Abstract
Several isolates of coelomycetous fungi with pigmented conidia were consistently isolated from diseased roots of Zea mays in irrigated plots monitored in the KwaZulu-Natal Province of South Africa. Based on their morphology, these isolates could be identified as representative of Stenocarpella macrospora, S. maydis, and Phaeocytostroma ambiguum. Although species of Stenocarpella are well-known as causal agents of cob and stalk rot and leaf blight of maize in South Africa, the occurrence and importance of P. ambiguum is less well documented and understood. To determine the role of P. ambiguum as a root pathogen of maize, pathogenicity tests were conducted under glasshouse conditions at 18 °C night and 28 °C day temperatures using a pasteurised soil, river sand and perlite medium and a 0.5 % sand-bran inoculum. Based on these results, P. ambiguum was shown to be a primary pathogen of maize, but to be less virulent than the positive control, S. maydis. Furthermore, to clarify the higher-level phylogeny of these fungal genera, isolates were subjected to DNA sequencing of the nuclear ribosomal DNA (ITS & LSU). Partial gene sequences of the translation elongation factor 1-alpha gene were added to confirm the species monophyly. To resolve the generic placement of Phaeocytostroma, additional species such as P. sacchari, P. plurivorum and P. megalosporum were also added to the analysis. Based on these results, Stenocarpella and Phaeocytostroma were shown to be two well defined genera, belonging to Diaporthales, Diaporthaceae, being closely allied to Phomopsis (Diaporthe). All three genera were also observed to form alpha as well as beta conidia, and although this phenomenon is well documented for Phomopsis and Phaeocytostroma, it is a new observation for Stenocarpella. In spite of the differences in conidial pigmentation, no support could be obtained for polyphyly in Diaporthaceae, suggesting that as observed in Botryosphaeriaceae (Botryosphaeriales), conidial pigmentation is not informative at the family level in Diaporthales.
Keywords: Diplodia, diplodiosis, Phaeocytostroma, phylogeny, Stenocarpella, systematics, Zea mays
INTRODUCTION
Soilborne diseases significantly reduce maize yields in irrigated systems where maize follows winter wheat in the KwaZulu-Natal Province of South Africa (Lamprecht et al. 2008). Over the years several fungi have been consistently isolated from maize plants with symptoms of crown and root rot in fields sampled in KwaZulu-Natal. The pathogenicity of these fungi remains unresolved.
Among the isolates obtained since 2006 in the present survey were several coelomycetous fungi with dematiaceous conidia, representing the genera Stenocarpella and Phaeocytostroma (Sutton 1980). Species of Stenocarpella are commonly isolated from diseased maize crops worldwide, especially during humid seasons (Odriozola et al. 2005). The two species reported from literature to be associated with cob and stalk rot and leaf blight of maize are S. macrospora and S. maydis (Marasas et al. 1979, Latterell & Rossi, 1983, Crous et al. 2006). According to Sutton and Waterston (1966) Diplodia maydis (S. maydis) can also infect roots and cause seedling blight. Cob rot develops at the base of the maize ear, growing up to its tip. After initial infection, maize grains appear less shiny and opaque-grey or somewhat brownish, leading to seedling blight, ear or stalk rot (Kellerman et al. 1991). Ear rot results in yield losses, reduced grain quality, and mycotoxins may accumulate in the grain (Rheeder et al. 1993). Species of Phaeocytostroma are commonly associated with stalk rots of different hosts (Sutton 1964, 1980, Holliday 1980), with P. ambiguum being reported from maize in Australia, France, North America, Serbia (Stovold et al. 1996), Mauritius, Tanzania (Sutton 1964), South Africa (Crous et al. 2000) and Yugoslavia (Lević & Petrović 1998).
Although S. macrospora and S. maydis have in the past been extensively published as species of Diplodia, Sutton (1980) placed them in Stenocarpella based on their distinct conidiogenesis, a fact supported by later molecular phylogenetic studies, which revealed these taxa to belong to the Diaporthales rather than the Botryosphaeriales (Crous et al. 2006). Their position within the order, however, remains unresolved. Similarly P. ambiguum was initially described as a species of Sphaeropsis (suggesting Botryosphaeriaceae), though nothing is known about the phylogenetic position of the genus Phaeocytostroma, and it is generally regarded as Ascomycota incertae sedis (<MycoBank.org>). Furthermore, although pathogenicity has been confirmed for South African isolates of S. maydis and S. macrospora on maize (Marasas & Van der Westhuizen 1979, Kellerman et al. 1991, Rheeder et al. 1993), this has not been done for P. ambiguum. The aim of the present study was thus to resolve the higher order phylogeny of Stenocarpella and Phaeocytostroma, and also determine the importance of P. ambiguum as pathogen on maize, when compared to S. maydis, which is regarded as an important pathogen of this host.
Materials and Methods
Isolates
Maize roots and crown pieces were surface sterilised in 1 % sodium hypochlorite for 1 min, rinsed twice in sterile distilled water and allowed to dry in a laminar flow bench. Pieces of tissue (5–10 mm) were placed on potato-dextrose agar (PDA) and water agar (WA) containing 0.02 % novostreptomycin. Petri dishes were incubated at 25 °C in the dark for 7 d. Fungi that developed were transferred to divided Petri dishes containing carnation leaf agar (WA with sterile carnation leaves; Fisher et al. 1981) in one half and PDA in the other. Plates containing colonies of Stenocarpella and Phaeocytostroma isolates were incubated at 20 °C under near-ultraviolet light radiation (12 h/d) for 21–28 d, when sporulating colonies could be positively identified. Colonies were sub-cultured onto 2 % PDA, 2 % malt extract agar, oatmeal agar (OA) and pine needle agar (PNA) (Crous et al. 2009), and incubated under continuous near-ultraviolet light at 25 °C to promote sporulation. To help resolve the generic position of Phaeocytostroma, isolates of additional species such as P. sacchari (CBS 275.34), P. plurivorum (CBS 113835) and P. megalosporum (CBS 284.65) were added to the analysis. Representative cultures obtained in this study are maintained in the culture collection of the Centraalbureau voor Schimmelcultures (CBS), Utrecht, the Netherlands (Table 1).
Table 1.
Collection details and DDBJ/EMBL/GenBank accession numbers of Phaeocytostroma and Stenocarpella isolates for which novel sequences were generated in this study.
| Species | Strain no. 1 | Substrate | Country | Collector | EMBL accession no. (ITS, LSU, TEF) 2 |
|---|---|---|---|---|---|
| Phaeocytostroma ambiguum | CPC 16775; Z171F | Zea mays | South Africa | S. Lamprecht | FR748034, —, FR748066 |
| CPC 16776; Z323C | Zea mays | South Africa | S. Lamprecht | FR748035, FR748095, FR748067 | |
| CPC 17071; Z113V | Zea mays | South Africa | S. Lamprecht | FR748036, —, FR748068 | |
| CPC 17072; Z155R | Zea mays | South Africa | S. Lamprecht | FR748037, FR748096, FR748069 | |
| CPC 17074; Z182Z | Zea mays | South Africa | S. Lamprecht | FR748038, FR748097, FR748070 | |
| CPC 17075; Z189Z | Zea mays | South Africa | S. Lamprecht | FR748039, FR748098, FR748071 | |
| CPC 17076; Z191AB | Zea mays | South Africa | S. Lamprecht | FR748040, FR748099, FR748072 | |
| CPC 17077; Z199Z | Zea mays | South Africa | S. Lamprecht | FR748041, FR748100, FR748073 | |
| CPC 17078; Z213H | Zea mays | South Africa | S. Lamprecht | FR748042, FR748101, FR748074 | |
| CPC 17079; Z225F | Zea mays | South Africa | S. Lamprecht | FR748043, FR748102, FR748075 | |
| CPC 17083; Z432W | Zea mays | South Africa | S. Lamprecht | FR748044, —, FR748076 | |
| P. megalosporum | CBS 284.65; IMI 092336 | Rice-field soil | India | — | FR748045, FR748103, FR748077 |
| P. plurivorum | CBS 113835; UPSC 2042 | Helianthus annuus | Portugal | — | FR748046, FR748104, FR748078 |
| P. sacchari | CBS 275.34 | — | Japan | — | FR748047, FR748105, FR748079 |
| Stenocarpella macrospora | CBS 117560; MRC 8615 | Rain damaged Bt maize hybrid, 2003-04 season | South Africa | J. Rheeder | FR748048, DQ377934, — |
| CPC 11863 | Zea mays | South Africa | P. Caldwell | FR748049, —, — | |
| S. maydis | CBS 117557; MRC 8612 | Bt maize hybrid from 2003-04 season | South Africa | J. Rheeder | FR748050, DQ377935, — |
| CBS 117558; MRC 8613 | Traditional/landrace maize from 2003/04 season | South Africa | J. Rheeder | FR748051, DQ377936, FR748080 | |
| CBS 117559; MRC 8614 | Commercial maize hybrid PAN-6043 from 2003-04 season | South Africa | J. Rheeder | FR748052, DQ377937, FR748081 | |
| CPC 16777; Z169F | Zea mays | South Africa | S. Lamprecht | FR748053, FR748106, FR748082 | |
| CPC 16778; Z178AB | Zea mays | South Africa | S. Lamprecht | FR748054, FR748107, FR748083 | |
| CPC 16779; Z181R | Zea mays | South Africa | S. Lamprecht | FR748055, FR748108, FR748084 | |
| CPC 16780; Z255K | Zea mays | South Africa | S. Lamprecht | FR748056, FR748109, FR748085 | |
| CPC 16781; Z255AD | Zea mays | South Africa | S. Lamprecht | FR748057, FR748110, FR748086 | |
| CPC 16782; Z255AE | Zea mays | South Africa | S. Lamprecht | FR748058, FR748111, FR748087 | |
| CPC 16784; Z401P | Zea mays | South Africa | S. Lamprecht | FR748059, FR748112, FR748088 | |
| CPC 16785; Z404K | Zea mays | South Africa | S. Lamprecht | FR748060, FR748113, FR748089 | |
| CPC 16786; Z410AA | Zea mays | South Africa | S. Lamprecht | FR748061, FR748114, FR748090 | |
| CPC 16787; Z422B | Zea mays | South Africa | S. Lamprecht | FR748062, FR748115, FR748091 | |
| CPC 16788; Z430D | Zea mays | South Africa | S. Lamprecht | FR748063, FR748116, FR748092 | |
| CPC 16789; Z434C | Zea mays | South Africa | S. Lamprecht | FR748064, FR748117, FR748093 | |
| CPC 17073; Z169F | Zea mays | South Africa | S. Lamprecht | FR748065, FR748118, FR748094 |
1CBS: CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands; CPC: Culture collection of P.W. Crous, housed at CBS; IMI: International Mycological Institute, CABI-Bioscience, Egham, Bakeham Lane, U.K.; MRC: Medical Research Council Fusarium Collection, Tygerberg, South Africa; UPSC: Uppsala University Culture Collection of Fungi, Botanical Museum University of Uppsala, Uppsala, Sweden.
2ITS: Internal transcribed spacers 1 and 2 together with 5.8S nrDNA; LSU: 28S nrDNA; TEF: partial translation elongation factor 1-alpha.
DNA phylogeny
Genomic DNA was extracted from mycelia taken from fungal colonies on MEA using the UltraCleanTM Microbial DNA Isolation Kit (Mo Bio Laboratories, Inc., Solana Beach, CA, USA). A part of the nuclear rDNA operon spanning the 3′ end of the 18S rRNA gene (SSU), the first internal transcribed spacer (ITS1), the 5.8S rRNA gene, the second ITS region (ITS2) and the first 900 bp at the 5′ end of the 28S rRNA gene (LSU) was amplified and sequenced as described by Cheewangkoon et al. (2008). Partial gene sequences for the translation elongation factor 1-alpha gene (TEF) were generated as described by Bensch et al. (2010). The generated ITS and LSU sequences were compared with other fungal DNA sequences from NCBI’s GenBank sequence database using a megablast search of the nr database; sequences with high similarity were added to the alignments. The Diaporthales LSU phylogeny of Tanaka et al. (2010) was used as starting point for Fig. 1 in this study. Novel sequences were lodged in the DDBJ/EMBL/GenBank nucleotide database (Table 1) and the alignments and phylogenetic trees in TreeBASE (<treebase.org>).
Fig. 1.
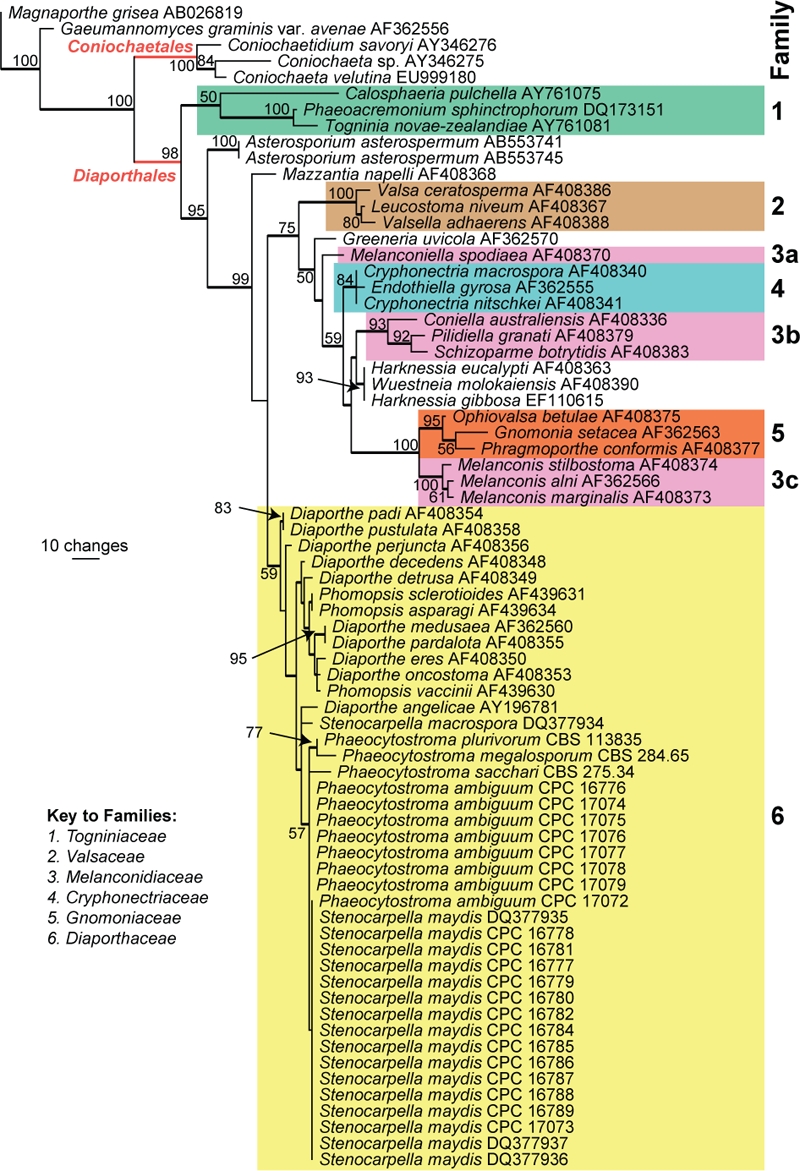
The first of 23 equally most parsimonious trees obtained from a heuristic search with 100 random taxon additions (PAUP v. 4.0b10). Bootstrap support values are shown at the nodes and strict consensus branches are thickened. Families are indicated in different coloured boxes. The tree was rooted to Gaeumannomyces graminis var. avenae (GenBank AF362556) and Magnaporthe grisea (GenBank AB026819).
Taxonomy
Wherever possible, 30 measurements (× 1000 magnification) were made of structures mounted in lactic acid, with the extremes of spore measurements given in parentheses. Colony colours (surface and reverse) were assessed after 1 mo on MEA, OA and PDA at 25 °C in the dark, using the colour charts of Rayner (1970).
Pathogenicity trial
Sand-bran inoculum was prepared according to Lamprecht (1986). Autoclaving times were adapted to 60 min on the first day followed by 30 min on two consecutive days. Ten plugs (2 mm diam) of each isolate were used to inoculate two 2 L flasks. Control flasks were inoculated with plugs of WA only. The inoculum was incubated for 11 d at 22 °C without being directly exposed to light. The mixture was shaken every fourth day to ensure even growth of the mycelium throughout the medium.
The pathogenicity trial was conducted in a glasshouse (18 °C night and 28 °C day temperatures) using plastic pots, 22.5 cm diam, with a holding capacity of 1 500 g planting medium. The planting medium was made up of equal amounts of soil, perlite and sand, which was pasteurised (30 min at 83 °C) and left for 3 d before being mixed with inoculum. An inoculum concentration of 0.5 % (wt/wt) was used. The inoculum was mixed with the planting medium and pots were watered and left to stand overnight in the glasshouse before being planted to 10 maize seeds (cv PHI 32D96B) the next day. Maize seeds were treated with hot water at 60 °C for 5 min (Daniels 1983) to ensure that clean seed was used. Pots were watered every alternate day to field capacity. Pathogenicity and relative virulence of each isolate were determined by calculating the percentage survival and plant growth (shoot length) as well as the percentage plants with crown and root rot severity using a 0–4 scale with 0 = no root rot, 1 = > 0–25 % root rot, 2 = > 25–50 % root rot, 3 = > 50–75 % root rot and 4 = > 75–100 % root rot, 3 wk after planting. To confirm the presence of the different fungi, re-isolations were made by plating 5 mm pieces of tissue excised from crowns and roots of plants with crown and root rot representatively selected from each treatment on PDA. The experimental design was a randomised block design with three replicates for each treatment.
Statistical analysis
Data were subjected to analysis of variance using SAS (v. 9.3, SAS Institute, Inc) and the Shapiro-Wilk test (Shapiro & Wilk 1965) was performed to test for normality. The Student’s t test for least significant differences were calculated to compare means at the 5 % significance level.
RESULTS
Phylogenetic analyses
Approximately 1 700 bases, spanning the ITS and LSU regions, and approximately 650 bp for TEF, were obtained. The LSU region was used in the phylogenetic analysis for the generic placement (Fig. 1) and ITS and TEF to determine species-level relationships (Figs 2, 3).
Fig. 2.
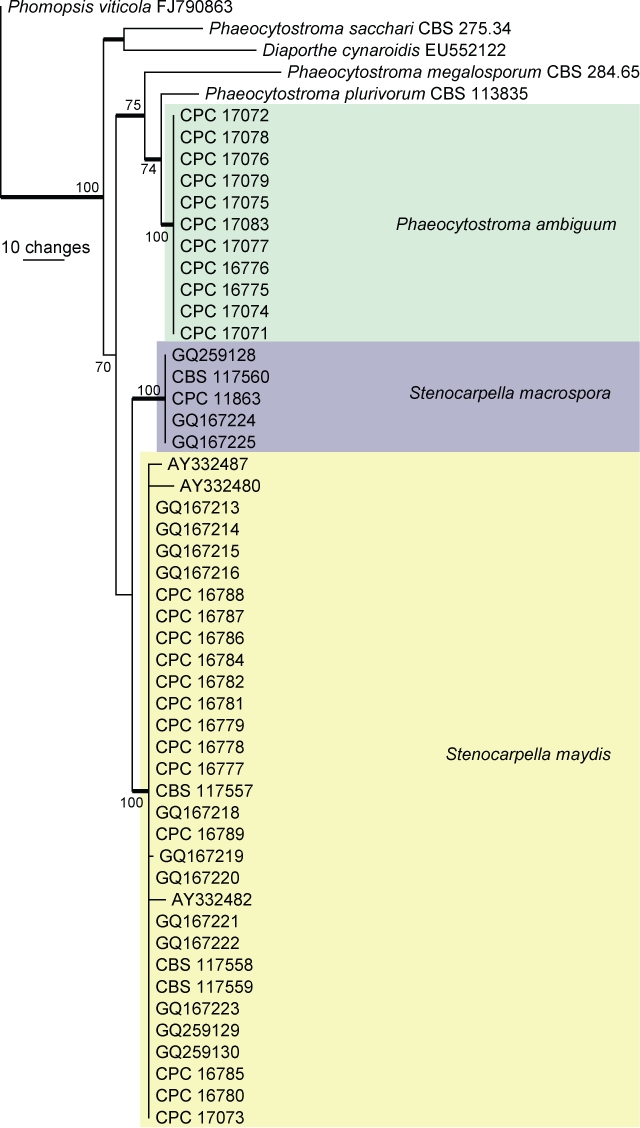
The first of two equally most parsimonious trees obtained from a heuristic search with 100 random taxon additions (PAUP v. 4.0b10). Bootstrap support values > 69 % are shown at the nodes and strict consensus branches are thickened. The three species from maize are indicated in different coloured boxes. The tree was rooted to Phomopsis viticola (GenBank FJ790863).
Fig. 3.
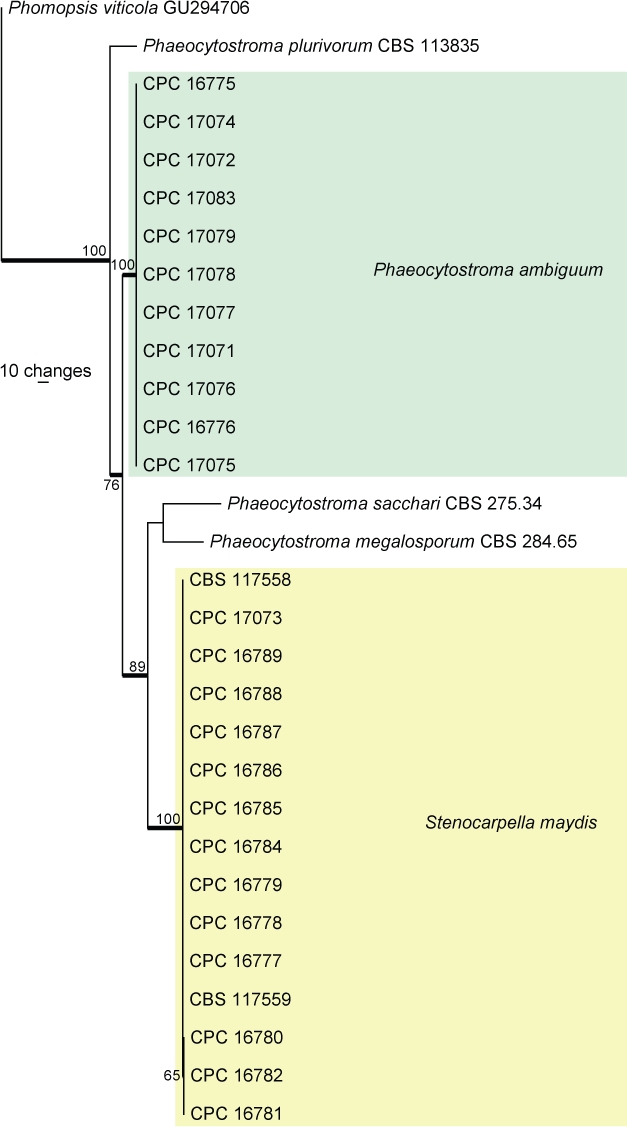
The first of two equally most parsimonious trees obtained from a heuristic search with 100 random taxon additions (PAUP v. 4.0b10). Bootstrap support values > 69 % are shown at the nodes and strict consensus branches are thickened. The two species from maize for which TEF sequences were available are indicated in different coloured boxes. The tree was rooted to Phomopsis viticola (GenBank GU294706).
The manually adjusted LSU alignment contained 72 taxa (including the two outgroup sequences) and, of the 836 characters used in the phylogenetic analysis, 179 were parsimony-informative, 54 were variable and parsimony-uninformative and 603 were constant. Twenty-three equally most parsimonious trees were retained from the heuristic search, the first of which is shown in Fig. 1 (TL = 578, CI = 0.516, RI = 0.834, RC = 0.430). The phylogenetic tree of the LSU region (Fig. 1) shows that Stenocarpella and Phaeocytostroma are embedded with the Diaporthaceae and could not be distinguished phylogenetically from Diaporthe.
The manually adjusted ITS alignment contained 52 taxa (including the outgroup sequence) and, of the 491 characters used in the phylogenetic analysis, 34 were parsimony-informative, 84 were variable and parsimony-uninformative and 373 were constant. Two equally most parsimonious trees were retained from the heuristic search, the first of which is shown in Fig. 2 (TL = 170, CI = 0.876, RI = 0.935, RC = 0.819). The phylogenetic tree of the ITS region (Fig. 2) shows that the sequences of species of Phaeocytostroma form a monophyletic lineage with a bootstrap support value of 75 % whereas the monophyletic lineage for Stenocarpella was poorly supported (51 %, not shown on tree).
The manually adjusted TEF alignment contained 30 taxa (including the outgroup sequence) and, of the 317 characters (due to the inclusion of a much shorter outgroup sequence compared to the length of the ingroup sequences) used in the phylogenetic analysis, 102 were parsimony-informative, 102 were variable and parsimony-uninformative and 113 were constant. Two equally most parsimonious trees were retained from the heuristic search, the first of which is shown in Fig. 3 (TL = 345, CI = 0.884, RI = 0.956, RC = 0.845). The phylogenetic tree of the TEF region (Fig. 3) shows very little intraspecific variation for S. maydis and P. ambiguum.
Taxonomy
Phaeocytostroma ambiguum (Mont.) Petr., Feddes Repert. 42: 457 (1927)
Basionym: Sphaeropsis ambigua Mont., Ann. Sci. Nat., Bot. 12: 308 (1849)
Synonyms: Phaeocytostroma istrica Petr., Ann. Mycol. 19: 45 (1921)
Phaeocytosporella zeae Stout, Mycologia 22: 280 (1930)
(Fig. 4)
Fig. 4.

Phaeocytostroma ambiguum (CPC 17079). A. Conidiomata on potato-dextrose agar. B, C. Conidiomata on pine needle agar. D–F. Conidiophores and paraphyses. G. Hyaline conidiophores giving rise to brown conidial mass. H, I. Alpha conidia. J. Conidiogenous cells giving rise to beta conidia. K. Beta conidia. Scale bars = 10 μm.
Conidiomata on PNA and OA immersed, initially solitary, but forming a stroma up to 2 mm diam, becoming multilocular with one to several clearly defined black necks extending above the stroma, up to 300 μm tall (after 2 wk, but becoming more elongated with age), up to 170 μm wide with terminal ostiole, up to 100 μm wide. Conidiomatal wall black, consisting of several layers of textura intricata to textura angularis, up to 40 μm wide; forming an inner pale brown to hyaline layer, up to 30 μm wide. Alpha conidiophores tightly aggregated, subcylindrical, branched in mid region, consisting of 2–3 supporting cells, giving rise to septate, cylindrical conidiogenous cells or paraphyses, 1–5-septate, 40–100 × 2–4 μm. Alpha conidiogenous cells hyaline, subcylindrical, terminal and lateral, 15–50 × 2–4 μm; apex with minute periclinal thickening and collarette. Paraphyses intermingled between conidiophores or arising from same conidiophores that give rise to conidiogenous cells, subcylindrical, hyaline, branched or not, 1–3 transversely septate, 40–120 × 2–4 μm; apex bluntly rounded. Alpha conidia medium brown, smooth, ellipsoid to pyriform (somewhat clavate on PNA), widest in middle of conidium, apex bluntly rounded, base truncate, (14–)15–16(–18) × (4.5–)5–6(–6.5) μm. Beta conidiophores interspersed among alpha conidiophores, hyaline, subcylindrical, branched, 1–3-septate, 10–30 × 2–4 μm; Beta conidiogenous cells phialidic, integrated, terminal and lateral, 10–20 × 2–3 μm. Beta conidia subcylindrical, straight to slightly curved, hyaline, smooth, widest in middle, tapering to acutely rounded apex; base truncate, 15–20 × 1.5–2 μm.
Culture characteristics: Colonies on OA flat, spreading with smooth margins and sparse aerial mycelium; surface flat, with a dull black layer and patches of flat white mycelium, forming a layer on the surface, covering the plate within 2 wk. On PDA similar, except that the black layer extends from the centre outwards, with the white layer in the outer region, less dense than on OA; reverse dull black in middle, pale white in outer region. On MEA appearing olivaceous-black due to woolly, grey aerial mycelium; reverse similar as on PDA.
Specimens examined: France ?: from stems of Zea mays, (PC 206294 – holotype). South Africa: KwaZulu-Natal, Winterton, Gourton farm, on roots of Zea mays, 2008, S. Lamprecht, (CBS H-20547 – epitypus hic designatus); culture ex-epitype CPC 17071 = CBS 128784.
Notes: The beta conidia described above for P. ambiguum were recently reported by Lević & Petrović (1998), and seem to be commonly produced by isolates of this species. Other taxa in the Diaporthaceae (Figs 1, 2), such as Phomopsis (Diaporthe) also produce beta conidia, suggesting that the putative link between Phaeocytostroma iliau and Clypeoporthe iliau (Barr 1978), could be correct.
Stenocarpella maydis (Berk.) B. Sutton, Coelomycetes: 432 (1980)
Basionym: Sphaeria maydis Berk., Hooker’s J. Bot., London 6: 15 (1847)
Synonyms: Additional synonyms are listed in Sutton (1980).
(Fig. 5)
Fig. 5.
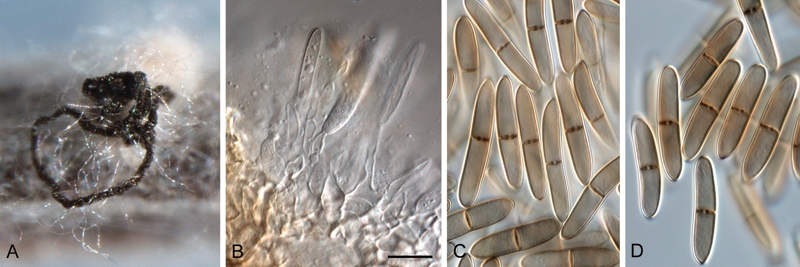
Stenocarpella maydis (CBS 117559). A. Conidioma with exuding black conidial cirrhus on pine needle agar. B. Conidiogenous cells giving rise to conidia. C, D. Conidia. Scale bar = 10 μm.
Specimens examined: South Africa: KwaZulu-Natal, Simdlangentsha, Bt Zea mays hybrid from 2003-04 season, J. Rheeder (ex-epitype CBS 117558 = MRC 8613, designated in Crous et al. 2006); ibid. CBS 117557 = MRC 8612; Hlabisa, commercial hybrid PAN-6043, MRC 8614 = CBS 117559.
Note: Conidia subcylindrical to narrowly ellipsoid, straight, curved, occasionally irregular, 0–2-septate, smooth-walled, pale brown, apex obtuse, base truncate, 15–34 × 5–8 μm (Sutton 1964).
Stenocarpella macrospora (Earle) B. Sutton, Mycol. Pap. 141: 202 (1977)
Basionym: Diplodia macrospora Earle, Bull. Torrey Bot. Cl. 24: 29 (1897)
Synonyms: Additional synonyms are listed in Sutton (1980).
(Fig. 6)
Fig. 6.

Stenocarpella macrospora (CPC 11863). A. Conidioma with exuding conidial mass on pine needle agar. B, C. Conidiogenous cells giving rise to conidia. D. Hyaline layer of conidiogenous cells giving rise to brown conidial mass. E, F. Alpha conidia. G. Conidiogenous cells giving rise to beta conidia. H. Beta conidia. Scale bars = 10 μm.
Specimens examined: South Africa: KwaZulu-Natal, Hlabisa, rain damaged Bt Zea mays hybrid, 2003-04 season, J. Rheeder (ex-epitype, CBS 117560 = MRC 8615, designated in Crous et al. 2006); KwaZulu-Natal, Zea mays kernels, 2005, P. Caldwell, CPC 11863 = CBS 128560.
Notes: Conidia subcylindrical to narrowly ellipsoid, straight, curved, occasionally irregular, 0–3-septate, smooth-walled, pale brown, apex obtuse, base truncate, 44–82 × 7.5–11.5 μm (Sutton 1964). Several cultures also formed hyaline, scolecosporous, curved beta conidia, which is a new observation for S. macrospora, but not uncommon in the Diaporthaceae (Fig. 6).
Pathogenicity trial
Stenocarpella maydis significantly reduced the survival of seedlings compared to the control and P. ambiguum (Table 2). Stenocarpella maydis isolates Z169F, Z178AB, Z181R, Z430D and Z434C significantly reduced seedling survival compared to the control, with the lowest survival rates recorded for Z178B, Z181R and Z430D (Table 3).
Table 2.
Survival, shoot length and crown and root rot recorded for maize seedlings inoculated with Phaeocytostroma ambiguum and Stenocarpella maydis under glasshouse conditions.
| Fungus | Survival (%) x | Shoot length (mm) x | Crown rot (%) x y | Root rot severity x z |
|---|---|---|---|---|
| Control | 100.0a | 489.7a | 0.00c | 0.00c |
| P. ambiguum | 97.6a | 446.0b | 45.1b | 1.40b |
| S. maydis | 74.8b | 367.7c | 67.2a | 2.59a |
xMeans within a column followed by the same letter do not differ significantly (P = 0.05)
yPercentage plants with crown rot
zRoot rot severity rated on a scale of 0–4 with 0 = no root rot, 1 = > 0–25 % root rot, 2 = >25–50 % root rot, 3 = > 50–75 % and 4 >75–100 % root rot.
Table 3.
Effect of different isolates of Phaeocytostroma ambiguum and Stenocarpella maydis on survival, plant growth (shoot length) and crown and root rot of maize seedlings under glasshouse conditions.
| Fungus | Isolate | Survival (%) x | Shoot length (mm) x | Crown rot (%) x y | Root rot severity x z |
|---|---|---|---|---|---|
| Control | 100.0a | 489.7ab | 0.0j | 0.00h | |
| P. ambiguum | Z113V | 100.0a | 416.9defg | 46.7def | 1.27g |
| Z171F | 100.0a | 442.9bcd | 60.0cd | 1.10g | |
| Z182Z | 96.7a | 434.1cd | 13.7hij | 1.14g | |
| Z189Z | 96.7a | 452.1abcd | 23.7ghi | 1.28g | |
| Z191AB | 100.0a | 422.6cdef | 100.0a | 2.00f | |
| Z199Z | 100.0a | 497.8a | 13.3hij | 1.13g | |
| Z213H | 96.7a | 487.9ab | 17.0hij | 1.07h | |
| Z222AS | 93.3ab | 496.4a | 14.2hij | 0.82g | |
| Z225F | 96.7a | 455.1abcd | 7.0ij | 1.21g | |
| Z323C | 100.0a | 375.8fgh | 100.0a | 2.30def | |
| Z432W | 93.3ab | 428.3cde | 100.0a | 2.04ef | |
| S. maydis | Z169F | 76.7bc | 381.4efgh | 100.0a | 2.21ef |
| Z178AB | 40.0e | 370.2gh | 50.6de | 2.83dc | |
| Z181R | 56.7de | 290.2j | 100.0a | 3.41b | |
| Z255K | 83.3abc | 376.6fgh | 77.8bc | 2.36def | |
| Z255AD | 100.0a | 377.9fgh | 73.3bc | 2.23ef | |
| Z401P | 83.3abc | 313.7ij | 59.7cd | 3.32bc | |
| Z404K | 86.7abc | 361.2hi | 100.0a | 2.58de | |
| Z410AD | 83.3abc | 420.6cdef | 27.8fgh | 2.20ef | |
| Z422B | 96.7a | 465.8abc | 38.5efg | 1.00g | |
| Z430D | 46.7e | 289.0j | 20.8ghi | 3.79ab | |
| Z434C | 70.0cd | 315.5ij | 90.5ab | 4.00a |
xMeans within a column followed by the same letter do not differ significantly (P = 0.05)
yPercentage plants with crown rot
zRoot rot severity rated on a scale of 0–4 with 0 = no root rot, 1 = > 0–25 % root rot, 2 = > 25–50 % root rot, 3 = > 50–75 % and 4 = >75–100 % root rot.
Both P. ambiguum and S. maydis caused significantly more crown and root rot, and growth reduction, than the control. However, S. maydis was the most virulent, causing significantly more crown and root rot and growth reduction than P. ambiguum (Table 2). Of the isolates included in this study, P. ambiguum isolates Z113V, Z182Z, Z191AB, Z323C and Z432W and all S. maydis isolates except Z422B significantly reduced plant growth (shoot length). The highest growth reductions were recorded for S. maydis isolates Z181R and Z430D, but growth reduction caused by these isolates did not differ significantly from that caused by isolates Z401P and Z434C. All isolates of both fungi caused significant crown rot compared to the control, except for P. ambiguum isolates Z182R, Z199Z, Z213H and Z222AS, and all isolates tested except Z213H (P. ambiguum) caused significant root rot. The highest root rot severities were recorded for isolates Z181R, Z401P, Z430D and Z434C (Table 3).
DISCUSSION
Stenocarpella maydis is well documented as a major cause of cob rot of maize (Ullstrup 1977), and the chief organism associated with diplodiosis (Kellerman et al. 1985). In contrast, S. macrospora has been seen as of less importance when compared to S. maydis (Virtanen et al. 1956). In Latin America and Africa, however, both pathogens have been regarded as important ear-rotting pathogens, because of their ability to produce toxins in infected grain, which may be used to feed livestock and poultry (Marasas et al. 1979). A later study by Latterell & Rossi (1983), however, produced results contradictory to those of Hoppe (1936), actually suggesting that S. macrospora was more virulent on young stalks than isolates of S. maydis. The contrasting results were partially explained by the fact that there may be strains with differing vigour within each species. In spite of their virulence, S. maydis is more commonly observed in the USA (Latterell & Rossi 1983), as well as South Africa (Marasas et al. 1979). Although commonly associated with root and stalk rot of maize, not much is known about the pathogenicity of P. ambiguum, other than the study by Stovold et al. (1996) in Australia. Its potential role as primary pathogen was, however, confirmed in the present study, though strains of P. ambiguum generally appeared to be less virulent than the strains of S. maydis tested (Tables 2, 3). Nevertheless, P. ambiguum should be considered as an important pathogen of maize, and certainly as part of a soilborne disease complex could result in significant damage to maize plants. Surveys conducted for a number of seasons in the KwaZulu-Natal province showed that the incidences of both fungi increase significantly towards the end of the growing season when maize plants are often subjected to moisture stress (Results not shown). Stovold et al. (1996) reported that while P. ambiguum can cause extensive infection of maize roots the fungus did not significantly affect the growth of plants under optimal conditions of soil moisture and nutrition. Although these fungi may overwinter in infected maize residue, from where they infect the roots, mesocotyl, crown and eventually the stalks of new plants, not much is known about their host specificity, and whether they could also be isolated from grasses that grow in the vicinity of maize fields.
Based on their pigmented conidia and Diplodia-like morphology, both Stenocarpella and Phaeocytostroma have in the past been suspected to be members of the Boytyosphaeriaceae, being initially described in genera such as Diplodia and Sphaeropsis. However, Crous et al. (2006) revealed Stenocarpella to belong to the Diaporthales, though the phylogenetic relationships of Phaeocytostroma remained obscure until the present study. From the taxa treated here (Figs 1, 2), it is clear that both anamorph genera are best allocated to the Diaporthales, Diaporthaceae. This is somewhat surprising, as their pigmented conidia suggests that they might represent a separate family within the Diaporthales. In spite of these differences, however, no support could be obtained for polyphyly in Diaporthaceae. These findings suggest that as observed earlier in the Botryosphaeriaceae (Botryosphaeriales) (Crous et al. 2006, Phillips et al. 2008), conidial pigmentation appears to be uninformative at the family level, while conidiogenesis, and the ability to produce both alpha and beta conidia, appear more informative at family level in Diaporthaceae (Diaporthales).
Acknowledgments
We thank the technical staff, Alta Schoeman, Almarie Van den Heever, Thabo Phasoana, Sheryldene Williams, Gregory Anthony and John Deysel (isolations, purifications and conducting the pathogenicity test), Arien van Iperen (cultures), Marjan Vermaas (photographic plates), and Mieke Starink-Willemse (DNA isolation, amplification and sequencing) for their invaluable assistance.
REFERENCES
- Barr ME. (1978) The Diaporthales in North America. Mycological Memoir 7: 1–232 [Google Scholar]
- Bensch K, Groenewald JZ, Dijksterhuis J, Starink-Willemse M, Andersen B, et al. (2010) Species and ecological diversity within the Cladosporium cladosporioides complex (Davidiellaceae, Capnodiales). Studies in Mycology 67: 1–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheewangkoon R, Crous PW, Hyde KD, Groenewald JZ, Toanan C. (2008) Species of Mycosphaerella and related anamorphs on Eucalyptus leaves from Thailand. Persoonia 21: 77–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Phillips AJL, Baxter AP. (2000) Phytopathogenic Fungi from South Africa. Stellenbosch: University of Stellenbosch, Department of Plant Pathology Press; [Google Scholar]
- Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO, et al. (2006) Phylogenetic lineages in the Botryosphaeriaceae. Studies in Mycology 55: 235–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Groenewald JZ, Samson RA. (eds) (2009) Fungal Biodiversity. [CBS Laboratory Manual Series no. 1.] Utrecht: Centraalbureau voor Schimmelcultures; [Google Scholar]
- Daniels BA. (1983) Elimination of Fusarium moniliforme from corn seed. Plant Disease 67: 609–611 [Google Scholar]
- Fisher NL, Burgess LW, Toussoun TA, Nelson PE. (1982) Carnation leaves as a substrate and for preserving cultures of Fusarium species. Phytopathology 72: 151–153 [Google Scholar]
- Holliday P. (1980) Fungus Disease of Tropical Crops. Cambridge: Cambridge University Press; [Google Scholar]
- Hoppe PE. (1936) Intraspecific and interspecific aversion in Diplodia. Journal of Agricultural Research 53: 671–680 [Google Scholar]
- Kellerman TS, Prozesky L, Anitra Schultz R, Rabie CJ, et al. (1991) Perinatal mortality in lambs of ewes exposed to cultures of Diplodia maydis (= Stenocarpella maydis) during gestation. Onderstepoort Journal of Veterinary Research 58: 297–308 [PubMed] [Google Scholar]
- Kellerman TS, Rabie CJ, Westhuizen GCA van der, Kriek NP, Prozesky L. (1985) Induction of diplodiosis, a neuromycotoxicosis, in domestic ruminants with cultures of indigenous and exotic isolates of Diplodia maydis. Onderstepoort Journal of Veterinary Research 52: 35–42 [PubMed] [Google Scholar]
- Lamprecht SC. (1986) A new disease of Medicago truncatula caused by Cylindrocladium scoparium. Phytophylactica 16: 189–193 [Google Scholar]
- Lamprecht SC, Farina MPW, Thibaud GR, Marais M, Habig JH, Bloem JF, Swart A. (2008) Soilborne diseases cause yield depression of maize in South Africa. Journal of Plant Pathology 90 (2, Suppl.): S2.412 (abstract). [Google Scholar]
- Latterell FM, Rossi AE. (1983) Stenocarpella macrospora (= Diplodia macrospora) and S. maydis (= D. maydis) compared as pathogens of corn. Plant Disease 67: 725–729 [Google Scholar]
- Lević J, Petrović T. (1998) Formation of α- and β-conidia by Phaeocytostroma ambiguum. Mycopathologia 140: 149–155 [DOI] [PubMed] [Google Scholar]
- Marasas WFO, Renburg SJ van, Mirocha CJ. (1979) Incidence of Fusarium species and the mycotoxins, deoxynivalenol and zearalenone, in corn produced in esophageal cancer areas in Transkei, southern Africa. Journal of Agricultural Food Chemistry 27: 1108–1112 [DOI] [PubMed] [Google Scholar]
- Marasas WFO, Westhuizen GCA van der. (1979) Diplodia macrospora: the cause of leaf blight and cob rot of maize (Zea mays) in South Africa. Phytophylactica 11: 61–64 [Google Scholar]
- Odriozola E, Odeón , Canton G, Clemente G, Escande A. (2005) Diplodia maydis: a cause of death of cattle in Argentina. New Zealand Veterinary Journal 53: 160–161 [DOI] [PubMed] [Google Scholar]
- Phillips AJL, Alves A, Pennycook SR, Johnston PR, Ramaley A, et al. (2008) Resolving the phylogenetic and taxonomic status of dark-spored teleomorph genera in the Botryosphaeriaceae. Persoonia 21: 29–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner RW. (1970) A Mycological Colour Chart. Kew: Commonwealth Mycological Institute; [Google Scholar]
- Rheeder JP, Marasas WFO, Schalkwyk DJ van. (1993) Incidence of Fusarium and Diplodia species in naturally infected grain of South African maize cultivars: a follow up study. Phytophylactica 25: 43–48 [Google Scholar]
- Shapiro SS, Wilk MB. (1965) An analysis of variance test for normality (complete samples). Biometrika 52: 591–611 [Google Scholar]
- Stovold GE, Newfield A, Priest MJ. (1996) Root and stalk rot of maize caused by Phaeocytostroma ambiguum recorded for the first time in New South Wales. Australasian Plant Pathology 25: 50–54 [Google Scholar]
- Sutton BC. (1964) Coelomycetes III. Annellolacinia gen nov., Aristastoma, Phaeocytostroma, Seimatosporium, etc. Mycological Papers 97: 1–42 [Google Scholar]
- Sutton BC. (1980) The Coelomycetes: Fungi Imperfecti with pycnidia, acervuli and stromata. Kew: Commonwealth Mycological Institute; [Google Scholar]
- Sutton BC, Waterston JM. (1966) Diplodia maydis. IMI Descriptions of Fungi and Bacteria. 84: 1–2 [Google Scholar]
- Tanaka K, Mel’nik VA, Kamiyama M, Hirayama K, Shirouzu T. (2010) Molecular phylogeny of two coelomycetous fungal genera with stellate conidia, Prosthemium and Asterosporium, on Fagales trees. Botany 88: 1057–1071 [Google Scholar]
- Ullstrup AJ. (1977) Diseases of corn. In: Corn and Corn Improvement (Sprague GF, ed.): 391–500 Madison, WI: American Society of Agronomy; [Google Scholar]
- Virtanen AI, Hietala PK, Wahlroos Ö. (1956) An antifungal factor in maize and wheat plants. Suomen Kemistilehti B 29: 143 [Google Scholar]


