Abstract
The skeletal elements of embryonic limb are prefigured by prechondrogenic condensation in which secreted molecules such as adhesion molecules and extracellular matrix have crucial roles. However, how the secreted molecules are controlled to organize the condensation remains unclear. In this study, we examined metabolic regulation of secretion in prechondrogenic condensation, using bioluminescent monitoring systems. We here report on ATP oscillations in the early step of chondrogenesis. The ATP oscillations depended on both glycolysis and mitochondrial respiration, and their synchronization among cells were achieved via gap junctions. In addition, the ATP oscillations were driven by Ca2+ oscillations and led to oscillatory secretion in chondrogenesis. Blockade of the ATP oscillations prevented cellular condensation. Furthermore, the degree of cellular condensation increased with the frequency of ATP oscillations. We conclude that ATP oscillations have a critical role in prechondrogenic condensation by inducing oscillatory secretion.
Keywords: prechondrogenic condensation, ATP oscillations, Ca2+ oscillations, secretion, bioluminescence
Prechondrogenic condensation prefigures skeletal elements of embryonic limbs in the early step of chondrogenesis by the formation of a cartilage template that is subsequently replaced by bone;1 therefore, it has been considered to be the most critical process in skeletal patterning.2 It was known that prechondrogenic condensation results from cellular aggregation and differential mitosis,3 but increased cellular packing in the initiation step of condensation is a consequence of aggregation rather than mitosis.4 Adhesion molecules and extracellular matrix (ECM) have central roles in cellular aggregation by mediating cell–cell adhesion and cell–ECM interactions.4 Thus, the secreted molecules such as adhesion molecules and ECM must be strictly controlled to determine the size and shape of condensations. However, little is known regarding how the secreted molecules are regulated to organize the condensations during chondrogenesis.
Secretion process depends on the intracellular ATP level because ATP is required for multiple steps of secretion process such as secretory vesicle transport, the priming process for membrane fusion, and supply of phosphate group.5, 6 Thus, energy metabolism can coordinate the secreted activity, independent of genetic control. Indeed, it has been known that insulin secretion in pancreatic β-cells is regulated by metabolic oscillations.7, 8 Thus, we postulated that the organizational mechanism for condensation may depend on metabolic regulation.
Bioluminescent reporters have been widely used to monitor the metabolic status, such as intracellular ATP level and oxygen level,8, 9, 10 and moreover is useful for long-term and continuous monitoring because of low toxicity and high biocompatibility.11 Thus, to investigate metabolic regulation of the secreted molecules in chondrogenesis, we established bioluminescent multi-reporter system to simultaneously monitor metabolic and secretory activities during chondrogenesis. In addition, we chose ATDC5 cells as in vitro chondrogenesis model system for study on prechondrogenic condensation because ATDC5 cells differentiate into cartilage nodules via natural condensation process which mimics in vivo prechondrogenic condensation,12 without need of manipulation to artificially enhance the cell-density like micromass cultures.13 Moreover, ATDC5 cells, which undergo chondrogenesis in monolayer culture, are suitable for bioluminescence monitoring because the bioluminescence technique currently has limited ability to quantify the signals in depth of the samples. In addition, some of the findings in ATDC5 cells were verified by the study using micromass culture of mesenchymal stem cells (MSCs). Here, we report for the first time on synchronized ATP oscillations in chondrogenesis. Our study demonstrates the critical role of ATP regulation for prechondrogenic condensation.
Results
ATP oscillations are generated in chondrogenesis
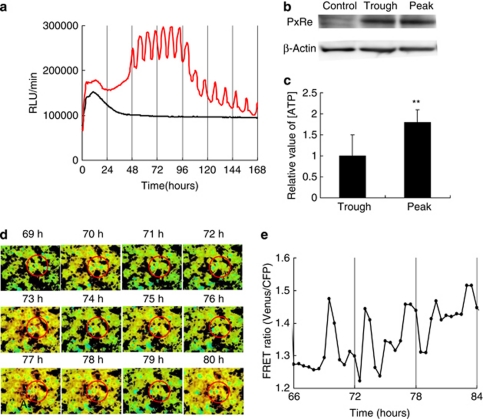
To monitor intracellular ATP level during chondrogenesis, we fused a gene of ATP-dependent Phrixothrix hirtus luciferase emitting red light (PxRe)14 to a constitutive ACTIN promoter (PACTIN-PxRe), and constructed ATDC5 cells transfected with PACTIN-PxRe. Bioluminescence monitoring showed that 2–4 days after chondrogenic induction, PACTIN-PxRe activity began to oscillate every 6–8 h and continued to oscillate for at least 3 days, despite being constant before the induction (Figure 1a). Our result that ATP concentration at the peak of the PACTIN-PxRe oscillations was significantly higher than that at the trough (Figure 1b), while the PxRe protein quantity was constant between the peak and the trough of the oscillations (Figure 1c), indicates that the PACTIN-PxRe oscillations reflect ATP oscillations. Moreover, imaging using a FRET-based ATP sensor15 confirmed that intracellular ATP level oscillates in chondrogenesis (Figures 1d and e; Supplementary Video 1).
Figure 1.
ATP oscillations are generated in chondrogenesis of ATDC5 cells. (a) Bioluminescence monitoring of PACTIN-PxRe activity in ATDC5 cells after changing the maintenance medium (black line) or the insulin-implemented medium (red line). (b) Protein expression levels of PxRe and β-actin at a trough and a peak of PACTIN-PxRe oscillations. Control: untransfected cells. (c) Relative value of ATP concentration at peak points and trough points of PACTIN-PxRe oscillations. Data represents the combined results from two independent experiments performed with six samples. (n=6, paired t test, **P<0.01). (d and e) Monitoring of cytoplasmic ATP level using a FRET-based ATP sensor. (d) Sequential images of the YFP/CFP emission ratio from 69 to 80 h after chondrogenic induction and (e) the time course of the YFP/CFP emission ratio inside the red circle from 66 to 84 h after chondrogenic induction
ATP oscillations are synchronized among cells, depending on gap junctions
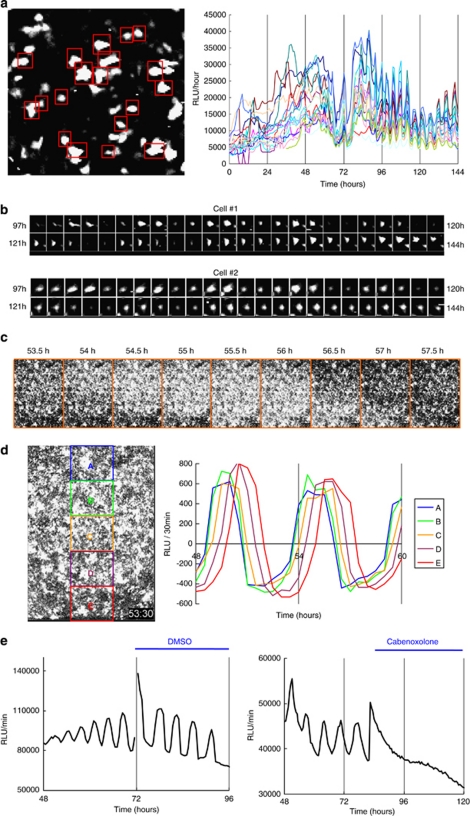
We then examined how ATP oscillations are induced in chondrogenesis. Single-cell imaging showed that PACTIN-PxRe activities in individual cells did not oscillate soon after chondrogenic induction but began to oscillate collectively at about 72 h, and then continued to oscillate robustly for at least 3 days (Figures 2a and b; Supplementary Video 2). This result reveals that the synchronized ATP oscillations resulted not from entrainment of autonomously oscillating cells, but from collective transition of cell populations from quiescence to the oscillations. In addition, low-magnification imaging showed that the PACTIN-PxRe oscillations propagated as waves with a velocity of 10–15 mm/h (Figures 2c and d; Supplementary Video 3). The synchronization of ATP oscillations among cells would be achieved by intercellular communication. We found that inhibition of gap junction by carbenoxolone eliminated the PACTIN-PxRe oscillations (Figure 2e). This result suggests that ATP oscillations are synchronized among cells via gap junctions-mediated intercellular communication.
Figure 2.
ATP oscillations are synchronized via gap junctions among ATDC5 cells in chondrogenesis. (a and b) Bioluminescence imaging at the single-cell level shows that PACTIN-PxRe intensities in individual cells start to oscillate collectively by intercellular synchronization during chondrogenesis. (a) Time course data of PACTIN-PxRe intensities of individual cells indicated by red squares after chondrogenic induction. (b) Sequential images of PACTIN-PxRe intensities in representative cells at 1-h intervals from 97 to 144 h after chondrogenic induction. (c and d) Bioluminescence imaging at a low magnification shows that synchronized oscillations of PACTIN-PxRe intensities propagate as intercellular waves during chondrogenesis. (c) Sequential images of PACTIN-PxRe intensities at 30 min intervals from 53.5 to 57.5 h after chondrogenic induction. (d) Detrended data of time course of PACTIN-PxRe intensities in individual areas indicated by coloured squares from 48 to 60 h after chondrogenic induction. (e) Effect of carbenoxolone treatment on PACTIN-PxRe oscillations during chondrogenesis
ATP oscillations depend on glycolysis and mitochondrial respiration
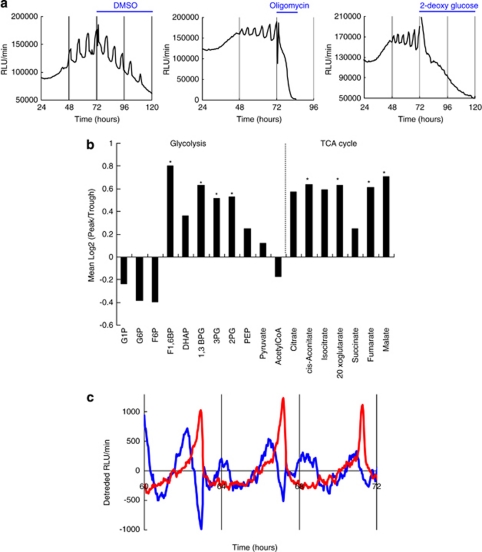
As ATP is produced mainly by glycolysis and oxidative phosphorylation, we investigated whether glycolysis and mitochondrial respiration are involved in ATP oscillations. We found that either glycolysis inhibitor, 2-deoxyglucose (2-DG), or mitochondrial ATPase inhibitor, oligomycin, suppressed PACTIN-PxRe oscillations (Figure 3a), indicating that the ATP oscillations depend on both glycolysis and mitochondrial respiration. We then quantified the concentration of metabolites involved in glycolysis and mitochondrial respiration at the peak and the trough of the PACTIN-PxRe oscillations. The ratio of the metabolite concentration at the peak compared with the concentration at the trough was defined as the peak/trough ratio. In the metabolite analysis, fructose 1,6-bisphosphate (F1,6BP), 1,3-bisphosphoglycerate, 3-phosphoglyceric acid, and 2-phosphoglyceric acid, which are involved in glycolysis; and cis-aconitate, 2-oxoglutaric acid, fumarate, and malate, which are included in mitochondrial respiration, showed significant changes between the peak and the trough of the oscillations (Figure 3b). This result suggests that both glycolysis and mitochondrial respiration may oscillate in chondrogenesis. In addition, the peak/trough ratio showed dramatic differences between F6P and F1,6BP, and between acetyl-CoA and citrate (Figure 3b), suggesting that biochemical reactions that are involved in conversion of F6P to F1,6BP and formation of citrate from acetyl-CoA and oxaloacetate may have key roles in generating the oscillations of glycolysis and mitochondrial respiration.
Figure 3.
ATP oscillations depend on both glycolysis and mitochondrial respiration in chondrogenesis of ATDC5 cells. (a) Treatment effect of 2-DG or oligomycin on PACTIN-PxRe oscillations. (b) Metabolite analysis shows the mean log ratio of concentration of metabolites involved in glycolysis and the TCA cycle in the peaks relative to the troughs of PACTIN-PxRe oscillations. Data represent the combined results from three independent experiments performed with two samples. (n=3, paired t test, *P<0.05). 1,3BPG, 1,3-bisphosphoglycerate; 1,3BPG, 1,3-bisphosphoglycerate; DHAP, dihydroxyacetone phosphate; G1P, glucose 1-phosphate; G6P, glucose 6-phosphate; F1,6BP, fructose 1,6-bisphosphate; F6P, fructose 6-phosphate; 2PG, 2-phosphoglycerate; PEP, phosphoenolpyruvate; 3PG, 3-phosphoglycerate. (c) Simultaneous monitoring of PACTIN-PxRe activity (red line) and PACTIN-ReBl activity (blue line) from 60 to 72 h after chondrogenic induction. The data were detrended by subtracting the 6 h running average from the raw data
To examine whether the metabolic pathways oscillate in chondrogenesis, we simultaneously monitored ATP production and oxygen consumption by co-transfection with both PACTIN-PxRe and a reporter carrying fused gene of oxygen-dependent Renilla luciferase emitting blue light (ReBl)10 to a constitutive ACTIN promoter (PACTIN-ReBl) in a luminometer with a multi-color filter system. As the ReBl substrate coelenterazine, in contrast to the stable PxRe substrate -luciferin, degrades rapidly over time, we established perfusion culture-based monitoring system to continuously supply coelenterazine for long-term bioluminescence monitoring during chondrogenesis. Our result showed that PACTIN-ReBl oscillated with twice the frequency of PACTIN-PxRe oscillations in chondrogenesis (Figure 3c). PACTIN-ReBl activity revealed the troughs at every peak of PACTIN-PxRe oscillations (Figure 3c), indicating that ATP production is coupled to oxygen consumption. This result strongly supports that oxidative phosphorylation in mitochondria oscillates in chondrogenesis. In addition, the PACTIN-ReBl oscillations showed other oscillatory components independent from the PACTIN-PxRe oscillations (Figure 3c), indicating oscillations of non-mitochondrial oxygen consumption in chondrogenesis. It is known that non-mitochondrial oxygen consumption supports glycolysis by recycling cytosolic NADH.16 Thus, the oscillations of non-mitochondrial oxygen consumption support that glycolysis oscillates in chondrogenesis.
ATP oscillations are driven by Ca2+ oscillations and induce oscillatory secretion in chondrogenesis
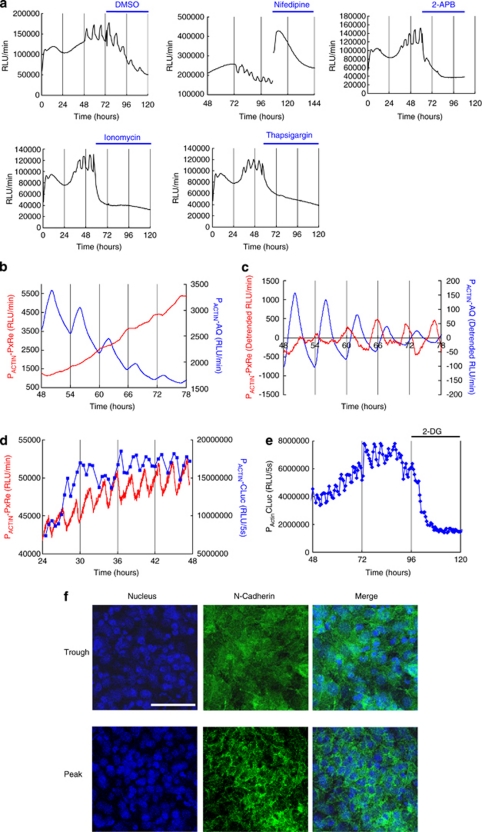
Ca2+ affects both glycolysis and mitochondrial respiration.7 This raises the possibility that Ca2+ is involved in the ATP oscillations. We found that either nifedipine, which blocks Ca2+ influx through a voltage-dependent Ca2+ channel (VDCC), or 2-aminoethoxydiphenyl borate (2-APB), which blocks Ca2+ influx through a store-operated channel (SOC), eliminated the PACTIN-PxRe oscillations (Figure 4a). Thus, the ATP oscillations depend on Ca2+ influx through both VDCC and SOC. In addition, the Ca2+ ionophore ionomycin and the intracellular Ca2+ pump inhibitor thapsigargin, both of which elevate intracellular Ca2+ levels, also eliminated ATP oscillations (Figure 4a). This result suggests that the ATP oscillations depend on Ca2+ dynamics rather than Ca2+ level. Our result, together with the fact that Ca2+ oscillations have been observed in a wide range of cell types,17 prompted us to postulate that Ca2+ oscillations drive ATP oscillations in chondrogenesis. Moreover, this hypothesis is strongly supported by our result that ATP oscillations depend on VDCC and SOC (Figure 4a) because Ca2+ oscillations are known to be driven by activating periodically VDCC through the repetitive membrane depolarizations or modulating release and uptake of Ca2+ at intracellular Ca2+ stores.17 The bioluminescent calcium-activated protein that emit blue light, Aequorin from Aequorea victoria (AqBl), is useful for monitoring Ca2+ level.18 Thus, to test our hypothesis, we monitored simultaneously both ATP and Ca2+ dynamics during chondrogenesis by transfecting both PACTIN-PxRe and the AqBl gene fused to an ACTIN promoter (PACTIN-AqBl) into ATDC5 cells. We found that intracellular Ca2+ level markedly oscillates before the induction of ATP oscillations, and ATP subsequently began to oscillate at the same frequency as Ca2+ oscillations but nearly in anti-phase to the Ca2+ oscillations in chondrogenesis, which is consistent with our hypothesis that Ca2+ oscillations drive ATP oscillations (Figures 4b and c). The anti-phase relationship between Ca2+ oscillations and ATP oscillations suggests that Ca2+ oscillations drive ATP oscillations through negative effects of Ca2+ on ATP level.
Figure 4.
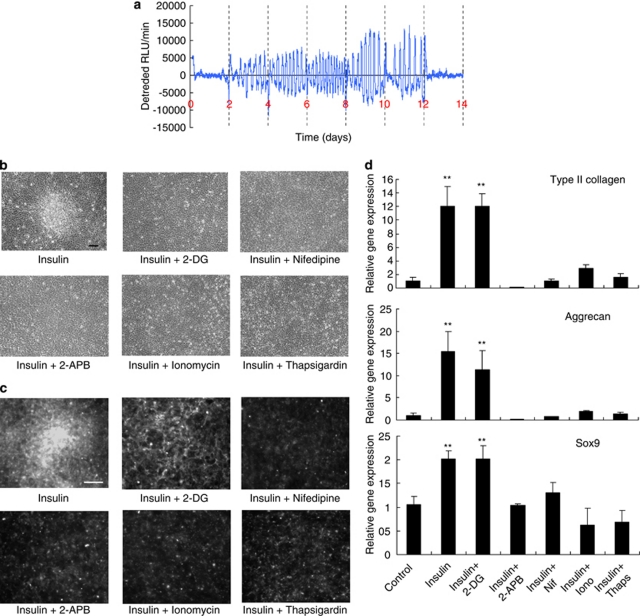
ATP oscillations are driven by Ca2+ oscillations, leading to oscillatory secretion in chondrogenesis of ATDC5 cells. (a) Effects of nifedipine, 2-APB, ionomycin, and thapsigargin treatment on insulin-induced PACTIN-PxRe oscillations. Time course analysis from (b) the raw data and (c) the detrended data taken with simultaneous monitoring of PACTIN-PxRe intensity (red line) and PACTIN-AqBl intensity (blue line) during chondrogenesis. (d) Time course data taken with simultaneous monitoring of PxRe intensity (red line) and secreted CLuc intensity (blue line) during perfusion with insulin-implemented medium. (e) Effect of 2-DG treatment on the oscillatory secretion of CLuc during perfusion with insulin-implemented medium. (f) Immunofluorescent staining of N-cadherin (green) at the peak and the trough of PxRe oscillations. Nuclei are stained blue by Hoechst 33342. Scale bars, 100 μm
Together with ATP, Ca2+ has key roles in the secretion process by promoting secretory vesicle fusion to the cellular membrane5 and reorganizing cytoskeleton acting as a barrier to the vesicles moving toward the membrane.19 Thus, our result that both ATP and Ca2+ oscillate in chondrogenesis suggests the possibility that secretory activity oscillates in chondrogenesis. To examine this possibility, we monitored secretory activity by using a reporter carrying the secreted Cypridina luciferase (CLuc)20 gene fused to ACTIN promoter during PACTIN-PxRe oscillations. We found that CLuc secretion oscillated in nearly same period as PACTIN-PxRe oscillations (Figure 4d), and 2-DG which eliminated the PACTIN-PxRe oscillations (Figure 3b) suppressed the CLuc oscillations (Figure 4e), indicating that ATP oscillations drive the oscillatory secretion in chondrogenesis. This suggests that the secreted molecules such as adhesion molecules can be regulated at the secretion level via the metabolic pathways during chondrogenesis.
It was known that N-cadherin has a key role in cellular condensation during chondrogenesis.21 Thus, we investigated whether ATP oscillations regulate the secretion of N-cadherin during chondrogenesis. We found that N-cadherin was localized at the cellular membranes in a peak phase of ATP oscillations, whereas N-cadherin was diffused in the whole cell in a trough phase (Figure 4f). This result indicates that N-cadherin secretion oscillates during ATP oscillations. This provides evidence that ATP oscillations induce oscillatory secretion of the adhesion molecules during chondrogenesis.
ATP oscillations are crucial for prechondrogenic condensation
To explore link between ATP oscillations and prechondrogenic condensation, we assessed whether the ATP oscillations are induced specifically in the condensation phase of chondrogenesis. Previous studies reported that ATDC5 cells differentiate into cartilage nodules that increase in size for about 2 weeks after chondrogenic induction and then cease to grow upon entering the maturation stages in the culture; the insulin-supplemented medium was changed every other day,12, 22 which was confirmed in our observation (Supplementary Figure 1). Under the same culture conditions, we examined when ATP oscillations start, continue, and stop. PACTIN-PxRe oscillations started 2–4 days after chondrogenic induction, continued, and finally disappeared at 12–14 days (Figure 5a). This result indicates that the ATP oscillations are induced in the condensation phase of chondrogenesis. We then examined how inhibition of ATP oscillations affects chondrogenesis. 2-DG, nifedipine, 2-APB, ionomycin, and thapsigargin, which suppressed the PxRe oscillations (Figures 3a and 4a), prevented the condensation even in the presence of insulin (Figure 5b). Using peanut agglutinin (PNA) that preferentially binds to highly glycosylated proteoglycans, we confirmed that prechondrogenic condensation was suppressed by all of these pharmacological treatments (Figure 5c). However, PNA staining showed that 2-DG treatment induced the formation of widely distributed proteoglycans matrix in the presence of insulin (Figure 5c), indicating that 2-DG treatment suppressed cellular condensation but did not suppress chondrogenic differentiation in the presence of insulin. In agreement with these results, nifedipine, 2-APB, ionomycin, and thapsigargin suppressed gene expression of chondrogenic makers such as type II collagen (COL2A1), aggrecan, and Sox9 in the presence of insulin, whereas 2-DG treatment did not suppress it (Figure 5d). These results suggest that the ATP oscillations are critical for cellular condensation, but are dispensable for chondrogenic differentiation. We found that 2-DG and 2-APB decreased cell proliferation by ∼25%, whereas nifedipine, ionomycin, and thapsigargin had little effect on the cell proliferation (Supplementary Figure 2). This result indicates that the inhibited condensation by the pharmacological treatments may be attributed in large part to suppression of cellular aggregation, not suppression of cellular proliferation. This suggests that blockade of ATP oscillations leads to the suppression of cellular aggregation. Thus, ATP oscillations have a critical role in cellular aggregation during prechondrogenic condensation.
Figure 5.
ATP oscillations have a critical role in prechondrogenic condensation during chondrogenesis of ATDC5 cells. (a) Bioluminescence monitoring of PACTIN-PxRe intensity in the culture; the insulin-supplemented medium was changed every other day for 2 weeks. (b and c) Cellular condensation and chondrogenic differentiation were examined with (b) phase contrast images and (c) staining images with FITC-labeled peanut agglutinin of ATDC5 cells 1 week after culturing in medium implemented with insulin plus 2-DG, nifedipine, 2-APB, ionomycin, and thapsigargin, respectively. Scale bars, 100 μm. (d) Effect of 2-DG, nifedipine (Nif), 2-APB, ionomycin (Iono), and thapsigargin (Thaps) on gene expression of type II collagen (COL2A1), aggrecan, and Sox9 1 week after chondrogenic induction. Data show mean±S.D. (n=4). Dunnett's test, **P<0.01 by ANOVA compared with Control (insulin−)
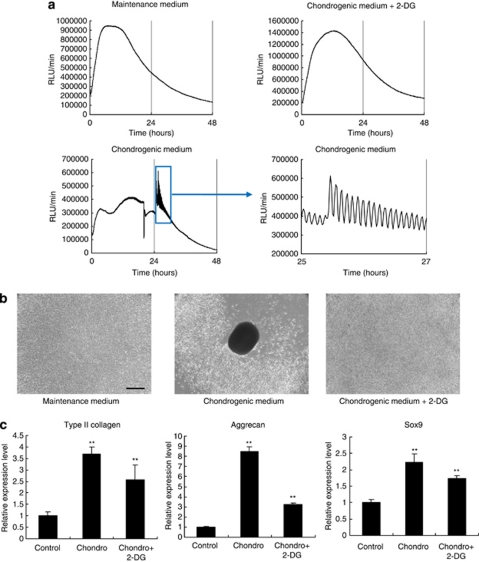
Micromass culture of MSCs is also known as a system to mimic prechondrogenic condensation.23 Thus, we examined whether ATP oscillations were required for prechondrogenic condensation in the micromass culture of primary mouse MSCs (mMSCs). We transfected mMSCs with PACTIN-PxRe and then induced chondrogenesis by incubating the mMSCs with the chondrogenic medium. Bioluminescence monitoring showed that PACTIN-PxRe activity did not oscillate in the maintenance medium, whereas the PACTIN-PxRe activity began to oscillate with a ∼5 min period within 1 day after chondrogenic induction (Figure 6a). In addition, 2-DG suppressed the induction of PACTIN-PxRe oscillations even in the chondrogenic medium. We also found that mMSCs differentiated into chondrocytes with the formation of a cellular condensation within 2 days after chondrogenic induction in the micromass culture with the chondrogenic medium (Figures 6b and c). However, when the micromass of mMSCs was treated with 2-DG in the chondrogenic medium, the cellular condensation was suppressed but chondrogenic differentiation proceeded (Figures 6b and c). These results in mMSCs demonstrate convincingly that ATP oscillations have a critical role in prechondrogenic condensation.
Figure 6.
ATP oscillations have a critical role for prechondrogenic condensation during chondrogenesis in micromass culture of mMSC. (a) Bioluminescence monitoring of PACTIN-PxRe activity in mMSCs after changing the maintenance medium, the chondrogenic medium, or chondrogenic medium implemented with 2-DG. (b) Condensation behaviours of mMSCs were examined with phase contrast images after 2 days of replacement with the maintenance medium, the chondrogenic medium, or chondrogenic medium implemented with 2-DG. Scale bars, 100 μm. (c) Gene expression analysis of type II collagen (COL2A1), aggrecan, and Sox9 in the micromass culture of mMSCs after 2 days of replacement with the maintenance medium, the chondrogenic medium (Chondro), or chondrogenic medium implemented with 2-DG (Chondro + 2-DG). Data show mean±S.D. (n=4). Dunnett's test, **P<0.01 by ANOVA compared with maintenance medium
Degree of prechondrogenic condensation increases with the frequency of ATP oscillations
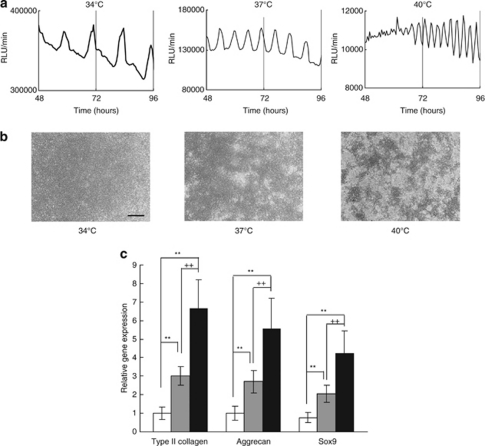
Many evidences have showed that the frequency of biological oscillations encode dynamical information to control cellular activity.24 Thus, we investigated the relationship between the frequency of ATP oscillations and the condensation behaviours. We found that frequency of ATP oscillations strongly depends on temperature from 34 to 40°C (Figure 7a). The average intervals between peaks of ATP oscillations were 10.43±1.37, 6.30±0.30, and 2.88±0.25 h at 34, 37, and 40°C, respectively (Figure 7a). The Q10 value of the oscillation rate calculated from the means was 5.33 (Supplementary Figure 3), which reveals strong temperature dependence. Cellular condensation was suppressed under the low frequency of ATP oscillations at 34°C even in the presence of insulin. In contrast, as the frequency of ATP oscillations increases with temperature rise from 37°C to 40°C, the degree of cellular condensation increases in chondrogenesis (Figure 7b). In addition, chondrogenic differentiation was also promoted with increasing temperature at 34–40°C (Figure 7c), which may be due to the increasing degree of cellular condensation. The positive correlation between the frequency of ATP oscillations and the degree of prechondrogenic condensation corroborates that ATP oscillations are crucial in prechondrogenic condensation. It was reported that mammalian cells show temperature compensation for cell division occurring over a range of temperature from 34 to 40°C.25 In agreement with the previous result, our result showed that temperature change from 34 to 40°C had little effect on proliferation of ATDC5 cells (Supplementary Figure 4). This result suggests that the enhanced condensation degree by the increased temperature may be due to the increased aggregation, not the increased proliferation. Therefore, we suggest that the increasing frequency of ATP oscillations facilitates prechondrogenic condensation by promoting cellular aggregation.
Figure 7.
The degree of cellular condensation increases with the frequency of ATP oscillations. (a) Bioluminescence monitoring of PACTIN-PxRe activities after chondrogenic induction (time=0 h) at 34°C, 37°C, and 40°C. (b) Condensation behaviours observed with phase contrast images at 34°C, 37°C, and 40°C. Scale bars, 500 μm. (c) Temperature effect on gene expression of type II collagen (COL2A1), aggrecan, and Sox9 at 34°C (white bars), 37°C (grey bars), and 40°C (black bars), respectively. Data show mean±S.D. (n=4). Tukey-Kramer test. **P<0.01 by ANOVA compared with 34°C. ++P < 0.01 by ANOVA compared with 37°C
Discussion
By using the newly developed bioluminescent multi-reporter system to monitor simultaneously changes in ATP, oxygen, Ca2+, and secretory activity, we have shown that ATP, oxygen, and Ca2+ oscillate, forming a interlinked oscillatory circuit, and lead to oscillatory secretion in the condensation phase of chondrogenesis. Our result has shown that Ca2+ oscillations drive ATP oscillations through negative effects on ATP level. Ca2+ can directly reduce the ATP level by activating ATP-consuming processes such as ions pumping and exocytosis.26 However, the negative effects of Ca2+ on ATP level may be mediated via glycolysis and mitochondrial respiration. This is supported by dependence of the ATP oscillations on glycolysis and mitochondrial respiration during chondrogenesis. Indeed, it is known that increase in Ca2+ level decreases glucose consumption presumably by inhibitory effect of Ca2+ on glycolytic enzymes27 and decreases mitochondrial ATP production by collapsing mitochondrial membrane potential.19, 28
In other hand, it has been known that conversion of F6P to F1,6BP by phosphofructokinase (PFK) drives glycolytic oscillations thorough its oscillatory activity because of positive feedback of its product, FBP or ADP,8, 29 and that citrate oscillations to originate from inhibition of its own synthesis drive mitochondrial oscillations,30 and citrate can link mitochondrial oscillations with glycolytic oscillations as a inhibitor of PFK activity.31 Thus, independent of Ca2+, PFK oscillatory activity and citrate oscillations can induce glycolytic and mitochondrial oscillations in chondrogenesis. This possibility is supported by our metabolite analysis data to show dramatic differences in the peak/trough ratio between F6P and F1,6BP and in that between acetyl-CoA and citrate. Therefore, we suggest that Ca2+-dependent and Ca2+-independent processes cooperatively lead to metabolic oscillations to underlie ATP oscillations in chondrogenesis. The strong temperature dependence of ATP oscillations can originate from highly temperature-sensitive properties of PFK activity and Ca2+ oscillations to drive the ATP oscillations.32, 33
Our result has shown the collective transition of cell populations from quiescence to synchronized ATP oscillations in chondrogenesis. This transition called a quorum-sensing transition, which is a sudden transition to collective behaviour, has been reported in many bacteria34 and Dictyostelium discoideum.35 It is known that when the concentration of a secreted signalling molecule reaches a threshold, the quorum-sensing transition is induced and encodes cell-density information in the frequency of the oscillations because the threshold is reached more quickly at higher cell densities.34, 35 Thus, although the signal molecules to synchronize ATP oscillations via quorum-sensing-type transition remain elusive, the synchronized ATP oscillations induced could encode information on cell density in the oscillations frequency, which may contribute to controlling the size and the shape of condensations in chondrogenesis. We also showed that synchronization of ATP oscillations is mediated by gap junctions. This is consistent with previous report that metabolic coupling is mediated by gap junctions.36 Although it is known that gap junctions are crucial in prechondrogenic condensation,37 their functions remain unclear. Our study suggests that gap junctions have a key role in prechondrogenic condensations by mediating the synchronized ATP oscillations.
Then, why are the synchronized ATP oscillations critical in prechondrogenic condensation? The synchronized ATP oscillations would induce cell populations to secrete simultaneously high levels of adhesion molecules and ECM in a periodic manner, which is corroborated by the result that N-cadherin secretion showed periodic pattern during the ATP oscillations. The synchronized secretion of the adhesion molecules and ECM can increase the chance of the adhesion molecules and ECM on one cell to encounter the complementary binding sites on another cell (trans-binding), which could promote cellular aggregation by increasing cell–cell adhesion sites and cell–matrix adhesion sites. Furthermore, the synchronized secretion of the adhesion molecules and ECM can promote to organize lateral clusters of adhesion molecules (cis-binding) that strengthen the cell–cell adhesions.38 Therefore, we suggest that the synchronized ATP oscillations have an important role in forming tightly packed condensations by enhancing the efficiency of the trans-binding and the cis-binding. This mechanism provides a reasonable explanation for why the degree of cellular condensation increases with the frequency of ATP oscillations. The increasing frequency of ATP oscillations would facilitate cellular aggregation by increasing the chance of the trans-binding and the cis-binding in this mechanism. Therefore, ATP oscillations may regulate the condensation size by modulating the oscillation frequency. Furthermore, our result that the condensation degree increases with the temperature increase seems to be consistent with previous observations that strong positive correlations exist between ambient temperature and limb size in mammals.39 Although these observations have long been reported, how temperature affects the limb growth remains unclear.40 Our suggestion that highly temperature-dependent ATP oscillations promote prechondrogenic condensation by increasing the oscillations frequency with increasing temperature can explain why limb growth is promoted with increasing temperature.
In addition to the function for prechondrogenic condensation, ATP oscillations can have a crucial role in energy supply for skeletal development because ATP is used as an energy source in cells. It is known that glycolytic oscillations are efficient in terms of maintaining a high ATP/ADP ratio.41 Therefore, the metabolic oscillations to underlie ATP oscillations may be useful for providing enough energy needed for the skeletal development, which has a high energy demand in a low-oxygen environment.42 Our finding of metabolic oscillations in chondrogenesis will provide new insight into molecular mechanisms to underlie skeletal development.
Materials and Methods
Cell culture and light microscopic observation
When ATDC5 cells maintained in Dulbecco's modified Eagle's medium-F12 (DMEM-F12) (Gibco, Tokyo, Japan) supplemented with 5% fetal bovine serum (FBS), 10 μg/ml human transferrin (Roche Molecular Biochemicals, Indianapolis, IN, USA) and 3 × 10−8 M sodium selenite (Sigma-Aldrich, St. Louis, MO, USA) reached confluency, the medium was replaced with the medium supplemented with 10 μg/ml insulin (Sigma-Aldrich), and 10 μg/ml insulin plus one of the following inhibitors: 1.5 mM 2-deoxyglucose (Sigma-Aldrich), 10 mg/ml of oligomycin (Sigma-Aldrich), 0.1 mM ionomycin (Sigma-Aldrich), 25 nM thapsigardin (Sigma-Aldrich), 40 mM nifedipine (Sigma-Aldrich), and 100 mM 2-aminoethoxydiphenyl borate (Calbiochem, San Diego, CA, USA), respectively, and then each medium was replaced every other day for 1 week. mMSCs (Invitrogen, Carlsbad, CA, USA), which are produced from bone marrow isolated from C57BL/6 mice, were maintained in DMEM-F12 with GlutaMAX-I supplemented with 10% MSC-qualified FBS (Invitrogen). For chondrogenesis, mMSCs were cultured in a high-density micromass (8 × 106 cells/ml) with the chondrogenic medium (Invitrogen). Microscopic observation was performed with a microscope (Nikon Eclipse TE300, Nikon, Tokyo, Japan).
Construction of reporter genes and transfection
The human actin promoter (–500/+101, donated by Toyobo, Osaka, Japan) was inserted into multiple cloning sites of vectors containing a Phrixothrix hirtus luciferase gene (Toyobo), Renilla luciferase gene (Promega, Madison, WI, USA), Cypridina luciferase gene (NEB, Ipswich, MA, USA), and Aequorin gene (synthesis from Bioneer (Daejeon, Korea) according to NCBI No. AAA27720), respectively. Then, the promoter region and the luciferase gene were inserted into retrovirus vectors (Clontech, Mountain View, CA, USA), respectively. FRET-based ATP probe gene (donated by Takaharu Nagai, Hokkaido University, Japan) was also inserted into the retrovirus vectors. ATDC5 cells were transfected using retrovirus infection and then were selected by G418 or puromycin. mMSCs were transiently transfected using Lipofectamine LTX (Invitrogen).
ATP concentration measurement
We used an ATP colorimetric assay kit (BioVision, Milpitas, CA, USA), which utilized the phosphorylation of glycerol to generate a product that was quantified using a colorimetric method. We performed two independent experiments with six samples and then analyzed the combined results.
Real-time monitoring using bioluminescent probes
After replacing with the medium including luciferin (0.1 mM) and chemicals, bioluminescence (relative light unit: RLU) was monitored using a dish-type luminescencer, Kronos, for 1 min at 1–30 min intervals. As coelenterazine, the substrate for both ReBl and AqBl, degrades rapidly in cell, simultaneous monitoring of both PxRe and ReBl or both PxRe and AqBl was performed with two peristaltic pumps by perfusing the medium (10 μg/ml insulin, 0.05 mM luciferin, 0.1 μM coelenterazine) at 1.0 ml/h. The intensities from PxRe (R) and from ReBl or AqBl (B) was calculated from the total RLU (F0) and the RLU that passed through a 620-nm long-pass filter (F1), the optical filter's transmission coefficient for ReBl and PxRe (κB and κR, respectively) using the following equation:
 |
Real-time monitoring of CLuc secretion was performed by perfusing the medium (10 μg/ml insulin and 0.05 mM luciferin) from bottles to the fraction collector at a flow rate of 1.0 ml/h, and collecting the fractions every 30 min. The CLuc activity was measured by mixing with Cypridina luciferin (1 μM). To monitor simultaneously CLuc secretion and PxRe activity, the fractions were collected during the monitoring of PxRe activity using Kronos and then the CLuc activity of the fractions was measured in the same way. Bioluminescence imaging was performed using a luminescence microscope, CellGraph (ATTO, Osaka, Japan), at a 1-h interval or at a 30 min interval for 1 week, immediately after being replaced by the medium with 0.3 mM luciferin and 10 μg/ml insulin.
FRET imaging
Wide-eld observations of the cells were performed on an inverted fluorescence microscopy (Keyence, Osaka, Japan) using a PlanApo VC60, 1.40 numerical aperture, oil-immersion objective (Nikon). Dual-emission ratio imaging of FRET-ATP probe was performed by filters: an 427/10 excitation lter, an FF458-Di01 dichroic mirror, and two emission lters (483/32 for CFP and 542/27 for YFP). Fluorescence emission was imaged by using a cooled charge-coupled device camera; the exposure times were 500 ms for CFP and YFP images. Cells were maintained on a microscope at 37 °C with a continuous supply of 95% air and 5% carbon dioxide mixture by using a stage-top incubator (Tokai Hit, Fujinomiya, Japan). Image analysis was performed using Aquacosmos (Hamamatsu Photonics, Hamamatsu, Japan). The YFP/CFP emission ratio was calculated by dividing pixel-by-pixel a YFP image with a CFP image.
Metabolite analysis by using CE-MS system
In each independent experiment, we prepared two samples of cultured ATDC5 cells transfected with PACTIN-PxRe and induced simultaneously the samples to differentiate into chondrocytes at confluence. When the PACTIN-PxRe oscillations reached the trough and the peak after the third or the fourth peak, respectively, we treated the samples for metabolite quantification. The measurement of extracted metabolites was performed by using a capillary electrophoresis (CE)-connected ESI-TOFMS system with electrophoresis buffer (Solution ID H3302-1021, Human Metabolome Technologies Inc., Tsuruoka, Japan). CE-TOFMS was carried out using an Agilent CE Capillary Electrophoresis System equipped with an Agilent 6210 Time of Flight mass spectrometer, Agilent 1100 isocratic HPLC pump, Agilent G1603A CE-MS adapter kit, and Agilent G1607A CE-ESI-MS sprayer kit (Agilent Technologies, Waldbronn, Germany). We performed three independent experiments and then analyzed the combined results.
Immunoblot
The ATDC5 cells at the peak or the trough of PACTIN-PxRe oscillations were prepared by M-PER extraction reagent (Pierce, Rockford, IL, USA). The primary antibodies used were: anti-actin antibody (1 : 5000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-PxRe antibody (1 : 100; donated by Yoshihiro Nakajima, AIST, Osaka, Japan). The primary antibodies were detected using horseradish peroxidase-conjugated anti-rabbit/mouse IgG antibody (1 : 500; Millipore, Billerica, MA, USA). Positive signals were visualized using an enhanced chemiluminescence detection system (Fujitsu, Tokyo, Japan).
Immunofluorescence staining
When PxRe oscillations of ATDC5 cells showed the peak or the trough after 2 day of chondrogenic induction, the cells were fixed with 4% paraformaldehyde for 15 min at room temperature, and then incubated with PBS containing 0.2% Triton X-100 (Sigma-Aldrich) for 15 min. Cells were then washed three times with PBS. After being blocked by 3% BSA in PBS for 60 min at room temperature, cells were incubated with rabbit anti-N-cadherin antibody (1 : 100; Santa Cruz Biotechnology) at 4°C overnight, washed three times with PBS, and then incubated with Alexa488-conjugated secondary antibody (1 : 1000; Invitrogen) for 60 min at room temperature in the dark. Nuclei were stained with Hoechst 33342 (Dojindo, Tokyo, Japan).
PNA staining
Primary cultures were fixed in 4% formalin for 30 min at room temperature, and FITC-labelled PNA (Sigma-Aldrich, 100 μg/ml in PBS) was added to cultures and incubated for 1 h at room temperature. After washing, the distribution of PNA-bound cells was visualized with fluorescence microscopy (Kyence).
qPCR analysis
The total RNA was isolated from the ATDC5 cells cultured under various conditions for 7 days using the RNeasy Mini Kit (Qiagen, Venlo, The Netherlands). The real-time PCR reactions were performed using a thermal cycler dice real time system (Takara Bio, Otsu, Japan). The primer sequences were as follows: collagen type II (COL2A1) forward primer 5′-AGGGCAACAGCAGGTTCACATAC-3′, collagen type II (COL2A1) reverse primer 5′-TGTCCACACCAAATTCCTGTTCA-3′ aggrecan forward primer 5′-AGTGGATCGGTCTGAATGACAGG-3′, aggrecan reverse primer 5′-AGAAGTTGTCAGGCTGGTTTGGA-3′ Sox9 forward primer 5′-CAGTACCCGCATCTGCAC-3′, and Sox9 reverse primer 5′-TCTCTTCTCGCTCTCGTT-3′ GAPDH forward primer 5′-TGTGTCCGTCGTGGATCTGA-3′, and GAPDH reverse primer 5′-TTGCTGTTGAAGTCGCAGGAG-3′.
Acknowledgments
This work was supported financially by the Matching Program for Innovations in Future Drug Discovery and Medical Care in Japan.
Glossary
- PACTIN
actin promoter
- PxRe
Phrixothrix hirtus luciferase emitting red light
- ReBl
Renilla luciferase emitting blue light
- AqBl
Aequorin emitting blue light
- CLuc
Cypridina luciferase
- 2-DG
2-deoxyglucose
- F1,6BP
fructose 1,6-bisphosphate
- VDCC
voltage-dependent Ca2+ channel
- SOC
store-operated channel
- MSCs
mesenchymal stem cells
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by A Verkhratsky
Supplementary Material
References
- Olsen BR, Reginato AM, Wang W. Bone development. Ann Rev Cell Dev Biol. 2000;16:191–220. doi: 10.1146/annurev.cellbio.16.1.191. [DOI] [PubMed] [Google Scholar]
- Mariani FV, Martin GR. Deciphering skeletal patterning: clues from the limb. Nature. 2003;423:319–325. doi: 10.1038/nature01655. [DOI] [PubMed] [Google Scholar]
- Hall BK, Miyake T. All for one and one for all: condensations and the initiation of skeletal development. BioEssays. 2000;22:138–147. doi: 10.1002/(SICI)1521-1878(200002)22:2<138::AID-BIES5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Hall BK, Miyake T. The membranous skeleton: the role of cell condensations in vertebrate skeletogenesis. Anat Embryol. 1992;186:107–124. doi: 10.1007/BF00174948. [DOI] [PubMed] [Google Scholar]
- Martin TFJ. Stages of regulated exocytosis. Trends Cell Biol. 1997;7:271–276. doi: 10.1016/S0962-8924(97)01060-X. [DOI] [PubMed] [Google Scholar]
- Jones PM, Persaud SJ. Protein kinases, protein phosphorylation, and the regulation of insulin secretion from pancreatic β-cells. Endocr Rev. 1998;19:429–461. doi: 10.1210/edrv.19.4.0339. [DOI] [PubMed] [Google Scholar]
- Kennedy RT, Kauri LM, Dahlgren GM, Jung SK. Metabolic oscillations in β-cells. Diabetes. 2002;51:S152–S161. doi: 10.2337/diabetes.51.2007.s152. [DOI] [PubMed] [Google Scholar]
- Ainscow EK, Rutter GA. Glucose-stimulated oscillations in free cytosolic ATP concentration imaged in single islet β-cells: Evidence for a Ca2+-dependent mechanism. Diabetes. 2002;51:S162–S170. doi: 10.2337/diabetes.51.2007.s162. [DOI] [PubMed] [Google Scholar]
- Koop A, Cobbold PH. Continuous bioluminescent monitoring of cytoplasmic ATP in single isolated rat hepatocytes during metabolic poisoning. Biochem J. 1993;295:165–170. doi: 10.1042/bj2950165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen T, Taylor CT, Lam F, Moncada S. Redistribution of intracellular oxygen in hypoxia by nitric oxide: Effect on HIF1α. Science. 2003;302:1975–1978. doi: 10.1126/science.1088805. [DOI] [PubMed] [Google Scholar]
- Song W, Edwin C, Zhen C.Molecular probes for bioluminescence imagingIn: Cheng Z (eds).Current Organic Synthesis Bentham Science Publishers: Oak Park, IL; 2011488–497. [Google Scholar]
- Shukunami C, Shigeno C, Atsumi T, Ishizeki K, Suzuki F, Hiraki Y. Chondrogenic differentiation of clonal mouse embryonic cell line ATDC5 in vitro: differentiation-dependent gene expression of parathyroid hormone (PTH)/PTH-related peptide receptor. J Cell Biol. 1996;133:2457–2468. doi: 10.1083/jcb.133.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrill CP, Archer CW, Wolpert L. Cell sorting and chondrogenic aggregate formation in micromass culture. Dev Biol. 1987;122:503–515. doi: 10.1016/0012-1606(87)90314-9. [DOI] [PubMed] [Google Scholar]
- Viviani VR. The origin, diversity and structure–function relationships of insect luciferases. Cell Mol Life Sci. 2002;59:1833–1850. doi: 10.1007/PL00012509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura H, Nhat KP, Togawa H, Saito K, Iino R, Kato-Yamada Y, et al. Visualization of ATP levels inside single living cells with fluorescence resonance energy transfer-based genetically encoded indicators. Proc Natl Acad Sci USA. 2009;106:15651–15656. doi: 10.1073/pnas.0904764106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herst PM, Tan AS, Scarlett DJ, Berridge MV. Cell surface oxygen consumption by mitochondrial gene knockout cells. Biochim Biophys Acta. 2004;1656:79–87. doi: 10.1016/j.bbabio.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Sneyd J, Keizer J, Sanderson MJ. Mechanisms of calcium oscillations and waves: a quantitative analysis. FASEB. 1995;9:1463–1472. doi: 10.1096/fasebj.9.14.7589988. [DOI] [PubMed] [Google Scholar]
- Kennedy ED, Rizzuto R, Theler JM, Pralong WF, Bastianutto C, Pozzan T, et al. Glucose-stimulated insulin secretion correlates with changes in mitochondrial and cytosolic Ca2+ in aequorin-expressing INS-1 cells. J Clin Invest. 1996;98:2524–2538. doi: 10.1172/JCI119071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmey PA. Phosphoinositides and calcium as regulators of cellular actin asssembly and disassembly. Ann Rev Physiol. 1994;56:169–191. doi: 10.1146/annurev.ph.56.030194.001125. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Kobayashi K, Yamagishi K, Enomoto T, Ohmiya Y. cDNA cloning and characterization of a secreted luciferase from the luminous Japanese ostracod, Cypridina noctiluca. Biosci Biotechnol Biochem. 2004;68:565–570. doi: 10.1271/bbb.68.565. [DOI] [PubMed] [Google Scholar]
- DeLise AM, Tuan RS. Alterations in the spatiotemporal expression pattern and function of N-cadherin inhibit cellular condensation and chondrogenesis of limb mesenchymal cells in vitro. J Cell Biochem. 2002;87:342–359. doi: 10.1002/jcb.10308. [DOI] [PubMed] [Google Scholar]
- Shukunami C, Ishizeki K, Atsumi T, Ohta Y, Suzuki F, Hiraki Y. Cellular hypertrophy and calcification of embryonal carcinoma-derived chondrogenic cell line ATDC5 in vitro. J Bone Mine Res. 1997;12:1174–1188. doi: 10.1359/jbmr.1997.12.8.1174. [DOI] [PubMed] [Google Scholar]
- Scharstuhl A, Schewe B, Benz K, Gaissmaier C, Bühring HJ, Stoop R. Chondrogenic potential of human adult mesenchymal stem cells is independent of age or osteoarthritis etiology. Stem Cells. 2007;25:3244–3251. doi: 10.1634/stemcells.2007-0300. [DOI] [PubMed] [Google Scholar]
- Enzyme kinetics and nonlinear biochemical amplification in response to static and oscillating magnetic fieldsIn: Walleczek J (ed).Self-Organized Biological Dynamics & Nonlinear Control Cambridge University Press: Cambridge; 2000193–216. [Google Scholar]
- Klevecz RR, King GA. Temperature compensation in the mammalian cell cycle. Exp Cell Res. 1982;140:307–313. doi: 10.1016/0014-4827(82)90119-7. [DOI] [PubMed] [Google Scholar]
- Detimary P, Gilon P, Henquin JC. Interplay between cytoplasmic Ca2+ and the ATP/ADP ratio: a feedback control mechanism in mouse pancreatic islets. Biochem J. 1998;333:269–274. doi: 10.1042/bj3330269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SK, Kauri LM, Qian WJ, Kennedy RT. Correlated oscillations in glucose consumption, oxygen consumption, and intracellular free Ca2+in single islets of Langerhans. J Biol Chem. 2000;275:6642–6650. doi: 10.1074/jbc.275.9.6642. [DOI] [PubMed] [Google Scholar]
- Magnus G, Keizer J. Model of β-cell mitochondrial calcium handling and electrical activity. II. Mitochondrial variables. Am J Physiol. 1998;274:C1174–C1184. doi: 10.1152/ajpcell.1998.274.4.C1174. [DOI] [PubMed] [Google Scholar]
- Tornheim K, Lowenstein JM. The purine nucleotide cycle: IV. Interactions with oscillations of the glycolytic pathway in muscle extracts. J Biol Chem. 1974;249:3241–3247. [PubMed] [Google Scholar]
- Williamson JR, Cooper RH. Regulation of the citric acid cycle in mammalian systems. FEBS Lett. 1980;117:K73–K85. doi: 10.1016/0014-5793(80)80572-2. [DOI] [PubMed] [Google Scholar]
- Kemp RG, Gunasekera D. Evolution of the allosteric ligand sites of mammalian phosphofructo-1-kinase. Biochemistry. 2002;41:9426–9430. doi: 10.1021/bi020110d. [DOI] [PubMed] [Google Scholar]
- Mair T, Warnke C, Tsuji K, Müller SS. Control of glycolytic oscillations by temperature. Biophys J. 2005;88:639–646. doi: 10.1529/biophysj.104.043398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J, Sowerby AJ, Finch SA, Fechner M, Stier A. Temperature dependence of Ca2+ wave properties in cardiomyocytes: implications for the mechanism of autocatalytic Ca2+ release in wave propagation. Biophys J. 1995;68:40–45. doi: 10.1016/S0006-3495(95)80196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- Gregor T, Fujimoto K, Masaki N, Sawai S. The onset of collective behaviour in social amoebae. Science. 2010;328:1021–1025. doi: 10.1126/science.1183415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilula NB, Reeves OR, Steinbach A. Metabolic coupling, ionic coupling and cell contacts. Nature. 1972;235:262–265. doi: 10.1038/235262a0. [DOI] [PubMed] [Google Scholar]
- Coelho CND, Kosher RA. Gap junctional communication during limb cartilage differentiation. Dev Biol. 1991;144:47–53. doi: 10.1016/0012-1606(91)90477-k. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Sivasankar S, Nelson WJ, Chu S. Resolving cadherin interactions and binding cooperativity at the single-molecule level. Proc Natl Acad Sci USA. 2009;106:109–114. doi: 10.1073/pnas.0811350106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JA. The influence of physical conditions in the genesis of species. Radic Rev. 1877;1:108–140. [Google Scholar]
- Harland SC. Effect of temperature on growth in weight and tail-length of inbred and hybrid mice. Nature. 1960;186:446. [Google Scholar]
- Termonia Y, Ross J. Oscillations and control features in glycolysis: numerical analysis of a comprehensive model. Proc Natl Acad Sci USA. 1981;78:2952–2956. doi: 10.1073/pnas.78.5.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwoodie SL. The role of hypoxia in development of the mammalian embryo. Dev Cell. 2009;17:755–773. doi: 10.1016/j.devcel.2009.11.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.