Abstract
Background
Diabetes is a chronic condition that significantly impacts quality of life. Poor glycemic control is associated with more diabetes complications, depression, and worse quality of life. The impact of glycemic variability on mood and quality of life has not been studied.
Methods
A descriptive exploratory design was used. Twenty-three women with type 2 diabetes wore a continuous glucose monitoring system for 72 h and completed a series of questionnaires. Measurements included (1) glycemic control shown by glycated hemoglobin and 24-h mean glucose, (2) glycemic variability shown by 24-h SD of the glucose readings, continuous overall net glycemic action (CONGA), and Fourier statistical models to generate smoothed curves to assess rate of change defined as “energy,” and (3) mood (depression, anxiety, anger) and quality of life by questionnaires.
Results
Women with diabetes and co-morbid depression had higher anxiety, more anger, and lower quality of life than those without depression. Certain glycemic variability measures were associated with mood and quality of life. The 24-h SD of the glucose readings and the CONGA measures were significantly associated with health-related quality of life after adjusting for age and weight. Fourier models indicated that certain energy components were significantly associated with depression, trait anxiety, and overall quality of life. Finally, subjects with higher trait anxiety tended to have steeper glucose excursions.
Conclusions
Data suggest that greater glycemic variability may be associated with lower quality of life and negative moods. Implications include replication of the study in a larger sample for the assessment of blood glucose fluctuations as they impact mood and quality of life.
Background
Depression is a serious problem affecting almost 25% of persons with diabetes, and the risk of depression is doubled in women compared with men with diabetes.1 A meta-analysis of 27 studies demonstrated that depression is significantly associated with hyperglycemia for both type 1 and type 2 diabetes.2 Moods such as anxiety and anger often accompany depression in persons with diabetes.3–5 Anxiety has been reported to be as high as 30–40% in persons with diabetes,6,7 with women having higher levels than men.8 Anxiety has also been associated with poor glycemic control.9,10 Anger has also been linked to depression11 and associated with poorer self-management and hyperglycemia for persons with diabetes.12,13
The role of blood glucose alterations and its impact on mood have not been well studied, although the literature suggests such relationships exist. For persons with type 1 diabetes, high glucose values have been reported to negatively impact mood.14,15 Tension and anger have been reported to be higher in type 1 diabetes individuals in the hyperglycemic range compared with those in the euglycemic or hypoglycemic range with continuous glucose monitoring (CGM).14 For adults with diabetes who take insulin, both low and high blood glucose values have been associated with negative moods. Low blood sugar has been associated with “nervousness,” whereas high blood sugar with “anger or sadness.”16 Recently, researchers have found no relationship between positive affect and glucose but noted that “on the days in which negative affect was higher than usual, the next morning's glucose was also higher than usual (“grumpy days” affect subsequent blood glucose levels).”17
For women with type 2 diabetes, negative moods have been reported to impact their day-to-day living and overall quality of life.18 Both depression and poor glycemic control have been associated with poor quality of life in persons with diabetes,19 and women have been reported to have poorer glycemic control and quality of life than men with diabetes.20–23
Although glycated hemoglobin (HbA1c) has been the standard for assessing glycemic control, glycemic variability may have an important role in the risk for complications of diabetes.24 Information regarding the role that glycemic variability may have on psychological functioning is limited.25 Research on whether persons who have depression and other mood disorders have greater glycemic variability has not been studied. The purpose of this study was to examine whether women with type 2 diabetes have greater glycemic variability and whether this impacts their quality of life.
Subjects and Methods
Design
This was a descriptive exploratory design to examine the relationship among mood, glycemic control/variability, and quality of life in women with type 2 diabetes and also to compare women who were depressed with women who were not depressed on these measures. This approach was used as there is limited information about these measures and how they mutually interact.
Subjects
Women who were 18–75 years old, diagnosed with type 2 diabetes for greater than 6 months, and able to perform fingerstick glucose testing four times daily were eligible to participate. Women who were part of an approved diabetes research database were sent a formal letter informing them about the study, and flyers were also placed at the clinics where diabetes patients receive care. The letter and the flyer informed women that the purpose of the study was to determine whether fluctuations in blood glucose were associated with changes in mood.
Procedures
Subjects who met study criteria had two scheduled visits. The first visit lasted about 2 h (data collection and monitoring device insertion), and the second visit about 20 min (for removal of the monitoring device). At the first visit, after the patients read and signed the informed consent document, the physical measurements of the participants were taken (height, weight, blood pressure, and HbA1c and glucose) by the study nurse. Then the Medtronic (Northridge, CA) MiniMed CGMS® Gold™ sensor was inserted, and women were instructed how to enter their daily blood glucose fingersticks (morning, lunch, dinner, and before bedtime) into the device for calibration. Women were blinded to the sensor reading. Because the device took 1 h to calibrate, women completed a questionnaire booklet during that time. The booklet included self-report tools to assess health, mood (depression, anxiety, anger), and quality of life. They were given a light snack during this time. At the completion of the 72-h period, subjects returned to the data collection site, and the CGMS sensor was removed. At that time, the condition of the patient's skin at the insertion site was examined for redness, swelling, or inflammation.
Measures
Mood
Depression, anxiety, and anger were measured using standard self-report tools: (1) Depression. The Center for Epidemiologic Studies Depression (CES-D) tool is 20 items and was used to measure depression. A cutoff score of ≥16 indicates significant depressive symptoms and depression. The tool has excellent internal reliability and validity.26 The CES-D and the Beck Depression Inventory have performed comparably as depression screening tools.27 (2) Anxiety. The State-Trait Anxiety Inventory is 40 items and was used to measure the temporary condition of “state anxiety” and the more long-standing quality of “trait anxiety.” The reliability and validity of this tool have been well established.28,29 (3) Anger. The State Trait Anger Expression Inventory is 44 items and was used to measure anger as an emotion (State Anger) and the predisposition to experience angry feelings as a personality trait (Trait Anger). Reliability and validity of the tool have been reported.30
Quality of life
The Ferrans and Powers31 Quality of Life Index–Diabetes III Version was used to assess quality of life. This questionnaire is 34 items that measures satisfaction and importance in four areas of life: (1) health and functioning, (2) social and economic, (3) psychological/spiritual, and (4) family that impact the quality of life for persons with diabetes. Reliability and validity of the tool have been well established.31,32
Glycemic control
This was assessed by two methods: (1) HbA1c (%) was measured using the DCA 2000 analyzer (Miles Diagnostic Division, Elkhart, IN). A fingerstick from the subject was collected by a trained nurse experienced with this methodology. This measurement of HbA1c is used in clinical practice.33 (2) The 24-h mean glucose was derived by the sample mean of the 288 glucose assessments collected via the sensor. These were generated by a sensor reading taken every 5 min. For every hour, there were 12 readings, yielding a total of 12×24=288 readings for each 24-h period.
Glucose variability
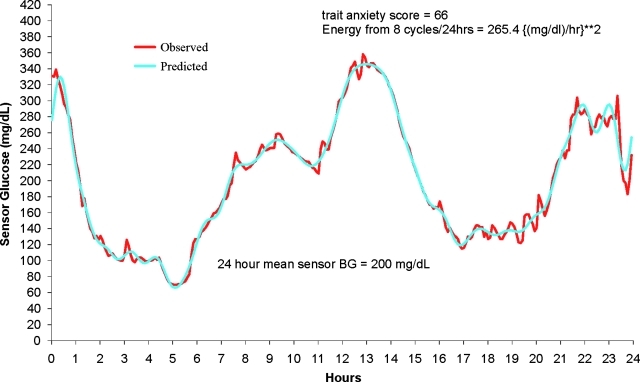
This was assessed by three methods: (1) The 24-h SD was derived by the SD of the 288 sensor readings and remains an effective, well-defined, and easy to calculate measure of glucose variability. (2) Continuous overall net glycemic action (CONGA) measures were introduced by McDonnell et al.34 Each CONGA(n) measure is the sample SD over time t of the difference between a sensor reading at time t and the reading at time t−(n×60 min). Use of CONGA analysis will quantify intraday fluctuations in glucose levels when continuous data points are available. This report studied CONGA1 through CONGA6. Higher CONGA values indicate more glycemic variability. (3) Energy spectrum and average energy over 24 h (E) were generated by the replacement of the raw 24-h curve with a smoothed curve using a discrete Fourier transform.35 This report marks the introduction of this new measure, CGM “energy,” that is currently not being captured by traditional measures of glycemic variability. Just as white light can be broken down into its component colors, so a 24-h CGM profile can be partitioned into component frequencies each with their own amplitudes. High frequencies mean shorter wavelengths and therefore steeper curves for a given amplitude. The energy at each frequency is proportional to the square of the frequency times amplitude, capturing the essence of increasing steepness with increasing frequency. This set of component energies for a 24-h CGM profiles we call the CGM energy spectrum. The average energy E is the sum of these component energies divided by 24 h with units (mg/dL-h)2. The SD is captured by just the amplitudes at each frequency and is only a measure of the variation about the 24 mean h (being proportional to the square root of the sum of the amplitudes). The SD does not require anything other than the sensor glucose readings, whereas E and its components requires a presmoothing by discrete Fourier transform, making the rate of change well defined. We used a Fourier approximation containing frequencies from 1 through 24 and the constant term (the 24-h mean glucose). An example of the discrete Fourier approximation to a 24-h CGM profiles is provided in Figure 1.
FIG. 1.
Observed and predicted (24-cycle Fourier approximateion) sensor glucose values for study participants. r2=0.984. BG, blood glucose.
Human subjects
The study protocol, including recruitment strategy, was approved by the Institutional Review Board where the study was undertaken. Participants were paid a stipend of $50 for the amount of time that they spent onsite for data collection (2.5 h) as well as the time that they spent taking and recording their blood glucose four times daily. There were no adverse events from the CGM.
Statistical analysis
Demographic, background, health data, and CGM parameters were summarized using descriptive statistics. Women were classified according to the CES-D-20 depression score (<16 for nondepressed and ≥16 for depressed). For each of the parameters, a P value was computed testing the hypothesis that the mean score was the same for the groups, and this used the two-sample t test. These P values were descriptive, with no adjustment for multiple comparisons due to the exploratory nature of the study.
Pearson correlation between each mood/anxiety score and each CGM measure summarized with its associated P value testing whether the underlying population correlation was zero. This test used the Fisher Z transformation and the normal distribution. The purpose of this step was to begin the screening for any apparent relationship between some aspect of the subject's glucose curve and measures of the woman's mental health. Pearson correlations may be confounded by factors other than the major variables under study. Because it was found that age and weight were factors affecting many of the mood/anxiety scales and some CGM measures, a second set of correlations was generated by removing the effects of age and weight. This was done by computing the partial correlations of each mood/anxiety scale with each CGM scale adjusting for age and weight. Again the nominal P values of these partial correlations were provided, testing the null hypothesis that the corresponding population partial correlation was 0.
The average energy subject's 24-h profile and the first 12 component energies were part of this correlation analysis. P values quoted for the 12 energy components' (partial) correlation with a given mood/anxiety score were adjusted for multiplicity in the following sense. The scores were randomly permuted 10,000 times among the 23 subjects, inducing a 0 correlation in all components, and the maximum absolute correlation among 12 components were obtained for each permutation. The P value was then based on the percentile rank of the observed correlation among the 10,000 maxima generated with no correlation. This bootstrap method had the effect of exacting a larger price for statistical significance because of the orthogonality of the energy scales.
Results
Women were compared according to their depression status (depressed vs. nondepressed) (Table 1). There were no significant differences between the groups on age, years with diabetes, body mass index, and HbA1c. The median age of women was 51 years (range, 40–67 years), and duration of diabetes was 10 years (2–26 years). The median body mass index was 37.7 kg/m2 (20.4–53.3 kg/m2). Regarding blood glucose control, the median HbA1c was 8.0% (6.0–13.0%). About 25% of these subjects had HbA1c levels at 7% or lower, so according to the cutoff of 7% about three-quarters of these subjects exhibited inadequate blood glucose control.
Table 1.
Characteristics of Participants
| |
Mean (SD) CES-D score |
||
|---|---|---|---|
| Variable | <16 | ≥16 | P value |
| Sample (n) | 11 | 12 | |
| Age (years) | 53.9 (9.6) | 51.8 (4.9) | 0.858 |
| Diabetes' duration (years) | 11.2 (9.2) | 11.6 (6.3) | 0.914 |
| BMI (kg/m2) | 38.46 (8.58) | 38.53 (10.68) | 0.986 |
| HbA1c (%) | 8.9 (2.4) | 8.0 (1.8) | 0.291 |
| CES-D | 7.2 (4.2) | 27.0 (9.8) | <0.001 |
| State Anxiety | 28.0 (9.1) | 47.3 (9.4) | <0.001 |
| Trait Anxiety | 27.5 (4.7) | 48.7 (8.3) | <0.001 |
| State Anger | 15.0 (0) | 20.3 (7.6) | 0.030 |
| Trait Anger | 15.4 (5.7) | 18.0 (5.5) | 0.203 |
| Quality of Life | 23.3 (4.7) | 19.1 (5.0) | 0.054 |
| Health Subscale | 22.0 (4.9) | 17.8 (6.3) | 0.092 |
| Social & Economic Subscale | 23.6 (5.6) | 19.8 (4.6) | 0.087 |
| Psychological Subscale | 24.9 (5.0) | 19.1 (7.7) | 0.044 |
| Family Subscale | 23.7 (6.3) | 22.4 (4.5) | 0.593 |
BMI, body mass index; CES-D, Center for Epidemiologic Studies Depression; HbA1c, glycated hemoglobin.
In comparing depressed and nondepressed women, there were statistically significant differences on mood measures (Table 1). Depressed women had higher depression scores (P<0.001), state and trait anxiety scores (P<0.001), and state anger scores (P=0.030). In terms of quality of life, depressed women reported an overall lower quality of life (P=0.054) and poorer psychosocial and spiritual well-being (P=0.044). There was a trend for depressed women to report worse health and functioning (P=0.092) and lower social and economic quality of life (P=0.087). The family quality of life measures were not different between the depressed and nondepressed groups.
In terms of the CGM data, the protocol period of 72 h yielded, for most subjects, two complete 24-h records, and all subjects had at least one complete 24-h record. Of the 23 subjects in this study, 18 subjects (78.3%) had two complete 24-h records, four subjects had one complete record from midnight to midnight, and one subject had one complete 24-h record after performing a time shift on the data. Hence, the 23 subjects generated 41 complete records, which became the sampling units for the subsequent analyses, and all subjects are represented in the CGM data analysis.
Two sets of summary glucose curves were obtained for each subject: one set was based on the average of all full 24-h curves available from each subject; the other set was based on the last available 24-h curve. The primary analyses were based on this second set, using the last full 24-h assessment, because this allowed time for the participant to become adjusted to the CGM device, as it is possible that the initial experience may have created a small amount of stress that could have impacted the variability. A decision was also made to use one continuous 24-h record to provide a better estimate of the glucose parameters rather than to use pieces of the records, which could have biased the estimates. Table 2 provides descriptive summaries for the CGM sensor glucose parameters for the depressed and nondepressed women. There were no significant differences between the groups on the glucose measures.
Table 2.
Descriptive Summaries from Patients' 24-h Continuous Glucose Monitoring CGMS Profiles Using the Last 24-h Profile (Midnight to Midnight) from Each Patient
| |
Mean (SD) CES-D score |
||
|---|---|---|---|
| Assessment | <16 | ≥16 | P value |
| 24-h mean (mg/dL) | 190.9 (59.2) | 159.4 (62.2) | 0.143 |
| 24-h SD (mg/dL) | 41.7 (25.1) | 41.6 (18.7) | 0.993 |
| CONGA (mg/dL) | |||
| CONGA1 | 32.0 (13.4) | 33.0 (11.2) | 0.857 |
| CONGA2 | 45.2 (22.3) | 46.2 (20.2) | 0.910 |
| CONGA3 | 53.8 (27.4) | 52.3 (25.5) | 0.896 |
| CONGA4 | 58.4 (30.0) | 55.7 (28.3) | 0.824 |
| CONGA5 | 61.3 (35.2) | 57.9 (31.0) | 0.807 |
| CONGA6 | 63.7 (41.7) | 58.1 (31.3) | 0.718 |
| 24-h average energy (mg/dL-h)2 | 46.5 (32.3) | 48.9 (25.4) | 0.848 |
| Energy (mg/dL-h)2 | |||
| 1 cycle/24 h | 43.1 (85.8) | 32.7 (36.7) | 0.705 |
| 2 cycles/24 h | 49.96 (52.20) | 49.19 (49.76) | 0.971 |
| 3 cycles/24 h | 68.8 (61.0) | 66.9 (88.0) | 0.955 |
| 4 cycles/24 h | 44.5 (43.2) | 68.9 (46.9) | 0.211 |
| 5 cycles/24 h | 141.5 (271.4) | 106.7 (103.6) | 0.684 |
| 6 cycles/24 h | 31.0 (30.8) | 54.7 (47.0) | 0.170 |
| 7 cycles/24 h | 50.4 (77.2) | 103.7 (176.9) | 0.368 |
| 8 cycles/24 h | 41.8 (35.9) | 70.5 (53.7) | 0.151 |
| 9 cycles/24 h | 44.3 (45.7) | 46.1 (44.4) | 0.925 |
| 10 cycles/24 h | 55.6 (97.3) | 36.8 (42.3) | 0.547 |
| 11 cycles/24 h | 71.6 (102.1) | 45.0 (32.0) | 0.399 |
| 12 cycles/24 h | 31.7 (30.0) | 57.5 (46.0) | 0.130 |
CES-D, Center for Epidemiologic Studies Depression; CONGA, continuous overall net glycemic action.
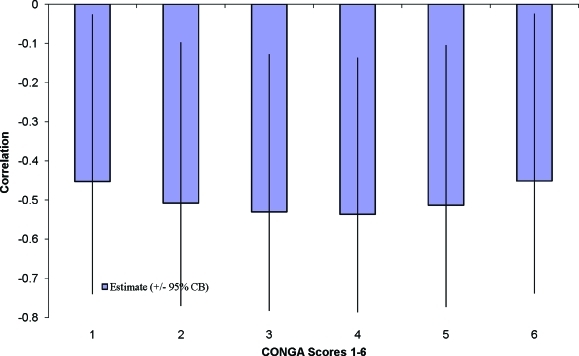
Pearson correlations indicated that 24-h mean blood glucose was not associated with quality of life or mood. After adjustment for age and weight, the 24-h SD of the glucose readings (r=− 0.47, P=0.033) and the CONGA1–CONGA6 measures were significantly associated with health-related quality of life (r=− 0.46, P=0.035 to r=− 0.55, P=0.011) (Fig. 2).
FIG. 2.
Partial correlation of the health and functioning quality of life with continuous overall net glycemic action (CONGA) 1–6 adjusted by age and weight. Note that all six CONGA correlations with health and functioning quality of life are statistically significant at P<0.05 by virtue of all exceeding the 95th percentile of the maximum of six correlations based on 10,000 simulations where the true correlations were 0. CB, confidence bound (same as confidence interval). Color images available online at www.liebertonline.com/dia
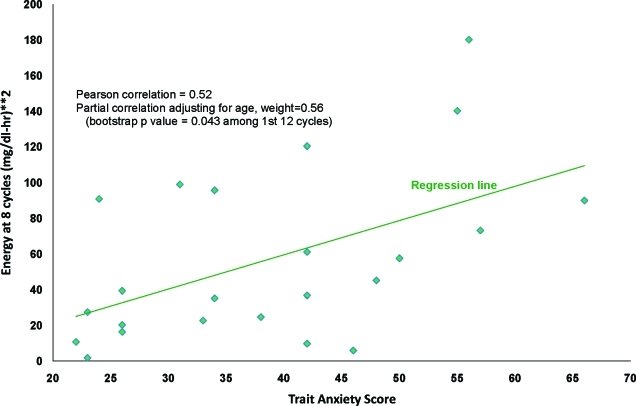
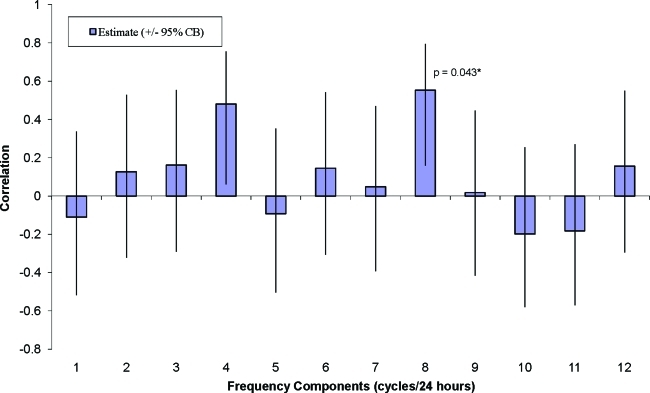
The average energy E was not associated with quality of life or mood. Pearson adjusted correlations indicated that certain energy components were significantly associated with depression (r=+ 0.54, P=0.012), trait anxiety (r=+0.56, P=0.008), and overall quality of life (r=−0.66, P=0.001). The eight-cycle (3-h) energy measure was associated with trait anxiety (bootstrap P=0.043) (Figs. 3 and 4). The eight-cycle (3-h) energy measure was also associated with depression, although it was not statistically significant (bootstrap P=0.094).
FIG. 3.
Energy at eight cycles per 24 h versus trait anxiety score. Color images available online at www.liebertonline.com/dia
FIG. 4.
Partial correlation of trait anxiety score with continuous glucose monitoring energy components adjusted by age and weight. *Randomly permuting the 23 subjects and computing the maximum correlations among the first 12 cycles (10,000 repetitions), only 4.3% of these simulated maxima exceeded the observed correlation at a frequency of eight cycles per 24 h. CB, confidence bound (same as confidence interval). Color images available online at www.liebertonline.com/dia
Discussion and Conclusions
The findings indicate that the depressed and not depressed groups were comparable on glycemic control. The results that the women with depression had greater anxiety, slightly higher anger levels, and lower quality of life were expected and consistent with previous research.1,4,6,12,20–22 Penckofer et al.18 reported that women experience multiple emotions that significantly impact on their quality of life. In addition, clinical practice has indicated that persons with diabetes who are depressed also have accompanying anxiety.5
The correlation of CONGA and the 24-h SD, traditional measures of glycemic variability, with health-related quality of life is an important finding of this exploratory study. Although previous research has indicated that glycemic control can significantly impact the patient's quality of life,36 research has not addressed how glycemic variability may impact on the patient's quality of life. In this study, the relationship between perceived health-related quality of life and glycemic variability was significant for women with type 2 diabetes. Researchers have reported on the importance of CGM on self-management for persons with diabetes.37 The current study found that when women with type 2 diabetes experienced more glycemic variability, they tended to report a poorer health-related quality of life. Although it is logical to assume that poor self-management may cause more glycemic variability, it is very possible that greater glycemic variability results in more frequent episodes of hyper- and hypoglycemia, which may contribute to depression as well as anxiety. As a consequence, one's ability to perform self-management can be more challenging and negatively impact his or her overall quality of life. Thus, additional research is needed to gain further insight into understanding these bidirectional relationships.
Finally, the relationship between a measure of glycemic energy (as distinct from variability) and mood is an interesting finding needing further research. This exploratory study demonstrated that trait anxiety was significantly associated with the 3-h glycemic energy cycle, and depression may be associated with this same index. It may be that a subtle assessment of the CGM glucose profile is required to determine whether important frequencies of large glucose excursions characterize subjects with anxiety and depression. In this study, the subjects with the higher trait anxiety scores tended to have steeper glucose excursions focused on 3 h in length (24 h/eight cycles=3 h).
Previous research using Fourier models for CGM profiles has indicated the importance in being able to relate the patient's reported symptoms (e.g., hypoglycemia) and blood glucose levels.35 Thus, the potential to relate fluctuations in blood glucose to alterations in the patient's mood has significant research and clinical implications. First, patients could understand the impact of blood glucose fluctuations on their emotions and overall well-being. Second, researchers could use it as a novel and feasible outcome measure for evaluating the effectiveness of behavioral interventions for treatment of disorders like anxiety or depression that are more common in persons with diabetes.
Limitations
Given the descriptive, exploratory nature of the study, the sample size was small, which is a limitation. The self-report measures may also be a limitation, but they were all obtained within the 72-h time period of CGM. Although persons with diabetes can have challenges in managing their health behaviors that may significantly impact glycemic variability, whether the relationships found in this study were due to depression or health behaviors resulting from depression is unknown. Because there were numerous psychological measures and methods of assessing glycemic control as well as variability, it is possible that the significance may have been due to multiple testing. Thus, recommendations for the future would include the replication with a larger sample with measurements done over multiple time points to validate these findings.
Summary
Exploring glycemic variability and the impact it could have on women is important and significant as it may explain why they are at greater risk for depression, poorer quality of life, and earlier cardiac morbidity and mortality. After adjusting for confounders of age and weight, the findings relative to glycemic variability and trait anxiety were significant, and those with depression were marginal. Anxiety and depression occur more often in women with diabetes than men with diabetes.8 The importance of these findings is that anxiety and depression may impact on diabetes self-care behaviors and quality of life, and glycemic variability may be a factor associated with these outcomes.
The use of CGM to improve glycemic control and reduce glycemic variability has been reported.38 More recently, reduction of glucose variability has been associated with a lower risk of hypo- and hyperglycemia.39 Fluctuations in blood glucose, both high and low, have been shown to impact mood; however, research is limited. The study of the impact of glycemic variability on psychological functioning is a fertile area for research.25 Using CGM to improve the mental and physical well-being of persons with diabetes has significant implications for both clinicians and researchers. Studies using CGM for evaluating diabetes self-management and for determining the benefit of both pharmacologic and nonpharmacologic therapies to improve glycemic control and reduce glycemic variability will be essential to enhance the quality of life for persons living with diabetes in the immediate future.
Acknowledgments
This work was funded by Loyola University Chicago, School of Nursing Research Funds and the National Institutes of Nursing Research (grant K23NR009240).
Author Disclosure Statement
P.S. is a Managing Member and M.M. at the time this study was performed was a member of Glucose Harmonics, which performed the statistical analyses. S.P., L.Q., M.B., and C.F. have no competing interests to declare.
References
- 1.Anderson RJ. Freedland KE. Clouse RE. Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24:1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 2.Lustman PJ. Anderson RJ. Freedland KE. de Groot M. Carney RM. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care. 2000;23:934–942. doi: 10.2337/diacare.23.7.934. [DOI] [PubMed] [Google Scholar]
- 3.Peyrot M. Rubin R. Levels and risk of depression and anxiety symptomatology among diabetic adults. Diabetes Care. 1997;20:585–590. doi: 10.2337/diacare.20.4.585. [DOI] [PubMed] [Google Scholar]
- 4.Chyun DA. Melkus GD. Katten DM. Price WJ. Davey JA. Grey N. Heller G. Wackers FJ. The association of psychological factors, physical activity, neuropathy, and quality of life in type 2 diabetes. Biol Res Nurs. 2006;7:279–288. doi: 10.1177/1099800405285748. [DOI] [PubMed] [Google Scholar]
- 5.Khuwaja AK. Lalani S. Dhanani R. Azam IS. Rafique G. White F. Anxiety and depression among outpatients with type 2 diabetes: a multi-centre study of prevalence and associated factors. Diabetol Metab Syndr. 2010;2:72. doi: 10.1186/1758-5996-2-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grigsby AB. Anderson RJ. Freedland KE. Clouse RE. Lustman PJ. Prevalence of anxiety in adults with diabetes: a systematic review. J Psychosom Res. 2002;53:1053–1060. doi: 10.1016/s0022-3999(02)00417-8. [DOI] [PubMed] [Google Scholar]
- 7.Lloyd CE. Dyer PH. Barnett AH. Prevalence of symptoms of depression and anxiety in a diabetes clinic population. Diabet Med. 2000;17:198–202. doi: 10.1046/j.1464-5491.2000.00260.x. [DOI] [PubMed] [Google Scholar]
- 8.Huang CJ. Chiu HC. Lee MH. Wang SY. Prevalence and incidence of anxiety disorders in diabetic patients: a national population-based cohort study. Gen Hosp Psychiatry. 2011;33:8–15. doi: 10.1016/j.genhosppsych.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Anderson RJ. Grigsby AB. Freedland KE. deGroot M. McGill JB. Clouse RE. Lustman PJ. Anxiety and poor glycemic control: a meta-analytic review of the literature. Int J Psychiatry Med. 2002;32:235–247. doi: 10.2190/KLGD-4H8D-4RYL-TWQ8. [DOI] [PubMed] [Google Scholar]
- 10.Wu SF. Huang YC. Liang SY. Wang TJ. Lee MC. Tung HH. Relationships among depression, anxiety, self-care behavior and diabetes education difficulties in patients with type 2 diabetes: a cross-sectional questionnaire survey. Int J Nurs Stud. 2011;48:1376–1383. doi: 10.1016/j.ijnurstu.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Pasquini M. Picardi A. Biondi M. Gaetano P. Morosini P. Relevance of anger and irritability in outpatients with major depressive disorder. Psychopathology. 2004;37:155–160. doi: 10.1159/000079418. [DOI] [PubMed] [Google Scholar]
- 12.Yi JP. Yi JC. Vitaliano PP. Weinger K. How does anger coping style affect glycemic control in diabetes patents? Int J Behav Med. 2008;15:167–172. doi: 10.1080/10705500802219481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DePalma MT. Rollison J. Camporese M. Psychosocal predictors of diabetes management. Am J Health Behav. 2011;35:209–218. doi: 10.5993/ajhb.35.2.8. [DOI] [PubMed] [Google Scholar]
- 14.Hermanns N. Scheff C. Kulzer B. Weyers P. Pauli P. Kubiak T. Haak T. Association of glucose levels and glucose variability with mood in type 1 diabetic patients. Diabetologia. 2007;50:930–933. doi: 10.1007/s00125-007-0643-y. [DOI] [PubMed] [Google Scholar]
- 15.Van Tilburg MAL. McCaskill CC. Lane JD. Edwards CL. Bethel A. Feinglos MN. Surwit RS. Depressed mood is a factor in glycemic control in type 1 diabetes. Psychosom Med. 2001;63:551–555. doi: 10.1097/00006842-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Gonder-Frederick LA. Cox DJ. Bobbit SA. Pennebaker JW. Mood changes associated with blood glucose fluctuations in insulin-dependent diabetes mellitus. Health Psychol. 1989;8:45–59. doi: 10.1037//0278-6133.8.1.45. [DOI] [PubMed] [Google Scholar]
- 17.Skaff MM. Mullan JT. Hoffman L. Mohr D. Almedia DM. Masharani U. Fisher L. Daily negative mood affects fasting glucose in type 2 diabetes. Health Psychol. 2009;28:265–272. doi: 10.1037/a0014429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penckofer S. Ferrans C. Velsor-Friedrich B. Savoy S. The psychological impact of living with diabetes: women's day to day experiences. Diabetes Educ. 2007;33:680–690. doi: 10.1177/0145721707304079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egede LE. Grubaugh AL. Ellis C. The effect of major depression on preventive care and quality of life among adults with diabetes. Gen Hosp Psychiatry. 2010;32:563–569. doi: 10.1016/j.genhosppsych.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Whittemore R. Melkus G. Grey M. Self-report of depressed mood and depression in women with type 2 diabetes. Issues Mental Health Nurs. 2004;25:243–260. doi: 10.1080/01612840490274750. [DOI] [PubMed] [Google Scholar]
- 21.Uden AL. Elofsson S. Andreasson A. Hillered E. Eriksson I. Brismar K. Gender differences in self-rated health, quality of life, quality of care, and metabolic control in patients with diabetes. Gender Med. 2008;5:162–180. doi: 10.1016/j.genm.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Ali S. Stone M. Skinner TC. Robertson N. Davies M. Khunti K. The association between depression and health-related quality of life in people with diabetes: a systematic literature review. Diabetes Metab Res Rev. 2010;26:75–89. doi: 10.1002/dmrr.1065. [DOI] [PubMed] [Google Scholar]
- 23.Verma SK. Luo N. Subramanian M. Sum CF. Stahl D. Liow PH. Chong SA. Impact of depression on health related quality of life in patients with diabetes. Ann Acad Med Singapore. 2010;39:913–917. [PubMed] [Google Scholar]
- 24.Hirsch IB. Glycemic variability: it's not just about A1C anymore! Diabetes Technol Ther. 2005;7:780–783. doi: 10.1089/dia.2005.7.780. [DOI] [PubMed] [Google Scholar]
- 25.Rausch JR. Measures of glycemic variability and links with psychological functioning. Curr Diabetes Rep. 2010;10:415–421. doi: 10.1007/s11892-010-0152-0. [DOI] [PubMed] [Google Scholar]
- 26.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 27.Zich JM. Attkinsson CC. Greenfield TK. Screening for depression in the primary clinics: the CES-D and the BDI. Int J Psychiatry Med. 1990;20:259–277. doi: 10.2190/LYKR-7VHP-YJEM-MKM2. [DOI] [PubMed] [Google Scholar]
- 28.Speilberger C. Gorsuch RL. Lushene R. Vagg PR. Jacobs GA. State-Trait Anxiety Inventory for Adults. Menlo Park, CA: Mind Garden Inc.; 1977. [Google Scholar]
- 29.Barnes L. Harp D. Jung W. Reliability generalization of scores on the Spieberger state-trait anxiety inventory. Educ Psychol Meas. 2002;62:603–618. [Google Scholar]
- 30.Spielberger C. State-Trait Anger Expression Inventory-2. Professional Manual. Lutz, FL: Psychological Assessment Resources; 1999. [Google Scholar]
- 31.Ferrans C. Powers M. Quality of Life Index. www.uic.edu/orgs/qli/ [Nov 27;2011 ]. www.uic.edu/orgs/qli/
- 32.Quinn L. Poradzisz M. Ferrans C. Psychometric properties of the Quality of Life Index: diabetes version [abstract] Diabetes. 1999;48(Suppl 1):A320. [Google Scholar]
- 33.Cholestech Corp. The Accuracy and Reproducibility of a Rapid, Fingerstick Method for Measuring A1c Certified by the NGSP. Technical Brief. Hayward, CA: Cholestech Corp.; 2002. [Google Scholar]
- 34.McDonnell CM. Donath SM. Vidmar SI. Werther GA. Cameron FJ. A novel approach to continuous glucose analysis utilizing glycemic variation. Diabetes Technol Ther. 2005;7:253–263. doi: 10.1089/dia.2005.7.253. [DOI] [PubMed] [Google Scholar]
- 35.Miller M. Strange P. Use of Fourier models for analysis and interpretation of continuous glucose monitoring glucose profiles. J Diabetes Sci Technol. 2007;1:630–638. doi: 10.1177/193229680700100506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubin R. Peyrot M. Quality of life and diabetes. Diabetes Metab Res Rev. 1999;15:205–218. doi: 10.1002/(sici)1520-7560(199905/06)15:3<205::aid-dmrr29>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 37.Fritschi S. Quinn L. Penckofer S. Surdyk P. Continuous glucose monitoring: the experience of women with type 2 diabetes. Diabetes Educ. 2010;36:250–257. doi: 10.1177/0145721709355835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robard D. Bailey T. Jovanovic L. Zisser H. Kaplan R. Garg SK. Improved quality of glycemic control and reduced glycemic variability with use of continuous glucose monitoring. Diabetes Technol Ther. 2009;11:717–723. doi: 10.1089/dia.2009.0077. [DOI] [PubMed] [Google Scholar]
- 39.Kohnert K-D. Heinke P. Vogt L. Zander E. Fritzsche G. Augstein P. Salzieder E. Reduced glucose variability is associated with improved quality of glycemic control in patients with type 2 diabetes: a 12-month observational study. J Endocrinol Metab. 2011;1:64–72. [Google Scholar]