 10.1023/a:1005373209964. [DOI] [PubMed] [Google Scholar]
- 48.Manns B, Burgess E, Hyndman M, Parsons H, Schaefer J, Scott-Douglas N. Hyperhomocyst(e)inemia and the prevalence of atherosclerotic vascular disease in patients with end-stage renal disease. Am J Kidney Dis. 1999;34:669–677. doi: 10.1016/S0272-6386(99)70392-6. [DOI] [PubMed] [Google Scholar]
- 49.Kelly P, Rosand J, Kistler J, Shih V, Silveira S, Plomaritoglou A, Furie K. Homocysteine, mthfr 677c-->t polymorphism, and risk of ischemic stroke: Results of a meta-analysis. Neurology. 2002;59:529–536. doi: 10.1212/wnl.59.4.529. [DOI] [PubMed] [Google Scholar]
- 50.Bachmann J, Tepel M, Raidt H, Riezler R, Graefe U, Langer K, Zidek W. Hyperhomocysteinemia and the risk for vascular disease in hemodialysis patients. J Am Soc Nephrol. 1995;6:121–125. doi: 10.1681/ASN.V61121. [DOI] [PubMed] [Google Scholar]
- 51.Clarke R, Lewington S, Donald A, Johnston C, Refsum H, Stratton I, Jacques P, Breteler M, Holman R. Underestimation of the importance of homocysteine as a risk factor for cardiovascular disease in epidemiological studies. J Cardiovasc Risk. 2001;8:363–369. doi: 10.1177/174182670100800605. [DOI] [PubMed] [Google Scholar]