Abstract
The genus Pseudovirgaria, based on P. hyperparasitica, was recently introduced for a mycoparasite of rust sori of various species of Frommeëlla, Pucciniastrum and Phragmidium in Korea. In the present study, an older name introduced by Saccardo based on European material, Rhinotrichum griseum, is shown to resemble P. hyperparasitica. Morphological study and ITS barcodes from fresh collections of R. griseum from Austria on uredinia and telia of Phragmidium bulbosum on Rubus spp. reveal that it is distinct from P. hyperparasitica. The status of the genus Rhinotrichum, introduced for a fungus occurring on dry wood, remains unclear. Pseudovirgaria grisea comb. nov. is therefore proposed for the mycoparasite occurring on rust fungi in Europe, and an epitype is designated from the recent collections.
Keywords: Dothideomycetes, ITS, LSU, Phragmidium bulbosum, Rubus caesius, Rubus fruticosus aggr.
INTRODUCTION
In addition to the well-known, widespread Sphaerellopsis filum (teleomorph Eudarluca caricis), which is a common mycoparasite of rust fungi (e.g. Eriksson 1966, Liesebach & Zaspel 2004), several genera of hyphomycetes occur on rust fungi, for example several species of Acrodontium, Itersonilia, Redbia, Spinulospora, Triposporina, and Tuberculina (Seifert et al. 2011). Some species of Cladosporium, such as C. tenuissimum and C. uredinicola, which have a wide host range and geographical distribution, are also rust-inhabiting (Pillay et al. 2005, Bensch et al. 2010). Ramularia anamorphs of Mycosphaerella, namely R. coleosporii, R. uredinis and R. uredinearum are well-known mycoparasites of uredinial and telial stages of numerous rusts (Braun 1998, Bartkowska 2007). Several genera of cercosporoid fungi, including Cladosporiella, Elletevera, Eriocercospora and Stenospora are reported as mycoparasites of rusts (Deighton 1969, Braun 1995). Another species with a more complex ecology is the entomogenous fungus Lecanicillium lecanii, mycoparasitic on coffee rust, Hemileia vastatrix, but which also has a mutualistic relationship with an ant (Azteca instabilis) that is associated with a scale insect (Coccus viridis), of which L. lecanii is a parasite (Vandermeer et al. 2009). A rather obscure monotypic genus is the recently described Pseudovirgaria, based on P. hyperparasitica, and known from several rust and host plant species in Korea (Arzanlou et al. 2007).
During a revision of species assigned to Oidium, we encountered the name Oidium griseum (syn. Rhinotrichum griseum), described by Saccardo (1877) from Italy on uredinia of Phragmidium spp. on Rubus caesius and R. fruticosus; an illustration was published by Saccardo in “Fungi Ital. I, fig. 63 (1877). We examined several syntypes and an additional sample accompanied by a drawing on the label. Although all collections are in very poor condition, traces of conidiophores and conidia (obovoid, often somewhat inequilateral, hyaline or almost so, 10–16 × 5–9 μm) were found, which proved to be sufficient to ascertain the identity of Rhinotrichum griseum. Morphologically, R. griseum closely resembled Pseudovirgaria hyperparasitica, presently only known from Korea, where it occurs on uredinia of various Frommeëlla, Pucciniastrum and Phragmidium spp. (Arzanlou et al. 2007). If these two taxa are synonymous, Saccardo’s species name (R. griseum) would have priority over the recently described P. hyperparasitica. Because of the poor condition of the type material of R. griseum, however, fresh collections were required to resolve this issue.
The aim of this study was thus to recollect “Rhinotrichum” griseum from Phragmidium spp. on Rubus caesius and R. fruticosus in Europe, to propose an epitype for this species, and compare it with Pseudovirgaria hyperparasitica.
MATERIALS AND METHODS
Isolates
Leaves bearing uredinia and telia of Phragmidium spp. on Rubus caesius and R. fruticosus were collected at two locations in Graz, Austria, and a fungus matching R. griseum was found on the specimens. Single conidial colonies were established from sporulating conidiophores on Petri dishes containing 2 % malt extract agar (MEA; Crous et al. 2009c) as described earlier (Crous et al. 1991). Colonies were sub-cultured onto potato-dextrose agar (PDA), oatmeal agar (OA), synthetic nutrient-poor agar (SNA), and incubated at 25°C under continuous near-ultraviolet light to promote sporulation. Reference strains are maintained in the CBS-KNAW Fungal Biodiversity Centre (CBS) in Utrecht.
DNA isolation, amplification and analyses
Genomic DNA was isolated from fungal mycelium grown on MEA, using the UltraCleanTM Microbial DNA Isolation Kit (MoBio Laboratories, Solana Beach, CA, USA) according to the manufacturer’s protocols. The primers V9G (de Hoog & Gerrits van den Ende 1998) and LR5 (Vilgalys & Hester 1990) were used to amplify part of the nuclear rDNA operon spanning the 3′ end of the 18S nrRNA gene (SSU), the internal transcribed spacer 1, the 5.8S nrRNA gene, the internal transcribed spacer 2 (ITS) and the first 900 bases at the 5′ end of the 28S nrRNA gene (LSU). The primers ITS4 (White et al. 1990) and LSU1Fd (Crous et al. 2009b) were used as internal sequence primers to ensure good quality sequences over the entire length of the amplicon. The PCR conditions, sequence alignment and subsequent phylogenetic analysis followed the methods of Crous et al. (2006, 2009a). Sequences were compared with the sequences available in NCBI’s GenBank nucleotide (nr) database using a megablast search and an alignment created manually. Alignment gaps were treated as new character states. Sequences derived in this study were lodged at GenBank, the alignment in TreeBASE (www.treebase.org/treebase/index.html), and taxonomic novelties in MycoBank (www.MycoBank.org; Crous et al. 2004).
Morphology
Morphological observations were based on preparations made in clear lactic acid from colonies sporulating on SNA. Observations were made with a Nikon SMZ1500 dissecting microscope, and with a Zeiss Axioscope 2 microscope using differential interference contrast (DIC) illumination. Colony characters and pigment production were noted after 1 mo of growth on MEA and OA (Crous et al. 2009c) incubated at 25°C. Colony colours (surface and reverse) were rated according to the colour charts of Rayner (1970).
RESULTS
Phylogeny
Approximately 1700 bases, spanning the ITS and LSU regions, were obtained from the sequenced DNA. The LSU region was not used in a phylogenetic analysis for the generic placement because a published placement is already available (Arzanlou et al. 2007) and ITS to determine species-level (Fig. 1). The manually adjusted ITS alignment contained 11 taxa (including the two outgroup sequences) and, of the 439 characters used in the phylogenetic analysis, 112 were parsimony-informative, 26 were variable and parsimony-uninformative and 301 were constant. Only two equally most parsimonious trees were retained from the heuristic search, the first of which is shown in Fig. 1 (TL = 155, CI = 0.981, RI = 0.977, RC = 0.959). The phylogenetic tree of the ITS region (Fig. 1) shows that the obtained sequences are distinct from Pseudovirgaria hyperparasitica. Sequences for isolates CPC 19126, 19128, 19130 and 19131 (= CBS 129276–129280) were deposited in GenBank under accession numbers JF957605–JF957614.
Fig. 1.
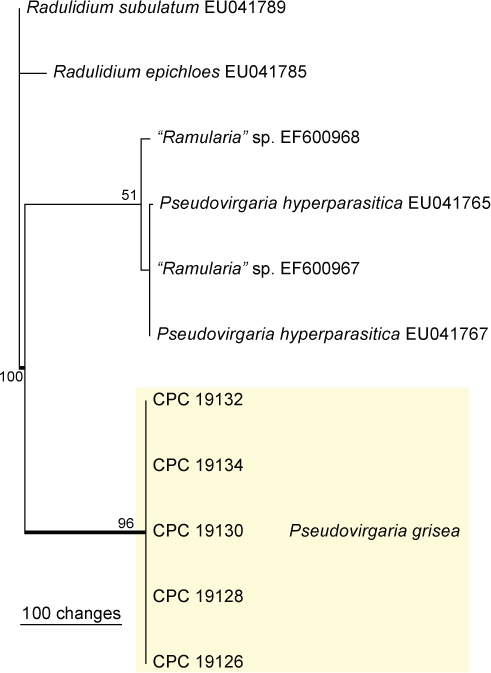
The first of two equally most parsimonious trees obtained from a heuristic search with 100 random taxon additions of the ITS sequence alignment. The scale bar shows 100 changes, and bootstrap support values from 1000 replicates are shown at the nodes. The novel sequences generated for this study are shown in the coloured block and branches present in the strict consensus tree are thickened. The tree was rooted to sequences of two Radulidium species.
Taxonomy
A careful comparison of cultures derived from Austrian material showed them to be morphologically identical to the type collection of Rhinotrichum griseum. Based on its phylogeny (Fig. 1) and morphology, a new combination and epitype are introduced for the European material below:
Pseudovirgaria grisea (Sacc.) U. Braun, Crous & Scheuer, comb. nov.
MycoBank MB560133
(Fig. 2)
Fig. 2.

Pseudovirgaria grisea. A–D. Colonies on telia of Phragmidium spp. (A = GZU 5957, B = GZU 5958, C = GZU 5870, D = GZU 5827). E–K. (GZU 5859). E. Colony sporulating on synthetic nutrient poor agar. F–I. Conidiophores giving rise to conidia. K. Conidia. Bars = 10 μm.
Basionym: Rhinotrichum griseum Sacc., Michelia 1: 87 (1877).
Synonyms: Oidium griseum (Sacc.) Linder, Lloydia 5: 184 (1942).
Rhinotrichella grisea (Sacc.) G. Arnaud, Bull. Trimestriel Soc. Mycol. France 69: 272 (1953).
In vivo: Colonies predominantly on telia (less so on uredinia), thin to moderately thick, loose, cobwebby, to dense, tomentose, pale to medium ochraceous-brown, or pale to medium greyish brown. Mycelium partly immersed in the rust sori, but mainly superficial, composed of branched hyphae with integrated conidiogenous cells; distinction between conidiophores and vegetative hyphae difficult and barely possible.
In vitro: Hyphae 2–5 μm wide, subhyaline to pale olivaceous, pale brownish to olivaceous in mass, thin-walled (≤ 0.5 μm), smooth, pluriseptate, occasionally slightly constricted at the septa. Conidiogenous cells integrated in creeping fertile threads, terminal or intercalary, 20–50 × 2–5 μm wide, subcylindrical to geniculate, subhyaline to pale olivaceous, thin walled, ≤ 0.5 μm, smooth, proliferation sympodial, with one to usually several conidiogenous loci per cell. Conidiogenous loci often crowded, causing slight swellings, up to 6 μm wide, subdenticulate, formed by the slightly bulging wall, convex, slightly narrowed towards the rounded apex, (0.5–)1.0(–1.5) μm diam and 0.5–1 μm high, wall of the loci unthickened, not or slightly darkened-refractive, in surface view visible as a minute circle. Conidia solitary, obovoid, mostly prominently inequilateral (one side flattened or only slightly convex, the other convex), (10–)12–15(–22) × (5–)6–7(–9) μm (av. 13 × 6.5 μm), aseptate, subhyaline, pale yellowish greenish to very pale olivaceous, wall ≤ 0.5 μm thick, smooth, apex slightly attenuated to broadly rounded, base rounded to abruptly attenuated towards a more or less conspicuous hilum, (0.5–)1(–1.5) μm diam, convex to truncate, unthickened, not to slightly darkened-refractive.
Culture characteristics: Colonies after 1 mo in the dark at 25°C spreading, erumpent with moderate aerial mycelium and feathery margins, reaching up to 25 mm diam; on MEA surface luteous to salmon, reverse sienna; on OA surface umber to olivaceous, with outer zones of apricot and dirty white, reverse luteous.
Type: Italy: Montello, on uredinia of Phragmidium sp. on Rubus caesius, Sept. 1876, P. A. Saccardo (PAD – lectotypus hic designatus). – Austria: Steiermark [Styria]: Graz city, Mariatrost distr., Leechwald, close to the area of the hospital “Landeskrankenhaus”, 400 m alt., 47°04′54″N, 15°27′43″E, quadrant [grid mapping unit] 8958/2, edge of a Querco-Carpinetum, on telia of Phragmidium bulbosum, on Rubus “fruticosus agg.”, 11 Nov. 2010, C. Scheuer 5859 (GZU – epitypus hic designatus; cultures ex-epitype CPC 19133, 19132 = CBS 129279); Iso-epitype material will be distributed in Mycotheca Graecensis.
Notes: Conidia of P. hyperparasitica are ovoid in vitro, often somewhat curved, (10–)13–15(–17) × (5–)6–7(–8) μm, and thus similar in size to those of P. grisea. Conidia of P. grisea tend to be more prominently inequilateral, and colonies on rust sori are medium ochraceous-brown, or pale to medium greyish brown, rather than rusty or cinnamon coloured as in P. hyperparasitica.
Additional specimens examined: Austria: Steiermark [Styria]: Graz city, Mariatrost distr., Leechwald, close to the area of the hospital “Landeskrankenhaus”, 400 m alt., 47°04′54″N, 15°27′43″E, quadrant [grid mapping unit] 8958/2, edge of a Querco-Carpinetum, on uredinia and telia of Phragmidium bulbosum, on Rubus “fruticosus agg.” [same locality and host as epitype], 4 Oct. 2010, leg. & det. C. Scheuer (# 5827, GZU); ibid., on telia, 8 Nov. 2010, leg. & det. C. Scheuer 5858 (GZU; cultures CPC 19130, 19131); ibid., (old, deteriorated material) on telia, 26 Jan. 2011, C. Scheuer 5870 (GZU; cultures CPC 19134, 19135); Geidorf distr., Holteigasse, car park beside the Botanical Garden of the university, 380 m alt., 47°04′56″N, 15°27′27″E, quadrant [grid mapping unit] 8958/2, roadside with tall herbs and Rubus caesius, on uredinia and telia of Phragmidium bulbosum, on Rubus caesius, 26 Sept. 2010, C. Scheuer 5817 (GZU; cultures CPC 19126, 19127; det. U. Braun, 1 Nov. 2010); ibid., on telia, 8 Nov. 2010, C. Scheuer 5857 (GZU; cultures CPC 19128, 19129). – Italy: Montello, on uredinia of Phragmidium microsorum; on Rubus caesius, Sept. 1876, P. A. Saccardo (PAD – syntype); on uredinia of Phragmidium on Rubus fruticosus, Selva, 1876, P. A. Saccardo (PAD – syntype); on uredinia of Phragmidium sp. on Rubus sp., Sept. 1876, P. A. Saccardo (PAD – syntype; marked as “type” by E. Hennebert, 15 Feb. 1965); on uredinia of Phragmidium sp. on Rubus caesius, Sept. 1875, P. A. Saccardo (PAD – authentic material, but not a type but with a drawing on the label).
DISCUSSION
Based on its unique morphology and phylogenetic analyses, the mycoparasitic hyphomycete found on uredinia of Fromeëlla and Phragmidium spp. in Korea was proposed as a new genus, Pseudovirgaria (Arzanlou et al. 2007). Pseudovirgaria may represent a novel family in the Dothideomycetes between the Pleosporales and Capnodiales (Arzanlou et al. 2007: fig. 1), but increased sampling is required for this clade before this relationship can be clearly resolved.
Rhinotrichum was introduced by Corda (1837) with R. simplex as the only species, described from dry wood. The identity of that species is still unclear, and Rhinotrichum is considered a doubtful genus (Hughes 1958, Carmichael et al. 1980, Seifert et al. 2011). Saccardo (1877) introduced the new species Rhinotrichum griseum which Linder (1942) assigned to Oidium s. lat. Arnaud (1953) assigned R. griseum to his new genus Rhinotrichella (nom. inval.), later validated with R. globulifera, isolated from Ganoderma basidiomes in Japan, as its type (de Hoog & Hermanides-Nijhof 1977). De Hoog (1972) mentioned that the type material of Rhinotrichum griseum was too scanty to observe the mode of conidial formation. When he later revised Rhinotrichella, he did not mention R. griseum again (de Hoog & Hermanides-Nijhof 1977). Rhinotrichella is unrelated to Pseudovirgaria and morphologically quite distinct. Thus, the generic name Pseudovirgaria is neither influenced nor threatened by the older names Rhinotrichum and Rhinotrichella.
Pseudovirgaria is morphologically similar to Virgaria, but has pale brown hyphae, conidia and conidiogenous cells. Furthermore, the conidiogenous loci of Pseudovirgaria are bulging and convex, in contrast to the more cylindrical denticles in Virgaria (Ellis 1971). Other morphological differences between Pseudovirgaria and similar genera such as Neoovularia and Pseudodidymaria are discussed by Arzanlou et al. (2007). Although Pseudovirgaria now comprises only two species, we are looking for additional collections on other rust fungi on different host plants to see if they might yield additional taxa.
Acknowledgments
We thank the technical staff, Arien van Iperen (cultures), Marjan Vermaas (photographic plates), and Mieke Starink-Willemse (DNA isolation, amplification and sequencing) for their invaluable assistance.
References
- Arnaud G. (1953) Mycologie concrète: genera II. Bulletin Trimestriel de la Société Mycologique de France 69: 265–306 [Google Scholar]
- Arzanlou M, Groenewald JZ, Gams W, Braun U, Shin H-D, Crous PW. (2007) Phylogenetic and morphotaxonomic revision of Ramichloridium and allied genera. Studies in Mycology 58: 57–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartkowska A. (2007) Parasitism of rust fungi spores by Ramularia species. Phytopatologia Polonica 43: 61–67 [Google Scholar]
- Bensch K, Groenewald JZ, Dijksterhuis J, Starink-Willemse M, Andersen B, Summerell BA, Shin H-D, Dugan FM, Schroers H-J, Braun U, Crous PW. (2010) Species and ecological diversity within the Cladosporium cladosporioides complex (Davidiellaceae, Capnodiales). Studies in Mycology 67: 1–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U. (1995) A monograph of Cercosporella, Ramularia and allied genera (phytopathogenic hyphomycetes). Vol. 1 Eching: IHW-Verlag; [Google Scholar]
- Braun U. (1998) A monograph of Cercosporella, Ramularia and allied genera (phytopathogenic hyphomycetes). Vol. 2 Eching: IHW-Verlag; [Google Scholar]
- Carmichael JW, Kendrick WB, Conners IL, Sigler L. (1980) Genera of Hyphomycetes. Edmonton: University of Alberta Press; [Google Scholar]
- Corda ACJ. (1837) Icones Fungorum hucusque Cognitorum. Vol. 1 Prague: JG Calve; [Google Scholar]
- Crous PW, Wingfield MJ, Park RF. (1991) Mycosphaerella nubilosa a synonym of M. molleriana. Mycological Research 95: 628–632 [Google Scholar]
- Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G. (2004) MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19–22 [Google Scholar]
- Crous PW, Groenewald JZ, Risède J-M, Simoneau P, Hyde KD. (2006) Calonectria species and their Cylindrocladium anamorphs: species with clavate vesicles. Studies in Mycology 55: 213–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ, Summerell BA, Wingfield BD, Wingfield MJ. (2009a) Co-occurring species of Teratosphaeria on Eucalyptus. Persoonia 22: 38–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schoch CL, Hyde KD, Wood AR, Gueidan C, Hoog GS de, Groenewald JZ. (2009b) Phylogenetic lineages in the Capnodiales. Studies in Mycology 64: 17–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Groenewald JZ, Samson RA. (eds) (2009c) Fungal Biodiversity. [CBS Laboratory Manual Series no. 1.] Utrecht: Centraalbureau voor Schimmelcultures; [Google Scholar]
- Deighton FC. (1969) Microfungi. IV: Some hyperparasitic hyphomycetes, and a note on Cercosporella uredinophila Sacc. Mycological Papers 118: 1–41 [Google Scholar]
- Ellis MB. (1971) Dematiaceous Hyphomycetes. Kew: Commonwealth Mycological Institute; [Google Scholar]
- Eriksson OE. (1966) On Eudarluca caricis (Fr.) O. Eriks., comb. nov., a cosmopolitan uredinicolous pyrenomycete. Botaniska Notiser 119: 33–69 [Google Scholar]
- Frank J, Crous PW, Groenewald JZ, Oertel B, Hyde KD, Phengsintham P, Schroers HJ. (2010) Microcyclospora and Microcyclosporella: novel genera accommodating epiphytic fungi causing sooty blotch on apple. Persoonia 24: 93–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoog GS de. (1972) The genera Beauveria, Isaria, Tritirachium and Acrodontium gen. nov. Studies in Mycology 1: 1–41 [Google Scholar]
- Hoog GS de, Gerrits van den Ende AHG. (1998) Molecular diagnostics of clinical strains of filamentous basidiomycetes. Mycoses 41: 183–189 [DOI] [PubMed] [Google Scholar]
- Hoog GS de, Hermanides-Nijhof EJ. (1977) The black yeasts and allied hyphomycetes. Studies in Mycology 15: 1–122 [Google Scholar]
- Hughes SJ. (1958) Revisiones hyphomycetum aliquot cum appendice de nominum rejiciendis. Canadian Journal of Botany 36: 724–836 [Google Scholar]
- Liesebach M, Zaspel I. (2004) Genetic diversity of the hyperparasite Sphaerellopsis filum on Melampsora willow rusts. Forest Pathology 34: 293–305 [Google Scholar]
- Linder DH. (1942) A contribution towards a monograph of the genus Oidium (fungi imperfecti). Lloydia 5: 165–207 [Google Scholar]
- Pillay L, McFarlane SA, Rutherford RS. (2005) A preliminary report on genetic diversity in populations of sugarcane rust in KwaZulu-Natal. Proceedings of the South African Sugarcane Technologists’ Association 79: 132–136 [Google Scholar]
- Rayner RW. (1970) A Mycological Colour Chart. Kew: Commonwealth Mycological Institute; [Google Scholar]
- Saccardo PA. (1877) Fungi italici autrographice delineati a Prof. P.A. Saccardo. Michelia 1: 73–100 [Google Scholar]
- Seifert K, Morgan-Jones G, Gams W, Kendrick B. (2011) The Genera of Hyphomycetes. [CBS Biodiversity Series no. 9] Utrecht: Centraalbureau voor Schimmelcultures; [Google Scholar]
- Vandermeer J, Perfecto I, Liere H. (2009) Evidence for hyperparasitism of coffee rust (Hemileia vastatrix) by the entomogenous fungus, Lecanicillium lecanii, through a complex ecological web. Plant Pathology 58: 636–641 [Google Scholar]
- Vilgalys R, Hester M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee J, Taylor J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (eds), PCR Protocols: a guide to methods and applications: 315–322 San Diego: Academic Press; [Google Scholar]


