Abstract
The American Diabetes Association now recommends hemoglobin A1c (HbA1c) screening for the diagnosis of diabetes. It has been reported that HbA1c levels underestimate glycemic levels in HIV-infected persons. We examined the performance of HbA1c as a screening test for diabetes in a group of HIV-infected people without diabetes. We conducted a retrospective cross-sectional cohort study among HIV-infected patients determining the sensitivity and specificity of HbA1c as a screening test compared to fasting blood glucose (FBG). The effect of treatment regimen on the relationship between HbA1c and FBG was assessed by multiple linear regressions. Twenty-two of the 395 patients included in the study were newly diagnosed with diabetes based on FBG≥126 mg/dL. Using a cutoff of HbA1c≥6.5%, HbA1c had a sensitivity of 40.9% and specificity of 97.5% for identification of incident diabetes. At an HbA1c level of 5.8% the product of sensitivity and specificity was maximized, with values of 88.8% and 77.5% respectively. Higher mean cell volume (MCV) values (p=0.02) and current use of a non-nucleoside reverse transcriptase inhibitors (NNRTIs; p=0.02) significantly increased the slope, while PI use significantly decreased the slope (p<0.001), of the linear regression of HbA1c compared to FBG. Tenofovir use did not significantly alter the slope or y-intercept of the line. Among HIV-infected nondiabetic patients, HbA1c is insensitive, although highly specific for diagnosing diabetes. Current antiretroviral (ART) use has significant and variable influence on the relationship between HbA1c and FBG. The use of HbA1c in conjunction with FBG may be the best modality to screen for diabetes.
Introduction
HIV-infected individuals who are currently receiving antiretroviral therapy (ART) appear to be at increased risk of developing diabetes mellitus.1–4 As a result, international guidelines recommend yearly screening of all HIV-infected individuals for diabetes.5,6
In 2009 the American Diabetes Association (ADA) introduced glycated hemoglobin A1c (HbA1c) as an alternative to fasting blood glucose (FBG) and 2-h oral glucose tolerance tests (OGTT) in screening for diabetes. They defined diabetes as having an HbA1c≥6.5%.7,8 As with FBG and OGTT, this cut-off was based on increasing risk of retinopathy.6 The use of HbA1c for screening (1) provides the clinician with the ability to estimate the average level of glycemia and (2) provides a measure that is accurate in a nonfasting state. ADA guidelines suggest HbA1c is an acceptable screening test unless there are abnormalities in erythrocyte structure (e.g., hemoglobinopathies) or turnover (e.g., pregnancy, significant bleeding, hemolysis, or iron deficiency anemia).
Reports of HbA1c levels in HIV-infected people with diabetes consistently demonstrated that HbA1c underestimated the level of glycemia.9–12 The reason for the discordant HbA1c-FBG relationship observed in HIV-infected patients is still unclear. The existing data on HbA1c in HIV-infected patients are in known diabetics for monitoring of glycemic control, prior to the widespread use of tenofovir. In this study we compared HbA1c and FBG as screening tests for the diagnosis of diabetes mellitus in the HIV-infected population.
Methods
Study design and participants
In 2009, providers at the Bellevue Virology Clinic began screening for diabetes using HbA1c as part of a quality improvement initiative. Charts of all HIV-infected patients 18 years of age or older who had one or more HbA1c values recorded in the hospital's electronic records between October 2009 and March 2010 were reviewed for inclusion in this retrospective cross-sectional cohort study. Patients were excluded from the study if they had underlying conditions or were on medications that alter either red blood cell life span or blood glucose levels. Conditions for exclusion included known diabetes (defined by current use of antihyperglycemic therapy), hemoglobinopathies, G6PD-deficiency, pregnancies, hospitalization or receipt of a transfusion within the prior 3 months, treatment with systemic corticosteroids, or absence of a FBG value in the 3 months prior to HbA1c testing. Approval for this study was received from the Institutional Review Board of the NYU School of Medicine, from Bellevue Hospital Center, and from the central office of the NY City Health and Hospital Corporation.
Measures
Demographic characteristics, current medications (including ART), most recent HIV viral load, CD4 count, and red blood cell indices were extracted from each patient's chart. The FBG used for comparison with the HbA1c was the average of FBG values obtained during the 3 months prior to HbA1c testing. At the study site, patients are asked if they are fasting prior to blood testing, and those in a nonfasting state are asked to return on a subsequent day for testing when they are fasting. FBG was measured in a Siemens ADVIA 2400 analyzer (Siemens Healthcare Diagnostics, Deerfield, IL) using the Hexokinase method, and HbA1c values were determined by the high-performance liquid chromatography (HPLC) assay of Primus diagnostics (Trinity Biotech, Bray, Ireland). Both tests were performed at Bellevue Hospital Center's Clinical Laboratory.
We defined FBG as elevated if it was≥126 mg/dL and HbA1c as elevated if it was≥6.5%, in accordance with laboratory cutoffs and recommendations of the ADA.7,8,13 We assessed the sensitivity and specificity of HbA1c as a screening test for diabetes using FBG as a gold standard. Additionally, patient variables (erythrocyte characteristics, race, and current ART) and their effect on both the test characteristics of HbA1c (sensitivity and specificity), and the relationship between HbA1c and FBG values were examined. The subset sensitivities and specificities were calculated using the accepted cut-point for diagnosing diabetes (HbA1c≥6.5%).
Statistical analyses
Data were analyzed using both SPSS version 19 (SPS Inc., Chicago, IL) and the R statistical environment.14 A receiver operating characteristic (ROC) curve was computed to evaluate the performance of HbA1c as a screening test for the full cohort using various cut-points. Pearson's uncorrected χ2 test was used to assess the significance of the differences among sensitivities and specificities of HbA1c level, for various subsets of the cohort.
The effect of erythrocyte characteristics, race, and current ART on the relationship between HbA1c and FBG were assessed by multiple linear regression. Regression models included FBG as the dependent variable and HbA1c as one independent variable, together with variables representing the characteristic under study and its interaction with HbA1c. A characteristic was said to affect the relationship between FBG and HbA1c if inclusion of the latter two terms in the model significantly reduced the regression's residual variance as judged by an appropriate F ratio. Additional F ratios were examined after a positive result to assess the significance of changes in slope or y-intercept. Model fit was assessed by graphic examination of residuals and influence statistics.
Results
Four hundred ninety HIV-infected patients had HbA1c results available during the study period, and 395 were included in the analysis. Of the 95 patients excluded, 39 were known to have diabetes and receiving antihyperglycemics, 22 had a hemoglobinopathy or G6PD deficiency, 3 were pregnant, 2 were receiving systemic corticosteroids, 40 had a recent hospitalization, and 19 had no FBG value in the 3 months preceding HbA1c testing. Baseline characteristics of the study population are displayed in Tables 1 and 2. The mean age of patients was 46.2 years, 77.5% were male, and the majority belonged to racial or ethnic minorities (46.8% black and 34.9% Hispanic).
Table 1.
Characteristics of Study Participants at Index Visit
| na | 395 |
| Demographics | |
| Age (mean±SD) | 46.2±10.9 |
| Gender (% male) | 77.5 |
| Race (%) | |
| Black | 46.8 |
| Hispanic | 34.9 |
| White | 7.3 |
| Asian/Pacific Islander | 7.8 |
| Other | 2.3 |
| Unknown | 0.8 |
| HIV disease characteristics | |
| CD4 count (mean±SD) | 445.0±250.4 |
| Viral load (% undetectable) | 66.8 |
| Laboratory measures | |
| HbA1c | |
| Meadian (IQR) | 5.5 (5.3–5.8) |
| Mean±SD | 5.6±0.8 |
| FBG | |
| Values per patientb (mean±SD) | 2.01±0.88 |
| Median (IQR) | 91 (84–99) |
| Mean±SD | 96.0±28.5 |
Number of participants.
Average number of FBG values per patient included in analysis.
SD, standard deviation; Hba1c; glycated hemoglobin A1c; IQR, interquartile range; FBG, fasting blood glucose.
Table 2.
Current Antiretrovirals of the 395 Study Participants at Index Visit
| na | |
|---|---|
| No antiretrovirals | 63 |
| Nucleoside/nucleotide reverse transcriptase inhibitor | 319 |
| Abacavir | 49 |
| Didanosine | 9 |
| Emtricitabine | 235 |
| Lamivudine | 72 |
| Stavudine | 4 |
| Tenofovir | 269 |
| Zidovudine | 41 |
| Non-nucleoside reverse transcriptase inhibitor | 136 |
| Efavirenz | 116 |
| Etravirine | 12 |
| Nevirapine | 8 |
| Protease Inhibitor | 188 |
| Ritonavirb | 181 |
| Atazanavir | 85 |
| Darunavir | 29 |
| Fosamprenavir | 17 |
| Indinavir | 2 |
| Lopinavir | 51 |
| Nelfinavir | 4 |
| Saquinavir | 2 |
| Tipranavir | 0 |
| Integrase inhibitor/raltegravir | 40 |
| Entry inhibitor | |
| Enfuvirtide | 1 |
| Maraviroc | 6 |
Number of participants receiving each treatment.
Of patients on protease inhibitors only 7 were not receiving ritonavir boosting; 4 patients taking nelfinavir, 2 taking atazanavir, and 1 taking fosamprenavir.
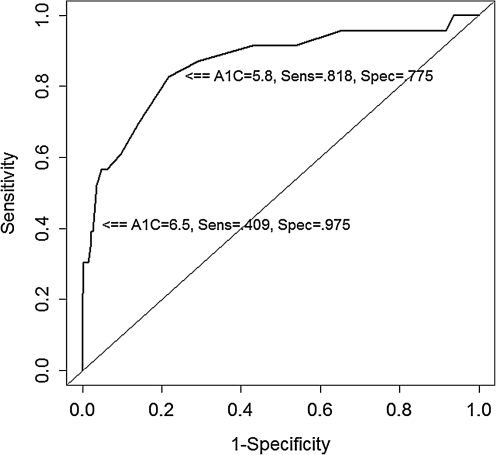
Of the 395 patients included in the study, 13 (3.3%) had an elevated FBG and normal HbA1c, 9 (2.3%) had an elevated HbA1c and a normal FBG, and 9 (2.3%) were concordant for elevations in both tests. Relative to the FBG gold standard, the sensitivity of HbA1c as a screening test for diabetes was 40.9% and the specificity was 97.5%. Based on the ROC curve the optimal cut-point, defined as the value that maximizes the product of sensitivity and specificity, was a HbA1c level of 5.8%, which was 81.8% sensitive and 77.5% specific (Fig. 1). Subgroup analysis by ART [categorized as either no ART or by receipt of regimen containing an nucleoside reverse transcriptase inhibitor (NRTI), non-nucleoside reverse transcriptase inhibitor (NNRTI), or protease inhibitor (PI)] showed no statistically significant effect on sensitivity or specificity (data not shown).
FIG. 1.
Receiver operating characteristic (ROC) curve for HbA1c levels as a diagnostic indicator for diabetes [defined as a fasting blood glucose (FBG)≥126 mg/dL]. A1c, glycated hemoglobin A1c (HbA1c); sens, sensitivity; spec, specificity.
Of the 13 patients with an elevated FBG and normal HbA1c, 4 were found to have been previously diagnosed with diabetes, 3 had normal FBG determinations on a subsequent visit, and 6 were new cases of diabetes and were started on either medical or lifestyle management. None of the 4 previously diagnosed diabetics were on antihyperglycemic therapy, 1 had a prior history of indinavir-induced diabetes, 1 had previous steroid-induced diabetes, and 2 were known diabetics off treatment. Of the 9 patients with an elevated HbA1c and a normal FBG, only 1 was found to have a prior history of elevated FBG levels, and 4 of the 9 had a subsequent HbA1c value<6.5%.
Multiple linear regression confirmed that mean cell volume (MCV) affected the relationship between FBG and HbA1c (F test for interaction between MCV and A1c=5.4, p=0.02) by increasing the slope of the regression curve. Hemoglobin level had no significant effect on this relationship, nor did racial identification as black versus nonblack (data not shown).
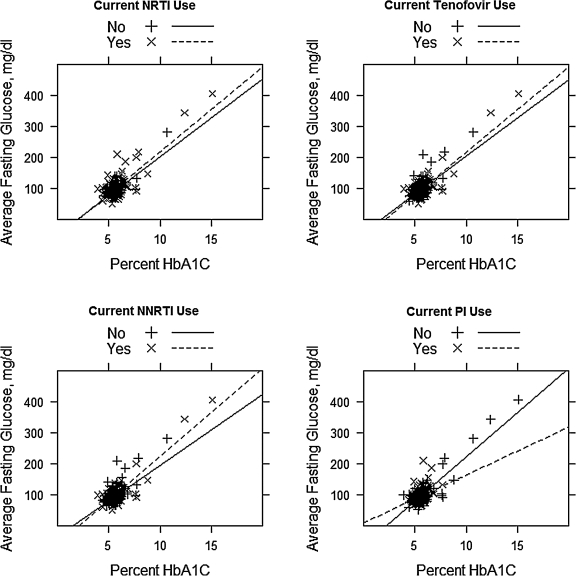
Current use of a PI was associated with a significantly decreased slope for the regression of FBG on HbA1c (F=17.02, p<0.001), suggesting that for patients receiving a PI, an elevated HbA1c may overestimate the actual level of glycemia (Fig. 2). Current use of an NNRTI was associated with both a significantly increased slope (F=5.16, p=0.02) and a significantly decreased y-intercept (F=6.69, p=0.01) for the regression of FBG on A1c, suggesting that elevated HbA1c values in these patients may underestimate the level of glycemia. Use of an NRTI reduced the y-intercept (F=5.44, p=0.02) but had no significant effect on the slope (F=0.64, p=0.42). Tenofovir use did not significantly impact the slope (F=1.23, p=0.27) nor the y-intercept (F=0.004, p=0.95) of the regression curve. We did not analyze the effects of other individual antiretrovirals because of the smaller numbers of patients receiving each agent.
FIG. 2.
Scatter plot and regression curves of fasting blood glucose and hemoglobin A1c (HbA1c) adjusted for current antiretroviral use. NRTI, nucleoside reverse transcriptase inhibitor; NNTRI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Discussion
Routine follow-up of HIV patients involves screening and monitoring for metabolic complications of ART including dyslipidemia and hyperglycemia. New guidelines from the American Diabetes Association recommend HbA1c as a screening test for diabetes. However previous studies have suggested HbA1c underestimates the level of glycemia in patients with HIV infection and diabetes.
In our cross-sectional cohort study we found that an HbA1c≥6.5% is insensitive, but highly specific in diagnosing diabetes among HIV-infected patients. The ideal cut-point on the ROC curve to yield the highest combination of sensitivity and specificity is 5.8%. This cut-point value is identical to that found in the general population through the NHANES cohort,15 and the sensitivity and specificity of the test was not significantly affected by antiretroviral regimen.
We identified 22 patients with discordant HbA1c and FBG values (13 false-negative, and 9 false-positive HbA1c values). Newly published recommendations from the ADA acknowledge that such conflicting laboratory tests are not an uncommon finding.8 The position statement cites unpublished NHANES data that indicates using a HbA1c cut-point of≥6.5% will diagnose one third fewer cases of diabetes compared to a FBG≥126 mg/dL. As of 2011 the ADA altered its recommendations to say that in cases of HbA1c and FBG discordance, the abnormal laboratory test should be repeated, and the diagnosis of diabetes should only be made if repeat testing is again above the diagnostic cut-point.8 Had we used these new recommendations to define diabetes in our study, we would have reduced the number of false-positive HbA1c values from 9 to 4, and decreased the number of false-negative tests from 13 to 12; resulting in an improved sensitivity and specificity of 42.9% and 98.7%, respectively.
Similar to previous studies,10–12 higher MCV resulted in lower HbA1c values than would be expected for the FBG level. Although thymidine analogues are well-documented to increase MCV16,17 we had only a small fraction of our patients on these agents (stavudine or zidovudine). Unlike previously published studies in the general population,18 our analysis did not show any significant effect of black race on the relationship between HbA1c and FBG. These results held even when including patients with either hemoglobinopathies or G6PD-deficiency (data not shown).
Our findings support the idea that HbA1c values are affected by current antiretroviral regimen. These findings are different from previous studies on diabetic patients that showed no statistically significant effect of PIs or NNRTIs on HbA1c levels.10,12 Our results suggest that HbA1c values in HIV-infected patients currently receiving PIs may overestimate, and those currently receiving NNRTIs may underestimate, the level of glycemia. In contrast tenofovir use did not appear to impact the relationship between HbA1c and FBG. The number of patients on abacavir was insufficient to determine an affect on the relationship between HbA1c and FBG as seen in prior studies.11
Limitations of our study include its small sample size, lack of long-term clinical outcomes, and use of fasting blood glucose (as opposed to OGTT) as the gold standard to which HbA1c was compared. Additionally FBG levels in our study were assumed to be fasting. Although the clinic procedure used at the study site attempts to ensure fasting blood testing, the retrospective use of routine laboratory testing may have allowed for the inclusion of nonfasting samples.
In conclusion, there is growing evidence about the variable accuracy of HbA1c as a screening test for diabetes. We observed that HbA1c is an insensitive, but highly specific screening test for diabetes in HIV-infected patients. The relationship of HbA1c to FBG is affected by specific antiretrovirals, but not enough to significantly alter the sensitivity or specificity of the test. More work is needed to determine the clinical significance and mechanism behind specific antiretroviral drug's impact on HbA1c. Despite the possible limits on its interpretation, the ability to use HbA1c to screen HIV-infected patients in a nonfasting state and estimate long-term glycemic levels makes it a practical screening modality, and we advocate the use of HbA1c and FBG in conjunction to guide the clinician in screening for diabetes.
Acknowledgments
This work was supported in part by the NIAID Grant No. 5 U01 A1069532. The manuscript was presented in part at the 18th Conference on Retroviruses and Opportunistic Infections, Boston, MA, February 27–March 2, 2011, and the American Conference for the Treatment of HIV, Denver, CO, April 7–April 9, 2011.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Brown T. Cole S. Li X, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the Multicenter AIDS Cohort Study. Arch Intern Med. 2005;165:1179–1184. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]
- 2.Tien P. Schneider M. Cole, et al. Antiretroviral therapy exposure and incidence of diabetes mellitus in the Women's Interagency HIV Study. AIDS. 2007;21:1739–1745. doi: 10.1097/QAD.0b013e32827038d0. [DOI] [PubMed] [Google Scholar]
- 3.Samaras K. Prevalence and pathogenesis of diabetes mellitus in HIV-1 infection treated with combined antiretroviral therapy. J Acquir Immune Defic Syndr. 2009;50:499–505. doi: 10.1097/QAI.0b013e31819c291b. [DOI] [PubMed] [Google Scholar]
- 4.Guaraldi G. Orlando G. Zona, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. 2011;53:1120–1126. doi: 10.1093/cid/cir627. [DOI] [PubMed] [Google Scholar]
- 5.Lundgren JD. Battegay M. Behrens G, et al. European AIDS clinical society (EASC) guidelines on the prevention and management of metabolic diseases in HIV. HIV Med. 2008;9:72–81. doi: 10.1111/j.1468-1293.2007.00534.x. [DOI] [PubMed] [Google Scholar]
- 6.Aberg JA. Kaplan JE. Libman H, et al. Primary care guidelines for the management of persons infected with human immunodeficiency virus: 2009 update by the HIV medicine association of the Infectious Disease Society of America. Clin Infect Dis. 2009;49:651–681. doi: 10.1086/605292. [DOI] [PubMed] [Google Scholar]
- 7.International Expert Committee. International Expert Committee Report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34:S62–S69. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polgreen P. Putz D. Stapleton JT. Inaccurate glycosylated hemoglobin A1C measurements in human immunodeficiency virus-positive patients with diabetes mellitus. Clin Infect Dis. 2003;37:e53–56. doi: 10.1086/376633. [DOI] [PubMed] [Google Scholar]
- 10.Diop ME. Bastard JP. Meunier N, et al. Inappropriately low glycated hemoglobin values and hemolysis in HIV-infected patients. AIDS Res Hum Retroviruses. 2006;22:1242–1247. doi: 10.1089/aid.2006.22.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim P. Woods C. Georgoff P, et al. A1C underestimates glycemia in HIV infection. Diabetes Care. 2009;32:1591–1593. doi: 10.2337/dc09-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glesby M. Hoover D. Shi Q, et al. Glycated haemoglobin in diabetic women with and without HIV infection: Data from the Women's Interagency HIV Study. Antivir Ther. 2010;15:571–577. doi: 10.3851/IMP1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 14.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austrai: R Foundation for Statistical Computing. 2011. www.R-project.org/ [Jan 3;2012 ]. www.R-project.org/
- 15.Buell C. Kerman D. Davidson M. Utility of A1C for diabetes screening in the 1999–2004 NHANES population. Diabetes Care. 2007;30:2233–2235. doi: 10.2337/dc07-0585. [DOI] [PubMed] [Google Scholar]
- 16.Steele RH. Keogh GL. Quin J. Fernando SL. Stojkova V. Mean cell volume (MCV) changes in HIV-positive patients taking nucleoside reverse transcriptase inhibitors (NRTIs): A surrogate marker for adherence. Int J STD AIDS. 2002;13:748–754. doi: 10.1258/095646202320753691. [DOI] [PubMed] [Google Scholar]
- 17.Geene D. Sudre P. Anwar D, et al. Causes of macrocytosis in HIV-infected Patients not treated with zidovudine: Swiss HIV Cohort Study. J Infect. 2000;40:160–163. doi: 10.1053/jinf.1999.0628. [DOI] [PubMed] [Google Scholar]
- 18.Ziemer DC. Kolm P. Weintraub WS, et al. Glucose-independent, black-white differences in hemoglobin A1c levels: A cross-sectional analysis of 2 studies. Ann Intern Med. 2010;152:770–777. doi: 10.7326/0003-4819-152-12-201006150-00004. [DOI] [PubMed] [Google Scholar]