Abstract
The transcription factor p73 is a member of the p53 family that can be expressed as at least 24 different isoforms with pro- or anti-apoptotic attributes. The TAp73 isoforms are expressed from an upstream promoter and are regarded as bona fide tumor suppressors; they can induce cell cycle arrest/apoptosis and protect against genomic instability. On the other hand, ΔNp73 isoforms lack the N-terminus transactivation domain; hence, cannot induce the expression of pro-apoptotic genes, but still can oligomerize with TAp73 or p53 to block their transcriptional activities. Therefore, the ratio of TAp73 isoforms to ΔNp73 isoforms is critical for the quality of the response to a genomic insult and needs to be delicately regulated at both transcriptional and post-translational level. In this review, we will summarize the current knowledge on the post-translational regulatory pathways involved to keep p73 protein under control. A comprehensive understanding of p73 post-translational modifications will be extremely useful for the development of new strategies for treating and preventing cancer.
Keywords: p73, post-translational modification, phosphorylation, acetylation, stability, cancer
Facts
p73 is expressed as multiple isoforms with opposing pro- and anti-apoptotic attributes.
p73 isoforms that contain the transactivation domain (TAp73) can induce cell cycle arrest and apoptosis.
p73 isoforms that lack the transactivation domain (ΔNp73) act as inhibitors of TAp73 and p53 function.
The ratio of pro- and anti-apoptotic p73 species is critical for the response to genomic insult.
Besides its functions in regulation of cell cycle arrest and apoptosis, p73 is a critical regulator of neural stem cell maintenance.
Open Questions
When and where each p73 isoform is expressed at protein level during development and adult life.
Other key molecular pathways that regulate TAp73:ΔNp73 ratio in different cancers.
How can the TAp73:ΔNp73 ratio be modulated for improved targeted therapy of different cancers?
Since its discovery in 1997, p73 became one of the most extensively studied genes, owing to the possibility to compensate for the loss of p53 function because of the remarkable homology between the two proteins.1 Indeed, subsequent research demonstrated that p73 can transactivate many p53 transcriptional targets efficiently and therefore there is substantial redundancy in the pro-apoptotic functions of p53 and p73.2, 3, 4 Therefore, inactivation of the pro-apoptotic functions of p73 is a key mechanism to provide selective advantage in cancers, but, augmentation of p73 activity in response to DNA damage is required to protect cells against tumorigenesis. Interestingly, p73 is rarely mutated in tumors, but elevated p73 levels are observed in several cancers including hepatocellular carcinomas, neuroblastomas, and the cancers of the lung, prostate, colon, breast and ovary.5, 6 This strongly suggests that other regulatory mechanisms that control p73 protein abundance and activity are deregulated in these tumors.
Protein–Protein Interactions
All p53 family members, p53, p63 and p73 are expressed as multiple isoforms.1, 5, 7 Use of alternative promoters (to generate the transcriptionally active TA and dominant negative ΔN isoforms) and extensive alternative splicing produces 24 different p73 isoforms with different abilities to induce or repress apoptosis (Figure 1).8, 9, 10 In addition to this complexity, presence of a polypyrimidine tract-binding protein motif in the second exon of p73 transcript suggests an IRES-dependent translation of another ΔNp73-like protein.11 ΔNp73- and ΔNp73-like proteins exhibit dominant negative activity toward the tumor suppressor functions of TAp73 (and also of p53), mostly via oligomerization, to comprise the transcriptional activity of the active tetramer.12, 13 In accordance with this inhibitory function, ΔNp73 confers chemoresistance in cancer cell lines14, 15 and ΔNp73 over-expression correlates with poor prognosis in primary tumors.12, 16 Other than the inhibitory role of ΔNp73 isoforms, the alternative spliced p73 variants can interact, via the oligomerization domain, to regulate each other's transcriptional activities.1, 8, 17 For example, it has been shown that co-expression of p73ɛ isoform is sufficient to impede p73β isoform-mediated expression of p21WAF1/CIP1.17
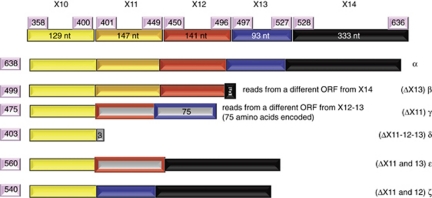
Figure 1.
Schematic representation of the extensive alternative splicing at the 3′ end of p73 transcript. Each exon is represented by a different color and changes in the open reading frame are represented as a frame in the color of the coding exon with grey color filling. For example, the β isoform is generated by splicing out exon 13, but exon 14 is read in a different frame, which results in an immature stop codon. Similarly, γ isoform is generated by splicing out exon 11, but exons 12 and 13 are transcribed from an alternative open-reading frame (ORF)
The significant homology between p53 and p73 (63% at DNA-binding domain, 29% at transactivation domain and 38% at tetramerization domain) initially raised the possibility that these protein can oligomerize and that p73 can potentially interact with other p53-binding proteins. Although both wild-type and mutant p53 were shown to interact with p73 in yeast two-hybrid assays, co-transfection-based experiments in tumor cell lines revealed that only mutant p53 can bind p73.1, 18 This binding resulted in reduced transcriptional activity of p73 and inhibition of ability of p73 to induce apoptosis. However, not all tumors with p73 over-expression harbor mutant p53, suggesting presence of other mechanisms to inhibit p73 activity.19
The other family member p6320, 21 also has key roles in regulation of p73 activity and stability. p63 and p73 share an extra α-helix, which is not present in p53, in their oligomerization domain and therefore can interact efficiently to form stable heterotetramers.22 The outcome of these interactions largely depends on the ratio between the pro- and anti-apoptotic family members. For example, ΔNp63 is over-expressed or amplified in >80% of squamous cell carcinomas where it blocks the transcriptional activity of p73 on pro-apoptotic promoters by possibly forming stable hetero-oligomers.23, 24
The key regulatory mechanism controlling p53 protein abundance and activity involves the ring finger ubiquitin ligase Mdm2.25, 26, 27 Over-expressed Mdm2 protein conveys its inhibitory effect by binding directly to p53 either to inhibit its transcriptional activity or to target it to proteasomal degradation.28, 29 Initially, Mdm2 appeared to be a perfect candidate to modulate p73 activity and stability. Indeed, succeeding work demonstrated that Mdm2 can bind to and inhibit the transcriptional activity of p73.30, 31, 32 However, unlike p53, p73 was stabilized following Mdm2 (and also the Mdm2-related protein Mdmx) binding.30 Similar to p53, p73 can transactivate Mdm2 expression. Therefore, a feedback-regulatory loop also exists in the p73-Mdm2 network, which relies only on the inhibitory function of Mdm2 to block p73-transcriptional activity and inhibit apoptosis, rather than modulating its steady-state levels.
Both p300 and CREB-binding protein (CBP) can interact with p73 and control its transcriptional activity, acting as transcriptional co-activators.33 The interaction between the N-terminal of p73 and CH1 domain of p300/CBP enhances the transcriptional activity of TAp73 isoforms. However, the N-terminal region of p73 is also key to its interaction with Mdm2 and therefore the competition between Mdm2 and p300/CBP for p73 binding is an important determinant of p73 transcriptional activity; that is, over-expression of Mdm2 results in dislocation of p300/CBP from p73 and loss of p73 transcriptional activity.34 Another example of competition-based control of p73 activity involves interaction of p73 with c-myc and MM1 (myc modulator 1). Similar to its influence on p53, c-myc is a potent inhibitor of p73 transcriptional activity.35 This inhibitory effect can be alleviated by co-expression of MM1, which can bind p73 at its C-terminus and prevent c-Myc-p73 interaction.
Other than p300, the most well-defined transcriptional co-activator of p73 is YAP; a WW domain protein that has strong transactivation activity but lacks a DNA-binding domain.36, 37 Expression of p73 together with YAP significantly improves its ability to induce transcription, even at levels where p73 expression alone is not sufficient to activate its target genes, such as Mdm2 and Bax.36 Activity of YAP is strictly controlled by phosphorylation by the pro-survival serine/threonine protein kinase Akt (protein kinase B).38, 39, 40 S127 phosphorylation of YAP by Akt promotes its localization to cytosol, where it can no longer act as a transcriptional co-activator. On the other hand, in response to pro-apoptotic signals, YAP is recruited to nuclear bodies by the promyelocytic leukemia protein (PML) to promote the transcriptional activity of p73. Interestingly, p73 expression is essential for the recruitment of YAP to PML-nuclear bodies following DNA damage as cells lacking p73 fail to do so.41
Interaction of p53 with viral oncoproteins is critical to its apoptotic functions. For example, the adenovirus E1B 55-kDa protein and polyomavirus SV40 T antigen inhibit p53 function by sequestering it in an inactive complex and the human papillomavirus E6 (HPV-E6) protein promotes its ubiquitin-dependent proteasomal degradation.42 Of interest, modulation of p73 activity and stability by viral oncoproteins differs largely from that of p53.32, 43 Although both E1B 55-kDa and SV40 T antigen fail to bind p73, HPV-E6 fails to mediate p73 degradation. However, HPV-E6 can still inactivate p73 by directly interacting with the TA domain and inhibiting its transcriptional activity.
Phosphorylation or acetylation of p73 following interaction with kinases and histone acetyltransferases (HATs) is also essential for the regulation of its activity and stability under normal conditions and, in particular, following DNA damage. These modifications lead to key changes in the portfolio of p73-interacting proteins mostly via altering its sub-cellular localization.
Phosphorylation and Acetylation-mediated Pathways
Accumulation of p53 in response to DNA damage is essential for activation of the response pathways. This is primarily achieved by phosphorylation of p53, which renders it resistant to Mdm2-mediated ubiquitination and enables its interaction with transcriptional co-activators.44 DNA damage-induced p53 phosphorylation is primarily mediated by the activation of serine/threonine kinases ataxia telangiectasia mutant (ATM) and Chk2. Although p73 is also targeted by Chk2 for phosphorylation,45 unlike p53, accumulation of p73 after DNA damage is primarily mediated by the non-receptor tyrosine kinase c-Abl.46, 47, 48, 49, 50 Following a genotoxic insult such as g-irradiation or cisplatin treatment, p73 interacts with c-Abl via its PxxP motif at the C-terminal homo-oligomerization domain and becomes phosphorylated predominantly at Tyr99, and also at Tyr121 and Tyr240.51 Activation of p73 by c-Abl in response to DNA damage is dependent on the presence of an intact mismatch repair system and involves the Mut L homolog-1 (MLH1). HCT116 cells that do not express MLH1 gene fail to activate the c-Abl-p73 pathway in response to cisplatin; a phenotype, which can be rescued by complementation with MLH1 expression.46
c-Abl-mediated p73 phosphorylation can be regarded as an initiator event to regulate a series of other modifications. One key regulatory p73-modification that is dependent on tyrosine phosphorylation is the acetylation of p73 by p300. p53 is the first non-histone protein that is identified as a substrate for HATs.52 Initial research to understand if p73 also serves as a target for lysine acetylation identified that interaction of p73 with the closely related transcriptional coactivator proteins p300 and CBP does not result in acetylation of p73 and that the acetylase-activity defective p300 mutant can still act as a co-activator for p73.53 Interestingly, the same group also showed that unlike full length TAp73α, the C-terminal fragment between amino acids 311–636 can be acetylated in vitro by p300. Indeed, the following year Costanzo et al.54 showed that p300, but not CBP or PCAF, can acetylate p73 only when cells are treated with the DNA-damaging agent doxorubicin. Of interest, although expression of non-acetylatable mutant of p73 failed to transactivate p53AIP to induce apoptosis, it had no effect on induction of p21WAF1/CIP1 expression, suggesting that acetylation is a critical regulatory mechanism to direct p73-mediated response to DNA damage.54 Interestingly, acetylation of p73 by p300 in response to DNA damage is regulated by the tyrosine kinase c-Abl, such that tyrosine99 phosphorylation is a prerequisite for p73 acetylation and fibroblasts from abl−/− mice fail to acetylate p73 following DNA damage.54
Another key kinase that is involved in regulation of p73 is p38.55 Remarkably, threonine phosphorylation of p73 upon DNA damage is also dependent on c-Abl activity. Following DNA damage, JNK/p38 MAPK pathway is activated by c-Abl,56 which is proceeded by phosphorylation of p73 by p38 at threonine residues adjacent to proline to promote its accumulation.
As summarized above, a relatively complicated network of different post-translational modifications merges to control p73 activity/stability and c-Abl lies at the heart of this network to initiate p73 acetylation and phosphorylation. A key regulator of this c-Abl-centered network is the prolyl isomerase PIN1 that specifically recognizes phosphorylated serine/threonine residues followed by proline and induces their substrates to undergo a conformational change. PIN1 binds to threonine-phosphorylated p73 upon DNA damage-induced c-Abl activation and enables its interaction with p300.57 In the absence of PIN1, p300 loses its activity to upregulate p73-dependent Bax expression in response to DNA damage. Intriguingly, interaction of p73 with PIN1 does not exclusively rely on DNA damage as the two proteins can also interact in non-stressed cells, suggesting that p73 is phosphorylated at Pin1consensus sites under normal conditions as well.
Indeed, p73 phoshorylation does not merely depend on DNA damage as it is phosphorylated during cell cycle by the cyclin-dependent kinases CDK2/CDK1 and by PKCδ.58, 59, 60 CDK2/CDK1-dependent p73 phosphorylation is predominantly achieved by interaction of p73 with cyclin A and cyclin B in G2 and M phases of the cell cycle, via its cyclin recognition motifs, and phosphorylation at threonine 86. This hampers the transcriptional activity of p73, possibly to inhibit its growth arrest properties at this key stage of cell cycle. In contrast, PKCδ-mediated phosphorylation of p73 at serine 388 activates the second TA domain of p73 (between amino acids 381–399) to regulate cell cycle progression, in a cell type-specific manner.59 This second TA domain is incapable of activating apoptosis-related genes and is regulated differentially throughout the cell cycle. PKCδ-mediated p73 phosphorylation is also important to augment its apoptotic functions in response to DNA damage. This is mediated by cleavage of PKCδ by caspase-3 to generate the constitutively active PKCδ-CF fragment, which can interact with and phosphorylate p73 at serine 289.61 Of interest, in response to stress, PKCδ is activated by c-Abl as well;62 therefore, serine phosphorylation of p73 by PKCδ is also indirectly regulated by c-Abl.
Modifications Leading to a Change in Subcellular Localization
Once phosphorylated by p38, p73 interacts with PML and consequently localizes to PML-nuclear bodies where it interacts with p300, homeodomain-interacting protein kinase 2 (HIPK2) and YAP, to promote its stability and transcriptional activity.41, 63, 64 Indeed, interaction of p73, YAP and p300 via PML is an important determinant of the selective activation of pro-apoptotic p73 targets in response to DNA damage.41 p73 ubiquitination is also significantly reduced following its interaction with PML and localization to PML-nuclear bodies.63 Apart from p38-mediated phosphorylation, c-Abl-mediated p73 phosphorylation also induces its sub-nuclear redistribution; following which, p73 translocates from the nucleocytoplasmic fraction to the nuclear matrix, potentially to become unavailable to ubiquitin ligases and escape proteasomal degradation.65
Interaction of p73 with the Protein Inhibitor of Activated STAT-1 (PIAS-1) also results in its localization to nuclear matrix and subsequent stabilization.66 However, due to sumo E3 ligase activity of PIAS-1, this interaction also results in sumoylation of p73 at K627 and its transcriptional inactivation.66, 67
Similar to p53, p73 has transcription-independent pro-apoptotic functions during apoptosis.68, 69 The transcription-deficient p73 mutant p73R293H (corresponding to the hotspot p53R273H mutant) can still efficiently induce apoptosis in response to TRAIL, but not etoposide, by a mechanism that involves localization of p73 to mitochondria and interaction with mitochondrial p53.69, 70 Remarkably, like the other family members, p73 is also targeted by caspases during apoptosis and caspase-cleaved p73 fragments localize to mitochondria to augment apoptosis.21, 69, 71
Unlike the two above-mentioned modifications to sub-cellular localization that augment p73 activity, neddylation of p73 by NEDD8 conjugation has an opposite effect.72 Once neddylated, p73 localizes to cytosol and therefore cannot function as a transcription factor. As interaction of p73 with Mdm2 is a prerequisite for its neddylation, only TAp73 isoforms are affected by this modification.
Relocalization of p73 to cytosol can also be induced by its interaction with the WW domain containing oxidoreductase protein Wwox.73 Although this interaction leads to loss of p73 transcriptional activity, its apoptotic activity is partially retained; further supporting a transcription-independent role of p73 in cell death.
Post-translational Modifications by Ubiquitin and Protein Stability
p73 protein stability is predominantly regulated by the ubiquitin-proteasome system.31, 74, 75 The first E3 ubiquitin ligase identified to ubiquitinate p73 and target it to proteasomal degradation is the HECT-domain E3 ubiquitin ligase Itch.76 Itch-mediated p73 degradation is predominantly controlled by two competition-based mechanisms. The first mechanism involves Nedd4-binding protein 1 (N4BP1); a WW-domain protein that can interact with Itch without leading to its ubiquitination.77 N4BP1 competes with p73 for Itch binding and therefore interaction of N4BP1 with Itch inhibits Itch-p73 binding. The other key mechanism is based on binding of YAP to the PPXY motif on p73.78 This motif is also used by Itch to interact with p73, therefore competition of YAP with Itch for the PPXY motif results in inactivation of Itch activity towards p73.
Itch is selective only for the α and β p73 isoforms (containing the PY motif interacting with Itch) of both TAp73 and ΔNp73.76, 79 However, upon genotoxic stress, the TAp73 and ΔNp73 isoforms are differentialy regulated. Interestingly, Dulloo et al.80 identified a selective ΔNp73 degradation pathway. This work indicated that a transcriptional target of TAp73 is potentially responsible for the degradation of ΔNp73 isoforms. Indeed, we recently identified a RING finger E3 ubiquitin ligase PIR2 (p73-Induced Ring finger protein 2, PIR2, also known as IBRDC2/Rnf144b) that is induced by TAp73 and selectively binds, ubiquitinates and degrades the ΔNp73 isoform. PIR2 is the only ubiquitin ligase, identified so far, that has differential specificity over the TAp73 and ΔNp73 isoforms. PIR2 is able to fine-tune the TAp73/ΔNp73 ratio and is critical for the regulation of the response to an apoptotic stimulus.81
Other mechanisms of differential regulation of TAp73 and ΔNp73 stability involves the stress-induced activation of c-jun,82 via YAP,83 and the antizyme 1 system.84
Implications of Post-translational Regulation of p73 Activity in Cancer
Oncogenic transformation of normal cells into cancer cells involves successive genetic changes that confer selective advantages to mediate survival and evade cell death.85 Cell death is initiated either by activation of cell surface receptors upon ligand binding (extrinsic pathway) or by activation of pro-apoptotic members of the Bcl-2 family (intrinsic pathway)86, 87, 88 and both pathways are mediated via sequential activation of specific cysteinyl aspartate proteinases, caspases, to cleave specific substrates after aspartate residues.89, 90, 91 p73, like the other members of the p53 family, has key roles in the regulation of both cell death pathways upon stress.26, 92, 93, 94, 95, 96
Chemoresistance is one of the major challenges in the field of tumor biology.97, 98, 99, 100 In cancers harboring mutant p53, inhibition of TAp73 pro-apoptotic activity is an important mechanism to adopt resistance to chemotherapy. Cancer cells achieve this predominantly by modulating the ratio between the pro- and anti-apoptotic p73 isoforms to escape death. Therefore, besides the differential transcriptional control of TA versus ΔNp73 expression, regulation of their function and stability via post-translational modifications, as summarized above, serves as a prompt and effective way to change this critical balance.
Concluding Remarks
The function of the guardian of the genome, p53, is often compromised in cancers. Due to the high structural and functional homology to p53, regulation of p73 activity or function represents a unique approach for targeted cancer therapy. The TAp73 isoforms can potentially be induced or activated to replace inactive p53 for induction of cell cycle arrest/apoptosis, or to inhibit metastatic mutant p53 function. Despite the similarities in gene structure and function, there are considerable differences in the post-translational control of p53 and p73 function, strongly suggesting that the upstream signals that regulate their post-translational modifications dictate their differential activities during development and malignant transformation. For example, although the E3-ubiquitin ligase mdm2 can mediate degradation of p53, it stabilizes p73, and although YAP binds p73 to augment its transcriptional activity, it cannot bind p53. A summary of p73-interacting proteins and p73 post-translational modifications are shown in Figures 2 and 3. A thorough characterization of molecular modifications of p73 and identification of similarities between the other family members will help to fill-in the missing pieces in the p53-p73 puzzle and lead to identification of better agents for targeted tumor therapy.
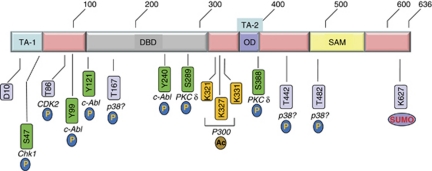
Figure 2.
Schematic representation of key post-translational modifications of p73. TA, transactivation domain; DBD, DNA-binding domain; OD, oligomerization domain; SAM, SAM domain; (P), phosphorylation; (Ac), acetylation; (SUMO), sumoylation
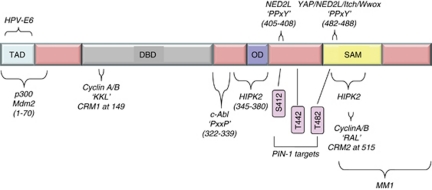
Figure 3.
Summary of p73-interacting proteins that modulate its activity/stability. p73 residues that are essential for interaction are indicated in apostrophes and the location of the domain is indicated in parenthesis. CRM, cyclin recognition motif; MMI, myc modulator 1
Glossary
- TAp73
transcriptionally active p73
- DNp73
dominant negative p73
- mdm2
mouse double minute 2
- CBP
CREB-binding protein
- CREB
cAMP response element-binding
- MM1
myc modulator 1
- YAP
yes-associated protein
- PML
promyelocytic leukemia protein
- HPV
human papilloma virus
- HAT
histone acetyltransferase
- ATM
ataxia telangiectasia mutant
- MLH1
Mut L homolog-1
- CDK
cyclin-dependent kinase
- PKC
protein kinase C
- HIPK2
homeodomain-interacting protein kinase 2
- PIAS-1
protein inhibitor of activated STAT-1
- TRAIL
TNF-related apoptosis-inducing ligand
- Wwox
WW domain containing oxidoreductase
- UPS
ubiquitin-proteasome system
- N4BP1
Nedd4-binding protein 1
- PIR2
p73-induced ring finger protein 2
The authors declare no conflict of interest.
Footnotes
Edited by G Melino
References
- Kaghad M, Bonnet H, Yang A, Creancier L, Biscan JC, Valent A, et al. Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell. 1997;90:809–819. doi: 10.1016/s0092-8674(00)80540-1. [DOI] [PubMed] [Google Scholar]
- Jost CA, Marin MC, Kaelin WG., Jr p73 is a simian [correction of human] p53-related protein that can induce apoptosis. Nature. 1997;389:191–194. doi: 10.1038/38298. [DOI] [PubMed] [Google Scholar]
- Ramadan S, Terrinoni A, Catani MV, Sayan AE, Knight RA, Mueller M, et al. p73 induces apoptosis by different mechanisms. Biochem Biophys Res Commun. 2005;331:713–717. doi: 10.1016/j.bbrc.2005.03.156. [DOI] [PubMed] [Google Scholar]
- Collavin L, Lunardi A, Del Sal G. p53-family proteins and their regulators: hubs and spokes in tumor suppression. Cell Death Differ. 2010;17:901–911. doi: 10.1038/cdd.2010.35. [DOI] [PubMed] [Google Scholar]
- Melino G, De Laurenzi V, Vousden KH. p73: Friend or foe in tumorigenesis. Nat Rev Cancer. 2002;2:605–615. doi: 10.1038/nrc861. [DOI] [PubMed] [Google Scholar]
- Stiewe T, Putzer BM. Role of p73 in malignancy: tumor suppressor or oncogene. Cell Death Differ. 2002;9:237–245. doi: 10.1038/sj.cdd.4400995. [DOI] [PubMed] [Google Scholar]
- Straub WE, Weber TA, Schafer B, Candi E, Durst F, Ou HD, et al. The C-terminus of p63 contains multiple regulatory elements with different functions. Cell Death Dis. 2010;1:e5. doi: 10.1038/cddis.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Laurenzi V, Costanzo A, Barcaroli D, Terrinoni A, Falco M, Annicchiarico-Petruzzelli M, et al. Two new p73 splice variants, gamma and delta, with different transcriptional activity. J Exp Med. 1998;188:1763–1768. doi: 10.1084/jem.188.9.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A, Walker N, Bronson R, Kaghad M, Oosterwegel M, Bonnin J, et al. p73-deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumors. Nature. 2000;404:99–103. doi: 10.1038/35003607. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Cano L, Herreros-Villanueva M, Fernandez-Alonso R, Ayuso-Sacido A, Meyer G, Garcia-Verdugo JM, et al. p73 deficiency results in impaired self renewal and premature neuronal differentiation of mouse neural progenitors independently of p53. Cell Death Dis. 2010;1:e109. doi: 10.1038/cddis.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayan AE, Roperch JP, Sayan BS, Rossi M, Pinkoski MJ, Knight RA, et al. Generation of DeltaTAp73 proteins by translation from a putative internal ribosome entry site. Ann NY Acad Sci. 2007;1095:315–324. doi: 10.1196/annals.1397.035. [DOI] [PubMed] [Google Scholar]
- Zaika AI, Slade N, Erster SH, Sansome C, Joseph TW, Pearl M, et al. DeltaNp73, a dominant-negative inhibitor of wild-type p53 and TAp73, is up-regulated in human tumors. J Exp Med. 2002;196:765–780. doi: 10.1084/jem.20020179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putzer BM, Tuve S, Tannapfel A, Stiewe T. Increased DeltaN-p73 expression in tumors by upregulation of the E2F1-regulated, TA-promoter-derived DeltaN′-p73 transcript. Cell Death Differ. 2003;10:612–614. doi: 10.1038/sj.cdd.4401205. [DOI] [PubMed] [Google Scholar]
- Bergamaschi D, Gasco M, Hiller L, Sullivan A, Syed N, Trigiante G, et al. p53 polymorphism influences response in cancer chemotherapy via modulation of p73-dependent apoptosis. Cancer Cell. 2003;3:387–402. doi: 10.1016/s1535-6108(03)00079-5. [DOI] [PubMed] [Google Scholar]
- Vossio S, Palescandolo E, Pediconi N, Moretti F, Balsano C, Levrero M, et al. DN-p73 is activated after DNA damage in a p53-dependent manner to regulate p53-induced cell cycle arrest. Oncogene. 2002;21:3796–3803. doi: 10.1038/sj.onc.1205465. [DOI] [PubMed] [Google Scholar]
- Muller M, Schilling T, Sayan AE, Kairat A, Lorenz K, Schulze-Bergkamen H, et al. TAp73/Delta Np73 influences apoptotic response, chemosensitivity and prognosis in hepatocellular carcinoma. Cell Death Differ. 2005;12:1564–1577. doi: 10.1038/sj.cdd.4401774. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Hijikata M, Takagi S, Chiba T, Shimotohno K. Transcriptional activities of p73 splicing variants are regulated by inter-variant association. Biochem J. 2001;356 (Part 3:859–866. doi: 10.1042/0264-6021:3560859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Como CJ, Gaiddon C, Prives C. p73 function is inhibited by tumor-derived p53 mutants in mammalian cells. Mol Cell Biol. 1999;19:1438–1449. doi: 10.1128/mcb.19.2.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyoung MP, Ellisen LW. p63 and p73 in human cancer: defining the network. Oncogene. 2007;26:5169–5183. doi: 10.1038/sj.onc.1210337. [DOI] [PubMed] [Google Scholar]
- Mitchell G, Fillinger J, Sittadjody S, Avila J, Burd R, Limesand K. IGF1 activates cell cycle arrest following irradiation by reducing binding of DeltaNp63 to the p21 promoter. Cell Death Dis. 2010;2010:e50. doi: 10.1038/cddis.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayan BS, Sayan AE, Yang AL, Aqeilan RI, Candi E, Cohen GM, et al. Cleavage of the transactivation-inhibitory domain of p63 by caspases enhances apoptosis. Proc Natl Acad Sci USA. 2007;104:10871–10876. doi: 10.1073/pnas.0700761104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutandin D, Lohr F, Niesen FH, Ikeya T, Weber TA, Schafer B, et al. Conformational stability and activity of p73 require a second helix in the tetramerization domain. Cell Death Differ. 2009;16:1582–1589. doi: 10.1038/cdd.2009.139. [DOI] [PubMed] [Google Scholar]
- Rocco JW, Leong CO, Kuperwasser N, DeYoung MP, Ellisen LW. p63 mediates survival in squamous cell carcinoma by suppression of p73-dependent apoptosis. Cancer Cell. 2006;9:45–56. doi: 10.1016/j.ccr.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Barton CE, Johnson KN, Mays DM, Boehnke K, Shyr Y, Boukamp P, et al. Novel p63 target genes involved in paracrine signaling and keratinocyte differentiation. Cell Death Dis. 2010;1:e74. doi: 10.1038/cddis.2010.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis M, Rothweiler F, Barth S, Cinatl J, van Rikxoort M, Loschmann N, et al. Adaptation of cancer cells from different entities to the MDM2 inhibitor nutlin-3 results in the emergence of p53-mutated multi-drug-resistant cancer cells. Cell Death Dis. 2011;2:e243. doi: 10.1038/cddis.2011.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster R, Timmer-Bosscha H, Bischoff R, Gietema JA, de Jong S. Disruption of the MDM2-p53 interaction strongly potentiates p53-dependent apoptosis in cisplatin-resistant human testicular carcinoma cells via the Fas/FasL pathway. Cell Death Dis. 2011;2:e148. doi: 10.1038/cddis.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Chiu SY, Hsu W. SUMO-specific protease 2 in Mdm2-mediated regulation of p53. Cell Death Differ. 2011;18:1005–1015. doi: 10.1038/cdd.2010.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- Momand J, Zambetti GP, Olson DC, George D, Levine AJ. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- Ongkeko WM, Wang XQ, Siu WY, Lau AW, Yamashita K, Harris AL, et al. MDM2 and MDMX bind and stabilize the p53-related protein p73. Curr Biol. 1999;9:829–832. doi: 10.1016/s0960-9822(99)80367-4. [DOI] [PubMed] [Google Scholar]
- Balint E, Bates S, Vousden KH. Mdm2 binds p73 alpha without targeting degradation. Oncogene. 1999;18:3923–3929. doi: 10.1038/sj.onc.1202781. [DOI] [PubMed] [Google Scholar]
- Dobbelstein M, Wienzek S, Konig C, Roth J. Inactivation of the p53-homologue p73 by the mdm2-oncoprotein. Oncogene. 1999;18:2101–2106. doi: 10.1038/sj.onc.1202512. [DOI] [PubMed] [Google Scholar]
- Zeng X, Li X, Miller A, Yuan Z, Yuan W, Kwok RP, et al. The N-terminal domain of p73 interacts with the CH1 domain of p300/CREB binding protein and mediates transcriptional activation and apoptosis. Mol Cell Biol. 2000;20:1299–1310. doi: 10.1128/mcb.20.4.1299-1310.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Chen L, Jost CA, Maya R, Keller D, Wang X, et al. MDM2 suppresses p73 function without promoting p73 degradation. Mol Cell Biol. 1999;19:3257–3266. doi: 10.1128/mcb.19.5.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Ozaki T, Nakagawa T, Miyazaki K, Takahashi M, Hosoda M, et al. Physical interaction of p73 with c-Myc and MM1, a c-Myc-binding protein, and modulation of the p73 function. J Biol Chem. 2002;277:15113–15123. doi: 10.1074/jbc.M111281200. [DOI] [PubMed] [Google Scholar]
- Strano S, Munarriz E, Rossi M, Castagnoli L, Shaul Y, Sacchi A, et al. Physical interaction with Yes-associated protein enhances p73 transcriptional activity. J Biol Chem. 2001;276:15164–15173. doi: 10.1074/jbc.M010484200. [DOI] [PubMed] [Google Scholar]
- Salah Z, Aqeilan RI. WW domain interactions regulate the Hippo tumor suppressor pathway. Cell Death Dis. 2011;2:e172. doi: 10.1038/cddis.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Totty NF, Irwin MS, Sudol M, Downward J. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol Cell. 2003;11:11–23. doi: 10.1016/s1097-2765(02)00776-1. [DOI] [PubMed] [Google Scholar]
- Chu KM, Minogue S, Hsuan JJ, Waugh MG. Differential effects of the phosphatidylinositol 4-kinases, PI4KIIalpha and PI4KIIIbeta, on Akt activation and apoptosis. Cell Death Dis. 2010;1:e106. doi: 10.1038/cddis.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B, Sen T, Asnaghi L, Valapala M, Yang F, Hose S, et al. betaA3/A1-Crystallin controls anoikis-mediated cell death in astrocytes by modulating PI3K/AKT/mTOR and ERK survival pathways through the PKD/Bit1-signaling axis. Cell Death Dis. 2011;2:e217. doi: 10.1038/cddis.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strano S, Monti O, Pediconi N, Baccarini A, Fontemaggi G, Lapi E, et al. The transcriptional coactivator Yes-associated protein drives p73 gene-target specificity in response to DNA Damage. Mol Cell. 2005;18:447–459. doi: 10.1016/j.molcel.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- Marin MC, Jost CA, Irwin MS, DeCaprio JA, Caput D, Kaelin WG., Jr Viral oncoproteins discriminate between p53 and the p53 homolog p73. Mol Cell Biol. 1998;18:6316–6324. doi: 10.1128/mcb.18.11.6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo F, Wahl GM. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat Rev Cancer. 2006;6:909–923. doi: 10.1038/nrc2012. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Prives C, Cordon-Cardo C. p73alpha regulation by Chk1 in response to DNA damage. Mol Cell Biol. 2003;23:8161–8171. doi: 10.1128/MCB.23.22.8161-8171.2003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gong JG, Costanzo A, Yang HQ, Melino G, Kaelin WG, Jr, Levrero M, et al. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature. 1999;399:806–809. doi: 10.1038/21690. [DOI] [PubMed] [Google Scholar]
- Yuan ZM, Shioya H, Ishiko T, Sun X, Gu J, Huang YY, et al. p73 is regulated by tyrosine kinase c-Abl in the apoptotic response to DNA damage. Nature. 1999;399:814–817. doi: 10.1038/21704. [DOI] [PubMed] [Google Scholar]
- Agami R, Blandino G, Oren M, Shaul Y. Interaction of c-Abl and p73alpha and their collaboration to induce apoptosis. Nature. 1999;399:809–813. doi: 10.1038/21697. [DOI] [PubMed] [Google Scholar]
- Meltser V, Ben-Yehoyada M, Reuven N, Shaul Y. c-Abl downregulates the slow phase of double-strand break repair. Cell Death Dis. 2010;1:e20. doi: 10.1038/cddis.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zeng L, Wang J, Chau JF, Lai KP, Jia D, et al. A positive role for c-Abl in Atm and Atr activation in DNA damage response. Cell Death Differ. 2011;18:5–15. doi: 10.1038/cdd.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai KK, Yuan ZM. c-Abl stabilizes p73 by a phosphorylation-augmented interaction. Cancer Res. 2003;63:3418–3424. [PubMed] [Google Scholar]
- Gu W, Roeder RG. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- Zeng X, Lee H, Zhang Q, Lu H. p300 does not require its acetylase activity to stimulate p73 function. J Biol Chem. 2001;276:48–52. doi: 10.1074/jbc.C000722200. [DOI] [PubMed] [Google Scholar]
- Costanzo A, Merlo P, Pediconi N, Fulco M, Sartorelli V, Cole PA, et al. DNA damage-dependent acetylation of p73 dictates the selective activation of apoptotic target genes. Mol Cell. 2002;9:175–186. doi: 10.1016/s1097-2765(02)00431-8. [DOI] [PubMed] [Google Scholar]
- Sanchez-Prieto R, Sanchez-Arevalo VJ, Servitja JM, Gutkind JS. Regulation of p73 by c-Abl through the p38 MAP kinase pathway. Oncogene. 2002;21:974–979. doi: 10.1038/sj.onc.1205134. [DOI] [PubMed] [Google Scholar]
- Cong F, Goff SP. c-Abl-induced apoptosis, but not cell cycle arrest, requires mitogen-activated protein kinase kinase 6 activation. Proc Natl Acad Sci USA. 1999;96:13819–13824. doi: 10.1073/pnas.96.24.13819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani F, Piazza S, Gostissa M, Strano S, Zacchi P, Mantovani R, et al. Pin1 links the activities of c-Abl and p300 in regulating p73 function. Mol Cell. 2004;14:625–636. doi: 10.1016/j.molcel.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Gaiddon C, Lokshin M, Gross I, Levasseur D, Taya Y, Loeffler JP, et al. Cyclin-dependent kinases phosphorylate p73 at threonine 86 in a cell cycle-dependent manner and negatively regulate p73. J Biol Chem. 2003;278:27421–27431. doi: 10.1074/jbc.M300251200. [DOI] [PubMed] [Google Scholar]
- Nyman U, Vlachos P, Cascante A, Hermanson O, Zhivotovsky B, Joseph B. Protein kinase C-dependent phosphorylation regulates the cell cycle-inhibitory function of the p73 carboxy terminus transactivation domain. Mol Cell Biol. 2009;29:1814–1825. doi: 10.1128/MCB.00585-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Choi K, Ryu SW, Kang SW, Choi C. TRAIL promotes caspase-dependent pro-inflammatory responses via PKCdelta activation by vascular smooth muscle cells. Cell Death Dis. 2011;2:e223. doi: 10.1038/cddis.2011.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Datta R, Shioya H, Li Y, Oki E, Biedermann V, et al. p73beta is regulated by protein kinase Cdelta catalytic fragment generated in the apoptotic response to DNA damage. J Biol Chem. 2002;277:33758–33765. doi: 10.1074/jbc.M110667200. [DOI] [PubMed] [Google Scholar]
- Yuan ZM, Utsugisawa T, Ishiko T, Nakada S, Huang Y, Kharbanda S, et al. Activation of protein kinase C delta by the c-Abl tyrosine kinase in response to ionizing radiation. Oncogene. 1998;16:1643–1648. doi: 10.1038/sj.onc.1201698. [DOI] [PubMed] [Google Scholar]
- Bernassola F, Salomoni P, Oberst A, Di Como CJ, Pagano M, Melino G, et al. Ubiquitin-dependent degradation of p73 is inhibited by PML. J Exp Med. 2004;199:1545–1557. doi: 10.1084/jem.20031943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Park JS, Um SJ. Identification and characterization of HIPK2 interacting with p73 and modulating functions of the p53 family in vivo. J Biol Chem. 2002;277:32020–32028. doi: 10.1074/jbc.M200153200. [DOI] [PubMed] [Google Scholar]
- Ben-Yehoyada M, Ben-Dor I, Shaul Y. c-Abl tyrosine kinase selectively regulates p73 nuclear matrix association. J Biol Chem. 2003;278:34475–34482. doi: 10.1074/jbc.M301051200. [DOI] [PubMed] [Google Scholar]
- Munarriz E, Barcaroli D, Stephanou A, Townsend PA, Maisse C, Terrinoni A, et al. PIAS-1 is a checkpoint regulator which affects exit from G1 and G2 by sumoylation of p73. Mol Cell Biol. 2004;24:10593–10610. doi: 10.1128/MCB.24.24.10593-10610.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minty A, Dumont X, Kaghad M, Caput D. Covalent modification of p73alpha by SUMO-1. Two-hybrid screening with p73 identifies novel SUMO-1-interacting proteins and a SUMO-1 interaction motif. J Biol Chem. 2000;275:36316–36323. doi: 10.1074/jbc.M004293200. [DOI] [PubMed] [Google Scholar]
- Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, et al. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11:577–590. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Sayan AE, Sayan BS, Gogvadze V, Dinsdale D, Nyman U, Hansen TM, et al. P73 and caspase-cleaved p73 fragments localize to mitochondria and augment TRAIL-induced apoptosis. Oncogene. 2008;27:4363–4372. doi: 10.1038/onc.2008.64. [DOI] [PubMed] [Google Scholar]
- John K, Alla V, Meier C, Putzer BM. GRAMD4 mimics p53 and mediates the apoptotic function of p73 at mitochondria. Cell Death Differ. 2011;18:874–886. doi: 10.1038/cdd.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayan BS, Sayan AE, Knight RA, Melino G, Cohen GM. p53 is cleaved by caspases generating fragments localizing to mitochondria. J Biol Chem. 2006;281:13566–13573. doi: 10.1074/jbc.M512467200. [DOI] [PubMed] [Google Scholar]
- Watson IR, Blanch A, Lin DC, Ohh M, Irwin MS. Mdm2-mediated NEDD8 modification of TAp73 regulates its transactivation function. J Biol Chem. 2006;281:34096–34103. doi: 10.1074/jbc.M603654200. [DOI] [PubMed] [Google Scholar]
- Aqeilan RI, Pekarsky Y, Herrero JJ, Palamarchuk A, Letofsky J, Druck T, et al. Functional association between Wwox tumor suppressor protein and p73, a p53 homolog. Proc Natl Acad Sci USA. 2004;101:4401–4406. doi: 10.1073/pnas.0400805101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer S, Ozaki T, Miyazaki K, Kato C, Hanamoto T, Nakagawara A. Protein stability and function of p73 are modulated by a physical interaction with RanBPM in mammalian cultured cells. Oncogene. 2005;24:938–944. doi: 10.1038/sj.onc.1208257. [DOI] [PubMed] [Google Scholar]
- Kikuchi H, Ozaki T, Furuya K, Hanamoto T, Nakanishi M, Yamamoto H, et al. NF-kappaB regulates the stability and activity of p73 by inducing its proteolytic degradation through a ubiquitin-dependent proteasome pathway. Oncogene. 2006;25:7608–7617. doi: 10.1038/sj.onc.1209748. [DOI] [PubMed] [Google Scholar]
- Rossi M, De Laurenzi V, Munarriz E, Green DR, Liu YC, Vousden KH, et al. The ubiquitin-protein ligase Itch regulates p73 stability. EMBO J. 2005;24:836–848. doi: 10.1038/sj.emboj.7600444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberst A, Malatesta M, Aqeilan RI, Rossi M, Salomoni P, Murillas R, et al. The Nedd4-binding partner 1 (N4BP1) protein is an inhibitor of the E3 ligase Itch. Proc Natl Acad Sci USA. 2007;104:11280–11285. doi: 10.1073/pnas.0701773104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Adamovich Y, Reuven N, Shaul Y. The Yes-associated protein 1 stabilizes p73 by preventing Itch-mediated ubiquitination of p73. Cell Death Differ. 2007;14:743–751. doi: 10.1038/sj.cdd.4402063. [DOI] [PubMed] [Google Scholar]
- Maisse C, Munarriz E, Barcaroli D, Melino G, De Laurenzi V. DNA damage induces the rapid and selective degradation of the DeltaNp73 isoform, allowing apoptosis to occur. Cell Death Differ. 2004;11:685–687. doi: 10.1038/sj.cdd.4401376. [DOI] [PubMed] [Google Scholar]
- Dulloo I, Sabapathy K. Transactivation-dependent and -independent regulation of p73 stability. J Biol Chem. 2005;280:28203–28214. doi: 10.1074/jbc.M501702200. [DOI] [PubMed] [Google Scholar]
- Sayan BS, Yang AL, Conforti F, Tucci P, Piro MC, Browne GJ, et al. Differential control of TAp73 and DeltaNp73 protein stability by the ring finger ubiquitin ligase PIR2. Proc Natl Acad Sci USA. 2010;107:12877–12882. doi: 10.1073/pnas.0911828107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh WH, Siddique MM, Boominathan L, Lin KW, Sabapathy K. c-Jun regulates the stability and activity of the p53 homologue, p73. J Biol Chem. 2004;279:44713–44722. doi: 10.1074/jbc.M407672200. [DOI] [PubMed] [Google Scholar]
- Danovi SA, Rossi M, Gudmundsdottir K, Yuan M, Melino G, Basu S. Yes-associated protein (YAP) is a critical mediator of c-Jun-dependent apoptosis. Cell Death Differ. 2008;15:217–219. doi: 10.1038/sj.cdd.4402226. [DOI] [PubMed] [Google Scholar]
- Dulloo I, Gopalan G, Melino G, Sabapathy K. The antiapoptotic DeltaNp73 is degraded in a c-Jun-dependent manner upon genotoxic stress through the antizyme-mediated pathway. Proc Natl Acad Sci USA. 2010;107:4902–4907. doi: 10.1073/pnas.0906782107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Fricker M, O'Prey J, Tolkovsky AM, Ryan KM. Phosphorylation of Puma modulates its apoptotic function by regulating protein stability. Cell Death Dis. 2010;1:e59. doi: 10.1038/cddis.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placzek WJ, Wei J, Kitada S, Zhai D, Reed JC, Pellecchia M. A survey of the anti-apoptotic Bcl-2 subfamily expression in cancer types provides a platform to predict the efficacy of Bcl-2 antagonists in cancer therapy. Cell Death Dis. 2010;1:e40. doi: 10.1038/cddis.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei WW, Zhang KH, Pan XC, Wang DM, Hu Y, Yang YN, et al. Histone deacetylase 1 and 2 differentially regulate apoptosis by opposing effects on extracellular signal-regulated kinase 1/2. Cell Death Dis. 2010;1:e44. doi: 10.1038/cddis.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S. Caspase function in programmed cell death. Cell Death Differ. 2007;14:32–43. doi: 10.1038/sj.cdd.4402060. [DOI] [PubMed] [Google Scholar]
- Seervi M, Joseph J, Sobhan PK, Bhavya BC, Santhoshkumar TR. Essential requirement of cytochrome c release for caspase activation by procaspase-activating compound defined by cellular models. Cell Death Dis. 2011;2:e207. doi: 10.1038/cddis.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirawan E, Vande Walle L, Kersse K, Cornelis S, Claerhout S, Vanoverberghe I, et al. Caspase-mediated cleavage of Beclin-1 inactivates Beclin-1-induced autophagy and enhances apoptosis by promoting the release of proapoptotic factors from mitochondria. Cell Death Dis. 2010;1:e18. doi: 10.1038/cddis.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchenko ND, Hanel W, Li D, Becker K, Reich N, Moll UM. Stress-mediated nuclear stabilization of p53 is regulated by ubiquitination and importin-alpha3 binding. Cell Death Differ. 2010;17:255–267. doi: 10.1038/cdd.2009.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasini R, Tsuchihara K, Wilhelm M, Fujitani M, Rufini A, Cheung CC, et al. TAp73 knockout shows genomic instability with infertility and tumor suppressor functions. Genes Dev. 2008;22:2677–2691. doi: 10.1101/gad.1695308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MK, Tong WM, Wang ZQ, Sabapathy K. Serine 312 phosphorylation is dispensable for wild-type p53 functions in vivo. Cell Death Differ. 2011;18:214–221. doi: 10.1038/cdd.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Bahlani S, Fraser M, Wong AY, Sayan BS, Bergeron R, Melino G, et al. P73 regulates cisplatin-induced apoptosis in ovarian cancer cells via a calcium/calpain-dependent mechanism. Oncogene. 2011;30:4219–4230. doi: 10.1038/onc.2011.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh WH, Nam SY, Sabapathy K. An essential role for p73 in regulating mitotic cell death. Cell Death Differ. 2010;17:787–800. doi: 10.1038/cdd.2009.181. [DOI] [PubMed] [Google Scholar]
- Wasik AM, Almestrand S, Wang X, Hultenby K, Dackland AL, Andersson P, et al. WIN55,212-2 induces cytoplasmic vacuolation in apoptosis-resistant MCL cells. Cell Death Dis. 2011;2:e225. doi: 10.1038/cddis.2011.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Nangia-Makker P, Balan V, Hogan V, Raz A. Calpain activation through galectin-3 inhibition sensitizes prostate cancer cells to cisplatin treatment. Cell Death Dis. 2010;1:e101. doi: 10.1038/cddis.2010.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidari N, Hicks MA, Harada H. GX15-070 (obatoclax) overcomes glucocorticoid resistance in acute lymphoblastic leukemia through induction of apoptosis and autophagy. Cell Death Dis. 2010;1:e76. doi: 10.1038/cddis.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar N, Li X, Padi SK, Zhang Q, Tang MS, Guo B. Downregulation of miR-205 and miR-31 confers resistance to chemotherapy-induced apoptosis in prostate cancer cells. Cell Death Dis. 2010;1:e105. doi: 10.1038/cddis.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]