Abstract
The genus Scedosporium and its relatives comprising microascalean anamorphs with slimy conidia were studied. Graphium and Parascedosporium also belong to this complex, while teleomorphs are found in Pseudallescheria, Petriella, Petriellopsis, and Lophotrichus. Species complexes were clearly resolved by rDNA ITS sequencing. Significantly different ecological trends were observed between resolved species aggregates. The Pseudallescheria and Scedosporium prolificans clades were the only lineages with a marked opportunistic potential to mammals, while Petriella species were associated primarily with soil enriched by, e.g. dung. A consistent association with bark beetles was observed in the Graphium clade. The ex-type strain of Rhinocladium lesnei, CBS 108.10 was incorrectly implicated by Vuillemin (1910) in a case of human mycetoma; its sequence was identical to that of the ex-type strain of Parascedosporium tectonae, CBS 127.84.
Keywords: Parascedosporium tectonae; Rhinocladium lesnei; Graphium putredinis; Doratomyces putredinis; Scedosporium; Pseudallescheria; Microascales, ecology
INTRODUCTION
This study aims to provide a phylogenetic overview of the relatives of the human-opportunistic genus Pseudallescheria. Members of this genus, producing Scedosporium and eventually Graphium (syn)anamorphs, are found in industrially or agriculturally enriched environments. They are frequently observed in subcutaneous and systemic infections. Among the infections caused is a unique clinical entity, the near-drowning cerebral abscess (Guarro et al. 2006). The species are also encountered as persistent colonizers of the respiratory tract of patients with cystic fibrosis (Lu et al. 2011). In contrast, monomorphic Graphium species have been reported from galleries of bark beetles in conifer wood (Jacobs et al. 2003). This study was undertaken to establish, whether these widely different ecologies show any phylogenetic consistency.
Gilgado et al. (2007) introduced the polymorphic generic name Parascedosporium Gilgado in the framework of a taxonomic revision of Scedosporium, its synanamorph Graphium and its teleomorphs Pseudallescheria, Petriella, and Petriellopsis. Parascedosporium was segregated from Scedosporium and its synnematous synanamorph Graphium on the basis of sympodial conidiogenesis of the Scedosporium morph, conidia being borne on large, blunt denticles, and annellidic in the Graphium morph. Scedosporium, consistently present in members of the teleomorph genera mentioned above, has percurrent conidiogenesis resulting in somewhat slimy conidia. The description of Parascedosporium provided by Gilgado et al. (2007) was based on an authentic strain of Graphium tectonae (Booth 1964), CBS 127.84, which they renamed Parascedosporium tectonae. The strain had been isolated from Tectonia grandis seeds in Jamaica and its relation to the plant-inhabiting species Graphium putredinis needed to be established. We uncovered the ex-type strain of Rhinocladium lesnei which appeared to be molecularly similar to P. tectonae, but, according to its original description, it was associated with a case of human mycetoma. These widely different origins of supposedly interrelated strains required a thorough re-evaluation of the material.
MATERIALS AND METHODS
Strains and sequences
Strains and sequences included in this study, as well as isolation data and GenBank accession numbers, are listed in Table 1. Sequences compromised those downloaded from GenBank (Jacobs et al. 2003, Okada et al. 1998, Paciura et al. 2010) supplemented with sequences generated from material in the CBS collection of fungus cultures and other sequences from CBS not yet incorporated into the public collection.
Table 1.
Strains used for phylogenetic tree construction. Syntypes are marked with (ST), epitypes with (ET), and ex-type strains with (T).
| Main clade | Current ID | Obsolete name | Strain numbers | Status | Cross reference numbers | Associated insect | Host/Source | Country | GenBank number |
|---|---|---|---|---|---|---|---|---|---|
| Graphium | G. basitruncatum | Graphium basitruncatum | CBS 320.72 | (T) | JCM 9300; MFC 2997 | Forest soil | Solomon Islands | AB038427 | |
| Graphium | G. basitruncatum | Graphium basitruncatum | JCM 8083 | Soil | Japan | AB038425 | |||
| Graphium | G. penicillioides | Graphium penicillioides | CBS 102630 | JCM 10496 | Populus nigra, wood core | Czech Republic | AB038430 | ||
| Graphium | G. penicillioides | Graphium penicillioides | CBS 102632 | (ET) | JCM 10498; PRM 842988 | Populus nigra, wood core | Czech Republic | AB038432 | |
| Graphium | G. penicillioides | Graphium penicillioides | JCM 10499 | Populus nigra, wood core | Czech Republic | AB038433 | |||
| Graphium | G.laricis | Graphium laricis | CMW 5598 | DAOM 229754 | Ips cembrae | Larix decidua | Scotland | AY148181 | |
| Graphium | G.laricis | Graphium laricis | CMW 5601 | (T) | DAOM 229757 | Ips cembrae | Larix decidua | Austria | AY148183 |
| Graphium | G.laricis | Graphium laricis | CMW 5603 | DAOM 229759 | Ips cembrae | Larix decidua | Austria | AY148182 | |
| Graphium | G.laricis | Graphium laricis | CBS 116195 | CMW 5602; DAOM 229758 | Ips cembrae | Larix decidua | Austria | AY148184 | |
| Graphium | G. pseudomiticum | Graphium pseudormiticum | AsaP. 18 | Ips typographus | Unknown | Sweden | FJ824623 | ||
| Graphium | G. pseudomiticum | Graphium pseudormiticum | CMW 503 | (T) | Orthotomicus erosus | Pinus sp. | South Africa | AY148186 | |
| Graphium | G. pseudomiticum | Graphium pseudormiticum | CMW 5611 | Tomicus minor | Pinus sylvestris | Austria | AY148185 | ||
| Graphium | G. pseudomiticum | Graphium pseudormiticum | CMW 12285 | Tsuga dumosa | China | FJ434981 | |||
| Graphium | P. putredinis | Rhinocladium lesnei | CBS 108.10 | (T) | dH 14860; MUCL 11709; MUCL 15754 | Human, foot mycetoma | France | HQ185347 | |
| Graphium | R. fimbriasporum | Graphium fimbriisporum | CBS 421.94 | CMW 3353; CMW 5607 | Ips typographus | Picea abies | Austria | AY148179 | |
| Graphium | R. fimbriasporum | Graphium fimbriisporum | CBS 422.94 | CMW 3352; CMW 5606 | Ips typographus | Picea abies | Austria | AY148178; AY148180 | |
| Graphium | R. fimbriasporum | Graphium fimbriisporum | CMW 5605 | (T) | Ips typographus | Picea abies | France | AY148177 | |
| Lophotrichus | L. fimeti | Pseudallescheria fimeti | CBS 129.78 | (T) | dH 14898 | Dung of goat | India | AY879799 | |
| Microascus | Microascus trigonosporus var. trigonosporus | Microascus trigonosporus var. trigonosporus | CBS 665.71 | NRRL A-8019 | Soil | USA | AM774156 | ||
| Parascedosporium | P. putredinis | Graphium calicioides | CBS 102085 | JCM 9765 | decayed wood | Japan | AB007686 | ||
| Parascedosporium | P. putredinis | Graphium pudretinis | CBS 102083 | JCM 8082 | Chrysalidocarpus lutescens | Japan | HQ185348 | ||
| Parascedosporium | P. putredinis | Graphium putredinis | HSAUP052348 | Unknown | FJ914685 | ||||
| Parascedosporium | P. putredinis | Graphium tectonae | CBS 100392 | dH 11150 | Bathroom flask | Netherlands | GQ 476983 | ||
| Parascedosporium | P. putredinis | Parascedosporium tectonae | CBS 118694 | Actinidia deliciosa, leaf lesions | New Zealand | AM749735; AM749149 | |||
| Parascedosporium | P. tectonae | Parascedosporium tectonae | CBS 127.84 | (T) | dH14863; JCM 9753 | Tectona grandis, seed | Jamaica | AY228113 | |
| Petriella | P. guttulata | Petriella guttulata | CBS 362.61 | (ST) | MUCL 9886; TRTC 33049; UAMH 3996 | Dung of partridge | Germany | AY879800 | |
| Petriella | P. setifera | Petriella setifera | CBS 265.64 | Unknown | AY882349 | ||||
| Petriella | P. setifera | Petriella setifera | CBS 347.64 | MUCL 8138 | Compost | Belgium | AY882346 | ||
| Petriella | P. setifera | Petriella setifera | CBS 391.75 | Tursiops truncatus, skin lesion | The Netherlands | AY882344 | |||
| Petriella | P. setifera | Petriella setifera | CBS 395.69 | Maize-field soil | Canada | AY882348 | |||
| Petriella | P. setifera | Petriella setifera | CBS 710.96 | FMR 5550 | Soil | Singapore | AY882347 | ||
| Petriella | P. sordida | Melanospora asymmetrica | CBS 297.58 | Compost soil | Germany | AY882359 | |||
| Petriella | P. sordida | Petriella guttulata | CBS 520.72 | Unknown | Germany | AY882355 | |||
| Petriella | P. sordida | Petriella setifera | ATCC 26490 | Unknown | AF043596 | ||||
| Petriella | P. sordida | Petriella setifera | CBS 385.87 | Human, nail | Finland | AY882345 | |||
| Petriella | P. sordida | Petriella sordida | CBS 124169 | dH 19097 | Bathroom flask | Netherlands | GQ426957 | ||
| Petriella | P. sordida | Petriella sordida | CBS 184.73 | (T) | Wood | Sweden | AY882360 | ||
| Petriellidium | P. desertorum | Petriellidium desertorum | CBS 489.72 | (T) | UAMH 3993 | Salt-marsh soil | Kuwait | AY879798 | |
| Pseudallescheria | P. africana | Pseudallescheria africana | CBS 311.72 | (T) | dH 14874 | Brown, sandy soil | Namibia | AY879797; AY228115; AJ888425 | |
| Pseudallescheria | P. angusta | Pseudallescheria angusta | CBS 254.72 | (T) | Unknown | AY228114; AJ888414 | |||
| Pseudallescheria | P. apiosperma | Graphium eumorphum | CBS 987.73 | JCM 9748 | Human, otitis externa | Czecho-slovakia | AY877352 | ||
| Pseudallescheria | P. apiosperma | Pseudallescheria apiosperma | CBS 117407 | (T) | FMR 8619; dH 14354 | Kereatitis | Brazil | AJ888416; AJ889584 | |
| Pseudallescheria | P. boydii | Pseudallescheria boydii | CBS 119707 | (T) | Unknown | HQ185312 | |||
| Pseudallescheria | P. minutispora | Scedosporium minutispora | CBS 116911 | (T) | Leukemia patient, subcutaneous mycosis of the leg | Hungary | HQ185354 | ||
| Pseudallescheria | S. aurantiacum | Scedosporium aurantiacum | CBS 116910 | (T) | FMR 8630; dH 14360 | Human, ulcer of ankle | Spain | AJ888440; AJ889597; AJ890133; AJ890219 | |
| Pseudallescheria | S. dehoogii | Scedosporium dehoogii | CBS 117406 | (T) | FMR 6921; dH 14338 | Garden soil | Spain | AJ888389; HQ185341 | |
| Scedosproium prolificans | S. prolificans | Scedosporium prolificans | CBS 100391 | Disseminated infection, AIDS patient with Burkitt lymphoma | Germany | AY882368 | |||
| Scedosproium prolificans | S. prolificans | Scedosporium prolificans | CBS 116906 | dH 14616; IHEM 14076 | Sputum of cystic fibrosis patient | France | HQ185323 | ||
| Scedosproium prolificans | S. prolificans | Scedosporium prolificans | CBS 452.89 | (T) | Human, blood | France | HQ185322 |
DNA extraction and sequencing
Strains were cultured for 10–14 d on malt extract agar (MEA); genomic DNA was extracted according to Möller et al. (1992). ITS sequence data spanned the entire internal transcribed spacer region and the partial 28S ribosomal gene (D1/D2), generated with primers V9G and LS266 (de Hoog & Gerrits van den Ende 1998) and sequenced with primers its5 and its4. PCR reaction mixtures (total volume 25 μL) contained 0.4 μM forward and backward primers, 0.185 mM of each deoxynucleoside triphosphate (GC Biotech, Alphen aan de Rijn, The Netherlands), 10-fold concentrated NH4 BioTaq Reaction buffer (GC Biotech), and 20 ng DNA. For performing the PCR reactions a Thermal cycler 2720 (Applied Biosystems, Foster City, U.S.A.) was used. PCR conditions were as follows: a) initial cycle 94 °C for 5 min, b) 35 cycles of 94 °C for 60 s, 53 °C 60 s, 72 °C for 120 s and c) final cycle of 10 min at 72 °C for 7 min. Strains were sequenced with an abi 3700xl instrument using BigDye Terminator Sequencing Kits (Applied Biosystems). Electropherograms were edited, using the Lasergene software package (DNAstar, www.dnastar.com).
Phylogenetic tree construction
Phylogenetic trees were calculated on the basis of 47 ITS sequences. Alignment was made automatically and adjusted manually using the Muscle package (www.ebi.ac.uk/Tools/muscle/index.html). A maximum likelihood ITS tree was calculated using Mega5 (www.megasoftware.net). The calculation parameters were 1000 bootstrap replicates with substitution model GTR + GI and heuristic search set to close-neighbour-interchange. Microascus trigonosporus was used as outgroup to root the tree.
Morphological characteristics
Morphological characteristics of strains CBS 192.61, CBS 102082, CBS 102083, CBS 18694, and CBS 108.10 were recorded. Strains were grown at room temperature under daylight on potato dextrose agar (PDA). Slide cultures were made of cultures in the early stages of conidiation, transferring a 5 mm-sized piece of the colony with agar to a fresh PDA plate, covering the transferred piece with a cover slip and incubating it at room temperature until growth on the cover slip could be observed. Slides were stained with Cotton blue. Microscopic features were photographed using a Nikon Eclipse 80i microscope with a Nikon digital sight DS-Fi1 camera.
RESULTS
Sequences of strains of the following groups were included in the phylogenetic analyses: (1) Petriella clade (P. sordida, P. guttulata, and P. setifera), (2) Petriellopsis africana, (3) Scedosporium prolificans, (4) Pseudallescheria clade (S. aurantiacum, P. desertorum, P. minutispora, S. dehoogii, P. boydii, P. angusta, and P. apiosperma), (5) Lophotrichus fimeti, and (6) Graphium clade (G. penicilliodes, G. fimbriisporum, G. pseudormiticum, and G. laricis). We were unable to locate ex-type strains of (a) G. bulbicola, (b) G. cuneiferum, (c) Nematographium stilboideum (previously known as G. stilboideum), and (d) G. fructicola.
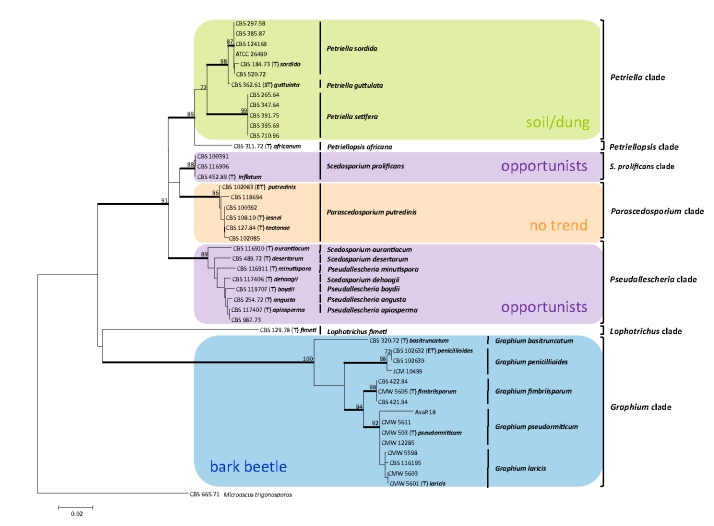
Bootstrap-supported branches (≥ 80) are indicated in bold in the ITS-tree (Fig. 1 and Table 1). Within the Petriella clade three branches were bootstrap-supported: (a) a branch of CBS 362.61 (ex-syntype of P. guttulata), (b) a branch of CBS 184.73 (ex-type Petriella sordida) including the strains identified in the GenBank and/or the CBS database as P. setifera (ATCC 26490, CBS 385.87), Melanospora asymmetrica (CBS 297.58), P. sordida (CBS 124169), and P. guttulata (CBS 520.72), (c) a third branch referred to as P. setifera. The latter branch did not contain any ex-type strain, but all strains attributed to this group (CBS 395.69, CBS 391.75, CBS 265.64, CBS 347.64, and CBS 710.96) were identified by GenBank and/or CBS database as P. setifera. Petriellopsis africana was represented only by its ex-type strain CBS 311.72. Sequences of strains of Scedosporium prolificans, CBS 452.89 (ex-type), CBS 116906, and CBS 100391 clustered in an isolated, bootstrap-supported clade which was the nearest neighbour of Parascedosporium.
Fig. 1.
Overview of treated members of Microascales including strains of Graphium, Pseudallescheria, Petriella, Lophotrichus, Petriellopsis, and Parascedosporium. The maximum likelihood ITS tree was calculated with 200 bootstrap replicates. Strains shaded in green represent strains isolated from dung, strains in shades of purple represent those with medical relevance and/or associated with hydrocarbon-polluted soil, and those shaded in blue are associated with trees and/or bark beetles, while strains shaded in orange do not have any apparent ecological trend. Bootstrap-supported branches (>80 %) are in bold and bootstrap values are indicated. Ex-type strains are marked with a bold (T), epitypes are marked with bold (ET), and syntypes with a bold (ST) after the strain number. The name used in the first description is given in italic bold type.
The Parascedosporium clade (96 % bootstrap support) compromised CBS 102085, CBS 118694, and CBS 102083 of Graphium putredinis (according to Okada et al. 1998), the ex-type of Rhinocladium lesnei (CBS 108.10), and the ex-type plus one additional sequence of Parascedosporium tectonae (CBS 127.84 and CBS 100392). Within the clade differences of maximally two bases were noted, but branches were statistically unsupported.
The bootstrap-supported Pseudallescheria clade was represented by ex-type strains of all currently recognized sibling species, including Scedosporium aurantiacum (CBS 116910), Petriellidium desertorum (CBS 489.72; according to latest taxonomic changes Pseudallescheria desertorum), Pseudallescheria minutispora (CBS 116911), Scedosporium dehoogii (CBS 117406), P. angusta (CBS 254.72), P. boydii (CBS 119707), and P. apiosperma (CBS 117407). Strain CBS 987.73 had been reported as causative agent of otitis externa. It was previously identified as Graphium eumorphum (Frágner & Hejzlar 1973), but the ITS sequence was 100 % identical to that of P. apiosperma, ex-type strain. Lophotrichus fimeti was represented exclusively by its ex-type strain (CBS 129.78).
The Graphium clade with 100 % bootstrap support included four bootstrap-supported branches. The first branch included the epitype of G. penicillioides (CBS 102632) and sequences of the G. penicillioides strains CBS 102630 and JCM 10499. The second branch contained a sequence of the ex-type of Stilbum basitruncatum (CBS 320.72; currently known as G. basitruncatum). The third branch was formed by strains of G. fimbriisporum (CMW 6505 ex-type strain, CBS 422.94, and CBS 421.94). The fourth bootstrap-supported subclade within the Graphium main clade included the ex-type strain of G. pseudormiticum (CMW 503) and the ex-type strain of G. laricis (CMW 5601), varying in ITS from each other by a single nucleotide change. Moreover, the sequences of the following strains were found in the same cluster: G. pseudormiticum (CMW 12285, CMW 5611, and AsaP. 18) and G. laricis (CBS 116195, CBS 5598, and CMW 5603). The two species G. pseudormiticum and G. laricis described by Jacobs et al. (2003) differed only by a single ITS nucleotide, which was similar to the G. laricis intra-species variation. Recently Cruywagen et al. (2010) distinguished the two species with TEF1 sequences.
The ex-type strain CBS 108.10 of Rhinocladium lesnei was originally reported from a human mycetoma (Vuillemin 1910). His original illustration is reproduced in Fig. 2. The drawings show a fungus with elongate cells producing one-celled sympodial conidia on hyphae, in addition to hyphae aggregating in large synnemata and producing a drop of mucous sympodial conidia at the apex (Fig. 2). The characteristic features of the mononematous anamorph are still exhibited in the authentic strain, CBS 108.10 (Fig. 3) and match with the descriptions and illustrations provided by Gilgado et al. (2007) for that of P. tectonae CBS 102083. We investigated the morphological characteristics of the strains CBS 192.61 (= G. putredinis), CBS 102083 (= G. putredinis), and CBS 118694 (= Parascedosporium tectonae). All isolates displayed characteristic solitary scedosporium-like conidiophores bearing lateral, cylindrical conidiogenous cells with 2–5 cylindrical denticles. Strains CBS 102082, CBS 118694, and CBS 102083 formed additional synnemata with an annellidic type of conidiation. The synnematous anamorph of R. lesnei was no longer produced, but Vuillemin (1910) illustrated it as being sympodial (Fig. 2). Hence, we cannot be certain of its identity and therefore treat R. lesnei as being doubtful.
Fig. 2.
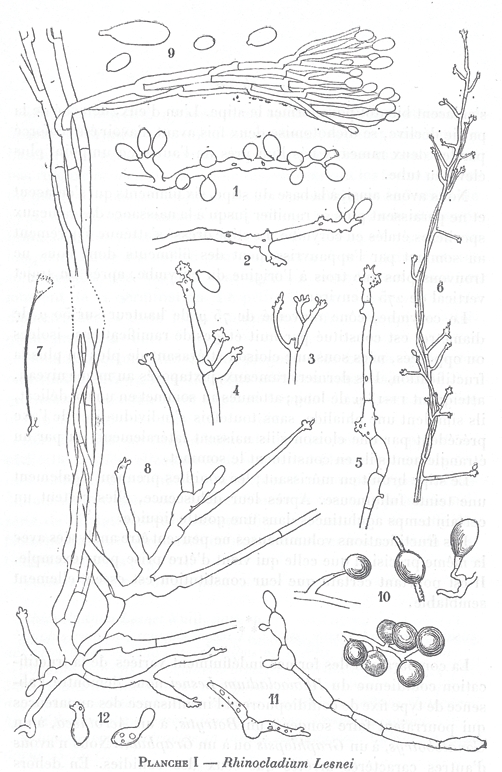
Reproduction of the original drawings of Rhinocladium lesnei (Vuillemin 1910).
Fig. 3.
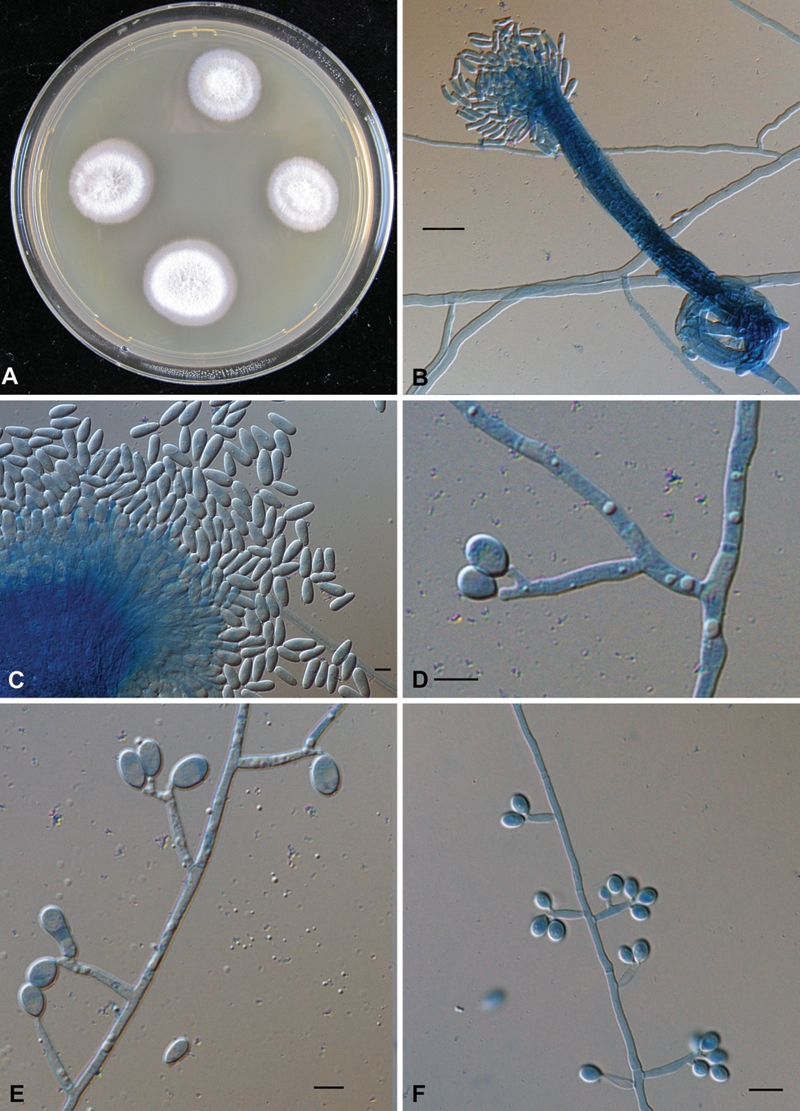
Macro- and micromorphological characteristics of Parascedosporium putredinis. A. Four day old culture on malt-extract agar (CBS 108.10). B. Graphium stage, with basal ring structure and conidia (CBS 320.72). C. Conidiogenous cells of the Graphium synnemata (CBS 102082). D. (CBS 118694). E. (CBS 118694). F. (CBS 102083): solitary conidiophores with lateral, cylindrical conidiogenous cells. Bar = 10 μm.
Graphium putredinis has previously been attributed to Doratomyces (Morton & Smith, 1963, Matsushima 1975), a microascalean genus with strongly hydrophobic conidia. This interpretation had been adopted in major databases such as Index Fungorum and MycoBank. However, Okada et al. (1998), following Hughes (1958), assigned it to the Pseudallescheria relationship comprising species with slimy conidia. The genus Doratomyces was introduced by Corda (1829), with a clearly recognizable description and illustration, rendering confusion with a mucous gaphium-like species unlikely. Gaphium cuneiferum is often listed as a synonym, although Hughes (1958), in the absence of authentic material, regarded this synonymy as doubtful. Hence we conclude that the nomenclature of the species at hand is as follows:
Parascedosporium putredinis (Corda) Lackner & de Hoog, comb. nov.
MyoBank MB519652
(Fig. 3)
Basionym: Stysanus putredinis Corda, – Icon. Fung, 3: 12 (1829).
Synonyms: Graphium putredinis (Corda) S. Hughes, – Can. J. Bot. 36: 772 (1958).
Doratomyces putredinis (Corda) F.J. Morton & G. Sm., Mycol. Pap. 86: 83 (1963); non sensu Matsushima (1975: 63).
Type: Czech Republic: Prague, on rotten stem of Echium sp., 1838, A.C. Corda (PR 155673 – holotype, slide ex-type DAOM 40745); Japan (Ogasawara, Chichijima, Komagari): isolated by G. Okada from Chrysalidocarpus lutescens in 1999, (CBS 102083 –epitypus hic designatus; JCM 8082 – isoepitypus).
? Stilbum cuneiferum Berk. & Broome, Ann. Mag. nat. Hist., ser 4 15: 33 (1875).
Synonyms: Sporocybe cuneifera (Berk. & Broome) Sacc., Syll. Fung. 4: 606 (1886).
Cephalotrichum cuneiferum (Berk. & Broome) Kuntze, Rev. Gen. Pl. 3: 453 (1898).
Graphium cuneiferum (Berk. & Broome) E.W. Mason & M.B. Ellis, Mycol. Pap. 56: 41 (1953).
Type: United Kingdom: on rotten stem of cabbage, (K(M) – holotype n.v.).
Rhinocladium lesnei Vuill., Bull. Séanc. Soc. Sci. Nancy 11: 143 (1910).
Sporotrichum lesnei (Vuill.) Castell. & Chalm., Man. Trop. Med., edn 3 : 1121 ( 1919).
Graphium lesnei (Vuill.) E.W. Mason, Mycol. Pap. 4: 94 (1937).
Type: France: isolated in 1910 by P. Vuillemin from pus of a mycetoma of human foot (CBS 108.10 – ex-holotype strain).
Graphium tectonae C. Booth, Mycol. Pap. 94: 5 (1964).
Parascedosporium tectonae (C. Booth) Gilgado et al., Int. J. Syst. Evol. Microbiol. 57: 2176 (2007).
Type: Jamaica: isolated from epicarp of Tectona grandis seed, July 1962, isol. C. Booth (IMI 95673d – holotype, n.v.; CBS 127.84 – ex-holotype strain).
The following description is of CBS 102083 on PDA after 14 d at 22 °C:
Colonies attaining about 50 mm diam, velvety, radially folded, grey, with numerous synnemata at the centre; reverse dark grey to black. Conidiophores when solitary, simple, undifferentiated, with lateral conidiogenous cells; conidiogenous cells cylindrical, 6–20 × 1.5–2.5 μm, hyaline, thin-walled, bearing 2–5 cylindrical denticles of up to 1 μm long; conidia obovoidal, 5–6 × 3–4 μm, smooth- and rather thick-walled. Synnemata erect, to 450 μm tall; stipe to 70 μm wide, olivaceous grey, apically splaying out and producing conidia in a slime droplet; conidiogenous cells percurrent, cylindrical, 10–37 × 1.5–2.5 μm; conidia (sub)cylindrical, 5.5–7.5 × 2.5–3.5 μm. Additional sessile conidia present in low frequency on undifferentiated hyphae. Maximum growth temperature was 37 °C.
Members of the Pseudallescheria clade, especially Scedosporium dehoogii including S. deficiens (Rainer & Kaltseis 2010), are frequently found in hydrocarbon-contaminated soils (Kaltseis et al. 2009). In temperate climates P. apiosperma, S. aurantiacum, and P. minutispora can be isolated from soils with increased nitrogen concentrations and lowered pH (Kaltseis et al. 2009). In arid areas, such as Australia and Spain, the member of the Pseudallescheria clade, most frequently isolated from environmental and clinical samples is S. aurantiacum (Rodriguez-Tudela et al. 2009). In contrast, P. boydii is the most frequently encountered agent in human infection (Kaltseis et al. 2009). In general, members of the Pseudallescheria clade tend to inhabit environments with increased human impact (agricultural soils, urban playgrounds, industrial areas, hydrocarbon-contaminated soils) and are also able to cause infections. In immunocompetent patients these infections are either traumatic or cerebral in almost drowned victims (Buzina et al. 2006), whereas in immunocompromised patients (e.g. transplant recipients) disseminated infections occur (Guarro et al. 2006). Strains of Scedosporium prolificans are frequently found in human infections and are regularly isolated from soil in arid areas of Australia (Heath et al. 2009), the USA (Spielberger et al. 1995), and Spain (Rodriguez-Tudela et al. 2009). Members of this species are known to carry multiple resistances against all available antifungal drugs (Guarro et al. 2006).
Petriellopsis africana was isolated only once from brown sandy soil; its ecology and virulence are unknown. Petriella strains are rarely associated with vertebrate infections. A cutaneous infection was described by Poelma et al. (1974) in a bottlenosed dolphin Tursiops truncatus; the causative agent was Petriella setifera (CBS 391.75). The type of P. guttulata (CBS 362.61) was obtained from dung of a partridge, and P. setifera (CBS 385.87) came from a human nail. All other Petriella strains were isolated from soil, dung, compost, and once from a bathroom jar (Table 1).
In contrast, all strains in the ITS Graphium clade were isolated from wood or forest soil. A consistent association with wood infested by bark insects seems likely. The three strains of G. fimbriisporum from France and Austria were associated with the Picea abies and the bark beetle Ips typographus. Jacobs et al. (2003) described G. laricis as exhibiting a specific host/insect association (Larix deciduas / Ips cembrae), verified in four strains (CBS 116195, CMW 5598, CMW 5601, and CMW 5603). The remaining strains in the same cluster (CMW 503, CMW 5611, and CMW 12285) were derived from trees and/or bark beetles, but did not share an exclusive association with a particular insect (Tomicus minor, Ips typographus, Orthomicus erosus) on a specific host tree (Pinus sp., Pinus sylvestris, Tsuga dumosa). Graphium penicillioides was isolated from a wood core of Populus nigra in the Czech Republic. In contrast, G. basitruncatum seems to be atypical as it was isolated twice from soil (Solomon Islands and Japan) and once from a leukemic patient (Kumar et al. 2007).
DISCUSSION
The opportunistic, multi-drug resistant species Scedosporium prolificans represents the closest relative of Parascedosporium putredinis as a sister clade (Fig. 1). In contrast to S. prolificans, P. putredinis was only once reported to be involved in a human infection (Vuillemin 1910, as Rhinocladium lesnei). This strain, CBS 108.10 originated from pus exuded from a fistula of a human foot mycetoma (Vuillemin 1910). No histopathology was provided, and no fungal grains were biopsied and cultured. Since 1910, P. putredinis has never been found in human infections, and the infection described by Vuillemin (1910) lacks histopathological proof; we therefore doubt the credibility of this being the causal agent of the clinical condition. Even though Scedosporium prolificans represents the nearest neighbour of P. putredinis, we found no evidence of P. putredinis being virulent to humans. Analyzing the origins and sources of isolation of strains attributed to P. putredinis (Table 1), no clear ecological trend is evident; some strains were isolated from living plant material, while others were isolated from soil, dung, or plant debris.
Other closely related fungi are those in the Petriella clade (i.e. Petriella setifera, P. guttulata, and P. sordida) and Petriellopsis africana. These strains were mainly isolated from dung and soil, with two exceptions: CBS 391.75 which caused a skin lesion in Tursiops truncatus kept in a Dolphinarium in The Netherlands (Table 1), and CBS 385.87 from a human nail, the latter possibly representing a contaminant. There is consequently not much evidence of any Petriella bearing intrinsic pathogenicity to vertebrates.
The Pseudallescheria clade encompasses the largest number of clinical strains, and is implemented in a wide spectrum of diseases (Guarro et al. 2006). Environmental isolates of this cluster are mostly found in soil and water enriched by agricultural or industrial pollution (Kaltseis et al. 2009). Recently, enhancement of isolation by using biodiesel fuel was noted for S. dehoogii (Eggertsberger et al., unpubl.) and for S. deficiens (Rainer & Kaltseis 2010); the latter species was described for one of the clusters within S. dehoogii. Scedosporium aurantiacum is generally more common in hot and arid areas such as Spain (Rodriguez-Tudela et al. 2009) and Australia (Heath et al. 2009), while the remaining species of the clade are prevalent in temperate climates.
Several clinical and animal cases attributed to Graphium species probably concerned misidentified strains. Graphium eumorphum, of which no type material is known to exist, was reported from a case of otitis externa (Frágner & Hejzlar 1973). The ITS sequence of the strain from this case, CBS 987.73 was found to be identical to the ex-type strain of P. apiosperma, CBS 117407. Käufer & Weber (1977) described a systemic infection with cerebral involvement in a dog caused by G. fructicola (Marchal & Marchal 1921) – but neither the original fungal material nor the veterinary specimen are known as preserved. The brain abscesses including histopathology reported by Käufer & Weber (1977) were similar to those caused by P. apiosperma and P. boydii in human infections, so a species of the Pseudallescheria clade may have been involved.
Kumar et al. (2007) reported a fungemia in a patient with acute leukemia caused by Graphium basitruncatum, which is the only example of a clinical strain in the clade with monomorphic Graphium species otherwise showing association with bark beetle communities (Fig. 1) (Romón et al. 2007). This specialized type of ecology is well known for members of the order Ophiostomatales (Carlier et al. 2006). Such fungal associations are often specific to a particular host insect. The beetles transport fungal cells in their mycangia and use fungal gardens as food source. Jacobs et al. (2003) and Paciura et al. (2010) reported on Graphium species as associates of bark beetles (Table 1); the species concerned all belong to a single clade (Fig. 1). In the study of Paciura et al. (2010), G. pseudormiticum was directly isolated from bark beetle galleries, providing strong evidence that the fungus is cultured by the beetles. Other authors recognized that strains of Graphium cause brown to black wood staining (Stauffer et al. 2001, Geldenhuis et al. 2004). With ITS sequencing (Fig. 1), G. pseudormiticum and G. laricis were nearly indistinguishable. Okada et al. (1998) erected G. laricis on the basis of its ecological niche rather than on sequence differences, because in their data (rDNA SSU) G. laricis was identical to G. pseudormiticum. Kirschner (1998) reported G. pseudormiticum to be associated with bark beetles of the genera Crypturgus, Dryocoetes, Hylurgops, Polygraphus, Trypodendron, Pityogenes, and Ips species on spruce, and Ips and Orthotomicus species on pine trees, suggesting a low degree of host-specialization. Nevertheless, association with bark beetles is consistent in the Graphium clade.
In conclusion, according to current knowledge, we find only evidence of vertebrate-pathogenicity for strains of the S. prolificans clade and the Pseudallescheria clade (mainly strains of P. apiosperma, P. boydii, and S. aurantiacum). In contrast, strains affiliated to the Graphium clade, in particular G. fimbriisporum, G. laricis, and G. pseudormiticum, inhabit niches in association with different kinds of bark beetles, while the majority of Petriella and Petriellopsis strains show an increased affinity towards soil, dung and compost. Parascedosporium putredinis does not exhibit any ecological specialization.
Acknowledgments
We are grateful to Kasper Luijstenburg for restoring the image of Rhinocladium lesnei and are indebted to Bert Gerrits van den Ende for phylogenetic tree construction. No humans or animals were used in this research, and no outside funding was received. All experiments on living material on Scedosporium / Pseudallescheria were performed according to the International Biosafety policies (biosafety level 2).
We report no conflicts of interest and are alone responsible for the content and writing the paper.
REFERENCES
- Booth C. (1964) Studies of pyrenomycetes. VII. Mycological Papers 94: 1–16 [Google Scholar]
- Buzina W, Feierl G, Haas D, Reinthaler FF, Holl A, Kleinert R, Reichenpfader B, Roll P, Marth E. (2006) Lethal brain abscess due to the fungus Scedosporium apiospermum (teleomorph Pseudallescheria boydii) after a near-drowning incident: case report and review of the literature. Medical Mycology 44: 473–477 [DOI] [PubMed] [Google Scholar]
- Carlier FX, Decock C, Jacobs K, Maraite H. (2006) Ophiostoma arduennense sp. nov. (Ophiostomatales, Ascomycota) from Fagus sylvetica in southern Belgium. Mycological Research 110: 801–810 [DOI] [PubMed] [Google Scholar]
- Corda ACJ. (1829) Die Pilze Deutschlands. In: Flora von Deutschland in Abbildungen nach der Naturmit Beschreibungen ( Sturm J. ed): 3 (2). Nürnberg: the authors; [Google Scholar]
- Cruywagen EM, de Beer ZW, Roux J, Wingfield MJ. (2010) Three new Graphium species from baobab trees in South Africa and Madagascar. Persoonia 25: 61–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frágner P, Hejzlar J. (1973) ‘Graphiosis’ – eine neue Erkrankung des Menschen? (‘Graphiosis’- a new human disease?). Česká Mykologie 27: 98–106 [Google Scholar]
- Geldenhuis MM, Roux J, Montenegro F, de Beer ZW, Wingfield MJ, Wingfield BD. (2004) Identification and pathogenicity of Graphium and Pesotum species from machete wounds on Schizolobium parahybum in Ecuador. Fungal Diversity 15: 137–151 [Google Scholar]
- Gilgado F, Gené J, Cano J, Guarro J. (2007) Reclassification of Graphium tectonae as Parascedosporium tectonae gen. nov., comb. nov., Pseudallescheria africana as Petriellopsis africana gen. nov., comb. nov. and Pseudallescheria fimeti as Lophotrichus fimeti comb. nov. International Journal of Systematic and Evolutionary Microbiology 57: 2171–2178 [DOI] [PubMed] [Google Scholar]
- Guarro J, Kantarcioglu AS, Horré R, Rodriguez-Tudela JL, Cuenca EM, Berenguer J, de Hoog GS. (2006) Scedosporium apiospermum: changing clinical spectrum of a therapy-refractory opportunist. Medical Mycology 44: 295–327 [DOI] [PubMed] [Google Scholar]
- Heath CH, Slavin MA, Sorrell TC, Handke R, Harun A, Phillips M, Nguyen Q, Delhaes L, Ellis D, Meyer W, Chen SCA. (2009) Population-based surveillance for scedosporiosis in Australia: epidemiology, disease manifestations and emergence of Scedosporium aurantiacum infection. Clinical Microbiology and Infection 15: 689–693 [DOI] [PubMed] [Google Scholar]
- Hoog GS de, Gerrits van den Ende AHG. (1998) Molecular diagnostics of clinical strains of filamentous basidiomycetes. Mycoses 41: 183–189 [DOI] [PubMed] [Google Scholar]
- Hughes SJ. (1958) Revisiones hyphomycetum aliquot cum appendice de nominibus rejiciendis. Canadian Journal of Botany 36: 127–836 [Google Scholar]
- Jacobs K, Kirisitis T, Wingfield MJ. (2003) Taxonomic re-evaluation of three related species of Graphium, based on morphology, ecology and phylogeny. Mycologia 95: 714–727 [DOI] [PubMed] [Google Scholar]
- Kaltseis J, Rainer J, de Hoog GS. (2009) Ecology of Pseudallescheria and Scedosporium species in human-dominated and natural environments and their distribution in clinical samples. Medical Mycology 47: 398–405 [DOI] [PubMed] [Google Scholar]
- Käufer I, Weber A. (1977) Graphium fructicola als Urache einer Systemmykose beim Hund. Mykosen 20: 39–46 [PubMed] [Google Scholar]
- Kirschner R. (1998) Diversität mit Borkenkäfern assoziierter filamentöser Mikropilze. PhD thesis, Biologische Fakultät. Eberhard-Karls-Universität Tübingen, Germany: [Google Scholar]
- Kumar D, Sigler L, Gibas CF, Mohan S, Schuh A, Medeiros BC, Peckham K, Humar A. (2007) Graphium basitruncatum fungemia in a patient with acute leukemia. Journal of Clinical Microbiology 45: 1644–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima T. (1975) Icones Microfungorum a Matsushima lectorum. Kobe: [Google Scholar]
- Möller EM, Bahnweg G, Sandermann H, Geiger HH. (1992) A simple and efficient protocol for isolation of high molecular weight DNA from filamentous fungi, fruit bodies, and infected plant tissues. Nucleic Acids Research 22: 6115–6116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton FJ, Smith G. (1963) The genera Scopolariopsis Bainier, Microascus Zukal, and Doratomyces Corda. Mycolological Papers 86: 1–96 [Google Scholar]
- Okada G, Seifert KA, Takematsu A, Yamaoka Y, Miyazaki S, Tubaki K. (1998) A molecular phylogenetic reappraisal of the Graphium complex based on 18S rDNA sequences. Canadian Journal of Botany 76: 1495–1506 [Google Scholar]
- Paciura D, Zhou XD, de Beer ZW, Jacobs K, Ye H, Wingfield MJ. (2010) Characterisation of the synnematous bark beetle-associated fungi from China, including Graphium carbonarium sp. nov. Fungal Diversity 40: 75–88 [Google Scholar]
- Poelma FG, de Vries GA, Blythe-Russell EA, Luyckx MHF. (1974) Lobomycosis in an Atlantic bottle-nosed dolphin in the Dolphinarium Harderwijk. Aquatic Mammals 13: 11–15 [Google Scholar]
- Rainer J, de Hoog GS, Wedde M, Gräser Y, Gilges S. (2000) Molecular variability of Pseudallescheria boydii, a neurotropic opportunist. Journal of Clinical Microbiologie 8: 3267–3273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainer J, Kaltseis J. (2010) Diversity in Scedosporium dehoogii (Microascaceae): S. deficiens sp. nov. Sydowia 62: 137–147 [Google Scholar]
- Rodriguez-Tudela JL, Berenguer J, Guarro J, Kantarcioglu AS, Horré R, de Hoog GS, Cuenca-Estrella M. (2009) Epidemiology and outcome of Scedosporium prolificans infection, a review of 162 cases. Medical Mycology 47: 359–370 [DOI] [PubMed] [Google Scholar]
- Romón P, Zhou XD, Iturrondobeitia JC, Wingfield MJ, Goldarazena A. (2007) Ophiostoma species (Ascomycetes: Ophiostomatales) associated with bark beetles (Coleoptera: Scolytinae) colonizing Pinus radiata in northern Spain. Canadian Journal of Microbiology 53: 756–767 [DOI] [PubMed] [Google Scholar]
- Spielberger RT, Tegtmeier BR, O’Donnell MR, Ito JI. (1995) Fatal Scedosporium prolificans (S. inflatum) fungemia following allogeneic bone marrow transplantation: report of a case in the United States. Clinical Infectious Diseases 21: 1067 [DOI] [PubMed] [Google Scholar]
- Stauffer C, Kirisits T, Nussbaumer C, Pavlin R, Wingfield MJ. (2001) Phylogenetic relationships between the European and Asian eight spined larch bark beetle populations (Coleoptera, Scolytidae) inferred from DNA sequences and fungal associates. European Journal of Entomology 98: 99–105 [Google Scholar]
- Vuillemin P. (1910) Description d’un type de chaque ordre de Conidiosporés. Bulletin des Séances du Société des Sciences de Nancy, Sér. 3, 11: 138–143 [Google Scholar]