Abstract
The phylogenetic placement of the monotypic dematiaceous hyphomycete genus Xanthoriicola was investigated. Sequences of the nLSU region were obtained from 11 specimens of X. physciae, which formed a single clade supported both by parsimony (91 %), and maximum likelihood (100 %) bootstraps, and Bayesian Posterior Probabilities (1.0). The closest relatives in the parsimony analysis were species of Piedraria, while in the Bayesian analysis they were those of Friedmanniomyces. These three genera, along with species of Elasticomyces, Recurvomyces, Teratosphaeria, and sequences from unnamed rock-inhabiting fungi (RIF), were all members of the same major clade within Capnodiales with strong support in both analyses, and for which the family name Teratosphaeriaceae can be used pending further studies on additional taxa.
Keywords: Ascomycota, Capnodiales, Friedmanniomyces, hyphomycetes, lichenicolous fungi, Piedrariaceae, rock inhabiting fungi
INTRODUCTION
The generic name Xanthoriicola was introduced for the species X. physciae (Hawksworth & Punithalingam 1973). This fungus appears to be obligately lichenicolous on Xanthoria parietina in Europe, and is also reported from Africa and Asia (Siefert et al. 2011). The fungus primarily occurs in the apothecia, growing through the hymenium, with broad cupulate enteroblastic conidiogenous cells generating conidia at the surface (Fig. 1). The conidia are dark brown, spherical, single-celled, and have a coarse, warted surface ornamentation. This fungus was illustrated by line drawings in Hawksworth & Punithalingam (1973), and photomicrographs and scanning electron micrographs are presented in Hawksworth (1979). The surfaces of infected apothecia become sooty black and so are easily seen in the field. Whole swards of the host lichen are rarely affected, so while deleterious to the host it does not destroy their populations. It has not been reported as growing in isolated culture, and experiments to inoculate fresh specimens of the host have proven unsuccessful (T.F. Preece, unpubl. data). Furthermore, no sexual state has been discovered or postulated by association with other fungi that occur on the same host lichen.
Fig. 1.
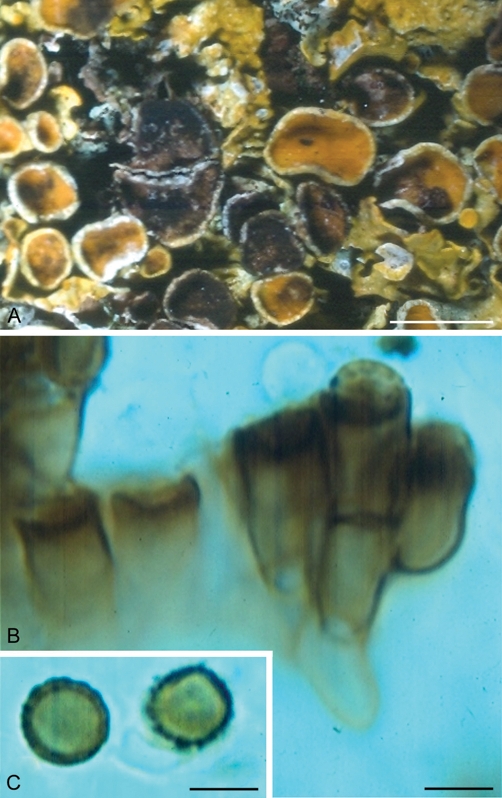
Xanthoriicola physciae (IMI 164974). A. Apothecia of Xanthoria parietina infected by the fungus. B. Conidiogenous cells in the upper part of the hymenium. C. Conidia. Bars A = 5 mm, B–C = 5 μm.
The fungus is particularly unusual in that the conidia are formed “semi-endogenously”, that is within the lower part of the collarette of the conidiogenous cells. This is a rare situation in hyphomycetous conidial fungi, and is otherwise seen only in Craspedodidymum, Cystodendron, Lambinonia, Metacapnodium, and some groups of Phialophora s. lat. (Ellis 1976, Seifert et al. 2011). In addition, some studies by Raman spectroscopy suggested that Xanthoriicola physciae might form scytonemin, a protective pigment only otherwise known in cyanobacteria (Preece 2009) – although that report now seems likely to have been a result of contamination from cyanobacteria growing on the surface of the hymenium.
Xanthoriicola physciae consequently appeared, on morphological grounds to occupy an isolated position amongst the conidial fungi. This investigation was initiated in order to determine its phlyogenetic relationships.
MATERIALS AND METHODS
Choice of additional taxa and outgroup
In addition to the Xanthoriicola specimens sequenced, 48 specimens of Dothideomycetes were included in the molecular study (Figs 2–3). The sampling was selected to include taxa that were close to our new sequences in GenBank, i.e. mainly conidial members of the Teratosphaeriaceae, together with other representatives of Capnodiales and Dothideales. Dothidea insculpta was used as outgroup.
Fig. 2.
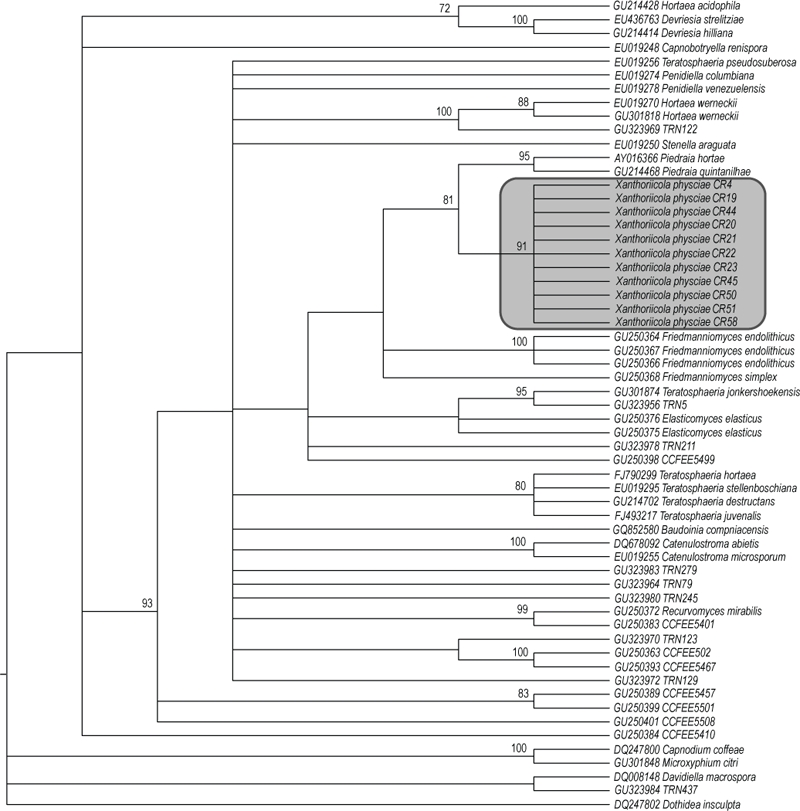
Consensus tree of 725 000 equally most parsimonious trees from the analysis of the nLSU dataset. Bootstrap values ≥ 70 % are indicated over branches. Species name and GenBank accession number are given for each terminal. Sequences from cultures which have not been named are referred to by culture reference numbers. The tinted box includes Xanthoriicola physciae.
Fig. 3.
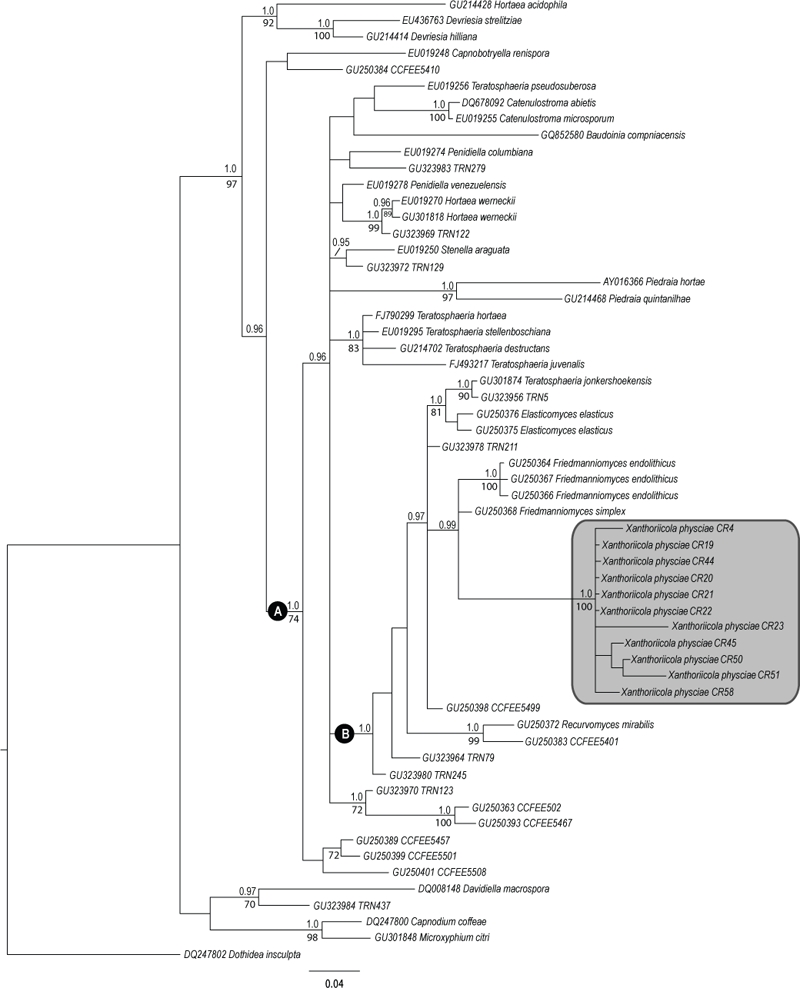
50 % majority rule Bayesian consensus tree with average branch lengths from the analysis of the nLSU dataset. Bayesian posterior probability values ≥ 0.95 are indicated over branches and ML bootstrap values ≥ 70 %, below branches. Branch lengths are scaled to the expected number of substitutions per site. A tinted box includes Xanthoriicola physciae. See the text for discussion of the clades distinguished as “A” and “B”.
Voucher information, and GenBank accession numbers of newly sequenced taxa are provided in Table 1.
Table 1.
Specimens of Xanthoriicola physciae from which sequence data were obtained, with details of reference collections where they are held and GenBank accession numbers.
| Specimen no. | Reference collection no. | GenBank accession no. | Country | Locality |
|---|---|---|---|---|
| CR4 | MAF-LICH 16882 | JN040487 | UK, England | St. Martins, Shropshire |
| CR19 | MAF-LICH 16883 | JN040488 | UK, England | Kinton, Oswestry, Shropshire |
| CR20 | MAF-LICH 16884 | JN040489 | UK, Wales | Kidwelly Quay, Carmarthenshire |
| CR21 | MAF-LICH 16885 | JN040490 | UK, England | Ashtead, Surrey |
| CR22 | MAF-LICH 16886 | JN040491 | UK, England | Headly Heath, Surrey |
| CR23 | MAF-LICH 16887 | JN040492 | UK, England | Ashtead, Surrey |
| CR44 | IMI 402504 | JN040493 | Germany | Regierungsbezirk Oberbayern, Bayern |
| CR45 | K(M)116894 | JN040494 | UK, Wales | Aferedw, Powys |
| CR50 | MAF-LICH 16888 | JN040495 | UK, England | Richmond upon Thames, Surrey |
| CR51 | MAF-LICH 16889 | JN040496 | UK, England | Tickhill, S. Yorkshire |
| CR58 | MAF-LICH 16890 | JN040497 | UK, England | Slapton Ley NNR, S. Devon |
DNA extraction
DNA was extracted directly from dried specimens. Fungi growing in the host apothecia were carefully excised with the point of a sterile scalpel blade to minimize as much as possible the obtaining of host tissue. Total DNA was extracted using the Qiagen DNeasy Plant MiniKit, according to the manufacturer’s instructions.
Amplification and sequencing
A fragment of ca. 1000 bp in the nLSU was amplified using the primers LR0R (R Vilgalys, www.biology.duke.edu/fungi/mycolab/primers.htm), LR5 (Vilgalys & Hester 1990), and also ones specifically designed in our laboratory to selectively amplify the DNA of Xanthoriicola physciae, avoiding that of the host. The primers we designed were: X158F (5′-GAGAGGATGCTTCTGGGCA-3′) and X756R (5′-CCGAAGCTCCCACCTCCGTT-3′). Primer combinations used were LR0R/LR5, LR0R/ X756R, and X158F/LR5PCR.
PCR amplifications were performed using Illustra™ Hot Start PCR beads, according to the manufacturer’s instructions, and using the settings in Hawksworth et al. (2010).
Before sequencing, the PCR products were purified using the Viogene PCR-M Clean-up System or the enzymatic method Exo-sap-IT©.
Sequence alignment and phylogenetic analysis
Sequences were aligned using MAFFT v. 6.611 (Katoh et al. 2002, Katoh & Toh 2008) using the procedures described in Wedin et al. (2009). The ambiguous regions in the alignment were identified and eliminated using Gblocks v. 0.91b (Castresana 2000).
Maximum parsimony and parsimony bootstrap analyses were performed using PAUP v. 4.0b10 (Swofford 2003) with the following settings: gaps were treated as “missing data”, 1 000 random addition sequence replicates, TBR branch swapping, steepest descent off, collapse branches if minimum length is 0, MulTrees on, and with 1000 trees allowed to be saved in each replicate. For the bootstrap analyses (Felsenstein 1985) we used: heuristic search settings identical with the above analysis, but with ten random addition replicates, 1000 bootstrap replicates, a full heuristic search, and retained groups with a frequency > 50 %. Parsimony-uninformative characters were excluded from these analyses.
Maximum likelihood analyses (ML) were achieved using the program Garli v. 0.951 (Zwickl 2006). Runs were terminated after 10 000 generations with no significant improvement in – lnL. Improvement values were set to 0.01 with a total improvement lower than 0.05 compared to the last topology recovered. Bootstrap support was assessed using 1 000 tree replicates under the same parameters as above.
We used the Bayesian method of Huelsenbeck et al. (2001) to analyse the data by Markov Chain Monte Carlo (MCMC) sampling as implemented in the software MrBayes v. 3.1.2 (Huelsenbeck & Ronquist 2001). Likelihood models were selected for each of the three gene regions using the Akaike Information Criterion (AIC) and the Bayesian Information Criterion (BIC) as implemented in jModeltest (Posada 2008). We used full likelihood optimization and selected from among only the 24 models implemented in MrBayes. Following this scheme, a GTR+I+G model was chosen for the nuclear LSU rDNA data using both criteria. The number of discrete gamma categories was kept at default four. Bayesian prior distributions included treating all tree topologies as equally likely, a uniform (0, 50) distribution for the gamma shape parameter, a uniform (0, 1) distribution for the proportion of invariable sites, and a flat (1, 1, 1, 1, 1, 1) Dirichlet for the rate matrix. Two parallel runs were performed, each with five chains, four of which were incrementally heated with a temperature of 0.15. The analysis was diagnosed for convergence every 100 000 generations, measured as the average standard deviation of splits across runs in the last half of the analysis. Every 100th tree was saved, and the first half of the run was discarded as burn-in.
RESULTS
We generated 11 new nLSU rDNA sequences (Table 1), which were aligned together with sequences already available in GenBank.
The matrix contained 775 characters from which 167 unambiguously aligned parsimony informative sites were used in the parsimony analysis. Our maximum parsimony analysis resulted in 725 000 most parsimonious trees of 644 steps, with CI = 0.393 and RI = 0.692.
The Bayesian analysis halted after 4 800 000 generations, when the average standard deviation of split frequencies across runs was then lower than 0.01 (= 0.0096). We considered the two runs to have converged and a majority rule consensus tree was constructed from the 48 000 trees of the stationary tree sample. Bayesian and ML analyses produced congruent phylogenies.
The 11 specimens of Xanthoriicola physciae formed a single clade supported both by parsimony bootstrap (91 %), ML bootstrap (100 %), and Bayesian Posterior Probabilities (1.0) (Figs 2–3).
Some incongruence was found between the results of parsimony analysis and the two other analysis methods used, and therefore two topologies are shown. The maximum parsimony reconstruction is shown in Fig. 2. Since no conflicts were found between the Bayesian and the ML topologies, only the Bayesian reconstruction is shown in Fig. 3, with ML bootstrap values added. When the maximum parsimony method was used, Piedraia species appeared as the closest relatives of Xathoriicola, together forming a clade with 81 % Bootstrap support (Fig. 2). However, the Bayesian and maximum likelihood methods both recovered Friedmanniomyces as the sister group of Xanthoriicola, although only with strong phylogenetic support by the Bayesian method (Bayesian posterior probability = 0.99; Fig. 3). In this analysis, Xanthoriicola and Friedmannimyces are included in a more inclusive monophyletic group together with Elasticomyces elasticus, Recurvomyces mirabilis, Teratosphaeria jonkershekensis, and diverse unnamed rock inhabiting fungi (Bayesian posterior probability = 1.0).
DISCUSSION
The systematic placement of the monotypic lichenicolous genus Xanthoriicola has remained obscure in the absence of any known sexual stage. Both the analyses we undertook place it in the same general area of the fungal phylogenetic tree, but with some differences as to the taxa revealed as the closest known relatives.
In the maximum parsimony tree (Fig. 2), the genus Piedraia forms the sister group. Piedraia comprises two known species both of which form minute ascomata on hair; P. hortae on human hair (“black piedra”) and P. quintanilhae on that of chimpanzees. The ascomata occur as black nodules on the hair, and have vermiform single-celled ascospores; the ascospores have whip-like extensions at both ends in P. hortae, but such extensions are absent in those of P. quintanilhae. No asexual state is known, and reports of one in Trichosporon are attributable to mixed infections. Excellent illustrations of P. hortae, and references to key literature, are provided by de Hoog et al. (2000). Sister to the Piedraia/Xanthoriicola clade in this analysis, but without bootstrap support, are two species of the hyphomycete genus Friedmanniomyces, which has no known sexual state.
Interestingly, in the Bayesian analysis (Fig. 3) it is the Friedmanniomyces species that appear as the sister group, with high support for the clade (0.99), the relationship to the Piedraia species being unresolved. This leads us to suspect that the relationship between Xanthoriicola and Piedraia, observed in the parsimony analyses, could be due to a long-branch attraction effect. Further analyses using additional molecular markers will be needed to ascertain this relationship. Friedmanniomyces endolithicus is found growing intermixed with cryptoendolithic lichen hyphae in the surface layers of sandstones in Antarctica. It has pale brown hyphae which give rise to chains of schizolytically produced doliiform conidia which are brown, 0(–3)-septate, and have truncated ends, and also forms multicellular balls of thick-walled brown cells (Seifert et al. 2011: pl 2D). In F. simplex, which occurs in similar situations, the conidia are darker brown, 1(–2)-celled, generally more elongated, with peculiar and often terminal chlamydospore-like cells, but no multicellular conidial balls. Both species grow in pure culture, and are described in detail by Selbmann et al. (2005).
Fungi in the clade labelled “B” in the Bayesian tree, with 1.0 support (Fig. 3) include two monotypic genera. Recurvomyces mirabilis which was also isolated from sandstone in Antarctica (Selbmann et al. 2008), but unlike the Friedmanniomyces species produces 0–1-septate, subhyaline to yellowish brown, thin-walled, elongate-ellipsoid conidia forming enteroblastically from the eponymous conidiophores which are characteristically often bent back towards the hyphae on which they arise. Elasticomyces elasticus was originally isolated from thalli of Usnea antarctica in Antarctica (Selbmann et al. 2008), but has since been found obtained from rock surfaces in the Alps, Andes, and Himalayas (Selbmann, pers. comm.). The conidium production in Elasticomyces is particularly distinctive. The pale to dark brown conidia are produced as arthrospores from long hyphae, the apices of which can continue to grow while the distal regions break up into 1-septate to multiseptate fragments; the conidium walls can be smooth or somewhat rough. Superb illustrations of this fungus and of Recurvomyces mirabilis are provided by Selbmann et al. (2008).
Also present in clade “B” (Fig. 3) are Teratosphaeria jonkershoekensis, in which no conidial state is known, and numerous unnamed sequences, apparently all derived from cultures or sequences obtained from rocks; rock-inhabiting fungi (RIF). Of especial interest because of its sister group position to the Friedmanniomyces/Xanthoriicola clade is CCFEE5499. That fungus was isolated from rock surfaces in the Alps and presents as a non-sporulating, dark, felty on the surface, crustose mycelium, with a morphology characteristic of RIF; ITS analyses show it to be distinct from F. endolithicus but it does fall in a separate group of fungi all isolated from rock surfaces (Selbmann, pers. comm.).
The clade labelled as “A” (with 1.0 bootstrap support), which includes Piedraia and the Friedmanniomyces/Xanthoriicola clade, also comprises the clinically important black yeast Hortaea werneckii (responsible for “tinea nigra” on human hands or more rarely feet; de Hoog et al. 2000) and several other fungi of which species of Teratosphaeria predominate (Fig. 3). Teratosphaeria had been synonymized with Mycosphaerella, but, was resurrected by Crous et al. (2007) and placed in the new family Teratosphaeriaceae, a sister family to Mycosphaerellaceae. The type species, T. fibrillosa, has a superficial stroma linking the ascomata (not seen in most other species), ascospores becoming brown and verruculose while in the ascus and with a mucous sheath, a multilayered endotunica in the ascus, pseudoparaphysoidal remnants disappearing with age, and ostiolar periphyses present in some other species. Around 60 species are now recognised, but some have no known anamorphs, and some no known teleomorph. The anamorphs have been referred to 12 different genera, including Hortaea and Pseudotaeniolina.
We note that two sterile filamentous lichens, Cystocoleus and Racodium, also proved to have affinities with Hortaea werneckii (Muggia et al. 2008). Indeed, Crous et al. (2009) found that Cystocoleus fell into the Teratosphaeriaceae clade, while Racodium was more basal and incertae sedis. LSU sequences for these genera were included in the preliminary analyses to look for a possible relation to Xanthoriicola, but both grouped with sequences of the Capnodiales acting as an outgroup and so were not included in the final analyses. However, it is nevertheless interesting to note that these lichenized fungi have some phylogenetic relationship to fungi able to colonise lichen and plant tissues, and rock surfaces. All these are under extreme environmental conditions of dryness, large temperature fluctuations, solar radiation, and nutrient shortage. The adaptation of these different fungi to such diverse ecological strategies merits further exploration.
We conclude that Xanthoriicola should, on the basis of the LSU analyses carried out here, be most appropriately referred to the same family as Piedraia and Teratosphaeria in Capnodiales. This clade has strong support in both the maximum parsimony (93 %) and Bayesian (1.0) analyses. We note that Piedrariaceae (Barr 1979) is an earlier family name than Teratosphaeriaceae (Crous et al. 2007), but we do not take that up here as we consider that the affinity with Piedraria may be accentuated by long-branch attraction, and also that much more taxon-sampling in this part of the fungal tree of life is necessary to be confident about familial circumscriptions.
This study shows that technical advances can facilitate progress in the resolution of systematic placements for lichenicolous fungi with no sexual state and currently of uncertain position, and also that an understanding of the relationships of these taxa has implications for phylogenetic reconstructions in the Dothideomycetes. Further, molecular phylogenetics, using DNA from specimens, has the power to resolve problematic generic and species concepts in lichenicolous fungi.
Acknowledgments
We are grateful to Tom F. Preece for access to the results of his unpublished experiments with this fungus, to Mark R.D. Seaward for comments on the scytonemin report, and to Laura Selbmann for her new information of Elasticomyces and the origin and morphology of isolate CCFEE5499. Tom F. Preece, R. Nigel Stringer, and Heidi Döring are thanked for collecting additional material for use in our study. This investigation was undertaken as a part of a research grant to DLH from the Ministerio de Educación y Ciencia of Spain (Proyectos I+D CGL 2008-01600).
REFERENCES
- Barr ME. (1979) A classification of Loculoascomycetes. Mycologia 77: 935–957 [Google Scholar]
- Castresana J. (2000) Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Molecular Biology and Evolution 17: 540–552 [DOI] [PubMed] [Google Scholar]
- Crous PW, Braun U, Groenewald JZ. (2007) Mycosphaerella is polyphyletic. Studies in Mycology 58: 1–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schoch CL, Hyde KD, Wood AR, Gueidan C, de Hooog GS, Groenewald JZ. (2009) Phylogenetic lineages in the Capnodiales. Studies in Mycology 64: 17–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hoog GS, Guarro J, Gené J, Figueras MJ. (2000) Atlas of Clinical Fungi. 2nd edn Utrecht: Centraalbureau voor Schimmelcultures; [Google Scholar]
- Ellis MB. (1976) More Dematiaceous Hyphomycetes. Kew: Commonwealth Mycological Institute; [Google Scholar]
- Hawksworth DL. (1979) The lichenicolous hyphomycetes. Bulletin of the British Museum (Natural History), Botany 6: 183–300 [Google Scholar]
- Hawksworth DL, Millanes AM, Wedin M. (2010) Roselliniella revealed as an overlooked genus of Hypocreales, with the description of a second species on parmelioid lichens. Persoonia 24: 12–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawksworth DL, Punithalingam E. (1973) New and interesting microfungi from Slapton, South Devonshire: Deuteromycotina. Transactions of the British Mycological Society 61: 57–69 [Google Scholar]
- Hibbett DS, Binder M, Bischoff JF, Blackwell M.et al [and 62 others] (2007) A higher-level phylogenetic classification of the Fungi. Mycological Research 111: 509–547 [DOI] [PubMed] [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T. (2002) MAFFT, a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30: 3059–3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Toh H. (2008) Recent developments in the MAFFT multiple sequence alignment program. Briefings in Bioinformatics 9: 286–298 [DOI] [PubMed] [Google Scholar]
- Muggia L, Hafellner J, Wirtz N, Hawksworth DL, Grube M. (2008) The sterile microfilamentous lichenized fungi Cystocoleus ebeneus and Racodium rupestre are relatives of plant pathogens and clinically important dothidealean fungi. Mycological Research 112: 51–57 [DOI] [PubMed] [Google Scholar]
- Preece TM. (2009) News about Xanthoriicola physciae. British Lichen Society Bulletin 104: 37 [Google Scholar]
- Seifert KA, Morgan-Jones G, Gams W, Kendrick WB. (2011) The Genera of Hyphomycetes. [CBS Biodiversity Series no. 9.] Utrecht: CBS-KNAW Fungal Biodiversity Centre; [Google Scholar]
- Selbmann L, de Hoog GS, Mazzaglia A, Friedmann EI, Onofri S. (2005) Fungi at the edge of life: cryptoendolithic black fungi from Antarctic desert. Studies in Mycology 51: 1–32 [Google Scholar]
- Selbmann L, de Hoog GS, Zucconi L, Isola D, Ruisi S, Gerrits van Ende AHG, Ruibal C, De Leo F, Urzi C, Onofri S. (2008) Drought meets acid: three new genera in a dothidealen clade of extremotolerant fungi. Studies in Mycology 61: 1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilgalys R, Hester M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedin M, Wiklund E, Jørgensen PM, Ekman S. (2009) Slippery when wet: phylogeny and character evolution in the gelatinous cyanobacterial lichens (Peltigerales, Ascomycetes). Molecular Phylogenetics and Evolution 53: 862–871 [DOI] [PubMed] [Google Scholar]
- Zwickl DJ. (2006) Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. PhD dissertation, The University of Texas at Austin; [Google Scholar]


