Abstract
Species in the present study were compared based on their morphology, growth characteristics in culture, and DNA sequences of the nuclear ribosomal RNA gene operon (including ITS1, ITS2, 5.8S nrDNA and the first 900 bp of the 28S nrDNA) for all species and partial actin and translation elongation factor 1-alpha gene sequences for Cladosporium species. New species of Mycosphaerella (Mycosphaerellaceae) introduced in this study include M. cerastiicola (on Cerastium semidecandrum, The Netherlands), and M. etlingerae (on Etlingera elatior, Hawaii). Mycosphaerella holualoana is newly reported on Hedychium coronarium (Hawaii). Epitypes are also designated for Hendersonia persooniae, the basionym of Camarosporula persooniae, and for Sphaerella agapanthi, the basionym of Teratosphaeria agapanthi comb. nov. (Teratosphaeriaceae) on Agapathus umbellatus from South Africa. The latter pathogen is also newly recorded from A. umbellatus in Europe (Portugal). Furthermore, two sexual species of Cladosporium (Davidiellaceae) are described, namely C. grevilleae (on Grevillea sp., Australia), and C. silenes (on Silene maritima, UK). Finally, the phylogenetic position of two genera are newly confirmed, namely Camarosporula (based on C. persooniae, teleomorph Anthracostroma persooniae), which is a leaf pathogen of Persoonia spp. in Australia, belongs to the Teratosphaeriaceae, and Sphaerulina (based on S. myriadea), which occurs on leaves of Fagaceae (Carpinus, Castanopsis, Fagus, Quercus), and belongs to the Mycosphaerellaceae.
Keywords: Anthracostroma, Camarosporula, Cladosporium, Mycosphaerella, phylogeny, Sphaerulina, taxonomy, Teratosphaeria
INTRODUCTION
The genus Mycosphaerella has in the past been recognised as one of the largest genera of ascomycetes, containing approx. 3000 species (Aptroot 2006), as well as several thousand asexual species (Crous et al. 2000, 2001, 2004b, c, 2006b, 2007, Crous & Braun 2003). Although Mycosphaerella has been linked to many different anamorph genera (Crous & Braun 2003, Crous et al. 2007), recent phylogenetic studies have shown that Mycosphaerella is actually polyphyletic (Crous et al. 2007, 2009a), with members belonging to many different genera, and even families, such as the Davidiellaceae (Braun et al. 2003, Schoch et al. 2006), Teratosphaeriaceae (Crous et al. 2007), Dissoconiaceae (Crous et al. 2009b), Mycosphaerellaceae and Schizothyriaceae (Aptroot 2006, Batzer et al. 2008, Crous 2009).
Members of the Mycosphaerella-complex are ecologically highly adaptable, and vary from being saprobic to fungicolous (Crous 2009). Mycosphaerella species are also among the most common and destructive plant pathogens known, causing serious diseases on many economically important crops (Farr et al. 1995, Crous & Braun 2003). Species are mainly foliicolous, although some are associated with stem cankers (Cortinas et al. 2006), fruit lesions (Pretorius et al. 2003) or blemishes, spots and specks (Batzer et al. 2008).
One of the largest barriers to understanding host specificity and speciation in this group of organisms, has been the relative unavailability of authentic cultures, as most of the species known to date, have been described without the deposit of associated ex-type cultures or DNA. The result is that the ecological behaviour of many species remains obscure (Crous & Groenewald 2005), while the phylogenetic position of many other pathogens and genera remains uncertain. During the course of the present study several potentially novel Mycosphaerella and Mycosphaerella-like species were collected, while potential epitype specimens were also collected for older, well-established names. The aim of this study was thus to describe these species, and also elucidate the phylogenetic relationship of genera such as Camarosporula (teleomorph Anthracostroma), and Sphaerulina within the Capnodiales.
MATERIALS AND METHODS
Isolates
Leaf and stem tissue bearing ascomata were soaked in water for approximately 2 h, after which they were placed in the bottom of Petri dish lids, with the top half of the dish containing 2 % malt extract agar (MEA; Oxoid, Hampshire, UK) (Crous et al. 2009c). Ascospore germination patterns were examined after 24 h, and single ascospore and conidial cultures established as described earlier (Crous et al. 1991, Crous 1998). To isolate asexual fungi, host tissues were incubated in moist chambers for up to 2 wk, and inspected daily for microfungi, and single conidial colonies established on MEA (Crous 2002). Colonies were subcultured onto 2 % potato-dextrose agar (PDA), synthetic nutrient-poor agar (SNA), MEA, and oatmeal agar (OA) (Crous et al. 2009c), and incubated under continuous near-ultraviolet light at 25 °C to promote sporulation. Nomenclatural novelties and descriptions were deposited in MycoBank (www.MycoBank.org; Crous et al. 2004a). All cultures obtained in this study are maintained in the open collection of the Centraalbureau voor Schimmelcultures (CBS-KNAW) and the working collection (CPC) of P.W. Crous in CBS (Table 1).
Table 1.
Collection details and GenBank accession numbers of isolates for which novel sequences were generated in this study.
| Species | Strain no. 1 | Substrate | Country | Collector(s) | GenBank Accession number (ITS, LSU, TEF, ACT) 2 |
|---|---|---|---|---|---|
| Camarosporula persooniae | CBS 112302 = CPC 3343 | Leaf spots on Persoonia sp. | Australia | P.W. Crous & B.A. Summerell | JF770447, JF770459, —, — |
| CBS 112494 = CPC 3350 | Leaf spots on Persoonia sp. | Australia | P.W. Crous & B.A. Summerell | JF770448, JF770460, —, — | |
| CBS 116258 = CPC 3344 | Leaf spots on Persoonia sp. | Australia | P.W. Crous & B.A. Summerell | JF770449, JF770461, —, — | |
| Cladosporium grevilleae | CBS 114271 = CPC 2913 | Leaves of Grevillea sp. | Australia | P.W. Crous & B.A. Summerell | JF770450, JF770462, JF770472, JF770473 |
| Cladosporium silenes | CBS 109082 | Stems of exposed Silene maritima | UK | A. Aptroot | EF679354, JF770463, EF679429, EF679506 |
| Mycosphaerella cerastiicola | CBS 115913 = CPC 11290 | Dead leaves and stems of Cerastium semidecandrum | Netherlands | A. Aptroot | JF770451, JF770464, —, — |
| Mycosphaerella etlingerae | CBS 129062 = CPC 12274 | Dead leaves of Etlingera elatior | Hawaii | W. Gams | JF770452, JF770465, —, — |
| CPC 12277 | Dead leaves of Etlingera elatior | Hawaii | W. Gams | JF770453, JF770466, —, — | |
| Mycosphaerella holualoana | CBS 129063 = CPC 12286 | Dead leaves of Hedychium coronarium | Hawaii | W. Gams | JF770454, JF770467, —, — |
| Sphaerulina myriadea | CBS 124646 = JCM 15565 | Leaves of Quercus dentata | Japan | K. Tanaka | JF770455, JF770468, —, — |
| Teratosphaeria agapanthi | CBS 129064 = CPC 18332 | Leaf spots on Agapanthus umbellatus | Portugal | P.W. Crous | JF770456, JF770469, —, — |
| CBS 129192 = CPC 18304 | Leaf spots on Agapanthus umbellatus | South Africa | P.W. Crous | JF770457, JF770470, —, — | |
| CPC 18266 | Leaf spots on Agapanthus umbellatus | South Africa | P.W. Crous | JF770458, JF770471, —, — |
1CBS: CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands; CPC: Culture collection of Pedro Crous, housed at CBS.
2ITS: Internal transcribed spacers 1 and 2 together with 5.8S nrDNA; LSU: partial 28S nrDNA; TEF: partial translation elongation factor 1-alpha gene; ACT: partial actin gene.
DNA phylogeny
Genomic DNA was extracted from mycelia of fungal colonies cultivated on MEA using the UltraCleanTM Microbial DNA Isolation Kit (Mo Bio Laboratories, Solana Beach, CA, USA). The Primers V9G (de Hoog & Gerrits van den Ende 1998) and LR5 (Vilgalys & Hester 1990) were used to amplify part of the nuclear rDNA operon spanning the 3′ end of the 18S nrRNA gene (SSU), the first internal transcribed spacer (ITS1), the 5.8S nrRNA gene, the second ITS region (ITS2) and the 5′ end of the 28S nrRNA gene (LSU). The primers ITS4 (White et al. 1990) and LSU1Fd (Crous et al. 2009b) were used as internal sequence primers to ensure good quality sequences over the entire length of the amplicon. To help resolve species of Cladosporium, the ITS region was supplemented with partial sequences of the translation elongation factor 1-α gene (EF-1α) using the primers EF1-728F (Carbone & Kohn 1999) and EF-2 (O’Donnell et al. 1998), and the actin gene (ACT) using the primers ACT-512F and ACT-783R (Carbone & Kohn 1999). The PCR amplifications were performed on a GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA, USA) in a total volume of 12.5 μL solution containing 10–20 ng of template DNA, 1 × PCR buffer, 2 mM MgCl2, 2.5 pmol for each primer, 60 μM of each dNTP (20 μM for EF-1α) and 0.5 U Taq DNA polymerase (Bioline Luckenwalde, Germany). For EF-1α, 0.7 μL dimethyl sulfoxide (DMSO) was added to the amplification reaction. PCR amplification conditions were set as follows: an initial denaturation temperature of 94 °C for 5 min, followed by 40 cycles of denaturation temperature of 94 °C for 45 s, primer annealing at 48 °C (52 °C for EF-1α) for 30 s, primer extension at 72 °C for 90 s and a final extension step at 72 °C for 6 min. The resulting amplicons were sequenced using the PCR primers and a BigDye Terminator Cycle Sequencing Kit v. 3.1 (Applied Biosystems) and analysed on an ABI Prism 3730xl DNA Sequencer (Perkin-Elmer, Norwalk, CN, USA).
The generated sequences were compared with other fungal DNA sequences from NCBI’s GenBank sequence database using a megablast search; sequences with high similarity were added to the alignment (LSU) or discussed in the species notes where applicable (ITS, EF-1α and ACT). The additional GenBank sequences were manually aligned using Sequence Alignment Editor v. 2.0a11 (Rambaut 2002). The phylogenetic analyses of the aligned sequence data were performed using PAUP (Phylogenetic Analysis Using Parsimony) v. 4.0b10 (Swofford 2003) and consisted of neighbour-joining analyses with the uncorrected (‘p’), the Kimura 2-parameter and the HKY85 substitution models and parsimony analyses. Alignment gaps were treated as missing data and all characters were unordered and of equal weight. Any ties were broken randomly when encountered. For parsimony analyses, alignment gaps were treated as a fifth (“new”) character state and all characters were unordered and of equal weight. Maximum parsimony analysis was performed using the heuristic search option with 100 random simple taxa additions and tree bisection and reconstruction (TBR) as the branch-swapping algorithm. Branches of zero length were collapsed and all multiple, equally parsimonious trees were saved. The robustness of the trees obtained was evaluated by 1 000 bootstrap replications (Hillis & Bull 1993). Tree length (TL), consistency index (CI), retention index (RI) and rescaled consistency index (RC) were calculated and the resulting trees were printed with TreeView v. 1.6.6 (Page 1996). New sequences were lodged in GenBank (Table 1) and the alignments and phylogenetic trees in TreeBASE (www.treebase.org).
Morphology
Culture characteristics were recorded from colonies grown on MEA, OA and PDA plates after 2–4 wk incubation in darkness at 25 °C. Colony colours (surface and reverse) were assessed using the colour charts of Rayner (1970). Preparations were mounted in lactic acid and studied under a light microscope (× 1000 magnification). The 95 % confidence intervals of spores were derived from 30 observations (unless stated otherwise), with extremes given in parentheses.
RESULTS
DNA phylogeny
Approximately 1700 bases, spanning the ITS and LSU regions, were obtained for isolates listed in Table 1. These two regions were analysed separately; ITS to determine species level relationships (presented in the species notes below, where applicable) and LSU for the generic placement. Approximately 300 and 220 bases were determined for EF-1α and ACT, respectively, and their respective blast results are discussed under the Cladosporium species notes below.
The manually adjusted LSU alignment contained 49 taxa (including the outgroup sequence) and, of the 825 characters used in the phylogenetic analysis, 147 were parsimony-informative, 103 were variable and parsimony-uninformative, and 575 were constant. Neighbour-joining analysis using the three substitution models on the sequence data yielded trees with similar topology and bootstrap support values. Twenty-five equally most parsimonious trees were obtained from the heuristic search, the first of which is shown in Fig. 1 (TL = 551, CI = 0.657, RI = 0.872, RC = 0.573). The phylogenetic tree of the LSU region (Fig. 1) showed three distinct groups of fungal isolates; the first was the Davidiellaceae clade (70 % bootstrap support), the second the Teratosphaeriaceae clade (98 % bootstrap support), and the third was the Mycosphaerellaceae clade (93 % bootstrap support).
Fig. 1.
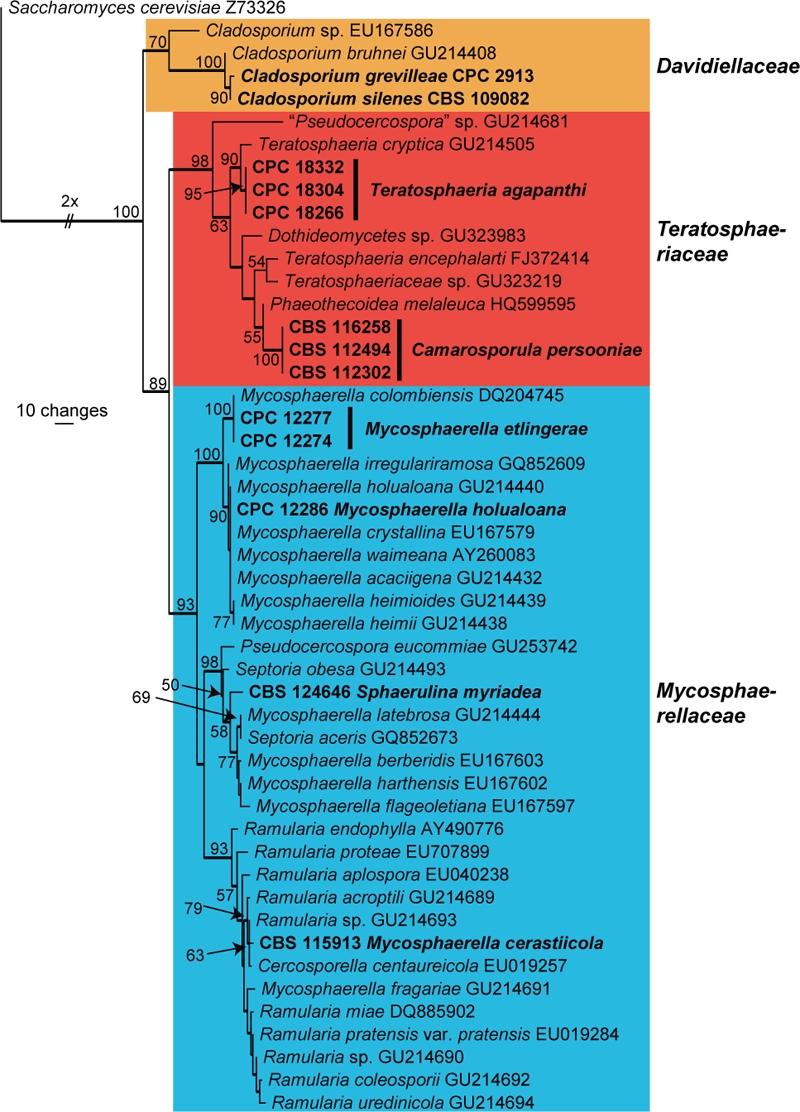
The first of 25 equally most parsimonious trees obtained from a heuristic search with 100 random taxon additions of the LSU sequence alignment using PAUP v. 4.0b10. The scale bar shows 10 changes, and bootstrap support values from 1 000 replicates are shown at the nodes. Thickened lines indicate the strict consensus branches and novel sequences are printed in bold face. The different families are indicated with coloured blocks. The tree was rooted to Saccharomyces cerevisiae (GenBank no. Z73326).
TAXONOMY
During the present study several novel taxa were delineated, or newly collected cultures linked to established names. These species are treated per family below:
Davidiellaceae
Cladosporium grevilleae Crous & Summerell, sp. nov.
MycoBank MB560082
Fig. 2.
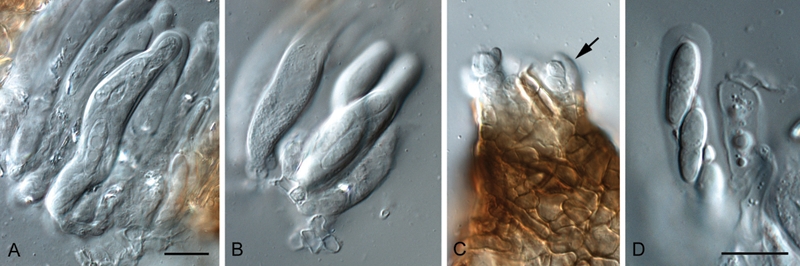
Cladosporium grevilleae (DAR 74881). A, B. Asci. C. Ostiolar region (arrowed). D. Ascus with ascospores. Bars = 10 μm.
Fig. 3.
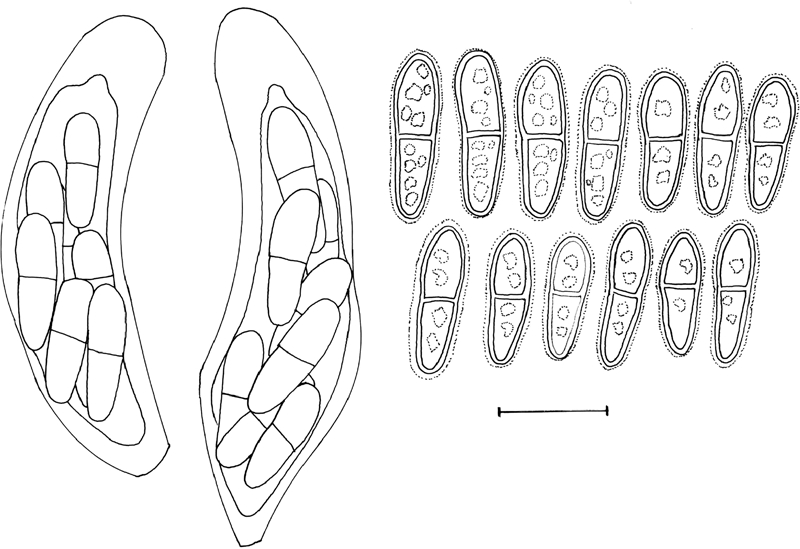
Cladosporium grevilleae (DAR 74881). Asci with ascospores. Ascospores showing sheath, and angular inclusions. Bar = 10 μm.
Etymology: Named after the host on which it was collected, Grevillea.
Asci fasciculati, bitunicati, subsessiles, obovoidei vel late ellipsoidei, octospori, 35–45 × 9–12 μm. Ascosporae tri- ad pluriseriatas, hyalinae, guttulatae cum inclusionibus angularibus, crassitunicatae, rectae vel leniter curvatae, fusoides-ellipsoideae, utrinque obtusae, mediane 1-septatae, (9–)11–12(–13) × 3.5–4(–4.5) μm.
Typus: Australia: New South Wales: Mount Annan Botanical Garden, on leaves of Grevillea sp., Aug. 1999, P.W. Crous & B.A. Summerell JT 974 (DAR 74881 – holotypus; cultures ex-type CPC 2913–2916 = CBS 114271).
In vivo: Leaf spots absent. Ascomata occurring in leaf litter, amphigenous, black, subepidermal, erumpent to superficial, globose, to 100 μm diam, with central, periphysate ostiole, 10–15 μm diam; wall of 3–4 layers of brown textura angularis. Asci aparaphysate, fasciculate, bitunicate with fissitunicate discharge, subsessile, obovoid to broadly ellipsoid, slightly curved, 8-spored, 35–45 × 9–12 μm, with visible apical apiculus. Ascospores tri- to multi-seriate, hyaline, guttulate with angular inclusions, thick-walled, straight to slightly curved, fusoid-ellipsoid with obtuse ends, medianly 1-septate, widest in middle of apical cell, slightly constricted at the septum, tapering towards both ends, but slightly more to lower end, (9–)11–12(–13) × 3.5–4(–4.5) μm; ascospores surrounded with a thin sheath when mounted in water, becoming brown and verruculose with age; ascospore germination with germ tubes parallel to the long axis of the spore, but distorting prominently (original spore cells up to 8 μm wide), germinating with numerous germ tubes, forming dense clusters of hyphae, but mostly remaining hyaline after 24 h on MEA.
Culture characteristics: Colonies after 2 wk at 24 °C spreading, reaching 15–20 mm diam. On MEA erumpent, with even, lobed margins; surface folded, with sparse aerial mycelium, olivaceous-grey; reverse iron-grey. On OA flat, with sparse aerial mycelium, and lobed, somewhat feathery margins; surface pale olivaceous-grey in middle, iron-grey in outer region. On PDA erumpent, with lobed, feathery margins; surface folded, with sparse aerial mycelium, grey-olivaceous; reverse iron-grey.
Notes: The genus Cladosporium 1816 is linked to teleomorphs that are placed in Davidiella 2003 (Braun et al. 2003, Schubert et al. 2007). In moving to a single nomenclature for pleomorphic fungi, we give preference to the oldest genus name, namely Cladosporium. Cladosporium grevilleae only forms the sexual state of the life-cycle. The ascospores are typical of a Cladosporium teleomorph, having thick walls, and angular inclusions (Aptroot 2006), becoming brown and verruculose with age. Presently we are not aware of any sexual or asexual species on Grevillea that represent this fungus, and thus we describe it here as new. Although all four loci supported the association of the species with Cladosporium, it did not match any of the Cladosporium sequences currently available on the GenBank nucleotide database (closest match on ITS was Davidiella macrospora GenBank EU167591 with 95 % identity, on EF-1α was Cladosporium myrtacearum GenBank HM148360 with 97 % identity and on ACT it was Cladosporium iranicum GenBank HM148599 with 89 % identity).
Cladosporium silenes Crous, sp. nov.
MycoBank MB560083
(Fig. 4)
Fig. 4.
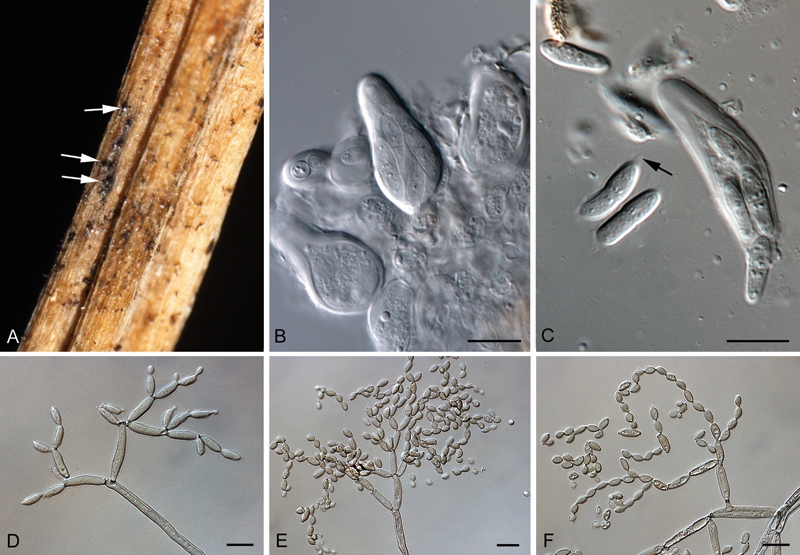
Cladosporium silenes (CBS H-19874). A. Ascomata on host tissue (arrows). B. Asci. C. Ascospores (arrow denotes mucoid appendage; A–C from Bensch et al. 2010). D–F. Conidiophores with conidial chains. Bars = 10 μm.
Etymology: Named after the host on which it was collected, Silene maritima.
Cladosporii cladosporioidis similis, sed conidiophoris brevioribus, non ramosis, cellulis conidiogenois longioribus, ramo-conidiis et conidiis intercalaribus brevioribus discernitur.
Typus: UK : Pembrokeshire: Skomer Island, stems of exposed Silene maritima, 22 Aug. 2000, A. Aptroot 49319) (CBS H-19874 – holotypus; culture ex-type CBS 109082).
In vivo: Ascomata occurring in exposed twigs, amphigenous, black to dark brown, subepidermal, to 70 μm diam, visible by an erumpent, central, periphysate ostiole, 5–10 μm; wall of 2–3 layers of red-brown textura angularis. Asci aparaphysate, fasciculate, bitunicate with fissitunicate discharge, subsessile, obovoid to broadly ellipsoid, straight to slightly curved, 8-spored, 25–35 × 10–12 μm, with visible apical apiculus. Ascospores tri- to multiseriate, hyaline, non-guttulate with angular inclusions, thick-walled, straight to slightly curved, fusoid-ellipsoid with obtuse ends, medianly 1-septate, widest in middle of apical cell, not to slightly constricted at the septum, tapering towards both ends, but slightly more to lower end, (10–)11–13(–14) × (3–)3.5(–4) μm; turning brown once discharged, and some containing remnants of a mucoid layer; germinating from both ends, distorting, becoming brown and finely verruculose.
Mycelium consisting of branched, septate, pale to medium brown 2(–4) μm wide hyphae, without any swellings and constrictions, smooth to minutely verruculose, walls unthickened. Conidiophores solitary, macronematous or micronematous, arising terminally from ascending hyphae or laterally, straight to somewhat flexuous, narrowly cylindrical to cylindrical-oblong, non-nodulose, not geniculate-sinuous, 15−100(−200) × 3−4(−4.5) μm, unbranched, pluriseptate, usually not constricted at septa, pale to medium olivaceous-brown, smooth to minutely verruculose, especially towards the base, walls unthickened, base sometimes swollen, up to 8 μm wide; micronematous conidiophores shorter, unbranched, 10−30 × 3−4 μm. Conidiogenous cells integrated, usually terminal, sometimes intercalary, cylindrical-oblong, not geniculate, non-nodulose, (10−)20−60 μm long, with up to three loci crowded at the apex, subdenticulate to denticulate, protuberant, 2(−2.5) μm diam, central dome mostly flat, somewhat thickened and darkened-refractive. Ramoconidia straight to slightly curved, cylindrical-oblong, 15−20(–25) ×3−4(–4.5) μm, aseptate, pale olivaceous-brown, concolorous with tips of conidiophores, smooth, base not cladosporioid, 2−2.5 μm wide, thickened, somewhat refractive. Secondary ramoconidia aseptate, smooth, pale olivaceous brown, cylindrical-oblong, (8–)10–15(–20) × 3.5–4 μm. Conidia numerous, catenate, in branched chains of up to 6 in the upper unbranched part, branching in all directions. Intercalary conidia limoniform, ellipsoid-ovoid, 7−8(−10) × (2−)2.5−3 μm, aseptate, with up to 3 distal hila. Small terminal conidia aseptate, subglobose, obovoid, ovoid to limoniform, 4−5(−6) × (2.5−)3(−3.5) μm; hila darkened and somewhat thickened, 0.5–1 μm diam.
Culture characteristics: Colonies after 2 wk at 24 °C spreading with moderate aerial mycelium and smooth, lobate margins, reaching 40 mm diam after 2 wk. On MEA surface pale olivaceous-grey to olivaceous-grey, reverse iron-grey. No OA surface grey-olivaceous to olivaceous-grey. On PDA surface grey-olivaceous, reverse olivaceous-grey.
Notes: Strain CBS 109082 represents an ascospore isolate, obtained from material of Silene maritima and initially identified as Mycosphaerella tassiana var. arthopyrenioides. Morphologically the CBS strain is similar to C. cladosporioides, but is a member of a distinct clade (C. cladosporioides s.lat. Lineage 3; see fig. 1, part A in Bensch et al. 2010). Cladosporium sileniae is the first sexual species known from the cladosporioides complex. Given that these ascomata are rather inconspicuous (immersed, approx. 100 μm diam), its not surprising that they have been largely overlooked in the past. Cladosporium sileniae differs from C. cladosporioides in having shorter, unbranched conidiophores, longer conidiogenous cells, shorter ramo- and intercalary conidia (Bensch et al. 2010).
Mycosphaerellaceae
Mycosphaerella cerastiicola Crous, sp. nov.
MycoBank MB560084
(Fig. 5)
Fig. 5.

Mycosphaerella cerastiicola (CBS 115913). A, B. Leaves with black ascomata and conidiomata. C, D. Asci. E, F. Ascospores. G. Conidiophores giving rise to conidia. H. Conidia. Bars = 10 μm.
Anamorph: Septoria-like
Etymology: Named after the host on which it was collected, Cerastium semidecandrum.
Ascosporae imbricatae, hyalinae, guttulatae, tenuitunicatae, rectae vel leniter curvatae, fusoides-ellipsoideae, constrictae ad septa mediana, utrinque attenuatae, (11–)15–20(–23) × (3–)3.5(–4) μm.
Typus: Netherlands: Flevoland: Noordoostpolder, near Urk, km block 20-26-24, coordinations 168,9, 523,5, on dyke of former sea, on dead leaves and stems of Cerastium semidecandrum, 2 May 2004, A. Aptroot (CBS H-20549 – holotypus; cultures ex-type CPC 11290 = CBS 115913; CPC 11291, 11292).
In vivo: Leaf spots absent. Ascomata amphigenous on leaves and stems, black, subepidermal, becoming erumpent, solitary, globose, up to 150 μm diam; central ostiole 5–10 μm diam; wall consisting of 2–3 layers of medium brown textura angularis. Asci aparaphysate, fasciculate, bitunicate with fissitunicate discharge, subsessile, narrowly ellipsoid to subcylindrical, straight to slightly curved, (2–)8-spored, 35–45 × 8–10 μm; apical chamber 1–1.5 μm diam. Ascospores bi- to tri-seriate, overlapping, hyaline, guttulate, thin-walled, straight to somewhat curved, fusoid-ellipsoidal with acutely rounded ends, widest at septum when immature, just above median septum when mature, constricted at the septum, tapering towards both ends, (11–)15–20(–23) × (3–)3.5(–4) μm; ascospores germinate from both ends, with germ tubes parallel to the long axis of the spore.
Septoria-like state developing on SNA and OA. Conidiomata pycnidial on host, pycnidial to sporodochial on agar, black under the dissecting scope, globose, immersed in agar to superficial; colonies also sporulating profusely on aerial mycelium via solitary loci, 1.5 μm diam, 1 μm tall, thickened along the rim. Stromata dark brown under the compound microscope, to 200 μm diam, 100 μm tall, consisting of dark brown, thickened, ovoid to globose cells, 5–10 μm diam; upper layers fertile, giving rise to a dense network of intermingled brown, hyphal-like elements, that in turn give rise to an upper layer of conidiophores. Conidiophores cylindrical, 0–1-septate, pale brown to hyaline at base, hyaline at apex, smooth, rarely branched at bulbous base, 20–30 × 2 μm. Conidiogenous cells terminal, hyaline, 10–15 × 1.5–2 μm, proliferating sympodially with flattened loci, 1.5–2 μm diam, unthickened and not darkened; on host material conidiogenous cells ampulliform, 10–15 μm long, 4–5 μm wide at bulbous base; sympodial or phialidic, with periclinal thickening visible. Conidia solitary, hyaline, smooth, guttulate, cylindrical with obtuse apex and truncate base, at times somewhat long obconically subtruncate on host material, 1–3-septate, straight to flexuous, (15–)50–60(–65) × (1.5–)2 μm (av. 55 × 2 μm) in vitro.
Culture characteristics: Colonies after 2 wk at 24 °C spreading, erumpent, with folded surface, splitting agar, with sparse aerial mycelium and smooth, lobate margins, reaching 15–20 mm diam. On MEA surface salmon to flesh to dirty white, reverse chestnut (centre), to apricot (margin). On OA surface olivaceous buff, fertile. On PDA surface dirty white with patches of olivaceous grey; reverse iron-grey in middle, salmon in outer region. Colonies on SNA developing a diffuse red pigment in agar.
Notes: Mycosphaerella cerastiicola is presently the only Mycosphaerella species known from Cerastium (Caryophyllaceae) (Aptroot 2006). Both ITS and LSU data (Fig. 1) support the placement of this genus within the Ramularia clade. A blast search using the ITS sequence obtains as closest hits (maximum identity of 97–98 %) an undescribed species of “Pseudocercosporella” (strains KACC 42395, KACC 42363, CPC 11297; GenBank EF600945, EF600956, GU214693), which appears to belong to this genus. The genus Ramularia has thus far been assumed to be monophyletic, and thus the description of M. cerastiicola with its Septoria-like to Pseudocercosporella-like anamorph is an enigma. Presently there is no bootstrap support for it to represent another genus than Mycosphaerella/Ramularia, raising the question if the anamorph could represent a possible Ramularia synanamorph? Further collections would be required to address this issue.
Mycosphaerella etlingerae Crous, sp. nov.
MycoBank MB560085
(Fig. 6)
Fig. 6.
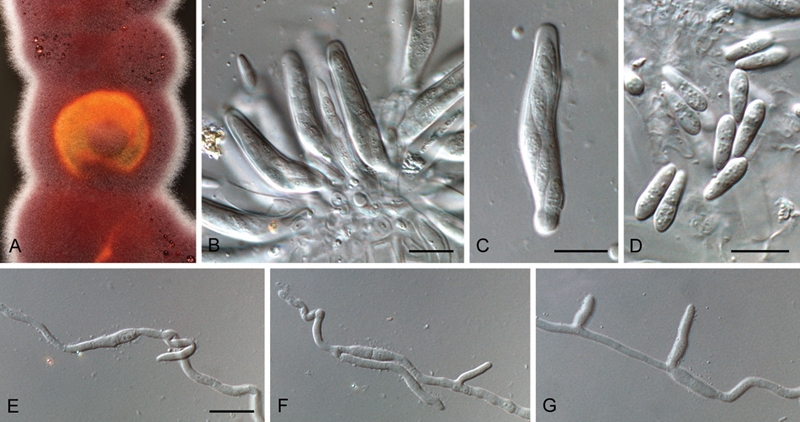
Mycosphaerella etlingerae (CPC 12274). A. Colony on malt extract agar. B, C. Asci. D. Ascospores. E–G. Germinating ascospores. Bars = 10 μm.
Etymology: Named after the host on which it was collected, Etlingera elatior.
Ascosporae imbricatae, hyalinae, granulatae, tenuitunicatae, rectae vel leniter curvatae, fusoides-ellipsoideae, mediane 1-septatae, leniter constrictae ad septa, (7–)9–10(–12) × 3(–3.5) μm.
Typus: USA: Hawaii: on dead leaves of Etlingera elatior, 14 Aug. 2005, W. Gams (CBS H-20550 – holotypus; cultures ex-type CBS 129062 = CPC 12274, 12275–12279).
In vivo: Leaf spots absent. Ascomata amphigenous, black, subepidermal, becoming erumpent, globose, up to 90 μm diam; central ostiole 5–10 μm diam; wall consisting of 2–3 layers of medium brown textura angularis. Asci aparaphysate, fasciculate, bitunicate with fissitunicate dehiscence, subsessile, narrowly ellipsoid to subcylindrical, straight to slightly curved, 8-spored, 35–45 × 6–7 μm. Ascospores bi- to tri-seriate, overlapping, hyaline, granular, thin-walled, straight to curved, fusoid-ellipsoidal with obtuse ends, widest in middle of apical cell, medianly 1-septate, slightly constricted at the septum, tapering towards both ends, but more prominently towards the lower end, (7–)9–10(–12) × 3(–3.5) μm; ascospores germinate from both ends, with germ tubes growing parallel to the long axis, developing lateral branches; ascospores remaining hyaline, becoming constricted, to 4 μm wide.
Culture characteristics: Colonies after 2 wk at 24 °C spreading, erumpent, surface irregular, folded, margin smooth, lobate, with sparse aerial mycelium, reaching 15 mm diam. On MEA surface pale mouse grey with patches of olivaceous grey and scarlet, with white margin; reverse iron-grey; red crystals produced in agar. On OA surface olivaceous grey. On PDA surface smoke-grey with patches of pale olivaceous-grey and scarlet.
Notes: No Mycosphaerella species have previously been reported from Etlingera elatior (Zingiberaceae), though six species have been reported on Zingiberaceae. These include M. alpiniae and M. alpiniicola on Alpinia in China, M. amomi on Amomum in China, M. hedychii on Hedychium in Brazil, M. zingiberi on Zingiber from China and Korea, and M. zingiberis on Zingiber from Japan (www.nt.ars-grin.gov/fungaldatabases). Other than for M. hedychii (ascospores 8–11 × 2 μm; Soares & Barreto 2008), the other species are somewhat obscure, and Aptroot (2006) could not trace any material for comparison. More than one species of Mycosphaerella was observed on the leaves of Etlingera elatior collected in Hawaii, but ascospores of M. etlingerae could be distinguished from other taxa by being fusoid-ellipsoidal, and widest in middle of the apical cell. Mycosphaerella etlingerae is 100 % identical to sequences of M. thailandica and M. colombiensis for both ITS and LSU. Mycosphaerella colombiensis, described from Eucalyptus leaf spots in Colombia, has ascospores that are obovoid, (11–)12–14(–15) × 3–3.5(-4) μm (Crous 1998), thus quite distinct from M. etlingerae. Mycosphaerella thailandica has ascospores that are fusoid-ellipsoidal, (9–)10–11(–12) × (2–) 2.5–3 μm, thus being very similar to those of M. etlingerae. However, M. thailandica causes a disease of Acacia in Thailand, and has a Pseudocercospora state in vivo and in vitro (Crous et al. 2004c), which is different from that observed in M. etlingerae.
Mycosphaerella holualoana Crous et al., Mycotaxon 78: 458 (2001).
(Fig. 7)
Fig. 7.
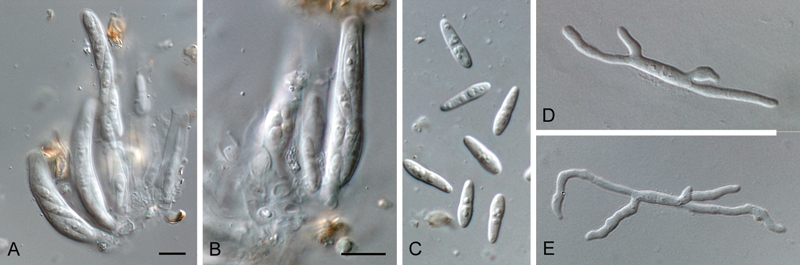
Mycosphaerella holualoana (CPC 12286). A, B. Asci. C. Ascospores. D, E. Germinating ascospores. Bars = 10 μm.
In vivo: Leaf spots absent. Ascomata amphigenous, black, subepidermal, becoming erumpent, aggregated in clusters, globose, to 70 μm diam; central ostiole 5–10 μm diam; wall consisting of 2–3 layers of medium brown textura angularis. Asci aparaphysate, fasciculate, bitunicate with fissitunicate dehiscence, subsessile, narrowly ellipsoid to subcylindrical, straight to slightly curved, 8-spored, 35–45 × 6–7 μm. Ascospores bi- to tri-seriate, overlapping, hyaline, prominently guttulate, thin-walled, straight, fusoid-ellipsoidal with obtuse ends, widest just above septum, medianly 1-septate, constricted at the septum, tapering towards both ends, but more prominently towards the lower end, (10–)11–12(–13) × (2.5–)3(–3.5) μm; ascospores germinate from both ends, with several germ tubes that are irregular in width and growth direction; ascospores becoming slightly constricted at septum, to 4 μm wide, remaining hyaline, smooth.
Culture characteristics: Colonies after 2 wk at 24 °C spreading, erumpent, surface folded with sparse aerial mycelium and even, lobate margin, reaching 20 mm diam. On MEA surface pale olivaceous grey with patches of olivaceous-grey; reverse olivaceous-grey to iron-grey. On OA surface olivaceous grey, reverse olivaceous-grey with patches of scarlet. On PDA surface grey-olivaceous to olivaceous grey, reverse iron-grey with patches of scarlet in centre.
Specimen examined: USA: Hawaii: on dead leaves of Hedychium coronarium, 14 Aug. 2005, W. Gams (CBS H-20551; cultures CBS 129063 = CPC 12286, 12287, 12288).
Notes: Mycosphaerella holualoana, which is part of the M. heimii-complex (Crous 1998) based on its LSU and ITS sequences, was initially described from leaf spots of Leucospermum spp. collected in Hawaii (Taylor et al. 2001). Ascospores were somewhat larger than in the present collection (12–15 × 2.5–3 μm), but the general shape, culture characteristics and germination patterns suggest that this is the same species, occurring on dead leaves of Hedychium coronarium. Another species of Mycosphaerella, M. hedychii, is also known from this host in Hawaii and Brazil (Stevens 1925, Soares & Barreto 2008). It differs from M. holualoana by being associated with brown leaf spots, 3–10 mm diam, having shorter asci (25–35 × 5–8 μm), and smaller ascospores (8–11 × 2 μm).
Sphaerulina myriadea (DC.) Sacc., Michelia 1(4): 399 (1878).
(Fig. 8)
Fig. 8.
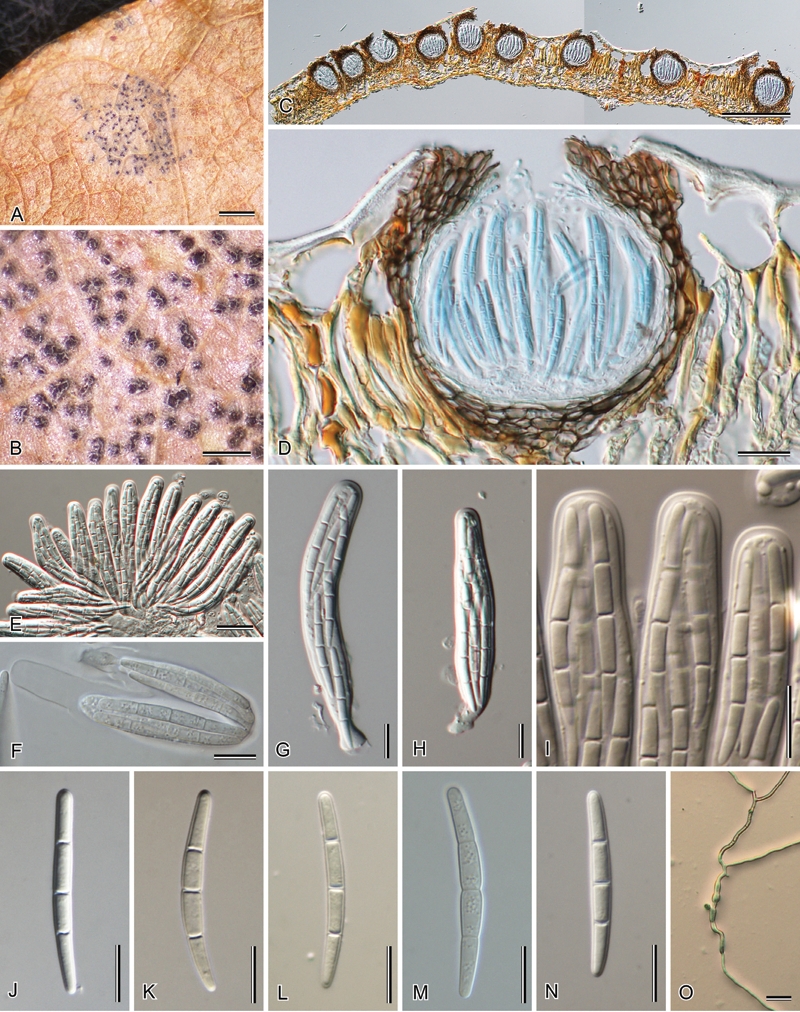
Sphaerulina myriadea (HHUF 29940). A. Leaf spot of Quercus dentata. B. Close-up of erumpent ascomata. C, D. Longitudinal section of ascomata. E. Fasciculate asci. F. Fissitunicate ascus. G, H. Asci. I. Apices of asci. J–N. Ascospores. O. Germinating ascospore. Bars: A = 1 mm; B, C = 200 μm; D, E, O = 20 μm; F–N = 10 μm.
Leaf spots epiphyllous, round to irregular shaped, grey to black, 1.2–8 mm diam (av. 3.8 mm). Ascomata pseudothecial, immersed, subepidermal, erumpent at the top, single to 2–3 grouped, globose in longitudinal section, glabrous, without prominent beak, 100–165 μm tall (av. 128 μm), 90–150 μm diam (av. 115 μm). Ostiole central, 7.5–15 μm wide, with hyaline inner periphyses of 2–2.5 μm wide. Ascomatal wall textura angularis in surface view; in longitudinal section 5–15 μm thick at side and base, composed of 2–4 layers of polygonal to subglobose brown cells (6–18 × 3.5–9 μm), 25–35 μm thick around apical ostiole. Interascal filaments not seen. Asci bitunicate in structure, discharge fissitunicate, clustered, arising from the centrum base of 12–15 μm thick, cylindrical to obclavate, rounded at apex, with or without a shallow apical chamber (ca. 0.5 μm high), with a knob-like stipe (7–10 μm long) or sessile, with 8 tri-seriate to bi-seriate ascospores, 57–82 × 10–13(–14.5) μm (av. 69.0 × 12.2 μm). Ascospores cylindrical, rounded at apex, slightly tapered at below, straight or slightly curved, 3-septate, with a primary septum nearly median (0.48–0.52, av. 0.50), hyaline, smooth, without sheath or appendages, (28–)30–38(–40.5) × (2.5–)3–3.5(–4) μm (av. 34.4 × 3.2 μm), L/W 9.7–12.4 (av. 10.8).
Culture characteristics: Colonies after 2 wk at 24 °C spreading, erumpent, with sparse aerial mycelium and feathery margins, reaching 8 mm diam. On MEA surface saffron, reverse luteous. On OA surface saffron. On PDA surface dirty white to pale luteous; reverse pale luteous.
Specimens examined: UK : sine loc., on leaves of Quercus robur, J.E. Vize [Microfungi Brit. Ex. No. 195] (ex IMI 57186, K(M) 167735). – Japan: Aomori: Tsugaru, Kidukuri, Bense-marsh (40°51′53″ N, 140°17′42″E), on leaves of Quercus dentata, 21 Apr. 2007, K. Tanaka 2243 (HHUF 29940; single ascospore culture CBS 124646 = JCM 15565). – Germany: Driesen, Lasch [Rabenhorst, Fungi Eur. no. 149] (L). – USA : California: Sequoia National Park. alt. 2590 m, on leaves of Castanopsis sempervirens, 18 Jun. 1931, H.E. Parks (BPI 623686); Lake Co., Hoberg’s Resort, on leaves of Quercus kelloggii, 15 May 1943, V. Miller (BPI 623707). Maryland: Marlboro, on leaves of Quercus alba, 26 Apr. 1929, C.L. Shear ( BPI 623705). Texas: Houston, on leaves of Quercus alba, 8 Apr. 1869, H.W. Ravenel (BPI 623704).
Notes: The genus Sphaerulina, which is based on S. myriadea with 3-septate ascospores, was distinguished from Mycosphaerella with 1-septate ascospores. These two genera have traditionally been separated on the basis of ascospore septation alone. However, several species with a Sphaerulina ascomatal anatomy and 3-septate ascospores are known that do not belong to Sphaerulina s. str., suggesting that ascospore septation alone is insufficiently robust to infer phylogenetic relatedness (Crous et al. 2003). The matter is further complicated as S. myriadea, which occurs on hosts in the Fagaceae, appears to be a species complex. Because of this, no epitype is designated here, pending further collections of authentic European material on Quercus from France.
In our study, S. myriadea clusters in Mycosphaerellaceae, as sister to Septoria s.str. (Fig. 1). Although the name Sphaerulina 1878 predates that of Mycosphaerella 1884, Mycosphaerellaceae have recently been shown to represent a generic complex (Crous et al. 2007, 2009a, b), in which Sphaerulina appears to represent a distinct lineage. Mycosphaerella is restricted to species with Ramularia anamorphs (Verkley et al. 2004), and remains distinct from Sphaerulina. A megablast search using the ITS sequence of S. myriadea places it with members of Mycosphaerella, but with a highest query coverage of 90 % for Mycosphaerella brassicicola (GenBank EU167607; Identities = 434/487 (89 %), Gaps = 37/487 (8 %)).
Teratosphaeriaceae
Camarosporula persooniae (Henn.) Petr., Sydowia 8: 99 (1954).
(Fig. 9)
Fig. 9.
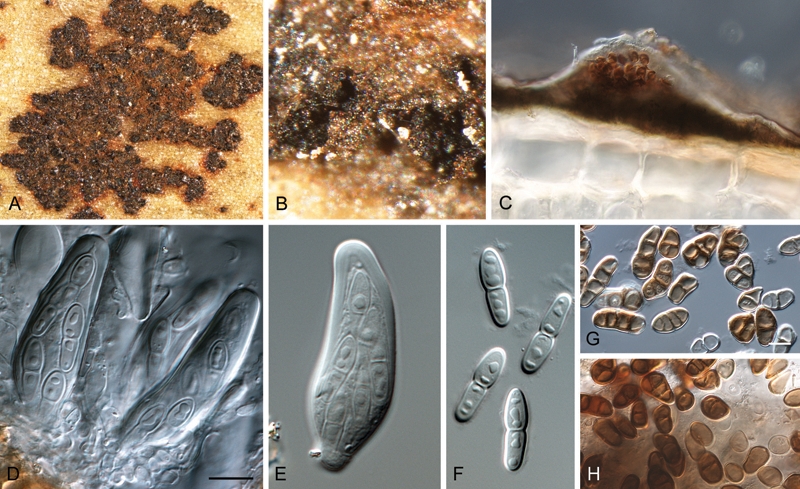
Camarosporula persooniae (CBS 116258). A. Leaf spot with ascostromata. B, C. Acervuli. D, E. Asci. F. Ascospores. G, H. Conidia. Bars = 10 μm.
Basionym: Hendersonia persooniae Henn., Hedwigia 40: 97 (1901).
Synonym: Dichomera persooniae (Henn.) Henn., Hedwigia 42: 87 (1903).
Teleomorph: Anthracostroma persooniae (Henn.) Petr., Sydowia 8: 97 (1954).
Basionym: Mycosphaerella persooniae Henn., Hedwigia 42: 81 (1903).
Synonyms: Sphaerella persooniae (Henn.) Sacc. & D. Sacc., Syll. Fung. 17: 639 (1905).
Pseudosphaerella persooniae (Henn.) Hansf., Proc. Linn. Soc. New South Wales 79: 123 (1954).
Leaf spots amphigenous, irregular, dark brown to black, specks of 0.5 mm, coalescing to larger spots to 1 cm diam. Ascostromata amphigenous, predominently epiphyllous, subcuticular, uni-to multi-locular, erumpent, solitary to aggregated, to 300 μm diam, with single, central, periphysate ostiole, 10–15 μm diam; wall consisting of brown, thick-walled, textura angularis; basal stroma on the epidermis consisting on a single layer of brown, thick-walled cells. Interascal filaments pseudoparaphysoids, filamentous, branched, anastomosed, hyaline, indistinct, constricted at septa, 3–5 μm. Asci sessile, stipitate, obovoid, 8-spored, 30–50 × 12–15 μm, with well developed apical chamber, 1–2 μm diam, and multi-layered endotunica. Ascospores fusoid-ellipsoid, hyaline, smooth, thick-walled, prominently guttulate, widest just above the median septum, prominently constricted at septum, (12–)13–15(–17) × (3–)4–5(–5.5) μm; becoming pale brown in older asci.
Conidiomata acervular, amphigenous, subcuticular, epidermal to subepidermal, separate or confluent, to 1 mm diam, composed of dark brown, thick-walled, globose to angular cells, dehiscing irregularly at the apex, visible as small black spots on superficial stromata. Conidiophores reduced to conidiogenous cells. Conidiogenous cells determinate, integrated, cylindrical to doliiform, hyaline to pale brown, smooth to finely verruculose, lining the conidiomatal cavity, 3–10 × 3–5 μm; minute periclinal thickening visible at apex, which in some cases appears to also proliferate percurrently. Conidia dictyoseptate, distoseptate, with 3 transverse septa and 1–2 vertical or oblique septa, thick-walled, finely verruculose, irregular or clavate to obclavate, with a broad truncate base, (10–)13–15(–17) × (5–)7–8(–10) μm.
Culture characteristics: Colonies after 2 wk at 24 °C spreading, erumpent with folded surface, and smooth, lobate margins, reaching 7 mm diam. On MEA surface umber to olivaceous grey; reverse iron-grey. On OA surface olivaceous rey. On PDA surface and reverse olivaceous grey.
Specimens examined: Australia: Western Australia: Perth, on Persoonia elliptica, 1900, Pritzel 104a (B – holotype of Hendersonia persooniae). New South Wales: Boudhi National Park, coastal understory, on leaves of Persoonia levis, 25 Sept. 1983, C. Liddell (K(M) 167734, ex IMI 281194); teleomorph and anamorph present; Ku-ring-gai Chase National Park, on leaves of Persoonia sp., 16 Nov. 1999, P.W. Crous & B.A. Summerell, epitype (CBS H-20548 – epitypus hic designatus of Hendersonia persooniae; cultures ex-epitype CPC 3344 = CBS 116258, CPC 3343 = CBS 112302).
Notes: The teleomorph genus Anthracostroma is based on a species initially described in Mycosphaerella as M. persooniae, which is in accordance to its Mycosphaerella-like morphology. Within the Teratosphaeriaceae this genus is distinct in that the ascomata are situated in a subcuticular stroma, and the asci are intermingled among numerous pseudoparaphyses. The Camarosporula anamorph is reminiscent of Dichomera, which is again allied to Botryosphaeriales (Barber et al. 2005, Crous et al. 2006a). Camarosporula is thus distinct within Teratosphaeriaceae. Both the phylogenetic analysis of the LSU sequences (Fig. 1) and the megablast searches of the ITS sequences place Phaeothecoidea melaleuca as closest sister species to Camarosporula persooniae.
Teratosphaeria agapanthi (Kalchbr. & Cooke) Crous, comb. nov.
MycoBank MB560086
(Fig. 10)
Fig. 10.

Teratosphaeria agapanthi (CPC 18304). A, B. Leaf spots and blight on Agapanthus. C. Circular aggregation of ascomata. D. Evenly distributed ascomata with ostiolar regions visible. E, G–J. Asci. F. Ostiole with periphyses (arrow). K. Ascospores. L. Germinating ascospores. Bars = 10 μm.
Basionym: Sphaerella agapanthi Kalchbr. & Cooke, Grevillea 9: 31 (1880).
Synonym: Mycosphaerella agapanthi (Kalchbr. & Cooke) Lindau, in Engler & Prantl, Natürlichen Pflanzenf. 1(1): 426 (1897).
In vivo: Leaf spots amphigenous, ellipsoid, large, developing where plants are grown in wet, shady areas, pale to medium brown, with a red-brown border, coalescing, becoming visible as leaf tip blight symptoms. Ascomata amphigenous, black, substomatal, erumpent, predominantly arranged in tight, round clusters, 2–6 mm diam; ascomata 80–150 μm diam, with central, periphysate ostiole, 10–15 μm diam; periphyses 0–1-septate, 10–15 × 1.5–2 μm; ascomatal wall of 3–4 layers of brown textura angularis. Interascal filaments absent. Asci fasciculate, bitunicate with fissitunicate dehiscence, subsessile, fusoid-ellipsoid, straight to slightly curved, 8-spored, 25–45 × 15–25 μm, with a small apical apiculus. Ascospores multi-seriate, hyaline, thick-walled, with angular cellular inclusions, fusoid-ellipsoid, medianly 1-septate, becoming constricted at the septum, widest in middle of apical cell, with rounded ends, granular, (17–)18–20(–21) × 4.5–5(–6) μm; ascospore germination irregular, from both ends or middle of the cell, parallel or at angle to the long axis; spore becoming brown, verruculose, 6–7 μm wide, but not swelling and distorting after 24 h on MEA.
Culture characteristics: Colonies after 14 d at 24 °C spreading, with sparse aerial mycelium and even, smooth, lobate margins, reaching 30 mm diam. On MEA surface dark mouse-grey, reverse greenish black. On OA surface olivaceous grey. On PDA surface olivaceous grey, reverse iron-grey.
Specimens examined: South Africa: Western Cape Province: on upper surface of dead leaves of Agapanthus umbellatus (Alliaceae), Kalchbrenner 1342 (K (M) – holotype); Kirstenbosch Botanical Garden, at entrance to lower gate, on leaf spots of A. umbellatus, 8 May 2010, P.W. Crous (CBS H-20552 – epitypus hic designatus; cultures ex-epitype CPC 18304–18305 = CBS 129192). – Portugal: Braga, Ria do Souto, on dead leaf of A. umbellatus, 8 June 2010, P.W. Crous (CBS H-20553; culture CPC 18332–18333= CBS 129064).
Notes: Ascospores in the holotype specimen were similar (17–21 × 5–6 μm) to those observed in the present collections. Aptroot (2006) regarded this as a species of Davidiella, but probably only observed immature asci, given that his observations referred to somewhat smaller ascospores (10–12 × 4–5 μm). Furthermore, he also refers to other African collections, which again match the ascospore dimensions on the type. These observations, together with its occurrence on Agapanthus in Portugal, suggest this species to probably be host-specific. Mycosphaerella agapanthi-umbellati, described from the same host in India, may be a later synonym, with asci 25–50 × 14 μm, and ascospores 14–25 × 4–7 μm (Corlett 1991). Both LSU and ITS data place T. agapanthi in Teratosphaeria (Fig. 1), with ITS sequences of T. considenianae (GenBank GQ852792), T. miniata (GenBank GQ852803) and T. stellenboschiana (GenBank GQ852825) having the highest identity (94 %) in a megablast search of the GenBank nucleotide database.
DISCUSSION
The present study resolves the phylogenetic position of several genera of which the classification has been the topic of past speculation. The genus Camarosporula (teleomorph Anthracostroma) is shown to belong to the Teratosphaeriaceae, which is not totally surprising given the fact that its teleomorph was originally described as Mycosphaerella persooniae. However, based on its unique ascostromata with erumpent ascomata, and acervular conidiomata with muriformly septate, brown conidia, Camarosporula appears to represent a distinct genus within this family, which is also supported by its DNA phylogeny (Fig. 1). The phylogenetic position of Sphaerulina, and potential taxonomic implications thereof has been discussed in detail elsewhere (Crous et al. 2003), as the genus predates Mycosphaerella. Results obtained in this study, however, have shown that while Mycosphaerella-like taxa with 3-septate ascospores have evolved more than once in the Mycosphaerellaceae (Crous et al. 2003), Sphaerulina s.str., typified by S. myriadea, represents a lineage embedded in the Pseudocercospora/Septoria clade. Although Sphaerulina may represent a genus in its own right, the present data are still insufficient to resolve its phylogeny within the Mycosphaerellaceae.
This study also introduces two species of Cladosporium that have teleomorphs, C. silenes which is a member of the C. cladosporioides complex (Bensch et al. 2010), and C. grevilleae which does not form an anamorph in culture. Furthermore, we managed to recollect a species associated with leaf spots of Agapanthus in South Africa, M. agapanthi, which Aptroot (2006) suspected to represent a possible species of Davidiella. As can be seen from its phylogenetic position (Fig. 1), however, it is a species of Teratosphaeria, which appears to be closely associated with its host, has been introduced into Europe (Portugal), and may also occur in other continents where this plant is cultivated.
Two species of Mycosphaerella known from leaf litter collected in Hawaii have been treated; M. etlingerae on Etlingera elatior, and M. holualoana on Hedychium coronarium. The latter species has until now been accepted as a leaf pathogen of Leucospermum in Hawaii (Taylor et al. 2001), and its occurrence on leaf litter of another host may suggest that more stringent plant hygiene practices need to be followed in Protea fields in Hawaii, as dead leaves of Hedychium appear to act as an alternate host for this fungus.
Although this study resolved several long standing questions related to the phylogeny of genera such as Camarosporula/Anthracostroma and Sphaerulina, it also raised some new questions. This is specifically true for the species described here as M. cerastiicola, collected on Cerastium semidecandrum in The Netherlands. While the teleomorph is rather odd in the sense that it can have less than eight ascospores, the anamorph is very peculiar in having a Septoria- or Pseudocercosporella-like morphology (pycnidia to sporodochia, and sympodial to phialidic proliferation, with conidia also formed individually on aerial mycelium). The oddity of this species lies in the fact that it clusters in the middle of the Ramularia clade, which has hitherto been accepted as monophyletic (Crous et al. 2009a, b). Whether Ramularia would eventually be reavealed as paraphyletic, or if this Septoria-like anamorph in fact represents a synanamorph of a Ramularia species, can only be resolved once more Mycosphaerella species with Ramularia-like anamorphs have been collected and subjected to DNA analysis.
Acknowledgments
We thank the technical staff, Arien van Iperen (cultures), Marjan Vermaas (photographic plates), and Mieke Starink-Willemse (DNA isolation, amplification and sequencing) for their invaluable assistance. Walter Gams and André Aptroot (both formerly CBS) are thanked for several of the collections treated here. The curators of B (Botanische Garten und Botanisches Museum, Berlin-Dahlem, Germany), BPI (USDA, Beltsville, USA), and Kew (UK) are acknowledged for making several valuable specimens available for study.
REFERENCES
- Aptroot A. (2006) Mycosphaerella and its anamorphs: 2. Conspectus of Mycosphaerella. CBS Biodiversity Series 5: 1–231 [Google Scholar]
- Barber PA, Burgess T, Hardy G, St J, Slippers B, Keane PJ, Wingfield MJ. (2005) Botryosphaeria species from Eucalyptus in Australia are pleoanamorphic, producing Dichomera synanamorphs in culture. Mycological Research 109: 1347–1363 [DOI] [PubMed] [Google Scholar]
- Batzer JC, Mercedes Diaz Arias M, Harrington TC, Gleason ML, Groenewald JZ, Crous PW. (2008) Four species of Zygophiala (Schizothyriaceae, Capnodiales) are associated with the sooty blotch and flyspeck complex on apple. Mycologia 100: 246–258 [DOI] [PubMed] [Google Scholar]
- Bensch K, Groenewald JZ, Dijksterhuis J, Starink-Willemse M, Andersen B, Summerell BA, Shin H-D, Dugan FM, Schroers H-J, Braun U, Crous PW. (2010) Species and ecological diversity within the Cladosporium cladosporioides complex (Davidiellaceae, Capnodiales). Studies in Mycology 67: 1–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U, Crous PW, Dugan F, Groenewald JG, Hoog SG de. (2003) Phylogeny and taxonomy of Cladosporium-like hyphomycetes, including Davidiella gen. nov., the teleomorph of Cladosporium s. str. Mycological Progress 2: 3–18 [Google Scholar]
- Carbone I, Kohn LM. (1999) A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556 [Google Scholar]
- Corlett M. (1991) An annotated list of the published names in Mycosphaerella and Sphaerella, Mycologia Memoir 18: 1–328 [Google Scholar]
- Cortinas MN, Crous PW, Wingfield BD, Wingfield MJ. (2006) Multi-gene phylogenies and phenotypic characters distinguish two species within the Colletogloeopsis zuluensis complex associated with Eucalyptus stem cankers. Studies in Mycology 55: 133–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW. (1998) Mycosphaerella spp. and their anamorphs associated with leaf spot diseases of Eucalyptus. Mycologia Memoirs 21: 1–170 [Google Scholar]
- Crous PW. (2002) Taxonomy and Pathology of Cylindrocladium (Calonectria) and allied genera. St Paul, MN: APS Press; [Google Scholar]
- Crous PW. (2009) Taxonomy and phylogeny of the genus Mycosphaerella and its anamorphs. Fungal Diversity 38: 1–24 [Google Scholar]
- Crous PW, Aptroot A, Kang JC, Braun U, Wingfield MJ. (2000) The genus Mycosphaerella and its anamorphs. Studies in Mycology 45: 107–121 [Google Scholar]
- Crous PW, Braun U. (2003) Mycosphaerella and its anamorphs. 1. Names published in Cercospora and Passalora. CBS Biodiversity Series 1: 1–571 [Google Scholar]
- Crous PW, Braun U, Groenewald JZ. (2007) Mycosphaerella is polyphyletic. Studies in Mycology 58: 1–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G. (2004a) MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19–22 [Google Scholar]
- Crous PW, Groenewald JZ. (2005) Hosts, species and genotypes: opinions versus data. Australasian Plant Pathology 34: 463–470 [Google Scholar]
- Crous PW, Groenewald JZ, Mansilla JP, Hunter GC, Wingfield MJ. (2004b) Phylogenetic reassessment of Mycosphaerella spp. and their anamorphs occurring on Eucalyptus. Studies in Mycology 50: 195–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ, Pongpanich K, Himaman W, Arzanlou M, Wingfield MJ. (2004c) Cryptic speciation and host specificity among Mycosphaerella spp. occurring on Australian Acacia species grown as exotics in the tropics. Studies in Mycology 50: 457–469 [Google Scholar]
- Crous PW, Groenewald JZ, Wingfield MJ, Aptroot A. (2003) The value of ascospore septation in separating Mycosphaerella from Sphaerulina in the Dothideales: a Saccardoan myth? Sydowia 55: 136–152 [Google Scholar]
- Crous PW, Kang JC, Braun U. (2001) A phylogenetic redefinition of anamorph genera in Mycosphaerella based on ITS rDNA sequence and morphology. Mycologia 93: 1081–1101 [Google Scholar]
- Crous PW, Schoch CL, Hyde KD, Wood AR, Gueidan C, Hoog GS de, Groenewald JZ. (2009a) Phylogenetic lineages in the Capnodiales. Studies in Mycology 64: 17–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO, Philips AJL, Alves A, Burgess T, Barber P, Groenewald JZ. (2006a) Phylogenetic lineages in the Botryosphaeriaceae. Studies in Mycology 55: 235–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Carnegie AJ, Wingfield MJ, Hunter GC, Burgess TI, Andjic V, Barber PA, Groenewald JZ. (2009b) Unravelling Mycosphaerella: do you believe in genera? Persoonia 23: 99–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Groenewald JZ, Samson RA. (eds) (2009c) Fungal Biodiversity. [CBS Laboratory Manual Series no. 1.] Centraalbureau voor Schimmelcultures, Utrecht: [Google Scholar]
- Crous PW, Wingfield MJ, Mansilla JP, Alfenas AC, Groenewald JZ. (2006b) Phylogenetic reassessment of Mycosphaerella spp. and their anamorphs occurring on Eucalyptus. II. Studies in Mycology 55: 99–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Park RF. (1991) Mycosphaerella nubilosa a synonym of M. molleriana. Mycological Research 95: 628–632 [Google Scholar]
- Farr DF, Bills GF, Chamuris GP, Rossman AY. (1995) Fungi on plants and plant products in the United States. St Paul, MN: APS Press; [Google Scholar]
- Hillis DM, Bull JJ. (1993) An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Systematic Biology 42: 182–192 [Google Scholar]
- Hoog GS de, Gerrits van den Ende AHG. (1998) Molecular diagnostics of clinical strains of filamentous Basidiomycetes. Mycoses 41: 183–189 [DOI] [PubMed] [Google Scholar]
- O’Donnell K, Kistler HC, Cigelnik E, Ploetz RC. (1998) Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proceedings of the National Academy of Sciences, USA 95: 2044–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RDM. (1996) TREEVIEW: An application to display phylogenetic trees on personal computers. Computer Applications in the Biosciences 12: 357–358 [DOI] [PubMed] [Google Scholar]
- Pretorius MC, Crous PW, Groenewald JZ, Braun U. (2003) Phylogeny of some cercosporoid fungi from Citrus. Sydowia 55: 286–305 [Google Scholar]
- Rambaut A. (2002) Sequence Alignment Editor. Version 2.0. Oxford: Department of Zoology, University of Oxford; [Google Scholar]
- Rayner RW. (1970) A Mycological Colour Chart. Kew: Commonwealth Mycological Institute; [Google Scholar]
- Schoch CL, Shoemaker RA, Seifert KA, Hambleton S, Spatafora JW, Crous PW. (2006) A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia 98: 1041–1052 [DOI] [PubMed] [Google Scholar]
- Schubert K, Groenewald JZ, Braun U, Dijksterhuis J, Starink M, Hill CF, Zalar P, Hoog GS de, Crous PW. (2007) Biodiversity in the Cladosporium herbarum complex (Davidiellaceae, Capnodiales), with standardisation of methods for Cladosporium taxonomy and diagnostics. Studies in Mycology 58: 105–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares DJ, Barreto RW. (2008) Fungal pathogens of the invasive riparian weed Hedychium coronarium from Brazil and their potential for biological control. Fungal Diversity 28: 85–96 [Google Scholar]
- Stevens FL. (1925) Hawaiian fungi. Bernice P. Bishop Museum Bulletin 19: 1–189 [Google Scholar]
- Swofford DL. (2003) PAUP*: phylogenetic analysis using parsimony (*and their methods). Version 4. Sunderland, MA: Sinauer Associates; [Google Scholar]
- Taylor JE, Crous PW, Palm ME. (2001) Foliar and stem fungal pathogens of Proteaceae in Hawaii. Mycotaxon 78: 449–490 [Google Scholar]
- Verkley GJM, Crous PW, Groenewald JZ, Braun U, Aptroot A. (2004) Mycosphaerella punctiformis revisited: morphology, phylogeny, and epitypification of the type species of the genus Mycosphaerella (Dothideales, Ascomycota). Mycological Research 108: 1271–1282 [DOI] [PubMed] [Google Scholar]
- Vilgalys R, Hester M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee J, Taylor J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (eds), PCR Protocols: a guide to methods and applications: 315–322 San Diego, CA: Academic Press; [Google Scholar]


