Abstract
Background:
Blood vessel mechanics has traditionally been of interest to researchers and clinicians. Changes in mechanical properties of arteries have been associated with various diseases.
Objective:
To provide a comprehensive review directed towards understanding the basic biomechanical properties of cerebral arteries under normal and diseased conditions.
Methods:
Literature review supplemented by personal knowledge.
Results:
The mechanical properties of vascular tissue may depend on several factors including macromolecular volume fraction, molecular orientation, and volume or number of cells such as smooth muscle cells. Mechanical properties of a blood vessel have been characterized using different methods such as in vitro tensile testing, non-invasive ultrasound examination, and mathematical models. Experiments are complicated by the variation in properties and content of materials that make up the vessel wall and more challenging as the size of the vessel of interest decreases. Therapeutic interventions aiming to alter the mechanical response are either pharmaceutical: including calcium channel blockers, angiotensin converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), and β-blockers; or, mechanical interventions such as angioplasty, stent placement, mechanical thrombectomy, or embolization procedures.
Conclusion:
It is apparent from the literature that macromolecular and cellular mechanics of blood vessels are not fully understood. Therefore, further studies are necessary to better understand contribution of these mechanisms to the overall mechanics of the vascular tissue.
Keywords: Vascular disease, arterial wall, viscoelastic properties, compliance, biomechanics
Introduction:
Blood vessel mechanics has traditionally been of interest to researchers and clinicians. Changes in mechanical properties of arteries have been associated with various diseases. Carotid intimal-medial thickness (IMT) can be used as a predictor of coronary heart disease and atherosclerotic disease.1 In a study conducted by Burke et al.,2 average IMT was thicker in patients with cardiovascular disease. Increase in stiffness of carotid arteries is believed to be responsible for a disproportionate increase in systolic and pulse pressure.3 Dijk et al.4 delineated the association of carotid stiffness with higher prevalence of carotid stenosis and stroke. The present review is directed towards understanding the basic biomechanical properties of cerebral arteries under normal and diseased conditions. The review discusses the potential for novel therapeutic strategies that modulate the biomechanical properties of cerebral arteries and therefore prevent or treat cerebrovascular diseases. Investigators have progressively shifted their focus from large vessels such as aorta, to smaller vessels such as coronary and cerebral arteries given the higher rate of diseases such as atherosclerosis and aneurysm observed in these arteries. The nonlinear stress-strain relationship of vascular tissue in response to biaxial or triaxial loading has presented a challenge to biomechanicians. Experiments are complicated by the variation in properties and content of materials that make up the vessel wall and more challenging as the size of the vessel of interest decreases.
The relationship between elastic storage and viscous dissipation of the energy transmitted to vessel wall during systole in cardiac cycle is not fully understood.5 Vessel wall consists of various macromolecules, smooth muscle cells (SMCs), endothelium, and fibroblasts. Correlations between volume fraction of these molecules and the mechanical properties of blood vessels have been developed for several vessels.6–8
Mechanical properties of a blood vessel have been characterized using different methods such as in vitro tensile testing, noninvasive ultrasound examination, and mathematical models. Each method provides some information that can help explain the properties and factors contributing to the mechanics of blood vessels; however, none these methods is ideal. In vitro tensile testing of the tissue determines viscoelastic properties, but by removing the tissue from the body the natural bioreactivity of the cells is affected. Noninvasive ultrasound devices measure compliance but yield a low percent of accuracy and are most often used to investigate more superficial vessels. Mathematical models are purely theoretical and do not account for all parameters observed under physiological conditions.
Macroscopic Structure:
Blood vessels are generally composed of three distinct layers. The inner most layer is the intima, the middle layer is the media, and the outermost layer is the adventitia. The intima consists of a single layer of endothelial cells that are bound to a basement membrane. The intima is separated from the media by an elastic lamella. The media consists of layers of elastic tissue and SMCs. The local hemodynamics dictate the thickness of these layers.9 Incorporated within the adventitia layer are elastic fibers and fibroblast cells.
Molecular Components:
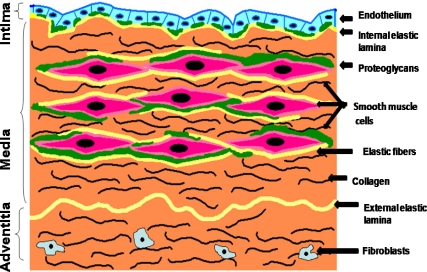
Arteries are composed of collagen fibers, elastin fibers, proteoglycans, smooth muscle, and endothelial cells. Different types of blood vessel have different arrangement and proportion of these components. Generally, single cell layer of endothelial cells in the intima is attached to internal elastic lamina of the medial layer via proteoglycans. Medial layer is mainly comprised of SMCs which are surrounded by proteoglycans, elastin fibers, and collagen fibers. Collagen fibers are predominately present in the adventitial layer (see Fig 1).
Figure 1:
Schematic representation of cross-section of arterial wall, illustrating the intimal, medial and adventitial layers and their molecular components.
Cerebral arteries have a greater smooth muscle concentration and are known to be more muscular in type compared to extracranial arteries. These arteries exhibit thinner media and adventitia layers with slightly thicker internal elastic lamina. They also have defects in the medial layer, which are normal and unique to the cerebral arteries. Cerebral autoregulation is another unique mechanism cerebral arteries posses, by which constant blood flow is maintained to the brain under varying conditions of systemic blood pressure. Autoregulation is maintained through the extensive network of arterioles in the cerebral circulation which control the blood flow into the capillary bed through constriction or dilation under increased or reduced systemic blood pressure. The autoregulatory capacity is able to maintain constant cerebral blood flow as long as mean arterial pressure varies from 50 to 150 mm Hg.
It has been observed that different blood vessels display various orientations of collagenous and elastic tissue structures. In addition, smooth muscle cells also show angular orientation with respect to the circumferential line producing oblique angles in the large vessels and no angular orientation in the smallest vessels. Collagen fibrils also appear to have a spiral or helical twist, which follows the fibrillar wrapping pattern around the vessel diameter.10,11 Type III collagen has been primarily associated with elastic fibers and is rarely found in the intimal layer. Type I collagen fibers are thicker than type III fibers and are found throughout the entire wall. They are also associated with SMC membranes.10 Both elastic and collagenous fibers display fibril patterns which run in radial and longitudinal directions in addition to the main fibril orientation.10,12 Under loading conditions, collagen fibrils show alignment along the direction of the applied load no matter what direction the load is applied in relation to the vessel axis.12
Mechanical Properties of Normal Blood Vessels:
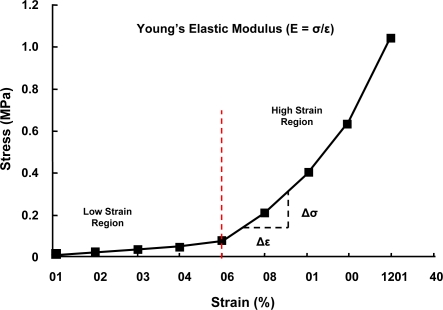
The mechanical properties of vascular tissue may depend on several factors including macromolecular volume fraction, molecular orientation, and volume or number of cells such as SMCs. Although blood vessels have been characterized as having an overall non-linear stress-strain behavior, when examined closely they appear to possess two distinct regions of relatively linear behavior as illustrated in Fig. 2. The region known as the lower modulus region appears to be the mode of operation for blood vessels under physiological pressures.13 The properties of vessel mechanics within this range are consistent with the loading mechanics of elastic fibers.14 As a result, the modulus, or stiffness of the vessel at the lower modulus region is expected to be in the range of 0.2–0.6 MPa. The lower modulus region tends to be linear up to about 50% strain in most vessels at which point the mechanics change to that of the upper modulus region. The upper modulus is also linear, but has a slope steeper than the lower modulus region thus indicating a stiffer response. The modulus of the upper region is measured to be ten folds higher then the lower modulus, about 2–6 MPa. The upper domain appears to be dominated by collagen fibers mechanics, but the mechanics is not consistent with other collagenous tissues.15 Modulus of a collagen molecule is approximately one order of magnitude lower than that calculated for other tissues, even when fibril orientation, polymer fraction, and macroscopic to molecular strain ratio are consistent.16 This seems to imply that collagen is associated with different macromolecules and /or smooth muscle than elastin.
Figure 2:
Stress-Strain curve of a typical artery. Elastin contributes to the mechanical properties of the lower region, while collagen plays a major role in the mechanical properties of the upper region.
The mechanics of blood vessel are considered to be viscoelastic.17 This means that a blood vessel tissue exhibits properties belonging to both viscous liquid and an elastic solid.18 Arteries and veins experience creep and relaxation when placed under load. In addition to time dependence, the responses are dependent on the magnitudes of the loads and strains placed on the tissue. These behaviors are consistent with energy dissipation that may be necessary during vascular overloading, therefore protecting tissues downstream from higher than normal pressures.
One study has shown that the elastic properties of blood vessels at basal blood pressure are due to elastin alone and that viscous properties are due to SMCs.8 Collagen has also been shown to contribute to the viscous properties of vessels.16,19,20 Collagen consists of flexible and rigid regions. When placed under tension, the flexible regions elongate first and contribute to the elastic segment of the stress-strain curve. Once the flexible regions are fully elongated the rigid regions prevent the collagen fibril from elongating further, which results in slippage of collagen fibrils past each other breaking the cross-links between them. It has also been shown that the rate at which the collagen is being stretched affects the slippage of these fibrils and changes the viscous response of the molecule.20 Values obtained for change in enthalpy (ΔH) in digital human flexor tendons have shown similarities with enthalpy changes obtained during creep tests of hyaluronic acid gels. The explanation has been that fibrillar slippage occurs between the fibrils and the ground substance in these tissues and is therefore the likely cause of viscous behavior in connective tissues.21
Other studies have focused on the uncrimping of collagen fibers throughout the expansion of the vessel wall with increased pressure and contribution of these phenomena to mechanical behavior.22,23 In addition, it has also been shown that alignment of collagenous networks causes an increase in elastic modulus.24 Other factors effecting the modulus of the tissue include, proteoglycan concentration and cross-linking.21,25,26 The use of the beta-adrenergic blocker propranolol not only lowered heart rate and blood pressure in propranolol fed aneurysm prone turkeys, it also induced an increase in elastin and collagen lysyl derived cross-links.25 Increasing the number of lysyl cross-links has been associated with an increase in vascular tissue tensile strength.
Viscoelastic behavior of blood vessels have been studied by performing tensile tests on different types of blood vessels.16,27 These studies were performed on human blood vessels and blood vessels from swine and canine. The load under tension at each strain increment was recorded for each vascular tissue. The tissue was allowed to relax at each strain increment to determine the viscous and elastic component of the tissue, with the viscous component measuring the energy dissipated during the relaxation, and the elastic component measuring the energy stored. The tests show that as the strain increases and the curve moves to the upper modulus region, the ratio of the viscous to elastic component increases as well.16 The ratio of the viscous to elastic component increases even more when strain rate is increased in these tests. From these tests it is evident that strain rate has a major effect on the viscous component of the tissue, while the elastic component is not affected by the rate of change. The strain to failure also increased for tissues that experienced higher strain rates. This observation suggests that some conformational changes or crystallization may be occurring at lower strain rates that otherwise does not occur at higher strain rates due insufficient time.
Tensile testing of vascular tissue has shortcomings due to the loss of muscle tone in an in vitro setting. SMCs are believed to be viable only up to two hours after its removal from the living organism, so in vitro testing will not be able to predict the contribution of the SMCs on the mechanical property of the blood vessel. Another factor to consider is the effect the testing techniques on tissue properties. Studies have shown care must be taken in sample preparation which itself may induce changes in the mechanical properties, as has been shown in human patellar tendon.28 Tensile tests require the tissue to be pulled apart while it is gripped by its ends, this can cause some deformation in the tissue structure at the gripping locations, which could results in premature failure. In many instances the tissue fails at the grips instead of the middle of the tissue where necking is occurring as a result of tension.
Compliance is a measure of vessel elasticity. A substantial work already has been carried out attempting to evaluate this parameter noninvasively, using pulse wave velocity,29 analyzing the diastolic pressure decay,30 or determining the stroke volume/pulse pressure index.31 These methods allow for determination of arterial compliance as a function of pressure. Because of the overall nonlinear elastic properties of arterial walls, measurements of compliance can be appropriately compared only if obtained over a range of pressures that elastic properties are linear. In 1991, a new approach was introduced utilizing a high-precision ultrasonic device to measure arterial compliance of peripheral arteries.32 The study confirmed that arterial compliance has a strong nonlinear dependency on intraarterial pressure and therefore has to be defined as a function of pressure.
Measurement of the arterial compliance can reveal mechanical properties such as elasticity of the vessel wall. However, the devices utilized for these measurements have some limitations. Most of these studies are performed on peripheral and surface arteries such as the brachial artery and the common carotid artery. Furthermore, these measurements do not elicit the viscous properties of blood vessels. Since these measurements are made under physiological pressures, the blood vessel is almost purely elastic and fails to reveal any of its unique energy dissipating mechanisms that are activated under higher pressures.
Mechanical Properties of Diseased Blood Vessels:
It is suggested in the literature that hyperplasia and / or hypertrophy, especially observed in hypertensive cases, contribute to changes in vascular mechanics.33–35 Hypertension results in an increase in collagen concentration and an increase in SMC cross-section. Changes in proteoglycan modulation which occur in hypertensive vessels could be a contributing factor to the development of these structural and functional modifications.36 These changes result in failure of the vessel at lower strain, and the region of the lower and the upper modulus subsequently have shorter duration. There may also be increase in the moduli of both the lower and the upper modulus regions. Alterations in the collagen and elastin content, and the effects on the mechanical properties of the blood vessels, have also been documented in the literature.37–41 Various mixtures of type I and type III collagen and elastin have been observed to be altered in different vessels.25,42 Drastic alterations of type III collagen and elastin are associated with several connective tissue diseases involving the vasculature including Ehlers-Danlos (vEDS) and Marfan’s syndromes.10,42 In a study conducted by Boutouyie et al.,43 it was shown that in (vEDS), also known as EDS type IV, an abnormally low intima-media thickness generates a higher wall stress at the site of the elastic artery and may increase the risk of arterial dissection and rupture.
Arterial dissection and aneurysm rupture have been the main focus of several studies in recent years. Calvet et al.44 observed an increased stiffness of the carotid wall in patients with spontaneous cervical artery dissection (sCAD). Arteries of sCAD patients undergo a higher level of circumferential wall stress than those of normal subjects. According to the principles of solid mechanics, the fatiguing effect of cyclic stress is dependent on the number of cycles and the amplitude of stress.45,46 Aging and circumferential wall stress may be considered as practical estimates of number of cycles and the amplitude of stress. Thus, the difference in circumferential wall stress of arteries in sCAD patients may explain the higher risk of dissection of the common carotid arteries.
Saccular (or sidewall) aneurysms have also been studied in humans to determine their viscoelastic properties.27 The tensile strength and viscoelastic parameters were obtained in the circumferential and longitudinal directions in the thick and thin parts of the aneurysm sac. These observations indicate that there are characteristic mechanical deterioration and steric inhomogeneities that accompany the loss of smooth muscle and the arrangement of connective tissue elements in the wall of an intracranial aneurysm.
In lathyrism, animal vessels show no angular orientation of SMCs, a lack of attachment of cells with lamellae, and an increase in collagen concentration.47 As a result, lathyritic animals show increased propensities for aneurysm and dissection formation. It may be concluded then that the mechanical properties of vascular tissues appear to be dependent not only on each component concentration but on how those components are organized.
The diameter and thickness of arterial wall increases with age. Increased amount of collagen and fragmentation of internal elastic membrane are also found.48,49 The age associated changes in structure eventually increases the stiffness of the arterial tree and myocardium, which, in turn result in functional changes. An important contributor to increased vascular and myocardial stiffness is increased collagen cross-linking due to age related formation of advanced glycosylation end-products (AGEs).48–52 Increased formation of AGEs is aggravated by events such as diabetes mellitus and hypertension.50 In addition to excessive cross-linking of collagen and elastin, AGEs may affect properties of different cell types, including endothelial cells and SMCs through interactions with their signal transduction receptors.53,54
Therapeutic Interventions:
Therapeutic interventions are aimed to alter the mechanical response of arteries. Pharmaceutical interventions include calcium channel blockers, angiotensin converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), and β-blockers, which treat hypertension. Mechanical interventions such as angioplasty, stent placement, mechanical thrombectomy, or embolization procedures are used for treatment of diseased arteries.
In patients with essential hypertension, numerous studies have shown a decrease in arterial stiffness with various classes of antihypertensive agents.55–63 Antihypertensive drugs could have both short-terms and long-term effects on arterial wall. The functional effects are both direct and indirect. Vascular smooth muscle relaxation is a direct effect of these drugs, occurring particularly in medium sized muscular arteries. The drugs reduce the stiffness of arteries indirectly by dilation of muscular arteries and decreasing arteriolar tone, which attenuates wave reflections and reduces mean arterial pressure (MAP). There is also growing evidence that these drugs cause structural alterations that may include vascular remodeling as well as changes in the distribution of elastin and collagen in the vessel wall.64
Administration of calcium channel blockers, ACEI, ARB, and β-blockers increases arterial distensibility and decreases pulse wave velocity (PWV) and arterial wave reflections. PWV is used to measure arterial stiffness65–67 and is an excellent predictor of the prognosis of hypertension.68,69 The effects of the drugs on the arterial wall are largely independent of blood pressure reduction. The changes induced by the drugs on the arterial wall vary depending on the type of artery and drug class.
While the strongest evidence for reduction of arterial stiffness is for ACEI, ARB and calcium channel blockers, the effect of β-blockers is less clearly demonstrated. ACEIs are more effective in reducing arterial stiffness than calcium channel blockers. The decrease in arterial IMT, aortic collagen, and persistence of these effects after discontinuation of therapy suggest that ACEI not only have functional effects on the vessel wall but may also promote vascular remodeling and structural changes. ACEI reduces aortic collagen by mechanism of action not involving bradykinin but blockade of angiotensin receptors instead.70 In some studies, ARB was added to therapy in poorly controlled hypertensive patients who were on ACEI among other antihypertensive drugs, and the results showed significant reduction in PWV and arterial wave reflection.63,71 Another study reported that angiotensin receptor genotypes were involved in an age-related increase in aortic stiffness in hypertensive patients.72 A further study reported that ARBs inhibit collagen accumulation in the aorta in an experimental model.70
Stent placement in blood vessels has become a common treatment for stenotic lesions and in some cases for aneurysms. Device failure and reduction in vessel compliance are significant clinical problems.73,74 The source of some failures may be due to the lack of understanding of the mechanics of blood vessels. A few studies have investigated the reduction in arterial compliance which occurs after stent deployment.75–77 Investigators are focusing on the interactions of stents with vessel wall in terms of vascular mechanics.78–80 The force exerted by the stent strains the vessel wall to increase lumen size, hence, after stent deployment the artery functions in a pre-strained state. Another words, the pre-strained artery is functioning at a higher strain region of the stress strain curve instead of the lower strain region normal arteries function at under physiological conditions. This shift causes the arteries to have a higher elastic modulus. In a recent study it was shown that elastic modulus of arteries increased with increased radial force of stent.16
Mathematical Models:
Constitutive equations have been used to model tissue mechanics by using various macromolecular elastic moduli and angular orientation. These model values range from 79.1 MPa up to 2.0 GPa for collagen and from 0.28 MPa to 0.58 MPa for elastin, and collagen angular orientations are between 15 and 20 degrees from the loading axes.8,22,23,81–83 These models have been designed to predict the contribution of mechanical behavior of the individual macromolecules that make up the vessel wall.
Most studies use constitutive equations known as strain energy functions to describe the mechanics of these tissues.84–92 These studies take into account the nonlinear behavior of vascular tissue, collagen fibril waviness, bending in the vascular wall, aging, and disease. The mathematical modeling of strain energy functions is based on continuum mechanics, which rely on Lagrangian interpolation methods to define the function from obtained set of data. The most favored configurations of these functions have been the polynomial and the exponential forms, Equation 1 and 2, respectively.
| (1) |
| (2) |
The symbol W represents the strain energy per unit of mass of the material; ρo is the mass density in the zero-stress state, ρoW is the strain energy per unit volume, ε denotes strain, and A, B, C, D, E, F, G, a, b, c are all constants. Taking the derivatives of these functions and interpolating them, gives the solution of stress of the material being tested. Other investigators have observed the helical nature of the fibrillar collagen and have explored the mechanics of the straightening behavior.22 Alternative solutions of the mechanics of these tissues use composite theory methods.22,82,83,93
Discussion:
All the current models of vessel wall in the literature use collagen moduli one to two orders of magnitude lower than the molecular modulus.94 Using these lower moduli, investigators have been able to accurately predict the mechanical behavior of vascular tissues. However, the published studies have failed to incorporate the higher moduli or discuss why using a lower modulus is sufficient.
After reviewing these studies, it appears that the molecular component contribution to mechanical properties is a complex combination of concentration, orientation, and other factors such as cross-links and fibrillar slippage. Mechanical properties of different arteries have been characterized; however, a universal model of how different components of vessel wall contribute to these properties has not been constructed. Therefore, it is impossible to accurately predict the mechanical response of an artery by merely knowing the components, concentration, and dimensions of the vessel.
Due to their structural differences, cerebral arteries are more prone to developing spasm, atherosclerosis, aneurysms, and other vascular diseases. The mechanism by which these diseases are developed is not well understood. These structural differences alter the mechanical properties of cerebral arteries. Better understanding of these structural disparities can give insight to the development of cerebrovascular diseases.
Studies have shown that SMCs contribute to the viscous response of the vessel wall and help the vessel dissipate energy.8 Cerebral arteries have a greater smooth muscle concentration and are more muscular in type compared to extracranial arteries. It would be premature, however, to assume that the reason cerebral arteries are more prone to aneurysms is due to the higher SMC concentration. Cerebral arteries have not been tested adequately to construct an accurate model of how all the different components of the vessel wall interact with each other. Mechanical testing of cerebral arteries plays a crucial role in understanding the mechanisms that lead to formation of the aneurysms and other arterial disease. Comparison of the mechanical properties derived from these studies with that of extracranial arteries, give insight to the differences in characteristics of the cerebral arteries, which help investigators understand the mechanisms involved.
Therapeutic interventions can be improved with a better understanding of mechanical behavior of arteries. Cardiovascular changes associated with aging and hypertension can be treated with new therapies devoid of antihypertensive effect influencing elastin and collagen function or structure that can reduce stiffness. Collagen cross-link breakers have recently been studied and have shown beneficial cardiovascular and renal effects in aging animals and human being.95
Conclusions:
In summation, the mechanical behavior of blood vessels can be attributed to, type I and type III collagen, elastin, proteoglycan, and SMC concentration and orientation. However, a relationship between collagen and elastin concentration and the high strain elastic modulus in vascular tissue has yielded mixed results with some studies showing an increase in high strain modulus with increasing collagen concentration and other reports showing the contrary. On the other hand, some correlation has been observed with low strain elastic modulus and elastin concentration.81,96 Additionally, viscous properties have been attributed to both extracellular matrix and SMCs.97,98 There also does not appear to be an agreement on the structure and connectivity of the molecular networks in the current models. While one study chose to use elastin smooth muscle series elements in parallel with collagen, most other studies see these three elements in parallel with each other.88 It is apparent from the literature that the macromolecular and cellular mechanics of blood vessels are not fully understood. Therefore, further studies are necessary to reveal the contribution of these mechanisms to the overall mechanics of the vascular tissue.
References:
- 1.O’Leary DH, Polak JF, Kronmal RA, et al. Thickening of the carotid wall. A marker for atherosclerosis in the elderly? Cardiovascular Health Study Collaborative Research Group. Stroke. 1996;27:224–231. doi: 10.1161/01.str.27.2.224. [DOI] [PubMed] [Google Scholar]
- 2.Burke GL, Evans GW, Riley WA, et al. Arterial wall thickness is associated with prevalent cardiovascular disease in middle-aged adults. The Atherosclerosis Risk in Communities (ARIC) Study. Stroke. 1995;26:386–391. doi: 10.1161/01.str.26.3.386. [DOI] [PubMed] [Google Scholar]
- 3.Safar ME, Blacher J, Mourad JJ, London GM. Stiffness of carotid artery wall material and blood pressure in humans: application to antihypertensive therapy and stroke prevention. Stroke. 2000;31:782–790. doi: 10.1161/01.str.31.3.782. [DOI] [PubMed] [Google Scholar]
- 4.Dijk JM, van der Graaf Y, Grobbee DE, Banga JD, Bots ML. Increased arterial stiffness is independently related to cerebrovascular disease and aneurysms of the abdominal aorta: the Second Manifestations of Arterial Disease (SMART) Study. Stroke. 2004;35:1642–1646. doi: 10.1161/01.STR.0000130513.77186.26. [DOI] [PubMed] [Google Scholar]
- 5.Wronski T, Persson PB, Seeliger E, Harnath A, Flemming B. Coupling of left ventricular and aortic volume elasticity in the rabbit. Am J Physiol Regul Integr Comp Physiol. 2000;279:R539–47. doi: 10.1152/ajpregu.2000.279.2.R539. [DOI] [PubMed] [Google Scholar]
- 6.He CM, Roach MR. The composition and mechanical properties of abdominal aortic aneurysms. J Vasc Surg. 1994;20:6–13. doi: 10.1016/0741-5214(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 7.Bank AJ, Wang H, Holte JE, et al. Contribution of collagen, elastin, and smooth muscle to in vivo human brachial artery wall stress and elastic modulus. Circulation. 1996;94:3263–3270. doi: 10.1161/01.cir.94.12.3263. [DOI] [PubMed] [Google Scholar]
- 8.Armentano RL, Barra JG, Levenson J, Simon A, Pichel RH. Arterial wall mechanics in conscious dogs. Assessment of viscous, inertial, and elastic moduli to characterize aortic wall behavior. Circ Res. 1995;76:468–478. doi: 10.1161/01.res.76.3.468. [DOI] [PubMed] [Google Scholar]
- 9.Stehbens WE, Lie JT. Vascular Pathology. New York: Chapman and Hall; 1995. [Google Scholar]
- 10.Rosenquist TH, Modis L. Spatial disorder of collagens in the great vessels, associated with congenital heart defects. Anat Rec. 1991;229:116–124. doi: 10.1002/ar.1092290113. [DOI] [PubMed] [Google Scholar]
- 11.Ishii T, Asuwa N. Spiraled collagen in the major blood vessels. Mod Pathol. 1996;9:843–848. [PubMed] [Google Scholar]
- 12.Bigi A, Ripamonti A, Roveri N. X-ray diffraction and scanning electron microscopy of bovine media aortic wall. Connect Tissue Res. 1977;5:37–39. doi: 10.3109/03008207709152610. [DOI] [PubMed] [Google Scholar]
- 13.Shadwick RE. Mechanical design in arteries. J Exp Biol. 1999;202:3305–3313. doi: 10.1242/jeb.202.23.3305. [DOI] [PubMed] [Google Scholar]
- 14.McCormack PD. Prediction of arterial wall failure under acceleration stress in high-performance aircraft. Aviat Space Environ Med. 1984;55:620–631. [PubMed] [Google Scholar]
- 15.Silver FH, Freeman JW, DeVore D. Viscoelastic properties of human skin and processed dermis. Skin Res Technol. 2001;7:18–23. doi: 10.1034/j.1600-0846.2001.007001018.x. [DOI] [PubMed] [Google Scholar]
- 16.Silver FH, Snowhill PB, Foran DJ. Mechanical behavior of vessel wall: a comparative study of aorta, vena cava, and carotid artery. Ann Biomed Eng. 2003;31:793–803. doi: 10.1114/1.1581287. [DOI] [PubMed] [Google Scholar]
- 17.Nerem RM. Tissue engineering a blood vessel substitute: the role of biomechanics. Yonsei Med J. 2000;41:735–739. doi: 10.3349/ymj.2000.41.6.735. [DOI] [PubMed] [Google Scholar]
- 18.Nichols WW, O’Rurke MF. McDonald’s Blood Flow in Arteries: Theoretical, experimental and Clinical principles. 4th ed. London: Arnold; 1998. p. 564. [Google Scholar]
- 19.Silver FH, Horvath I, Foran DJ. Viscoelasticity of the vessel wall: the role of collagen and elastic fibers. Crit Rev Biomed Eng. 2001;29:279–301. doi: 10.1615/critrevbiomedeng.v29.i3.10. [DOI] [PubMed] [Google Scholar]
- 20.Silver FH, Ebrahimi A, Snowhill PB. Viscoelastic properties of self-assembled type I collagen fibers: molecular basis of elastic and viscous behaviors. Connect Tissue Res. 2002;43:569–580. [PubMed] [Google Scholar]
- 21.Cohen RE, Hooley CJ, McCrum NG. Viscoelastic creep of collagenous tissue. J Biomech. 1976;9:175–184. doi: 10.1016/0021-9290(76)90002-6. [DOI] [PubMed] [Google Scholar]
- 22.Ling SC, Chow CH. The mechanics of corrugated collagen fibrils in arteries. J Biomech. 1977;10:71–77. doi: 10.1016/0021-9290(77)90070-7. [DOI] [PubMed] [Google Scholar]
- 23.Kwan MK, Woo SL. A structural model to describe the nonlinear stress-strain behavior for parallel-fibered collagenous tissues. J Biomech Eng. 1989;111:361–363. doi: 10.1115/1.3168392. [DOI] [PubMed] [Google Scholar]
- 24.Stromberg DD, Wiederhielm CA. Viscoelastic description of a collagenous tissue in simple elongation. J Appl Physiol. 1969;26:857–862. doi: 10.1152/jappl.1969.26.6.857. [DOI] [PubMed] [Google Scholar]
- 25.Boucek RJ, Gunja-Smith Z, Noble NL, Simpson CF. Modulation by propranolol of the lysyl cross-links in aortic elastin and collagen of the aneurysm-prone turkey. Biochem Pharmacol. 1983;32:275–280. doi: 10.1016/0006-2952(83)90555-5. [DOI] [PubMed] [Google Scholar]
- 26.Purslow PP, Wess TJ, Hukins DW. Collagen orientation and molecular spacing during creep and stress-relaxation in soft connective tissues. J Exp Biol. 1998;201:135–142. doi: 10.1242/jeb.201.1.135. [DOI] [PubMed] [Google Scholar]
- 27.Toth M, Nadasy GL, Nyary I, et al. Sterically inhomogenous viscoelastic behavior of human saccular cerebral aneurysms. J Vasc Res. 1998;35:345–355. doi: 10.1159/000025604. [DOI] [PubMed] [Google Scholar]
- 28.Atkinson TS, Ewers BJ, Haut RC. The tensile and stress relaxation responses of human patellar tendon varies with specimen cross-sectional area. J Biomech. 1999;32:907–914. doi: 10.1016/s0021-9290(99)00089-5. [DOI] [PubMed] [Google Scholar]
- 29.Steptoe A, Smulyan H, Gribbin B. Pulse wave velocity and blood pressure change: calibration and applications. Psychophysiology. 1976;13:488–493. doi: 10.1111/j.1469-8986.1976.tb00866.x. [DOI] [PubMed] [Google Scholar]
- 30.Simon AC, Laurent S, Levenson JA, Bouthier JE, Safar ME. Estimation of forearm arterial compliance in normal and hypertensive men from simultaneous pressure and flow measurements in the brachial artery, using a pulsed Doppler device and a first-order arterial model during diastole. Cardiovasc Res. 1983;17:331–338. doi: 10.1093/cvr/17.6.331. [DOI] [PubMed] [Google Scholar]
- 31.Ventura H, Messerli FH, Oigman W, et al. Impaired systemic arterial compliance in borderline hypertension. Am Heart J. 1984;108:132–136. doi: 10.1016/0002-8703(84)90555-6. [DOI] [PubMed] [Google Scholar]
- 32.Perret F, Mooser V, Hayoz D, et al. Evaluation of arterial compliance-pressure curves. Effect of antihypertensive drugs. Hypertension. 1991;18:II77–83. doi: 10.1161/01.hyp.18.4_suppl.ii77. [DOI] [PubMed] [Google Scholar]
- 33.Cox RH. Basis for the altered arterial wall mechanics in the spontaneously hypertensive rat. Hypertension. 1981;3:485–495. doi: 10.1161/01.hyp.3.4.485. [DOI] [PubMed] [Google Scholar]
- 34.Cox RH. Changes in arterial wall properties during development and maintenance of renal hypertension. Am J Physiol. 1982;242:H477–84. doi: 10.1152/ajpheart.1982.242.3.H477. [DOI] [PubMed] [Google Scholar]
- 35.Cox RH. Arterial wall mechanics and composition and the effects of smooth muscle activation. Am J Physiol. 1975;229:807–812. doi: 10.1152/ajplegacy.1975.229.3.807. [DOI] [PubMed] [Google Scholar]
- 36.Castro CM, Cruzado MC, Miatello RM, Risler NR. Proteoglycan production by vascular smooth muscle cells from resistance arteries of hypertensive rats. Hypertension. 1999;34:893–896. doi: 10.1161/01.hyp.34.4.893. [DOI] [PubMed] [Google Scholar]
- 37.Dobrin PB, Schwarcz TH, Mrkvicka R. Longitudinal retractive force in pressurized dog and human arteries. J Surg Res. 1990;48:116–120. doi: 10.1016/0022-4804(90)90202-d. [DOI] [PubMed] [Google Scholar]
- 38.Dobrin PB, Canfield TR. Elastase, collagenase, and the biaxial elastic properties of dog carotid artery. Am J Physiol. 1984;247:H124–31. doi: 10.1152/ajpheart.1984.247.1.H124. [DOI] [PubMed] [Google Scholar]
- 39.Fischer GM, Swain ML, Cherian K. Increased vascular collagen and elastin synthesis in experimental atherosclerosis in the rabbit. Variation in synthesis among major vessels. Atherosclerosis. 1980;35:11–20. doi: 10.1016/0021-9150(80)90023-4. [DOI] [PubMed] [Google Scholar]
- 40.Iwatsuki K, Iijima F, Chiba S. Reduction of blood pressure and vascular collagen in the spontaneously hypertensive rat by elastase. Arch Int Pharmacodyn Ther. 1983;263:63–73. [PubMed] [Google Scholar]
- 41.Minten J, Verheyen A, Cornelissen F, et al. Correlation between mechanical properties and wall composition of the canine superior vena cava. Arch Int Physiol Biochim. 1986;94:349–362. doi: 10.3109/13813458609071435. [DOI] [PubMed] [Google Scholar]
- 42.Dunmore PJ, Roach MR. The effects of age, vessel size, and Ehlers-Danlos type IV syndrome on the waviness index of arteries. Clin Invest Med. 1990;13:67–70. [PubMed] [Google Scholar]
- 43.Boutouyrie P, Germain DP, Fiessinger JN, et al. Increased carotid wall stress in vascular Ehlers-Danlos syndrome. Circulation. 2004;109:1530–1535. doi: 10.1161/01.CIR.0000121741.50315.C2. [DOI] [PubMed] [Google Scholar]
- 44.Calvet D, Boutouyrie P, Touze E, et al. Increased stiffness of the carotid wall material in patients with spontaneous cervical artery dissection. Stroke. 2004;35:2078–2082. doi: 10.1161/01.STR.0000136721.95301.8d. [DOI] [PubMed] [Google Scholar]
- 45.Nichols WW, Edwards DG. Arterial elastance and wave reflection augmentation of systolic blood pressure: deleterious effects and implications for therapy. J Cardiovasc Pharmacol Ther. 2001;6:5–21. doi: 10.1177/107424840100600102. [DOI] [PubMed] [Google Scholar]
- 46.Fung YC, Liu SQ. Elementary mechanics of the endothelium of blood vessels. J Biomech Eng. 1993;115:1–12. doi: 10.1115/1.2895465. [DOI] [PubMed] [Google Scholar]
- 47.Azzi G, Safars M, Viljanen-Tarifa E, Voros E, Robert AM. [Effect of benzquercin on the connective tissue of lathyritic mice. Optic and electron microscopic study] Pathol Biol (Paris) 1995;43:448–460. [PubMed] [Google Scholar]
- 48.Lakatta EG. Cardiovascular aging in health. Clin Geriatr Med. 2000;16:419–444. doi: 10.1016/s0749-0690(05)70021-5. [DOI] [PubMed] [Google Scholar]
- 49.Susic D, Nunez E, Hosoya K, Frohlich ED. Coronary hemodynamics in aging spontaneously hypertensive and normotensive Wistar-Kyoto rats. J Hypertens. 1998;16:231–237. doi: 10.1097/00004872-199816020-00014. [DOI] [PubMed] [Google Scholar]
- 50.Lee AT, Cerami A. Role of glycation in aging. Ann N Y Acad Sci. 1992;663:63–70. doi: 10.1111/j.1749-6632.1992.tb38649.x. [DOI] [PubMed] [Google Scholar]
- 51.Norton GR, Candy G, Woodiwiss AJ. Aminoguanidine prevents the decreased myocardial compliance produced by streptozotocin-induced diabetes mellitus in rats. Circulation. 1996;93:1905–1912. doi: 10.1161/01.cir.93.10.1905. [DOI] [PubMed] [Google Scholar]
- 52.Vasan S, Zhang X, Zhang X, et al. An agent cleaving glucose-derived protein crosslinks in vitro and in vivo. Nature. 1996;382:275–278. doi: 10.1038/382275a0. [DOI] [PubMed] [Google Scholar]
- 53.Vlassara H. The AGE-receptor in the pathogenesis of diabetic complications. Diabetes Metab Res Rev. 2001;17:436–443. doi: 10.1002/dmrr.233. [DOI] [PubMed] [Google Scholar]
- 54.Basta G, Lazzerini G, Massaro M, et al. Advanced glycation end products activate endothelium through signal-transduction receptor RAGE: a mechanism for amplification of inflammatory responses. Circulation. 2002;105:816–822. doi: 10.1161/hc0702.104183. [DOI] [PubMed] [Google Scholar]
- 55.Barenbrock M, Spieker C, Hoeks AP, Zidek W, Rahn KH. Effect of lisinopril and metoprolol on arterial distensibility. Hypertension. 1994;23:I161–3. doi: 10.1161/01.hyp.23.1_suppl.i161. [DOI] [PubMed] [Google Scholar]
- 56.De Cesaris R, Ranieri G, Filitti V, Andriani A. Large artery compliance in essential hypertension. Effects of calcium antagonism and beta-blocking. Am J Hypertens. 1992;5:624–628. doi: 10.1093/ajh/5.9.624. [DOI] [PubMed] [Google Scholar]
- 57.Savolainen A, Keto P, Poutanen VP, et al. Effects of angiotensin-converting enzyme inhibition versus beta-adrenergic blockade on aortic stiffness in essential hypertension. J Cardiovasc Pharmacol. 1996;27:99–104. doi: 10.1097/00005344-199601000-00016. [DOI] [PubMed] [Google Scholar]
- 58.Chen CH, Ting CT, Lin SJ, et al. Different effects of fosinopril and atenolol on wave reflections in hypertensive patients. Hypertension. 1995;25:1034–1041. doi: 10.1161/01.hyp.25.5.1034. [DOI] [PubMed] [Google Scholar]
- 59.Pancera P, Arosio E, Arcaro G, et al. Haemodynamic parameters in hypertensive patients: changes induced by lacidipine and nifedipine. J Hypertens Suppl. 1989;7:S284–5. doi: 10.1097/00004872-198900076-00138. [DOI] [PubMed] [Google Scholar]
- 60.Topouchian J, Asmar R, Sayegh F, et al. Changes in arterial structure and function under trandolapril-verapamil combination in hypertension. Stroke. 1999;30:1056–1064. doi: 10.1161/01.str.30.5.1056. [DOI] [PubMed] [Google Scholar]
- 61.Topouchian J, Brisac AM, Pannier B, et al. Assessment of the acute arterial effects of converting enzyme inhibition in essential hypertension: a double-blind, comparative and crossover study. J Hum Hypertens. 1998;12:181–187. doi: 10.1038/sj.jhh.1000581. [DOI] [PubMed] [Google Scholar]
- 62.Benetos A, Cambien F, Gautier S, et al. Influence of the angiotensin II type 1 receptor gene polymorphism on the effects of perindopril and nitrendipine on arterial stiffness in hypertensive individuals. Hypertension. 1996;28:1081–1084. doi: 10.1161/01.hyp.28.6.1081. [DOI] [PubMed] [Google Scholar]
- 63.Mahmud A, Feely J. Favourable effects on arterial wave reflection and pulse pressure amplification of adding angiotensin II receptor blockade in resistant hypertension. J Hum Hypertens. 2000;14:541–546. doi: 10.1038/sj.jhh.1001053. [DOI] [PubMed] [Google Scholar]
- 64.Mahmud A, Feely J. Antihypertensive drugs and arterial stiffness. Expert Rev Cardiovasc Ther. 2003;1:65–78. doi: 10.1586/14779072.1.1.65. [DOI] [PubMed] [Google Scholar]
- 65.Arnett DK, Evans GW, Riley WA. Arterial stiffness: a new cardiovascular risk factor? Am J Epidemiol. 1994;140:669–682. doi: 10.1093/oxfordjournals.aje.a117315. [DOI] [PubMed] [Google Scholar]
- 66.Lehmann ED. Clinical value of aortic pulse-wave velocity measurement. Lancet. 1999;354:528–529. doi: 10.1016/S0140-6736(99)00179-8. [DOI] [PubMed] [Google Scholar]
- 67.Kubo T, Miyata M, Minagoe S, et al. A simple oscillometric technique for determining new indices of arterial distensibility. Hypertens Res. 2002;25:351–358. doi: 10.1291/hypres.25.351. [DOI] [PubMed] [Google Scholar]
- 68.Blacher J, Asmar R, Djane S, London GM, Safar ME. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension. 1999;33:1111–1117. doi: 10.1161/01.hyp.33.5.1111. [DOI] [PubMed] [Google Scholar]
- 69.Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 70.Benetos A, Levy BI, Lacolley P, et al. Role of angiotensin II and bradykinin on aortic collagen following converting enzyme inhibition in spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol. 1997;17:3196–3201. doi: 10.1161/01.atv.17.11.3196. [DOI] [PubMed] [Google Scholar]
- 71.Klingbeil AU, John S, Schneider MP, et al. AT1-receptor blockade improves augmentation index: a double-blind, randomized, controlled study. J Hypertens. 2002;20:2423–2428. doi: 10.1097/00004872-200212000-00022. [DOI] [PubMed] [Google Scholar]
- 72.Lajemi M, Labat C, Gautier S, et al. Angiotensin II type 1 receptor-153A/G and 1166A/C gene polymorphisms and increase in aortic stiffness with age in hypertensive subjects. J Hypertens. 2001;19:407–413. doi: 10.1097/00004872-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 73.Chan P. Developments in restenosis. J Renin Angiotensin Aldosterone Syst. 2002;3:145–149. doi: 10.3317/jraas.2002.033. [DOI] [PubMed] [Google Scholar]
- 74.Naslund TC, Becker SY. Technical success from endovascular aneurysm repair in the post-marketing era: a multicenter prospective trial. Ann Vasc Surg. 2003;17:35–42. doi: 10.1007/s10016-001-0333-z. [DOI] [PubMed] [Google Scholar]
- 75.Moore J, Jr, Berry JL. Fluid and solid mechanical implications of vascular stenting. Ann Biomed Eng. 2002;30:498–508. doi: 10.1114/1.1458594. [DOI] [PubMed] [Google Scholar]
- 76.Vernhet H, Demaria R, Juan JM, et al. Arterial stenting and overdilation: does it change wall mechanics in small-caliber arteries? J Endovasc Ther. 2002;9:855–862. doi: 10.1177/152660280200900620. [DOI] [PubMed] [Google Scholar]
- 77.Finet G, Weissman NJ, Mintz GS, et al. Mechanism of lumen enlargement with direct stenting versus predilatation stenting: influence of remodelling and plaque characteristics assessed by volumetric intracoronary ultrasound. Heart. 2003;89:84–90. doi: 10.1136/heart.89.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leung DA, Spinosa DJ, Hagspiel KD, Angle JF, Matsumoto AH. Selection of stents for treating iliac arterial occlusive disease. J Vasc Interv Radiol. 2003;14:137–152. doi: 10.1097/01.rvi.0000058316.82956.56. [DOI] [PubMed] [Google Scholar]
- 79.Conti JC, Strope ER. Radial compliance of natural and mock arteries: how this property defines the cyclic loading of deployed vascular stents. Biomed Sci Instrum. 2002;38:163–172. [PubMed] [Google Scholar]
- 80.Squire JC, Rogers C, Edelman ER. Measuring arterial strain induced by endovascular stents. Med Biol Eng Comput. 1999;37:692–698. doi: 10.1007/BF02513369. [DOI] [PubMed] [Google Scholar]
- 81.Cox RH. Passive mechanics and connective tissue composition of canine arteries. Am J Physiol. 1978;234:H533–41. doi: 10.1152/ajpheart.1978.234.5.H533. [DOI] [PubMed] [Google Scholar]
- 82.Ault HK, Hoffman AH. A composite micromechanical model for connective tissues: Part II--Application to rat tail tendon and joint capsule. J Biomech Eng. 1992;114:142–146. doi: 10.1115/1.2895438. [DOI] [PubMed] [Google Scholar]
- 83.Ault HK, Hoffman AH. A composite micromechanical model for connective tissues: Part I--Theory. J Biomech Eng. 1992;114:137–141. doi: 10.1115/1.2895437. [DOI] [PubMed] [Google Scholar]
- 84.Bussy C, Boutouyrie P, Lacolley P, Challande P, Laurent S. Intrinsic stiffness of the carotid arterial wall material in essential hypertensives. Hypertension. 2000;35:1049–1054. doi: 10.1161/01.hyp.35.5.1049. [DOI] [PubMed] [Google Scholar]
- 85.Hudetz AG, Monos E. Characterization of anisotropic elastic properties of the arteries by exponential and polynomial strain energy functions. Acta Physiol Acad Sci Hung. 1981;57:111–122. [PubMed] [Google Scholar]
- 86.Wu SG, Lee GC. On nonlinear viscoelastic properties of arterial tissue. J Biomech Eng. 1984;106:42–47. doi: 10.1115/1.3138455. [DOI] [PubMed] [Google Scholar]
- 87.Tanaka E, Yamada H. Inelastic constitutive modeling for blood vessels based on viscoplasticity. Front Med Biol Eng. 1990;2:177–180. [PubMed] [Google Scholar]
- 88.Wuyts FL, Vanhuyse VJ, Langewouters GJ, et al. Elastic properties of human aortas in relation to age and atherosclerosis: a structural model. Phys Med Biol. 1995;40:1577–1597. doi: 10.1088/0031-9155/40/10/002. [DOI] [PubMed] [Google Scholar]
- 89.Fung YC, Liu SQ. Determination of the mechanical properties of the different layers of blood vessels in vivo. Proc Natl Acad Sci U S A. 1995;92:2169–2173. doi: 10.1073/pnas.92.6.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xie J, Zhou J, Fung YC. Bending of blood vessel wall: stress-strain laws of the intima-media and adventitial layers. J Biomech Eng. 1995;117:136–145. doi: 10.1115/1.2792261. [DOI] [PubMed] [Google Scholar]
- 91.Brossollet LJ, Vito RP. An alternate formulation of blood vessel mechanics and the meaning of the in vivo property. J Biomech. 1995;28:679–687. doi: 10.1016/0021-9290(94)00119-o. [DOI] [PubMed] [Google Scholar]
- 92.Zhou J, Fung YC. The degree of nonlinearity and anisotropy of blood vessel elasticity. Proc Natl Acad Sci U S A. 1997;94:14255–14260. doi: 10.1073/pnas.94.26.14255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gosline JM, Shadwick RE. The mechanical properties of fin whale arteries are explained by novel connective tissue designs. J Exp Biol. 1996;199:985–997. doi: 10.1242/jeb.199.4.985. [DOI] [PubMed] [Google Scholar]
- 94.Sasaki N, Odajima S. Stress-strain curve and Young’s modulus of a collagen molecule as determined by the X-ray diffraction technique. J Biomech. 1996;29:655–658. doi: 10.1016/0021-9290(95)00110-7. [DOI] [PubMed] [Google Scholar]
- 95.Susic D, Varagic J, Ahn J, Frohlich ED. Collagen cross-link breakers: a beginning of a new era in the treatment of cardiovascular changes associated with aging, diabetes, and hypertension. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4:97–101. doi: 10.2174/1568006043481347. [DOI] [PubMed] [Google Scholar]
- 96.Psaila JV, Melhuish J. Viscoelastic properties and collagen content of the long saphenous vein in normal and varicose veins. Br J Surg. 1989;76:37–40. doi: 10.1002/bjs.1800760112. [DOI] [PubMed] [Google Scholar]
- 97.Armentano RL, Graf S, Barra JG, et al. Carotid wall viscosity increase is related to intima-media thickening in hypertensive patients. Hypertension. 1998;31:534–539. doi: 10.1161/01.hyp.31.1.534. [DOI] [PubMed] [Google Scholar]
- 98.Boutouyrie P, Boumaza S, Challande P, Lacolley P, Laurent S. Smooth muscle tone and arterial wall viscosity: an in vivo/in vitro study. Hypertension. 1998;32:360–364. doi: 10.1161/01.hyp.32.2.360. [DOI] [PubMed] [Google Scholar]