Abstract
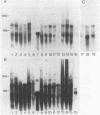
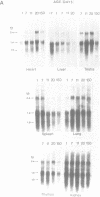
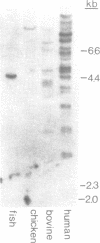
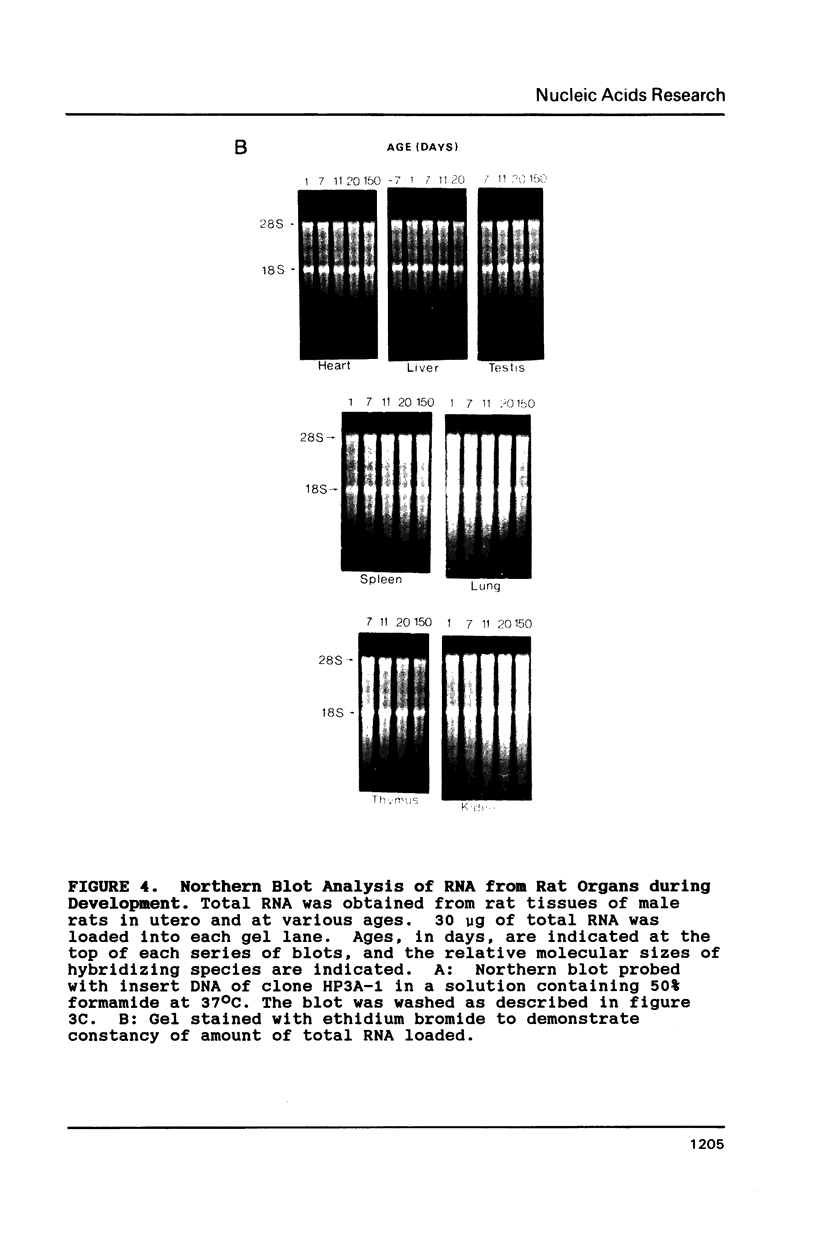
From a human placental lambda gt11 cDNA library, we have isolated a cDNA clone that encodes the entire 215-residue amino acid sequence of HMG-1. Analysis of an internal sequence similarity suggests that the DNA-binding domains of HMG-1 are separated by a rather long and flexible linker segment. Southern blotting of DNA digested with BamHI indicated a highly variable number of genes (or pseudogenes) for HMG-1 in different species. Characterization of HMG-1 mRNA expression by Northern blotting showed that three mRNA species of approximately 1.0, 1.4 and 2.4 kb were expressed in all mammalian organs and cell lines examined. These included several rat organs at different stages of development. Northern analysis also suggested the occurrence of HMG-1 mRNA in an invertebrate and a plant species.
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonne-Andrea C., Harper F., Sobczak J., De Recondo A. M. Rat liver HMG1: a physiological nucleosome assembly factor. EMBO J. 1984 May;3(5):1193–1199. doi: 10.1002/j.1460-2075.1984.tb01950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci L. R., Brock W. A., Goldknopf I. L., Meistrich M. L. Characterization of high mobility group protein levels during spermatogenesis in the rat. J Biol Chem. 1984 Jul 25;259(14):8840–8846. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dodgson J. B., Browne D. L., Black A. J. Chicken chromosomal protein HMG-14 and HMG-17 cDNA clones: isolation, characterization and sequence comparison. Gene. 1988 Mar 31;63(2):287–295. doi: 10.1016/0378-1119(88)90532-x. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C. Anionic regions in nuclear proteins. J Cell Biol. 1987 Oct;105(4):1479–1482. doi: 10.1083/jcb.105.4.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Haggren W., Kolodrubetz D. The Saccharomyces cerevisiae ACP2 gene encodes an essential HMG1-like protein. Mol Cell Biol. 1988 Mar;8(3):1282–1289. doi: 10.1128/mcb.8.3.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isackson P. J., Fishback J. L., Bidney D. L., Reeck G. R. Preferential affinity of high molecular weight high mobility group non-histone chromatin proteins for single-stranded DNA. J Biol Chem. 1979 Jul 10;254(13):5569–5572. [PubMed] [Google Scholar]
- Javaherian K., Liu J. F., Wang J. C. Nonhistone proteins HMG1 and HMG2 change the DNA helical structure. Science. 1978 Mar 24;199(4335):1345–1346. doi: 10.1126/science.628842. [DOI] [PubMed] [Google Scholar]
- Kohlstaedt L. A., King D. S., Cole R. D. Native state of high mobility group chromosomal proteins 1 and 2 is rapidly lost by oxidation of sulfhydryl groups during storage. Biochemistry. 1986 Aug 12;25(16):4562–4565. doi: 10.1021/bi00364a016. [DOI] [PubMed] [Google Scholar]
- Landsman D., Bustin M. Chromosomal proteins HMG-14 and HMG-17. Distinct multigene families coding for similar types of transcripts. J Biol Chem. 1986 Dec 5;261(34):16087–16091. [PubMed] [Google Scholar]
- Landsman D., Srikantha T., Bustin M. Single copy gene for the chicken non-histone chromosomal protein HMG-17. J Biol Chem. 1988 Mar 15;263(8):3917–3923. [PubMed] [Google Scholar]
- Lee K. L., Pentecost B. T., D'Anna J. A., Tobey R. A., Gurley L. R., Dixon G. H. Characterization of cDNA sequences corresponding to three distinct HMG-1 mRNA species in line CHO Chinese hamster cells and cell cycle expression of the HMG-1 gene. Nucleic Acids Res. 1987 Jul 10;15(13):5051–5068. doi: 10.1093/nar/15.13.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan A. D. Tests for comparing related amino-acid sequences. Cytochrome c and cytochrome c 551 . J Mol Biol. 1971 Oct 28;61(2):409–424. doi: 10.1016/0022-2836(71)90390-1. [DOI] [PubMed] [Google Scholar]
- Paonessa G., Frank R., Cortese R. Nucleotide sequence of rat liver HMG1 cDNA. Nucleic Acids Res. 1987 Nov 11;15(21):9077–9077. doi: 10.1093/nar/15.21.9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentecost B. T., Wright J. M., Dixon G. H. Isolation and sequence of cDNA clones coding for a member of the family of high mobility group proteins (HMG-T) in trout and analysis of HMG-T-mRNA's in trout tissues. Nucleic Acids Res. 1985 Jul 11;13(13):4871–4888. doi: 10.1093/nar/13.13.4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentecost B., Dixon G. H. Isolation and partial sequence of bovine cDNA clones for the high-mobility-group protein (HMG-1). Biosci Rep. 1984 Jan;4(1):49–57. doi: 10.1007/BF01120823. [DOI] [PubMed] [Google Scholar]
- Reeck G. R., Isackson P. J., Teller D. C. Domain structure in high molecular weight high mobility group nonhistone chromatin proteins. Nature. 1982 Nov 4;300(5887):76–78. doi: 10.1038/300076a0. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Pescador R., Urdea M. S. Use of unpurified synthetic deoxynucleotide primers for rapid dideoxynucleotide chain termination sequencing. DNA. 1984 Aug;3(4):339–343. doi: 10.1089/dna.1.1984.3.339. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankoff D. Matching sequences under deletion-insertion constraints. Proc Natl Acad Sci U S A. 1972 Jan;69(1):4–6. doi: 10.1073/pnas.69.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigler P. B. Transcriptional activation. Acid blobs and negative noodles. Nature. 1988 May 19;333(6170):210–212. doi: 10.1038/333210a0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda K., Kikuchi M., Mori K., Waga S., Yoshida M. Primary structure of non-histone protein HMG1 revealed by the nucleotide sequence. Biochemistry. 1988 Aug 9;27(16):6159–6163. doi: 10.1021/bi00416a050. [DOI] [PubMed] [Google Scholar]
- Walker J. M., Gooderham K., Hastings J. R., Mayes E., Johns E. W. The primary structures of non-histone chromosomal proteins HMG 1 and 2. FEBS Lett. 1980 Dec 29;122(2):264–270. doi: 10.1016/0014-5793(80)80453-4. [DOI] [PubMed] [Google Scholar]
- Watson D. C., Dixon G. H. Amino acid sequence homologies between the high-mobility-group proteins, HMG-T from trout testis and HMG-1 and -2 from calf thymus: is the poly-aspartic-glutamic acid polypeptide within the main chain? Biosci Rep. 1981 Feb;1(2):167–175. doi: 10.1007/BF01117014. [DOI] [PubMed] [Google Scholar]
- Wen L., Tweten R. K., Isackson P. J., Iandolo J. J., Reeck G. R. Ionic interactions between proteins in nonequilibrium pH gradient electrophoresis: histones affect the migration of high mobility group nonhistone chromatin proteins. Anal Biochem. 1983 Jul 15;132(2):294–304. doi: 10.1016/0003-2697(83)90011-8. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- de Haën C., Swanson E., Teller D. C. The evolutionary origin of proinsulin. Amino acid sequence homology with the trypsin-related serine proteases detected and evaluated by new statistical methods. J Mol Biol. 1976 Sep 25;106(3):639–661. doi: 10.1016/0022-2836(76)90256-4. [DOI] [PubMed] [Google Scholar]