Overexpession of neuroglobin prevents mitochondrial oxidative stress, apoptosis, and tissue damage in retinal ischemia.
Abstract
Purpose.
Neuroglobin (Ngb) is a vertebrate globin that is predominantly expressed in the retina and brain. To explore the role of Ngb in retinal neuroprotection during ischemia reperfusion (IR), the authors examined the effect of Ngb overexpression in the retina in vivo by using Ngb-transgenic (Ngb-Tg) mice.
Methods.
Retinal IR was induced in Ngb overexpressing Ngb-Tg mice and wild type (WT) mice by cannulating the anterior chamber and transiently elevating the IOP for 60 minutes. After Day 7 of reperfusion, the authors evaluated Ngb mRNA and protein expression in nonischemic control as well as ischemic mice and its effect on retinal histology, mitochondrial oxidative stress, and apoptosis, using morphometry and immunohistochemistry, quantitative PCR analysis and Western blot techniques.
Results.
Ngb-Tg mice without ischemia overexpress Ngb mRNA 11.3-fold (SE ± 0.457, P < 0.05) higher than WT control mice, and this overexpression of Ngb protein was localized to the mitochondria of the ganglion cells, outer and inner plexiform layers, and photoreceptor inner segments. This overexpression of Ngb is associated with decreased mitochondrial DNA damage in Ngb-Tg mice with IR in comparison with WT. Ngb-Tg mice with IR also revealed significant preservation of retinal thickness, significantly less activated caspase 3 protein expression, and apoptosis in comparison with WT mice.
Conclusions.
Neuroglobin overexpression plays a neuroprotective role against retinal ischemia reperfusion injury due to decreasing of mitochondrial oxidative stress-mediated apoptosis.
Neuroglobin (Ngb) is an oxygen-binding heme (151-amino acid) protein with a predicted molecular mass of approximately 17 kDa and is expressed in all vertebrates. It is related to myoglobin and hemoglobin, and is found predominantly in neurons.1–4 In the retina, neuroglobin is expressed at a concentration 100-fold higher than in the brain.5,6 However, its role in the retina is not known. The studies investigating the brain ischemia showed the neuroprotective effect of Ngb against ischemic damage. In these studies, neuroglobin overexpression results in decreased apoptosis and neuronal cell death during hypoxic cortical neuronal injury and smaller cerebral infarct volumes during transient cerebral ischemia.7,8 Conversely, the knock-down of Ngb in vitro has been associated with increased ischemic damage in cortical neuronal cells.9,10 However, the mechanism by which neuroglobin mediates such neuroprotection has not been elucidated. Several authors found Ngb in close association with mitochondrial functions, scavenging of reactive oxygen and nitrogen species as well as with enhancing of the oxygen supply.5–7,10–13
Ischemic injury is an essential feature that underlies the pathogenesis of many ophthalmic disorders such as retinal vascular occlusions, diabetic retinopathy, ischemic optic neuropathy, and glaucoma, and is a common cause of visual impairment and blindness.14 At the cellular level, ischemic injury primarily results from subsequent reperfusion, which activates a cascade of events leading to mitochondrial oxidative stress-mediated apoptosis.14–18 The high intraocular pressure (IOP) model of ocular ischemia in mice induced by transiently elevating the IOP above ocular perfusion pressure is a well recognized animal model of retinal ischemia that can be used to study the effects of ischemic reperfusion injury.14,19
To explore the role of Ngb in retinal neuroprotection, we examined the effects of neuroglobin overexpression in the retina during ischemia reperfusion in vivo by using novel Ngb-overexpressing transgenic (Ngb-Tg) mice in comparison with wild type (WT) BDF1 mice. Here, we determined the Ngb expression in Ngb-Tg mice in comparison with WT mice and demonstrate the close association of Ngb with mitochondria in the retina. In addition, we report that Ngb overexpression in vivo plays a neuroprotective role in retinal ischemia by preventing mitochondrial oxidative stress leading to decreased activated caspase 3 and apoptosis.
Methods
Animals
BDF1 (WT) mice were obtained from Charles River Laboratories International (Wilmington, MA) and Ngb-Tg mice were kindly provided by David A. Greenberg (Buck institute for Age Research, Novato, CA).20 Animal care and use was in compliance with institutional guidelines and with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Induction of Ischemia-Reperfusion
Mice were anesthetized with intramuscular ketamine chloride 80 mg/kg and xylazine 4 mg/kg. Ischemia reperfusion (IR) was performed using techniques previously described.21 Briefly, the anterior chamber of the left eye was cannulated with a 30-gauge infusion needle connected to a normal saline reservoir elevated to a height of 1.5 m to maintain an intraocular pressure of 110 mm Hg for 60 minutes. Retinal ischemia was confirmed by whitening of the iris and loss of red reflex and subsequent reperfusion by the return of the red reflex. The contralateral right eye served as a nonischemic control. Previous experiments with a sham procedure in the contralateral eye as nonischemic control did not show any difference in data.
Histologic Examination
Twelve WT and 12 Ngb-Tg mice were euthanized on Day 7 of reperfusion and 12 eyes from each group (WT-IR and Ngb-IR) were enucleated. The contralateral right eye served as a nonischemic control (WT-C, Ngb-C). Additionally 6 WT and 6 Ngb-Tg mice were euthanized on day 45 of reperfusion. All eyes were formalin-fixed and paraffin-embedded. Five-micrometer thick sections were stained with hematoxylin and eosin (H&E) and morphometric analysis was performed to quantify ischemic injury. Five sections were selected randomly in each eye. Total retinal thickness (from internal to outer limiting membrane, ILM-OLM) was measured in four adjacent areas of the inferior retina within 1 mm of the optic nerve, and the mean value was calculated. In a similar manner, the individual layers, inner nuclear layer (INL), inner plexiform layer (IPL), outer nuclear layer (ONL), outer plexiform layer (OPL), and ganglion cell layer (GCL) were also measured and the mean value calculated.
Statistical analysis comparing the differences among controls and two ischemic groups was performed separately in WT and Ngb-Tg mice using Mann-Whitney tests with P values <0.05 considered significant.
Ngb Gene Expression by Real-Time Polymerase Chain Reaction
The retinas of six mice from each group (nonischemic WT control mice [WT-C], WT mice with ischemia [WT-IR], nonischemic Ngb-Tg control mice [Ngb-C], and Ngb-Tg mice with ischemia [Ngb-IR]) were obtained on Day 7 of reperfusion. RNA was extracted using reagent (Trizol, Invitrogen, Carlsbad, CA) from each group of retinas. The cDNA template was generated using the reverse transcription kit (Omniscript; Qiagen, Valencia, CA). Real-time polymerase chain reaction (PCR) was performed to detect gene expression levels of Ngb. Each 25 uL PCR reaction mixture contained a master mix (SYBR green I; Bio-Rad Laboratories, Hercules, CA); 0.5 μM gene-specific primers for Ngb and the cDNA template. In the quantification analysis, glyceraldehyde-3-phosphatedehydrogenase (GAPDH) was used as a normalizing gene. PCR reactions for each gene in each experiment were performed in triplicate on each cDNA template, along with triplicate reactions of the housekeeping gene GAPDH. Dissociation melting curve analysis was used to check the specificity of PCR amplification products. The threshold cycle (Ct) difference between the experimental and control groups, for each gene in each tissue, was calculated and normalized to GAPDH, and the increase (x-fold) in mRNA expression was determined by the 2-ΔΔCt method as we had published previously.22,23 Statistical analysis of ΔΔCt was performed with ANOVA followed by Tukey-Kramer multiple comparison test for three independent samples, with significance set as P < 0.05 using statistical software (InStat; GraphPad, San Diego, CA).
Localization of Neuroglobin with Mitochondria
Seven-micrometer cryosections were obtained from 12 retinas of each group (WT-C, WT-IR, Ngb-C, and Ngb-IR) on day 7 of reperfusion. To localize Ngb with mitochondria, the retinal cryosections were incubated overnight at 4°C with a rabbit polyclonal anti-Ngb (1:200, Santa Cruz Biotechnology, Santa Cruz, CA) and mouse monoclonal Cytochrome c oxidase, subunit IV (COX IV, 1:200, Santa Cruz Biotechnology). The secondary antibodies used (1:200) were Cy2-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) and Texas Red dye–conjugated donkey anti-mouse IgG (Jackson ImmunoResearch Laboratories, Inc.) Isotype controls and PBS-replaced primary antibody were used as the negative controls. All experiments were performed in triplicate and all sections were viewed using confocal microscopy (Carl Zeiss, Oberkochen, Germany).
Detection of Mitochondrial Oxidative DNA Damage and Detection of Apoptotic Cells (TUNEL) in Retinal Ischemia Reperfusion Injury
Seven-micrometer cryosections were obtained from 12 retinas of each group (WT-C, WT-IR, Ngb-C, and Ngb-IR). 8-Hydroxy-dequanosine (8-OHdG) was detected using polyclonal anti-8OHdG (1:100, Chemicon, Billerica, MA) and colocalized with mouse monoclonal COX IV (1:100, Santa Cruz Biotechnology). The secondary antibodies used (1:200) were Cy2-conjugated donkey anti-mouse IgG and Texas Red dye-conjugated donkey anti-goat IgG. Isotype controls and PBS-replaced primary antibody were used as negative controls. All experiments were performed in triplicate and all sections were viewed using confocal microscopy (Carl Zeiss).
The TUNEL procedure was performed with an apoptosis detection kit (In Situ Cell Death Detection Kit, TMR red; Roche Diagnostics, Indianapolis, IN) according to the manufacturer's instructions. Label solution was used in place of the enzyme solution as the negative control, and positive control slides were treated with (DNase I recombinant; Roche Diagnostics). Staining was performed in triplicate and viewed with confocal microscopy.
Detection of Mitochondrial DNA Damage through Quantitative PCR
Total cellular DNA was isolated with a DNA kit (PureLink DNA kit; Invitrogen) as described by the manufacturer and the DNA concentration was determined using a high-sensitivity kit (Quanti-IT High Sensitivity DNA Assay Kit; Invitrogen) designed for the fluorometer (Qubit Fluorometer; Invitrogen). The quantitative PCR (qPCR) assay was performed as previously described.24,25 Briefly, PCR amplification was done using a kit (GeneAmp XL PCR kit; Applied Biosystems, Foster City, CA) on a PCR system (GeneAmp PCR system 9700; Applied Biosystems). The qPCR assay is based on the principle that the lesions such as oxidative DNA damage will block the thermostable DNA polymerase on the DNA template leading to a decrease in amplification of the fragment of interest. The reaction mixtures contained 15 ng of genomics DNA. The reagent conditions for the qPCR have been described previously.25 The primer sequences for the 10 kb mitochondrial genome were 5′-GCC AGC CTG ACC CAT AGC CAT ATT AT-3′ (sense) and 5′-GAG AGA TTT TAT GGG TGT ATT GCG G-3′ (antisense). Normalization for variations in amplification of the mitochondria due to possible changes in mitochondrial DNA steady state levels was performed by amplifying a small, 117 base pair (bp) mitochondrial fragment (sense, 5′-CCC AGC TAC TAC CAT CAT TCA AGT-3′ and antisense, 5′-GAT GGT TTG GGA GAT TGG TTG ATG-3′) which is independent of damage and would be expected to yield similar qPCR (short) concentrations.
As previously described, DNA lesion frequencies were calculated using the Poisson equation which assumes a random distribution of DNA lesions and is defined as f(x) = e−λλx/x!, where x = 0, for zero class molecules (molecules with no damage), and λ is the average lesion frequency.24–26 The amplification is directly proportional to the fraction of undamaged DNA templates. Therefore, the average lesion frequency per strand can be calculated as λ = −lnAt/Ao, where At represents the amount of amplification of the damaged template and Ao is the amount of amplification product from undamaged DNA. The amplification of At is also normalized to the amplification of Ao and expressed as the relative amplification ratio, At/Ao.24–26 Statistical analysis was performed with a Student's t-test with P values <0.05 considered significant.
Western Blot Analysis of Ngb and Caspase 3
Twelve retinas from each group (WT-C, WT-IR, Ngb-C, and Ngb-IR) on Day 7 of reperfusion were obtained. In addition, seven retinas from WT animals after 12 hours and seven retinas after 1 day of reperfusion were dissected out. All retinas were homogenized and lysed in protein extraction buffer (M-PER; Thermo Scientific, Waltham, MA) containing protease inhibitors (Calbiochem, San Diego, CA). The homogenates were centrifuged at 13,000 rpm for 20 minutes at 4°C. Protein quantification of the supernatant was determined using bovine serum albumin as the standard (Bio-Rad Laboratories). Equal amounts of protein samples were loaded and run on SDS-PAGE (4%–15% Tris-HCL polyacrylamide ready gels, Bio-Rad Laboratories). After electrophoresis, proteins were transferred onto polyvinylidene difluoride membranes (Bio-Rad Laboratories) using a transblot semidry system. The membranes were blocked using 5% skim milk and then probed with a polyclonal anti-Ngb (1:200, Santa Cruz Biotechnology) overnight a 4°C. In addition, activated caspase 3 was detected by probing the membranes with polyclonal anti-caspase 3 (1: 500, Santa Cruz Biotechnology). After incubation for 45 minutes with the secondary antibody tagged with horseradish peroxidase (anti-rabbit, Santa Cruz Biotechnology), signals were detected by chemiluminsecence system (Thermo Scientific). Equal protein loading of retinal lysates from each group was confirmed by reprobing blots with a monoclonal antibody to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and β-actin. The differences in expression levels were determined by scanning gels and determining the integrated density of the bands using ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html). Data are expressed as normalized ratios to GAPDH. The experiments were performed in triplicate, each time using different sets of animals. Statistical analysis evaluating three different blots was performed with a Student's t-test with P values <0.05 considered significant. WT control eyes were compared with WT-IR as well as with Ngb-C. In separate analysis the Ngb-Tg control eyes (Ngb-C) were compared with eyes after IR injury (Ngb-IR).
Results
Effect of Ngb Overexpression on Retinal Histology after IR
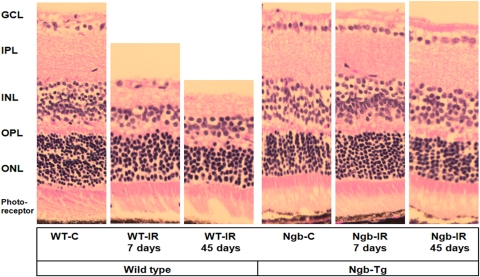
Histopathological changes of the WT retina on Day 7 of IR revealed marked thinning and atrophy, especially in the GC, IPL, and INL. (Fig. 1, Table 1). The quantitative morphometry of the total retinal thickness (ILM-OLM) showed a significant decrease in WT mice with ischemia (WT-IR) on Day 7 after reperfusion (188.7 ± 6.2 μm) and further decrease on Day 45 after reperfusion (145.6 ± 12.8 μm) when compared with WT-C (242.7 μm, P < 0.05 and P < 0.01 respectively). In addition, the thickness of the each individual retinal layer measured in WT mice on Day 45 after reperfusion also showed statistically significant reduction when compared with controls (WT-C). In Ngb-TG mice a significant increase of the total retinal thickness (ILM-OLM) was observed on Day 7 after ischemia (Ngb-IR, 222.0 ± 10.8 μm) compared with Ngb-C (200.0 ± 34 μm, P < 0.05). However on Day 45 after reperfusion there was no significant differences in the total retinal thickness as well as thickness of each retinal layer and the cell numbers in these layers in Ngb-Tg mice with IR compared with the Ngb-Tg control mice eyes (Table 1).
Figure 1.
The histopathologic changes of the retina in WT and Ngb-Tg mice at Day 7 and Day 45 after IR injury. The total retinal thickness is decreased in ischemic eyes of WT (WT-IR) mice compared with control eyes (WT-C) on both Day 7 and Day 45 post IR. The retinal thickness in Ngb-Tg mice with ischemia (NGB-IR) on Day 7 significantly increased compared with nonischemic eye (NGB-C), however on Day 45 after IR, no significant changes in the retinal thickness was observed compared with Ngb-C. H&E staining magnification, ×400.
Table 1.
Morphometric Analysis of Retinal Thickness in WT and Ngb-Tg Mice at Day 7 and Day 45 after IR Injury
| Retinal Layers | WT-C | WT-IR (7 Days) | WT-IR (45 Days) | Mann-Whitney | Ngb-C | Ngb-IR (7 Days) | Ngb-IR (45 Days) | Mann-Whitney |
|---|---|---|---|---|---|---|---|---|
| ILM-OLM, μm | 242.7 ± 48.0 | 188.7 ± 6.2 | 145.6 ± 12.8 | P < 0.05* | 200.0 ± 34 | 222 ± 10.8 | 192.3 ± 18.6 | P < 0.05* |
| P < 0.01† | NS† | |||||||
| ONL, μm | 56.4 ± 5.8 | 48.4 ± 2.4 | 34.2 ± 5.2 | P < 0.01* | 56.8 ± 8.7 | 59.8 ± 6.2 | 54.2 ± 7.5 | NS |
| P < 0.01† | ||||||||
| OPL, μm | 17.5 ± 3.9 | 15.9 ± 1.3 | 14.2 ± 3.4 | NS | 16.5 ± 4 | 17 ± 3.3 | 15.9 ± 3.5 | NS |
| P < 0.05† | ||||||||
| INL, μm | 56.6 ± 9.3 | 45.4 ± 3.0 | 40.2 ± 6.3 | P < 0.01* | 55.8 ± 8.5 | 53.7 ± 1.5 | 53.5 ± 7.5 | NS |
| P < 0.01† | ||||||||
| IPL, μm | 61.3 ± 7.7 | 46.0 ± 3.5 | 38.5 ± 4.9 | P < 0.01* | 58.45 ± 2.2 | 56.8 ± 2.25 | 55.8 ± 6.8 | NS |
| P < 0.01† | ||||||||
| ONL, cells | 345 | 217 | 185 | P < 0.01* | 350 | 300 | 296 | NS |
| P < 0.01† | ||||||||
| INL, cells | 152 | 106 | 84 | P < 0.01* | 149 | 145 | 139 | NS |
| P < 0.01† | ||||||||
| GCL, cells | 21 | 15 | 6 | P < 0.05* | 19 | 22 | 17 | NS |
| P < 0.001† |
Quantitative morphometry of total retinal thickness (ILM-OLM) and the thickness of individual retinal layers revealed that the thickness of the retinas in the Ngb mice with ischemia (Ngb-IR) was preserved in comparison with the retinas of the wild type mice with ischemia (WT-IR) which was significantly thinner (P < 0.05). The statistical analysis compared the difference between control eye and IR eye at Day 7 of reperfusion (*) and between control eye and IR eye at Day 45 of reperfusion (†). WT and Ngb-Tg mice were analyzed separately. NS, not significant.
Neuroglobin Overexpression in Ngb-Tg Mice and Effect of IR on Expression
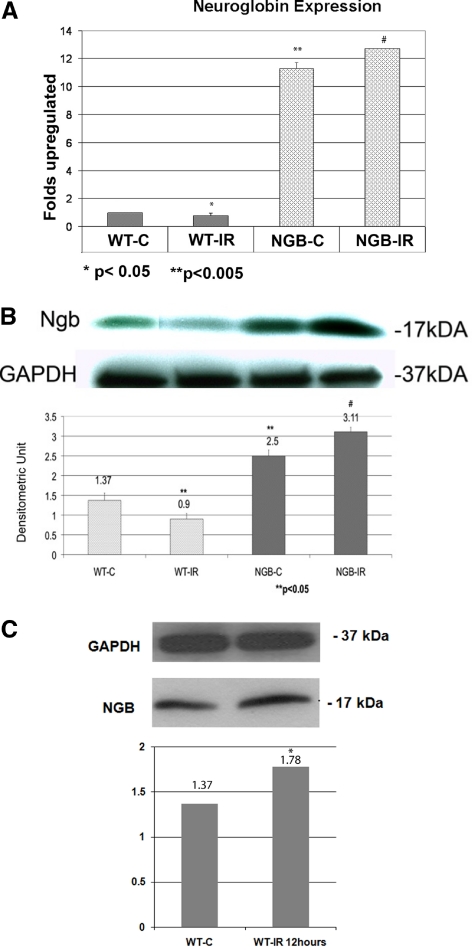
In Ngb-Tg mice, Ngb mRNA was overexpressed 11.3-fold (SE ± 0.457, P < 0.05) higher than normal WT mice. The differences of the Ngb gene expression in WT and Ngb-Tg animals are presented in Figure 2A. After Day 7 of reperfusion, Ngb mRNA expression significantly decreased in WT mice (WT-IR) to 0.77-fold (SE ± 0.32, P < 0.05) when compared with WT-C. In Ngb-Tg mice the significant upregulation of the Ngb mRNA expression was observed on Day 7 after reperfusion (1.42-fold, SE ± 0.171, P < 0.05) when compared with Ngb-Tg control mice. Increased gene expression of Ngb mRNA in the retina of Ngb-Tg mice correlated with the increased protein expression as revealed by the Western blot analysis. A significantly higher amount of the Ngb protein could be detected in control eyes of Ngb-Tg animals (Ngb-C) in comparison with WT-C eyes (2.5 to 1.37 densitometric unit respectively, P < 0.05). On Day 7 after reperfusion (Fig. 2B), in the Ngb-Tg mice with ischemia (Ngb-IR) there was a significant increase in the levels of Ngb protein (to 3.11 densitometric units, P < 0.05) compared with Ngb-Tg control eyes. However in contrast, WT mice with ischemia demonstrated a significant decrease in Ngb protein on Day 7 of reperfusion (to 0.9 densitometric units, P < 0.05) when compared with WT controls (Fig. 2B), whereas the retinas of WT mice after 12 hours of ischemia (Fig. 2C) showed a significant transient increase of the Ngb protein (1.78 densitometric units, P < 0.05) compared with the WT controls. The protein level of Ngb was significantly decreased subsequently after 1 day of reperfusion in the WT mice compared with WT controls (0.42 densitometric units, P < 0.05, data not shown).
Figure 2.
(A) Gene expression of Ngb mRNA was significantly overexpressed (11.28-fold increase, **P < 0.005) in Ngb-Tg mice without ischemia (NGB-C) in comparison with WT mice without ischemia (WT-C). There was a significant difference (*P < 0.05) in Ngb mRNA expression between WT-C and WT mice with ischemia (WT-IR). Similarly, there was a significant difference (#P < 0.05) between NGB-C mice and Ngb-Tg mice with ischemia (NGB-IR). mRNA was quantitated by real-time PCR analysis using gene-specific primers and was normalized to GAPDH. The relative multiple of change in mRNA expression was determined using the 2-ΔΔCt method. Experiments were conducted in triplicate. Statistical analysis was performed using t-tests. *P < 0.05; **P < 0.005: WT control eyes compared with WT-IR and Ngb control eyes. #P < 0.05: Ngb-Tg control eyes compared with Ngb-Tg IR eyes. (B) Western blot analysis of the retinal protein lysates revealed that the Ngb protein was significantly overexpressed (densitometric unit, 2.5; **P < 0.05) in Ngb-Tg mice without ischemia (NGB-C) in comparison with wild type control mice without ischemia (WT-C). The Ngb protein level was decreased in WT mice with ischemia (WT-IR) in comparison with WT mice without ischemia (WT-C) (**P < 0.05). In contrast the Ngb protein levels were significantly increased (P < 0.05) in Ngb-Tg mice with ischemia (NGB-IR) in comparison with Ngb-Tg control mice (NGB-C). The eyes with IR were compared with control eyes in WT and Ngb-Tg mice separately. Statistical analysis was performed using t-tests.**P < 0.05; WT control eyes compared with WT IR and Ngb control eyes. #P < 0.05: Ngb-Tg control eyes compared with Ngb-Tg IR eyes. (C) Western blot analysis of the retinal protein lysates revealed that Ngb protein was significantly overexpressed (densitometric unit, 1.78; *P < 0.05) in WT mice 12 hours after reperfusion (WT-IR) in comparison with WT control eyes without ischemia (WT-C).
Localization of Ngb in the Retina and Its Association with Mitochondria
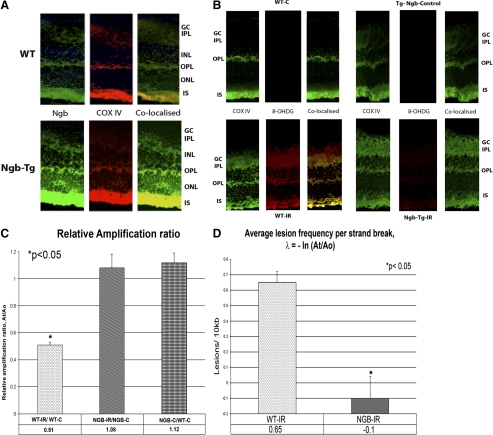
Immunostaining of 12 nonischemic WT control retinas showed the presence of Ngb staining (green) in the inner photoreceptor segments, IPL, OPL, and GC layer (Fig. 3A). Using the antibodies against COX IV, Ngb was found to colocalize with the mitochondria. The distribution of the Ngb staining did not differ between WT and Ngb-Tg control mice, however there was increased staining of Ngb in the Ngb-Tg mice.
Figure 3.
(A) Localization of Ngb (green) with mitochondrial marker, COX IV (red) in WT mice and Ngb-Tg mice. Ngb protein is localized in the inner segments (IS) of the photoreceptors, outer plexiform layer (OPL), inner plexiform layer (IPL), and ganglion cell (GC) layers, and is colocalized with the mitochondria distribution (COX IV) in the retina. (B) Immunostaining for 8-OHDG, a well recognized marker of oxidative DNA damage. 8-OHDG immunostaining demonstrating DNA damage and oxidative stress was localized in the GCL, INL, ONL, and photoreceptors of WT mice with ischemia (WT-IR). In contrast, no staining for 8-OHDG was detected in Ngb-Tg with IR indicating the absence of DNA damage and oxidative stress in these mice. Colocalization of 8-OHDG with a mitochondrial marker, COX IV demonstrates that the oxidative stress is in the mitochondria. (C) Measurement of mitochondrial DNA damage using quantitative PCR. The relative amplification of the 10 kb mitochondrial fragment comparing the amplicon amounts of NGB-IR/NGB-C and WT-IR/WT-C after normalizing with the 117 bp small mitochondrial fragment revealed a 52.8% decrease (*P < 0.05) in relative amplification ratio between WT-IR and NGB-IR. There was no significant difference between the relative amplification ratio of NGB-C/WT-C mice without ischemia and NGB-IR/NGB-C mice with ischemia. Results are expressed as mean ± SEM values for three QPCRs performed for each DNA per group, n = 6 mice per group. *P ≤ 0.05 was considered significant. (D) Decreased mitochondrial lesions in Ngb-Tg mice with IR. Average mitochondrial DNA damage per 10 kb DNA revealed a significant decrease in mitochondrial lesions (−0.1 lesion/10 kb, *P < 0.05) in Ngb-Tg mice with ischemia (NGB-IR) in comparison with WT mice with ischemia (WT-IR). The average lesion frequency per strand can be calculated as λ = −lnAt/Ao, where At represents the amount of amplification of the damaged template and Ao is the amount of amplification product from undamaged DNA. The amplification of At is also normalized to the amplification of Ao and expressed as the relative amplification ratio, At/Ao. Statistical analysis was performed with Student's t-test with *P < 0.05 considered significant.
Mitochondrial Oxidative Stress
In WT mice with ischemia, 8-OHDG, DNA damage could be detected in the ganglion cell layer, INL, and ONL. In addition, colocalization of 8-OHDG with mitochondrial COX IV was seen in the ganglion cell, INL, and ONL of WT mice with ischemia indicating that this oxidative stress is mitochondrial in nature. In contrast there was no immunostaining of 8-OHDG, in Ngb-Tg mice with ischemia, indicating the absence of DNA damage and oxidative stress in Ngb-Tg mice with ischemia (Fig. 3B). The presence of mitochondrial oxidative DNA damage was further confirmed using a quantitative PCR assay. By comparing the relative efficiency of amplification of the large mitochondrial fragment 10 kb, and normalizing this to the amplification of the smaller 117 bp fragments. The relative amplification of the 10 kb mitochondrial fragment comparing the amplicon amounts of Ngb-IR and WT-IR mice revealed a 52.8% decrease (P < 0.05) in relative amplification ratio. There was no statistical difference between the relative amplification ratio of Ngb-C/WT-C mice without ischemia (Fig. 3C). The average mitochondrial DNA lesion per 10 kb DNA, determined as: λ = −lnAt/Ao, also revealed a significant decrease in DNA lesions (−0.1 lesion/10 kb, P < 0.05) in Ngb-Tg mice with ischemia in comparison with WT mice with ischemia (Fig. 3D).
Apoptosis
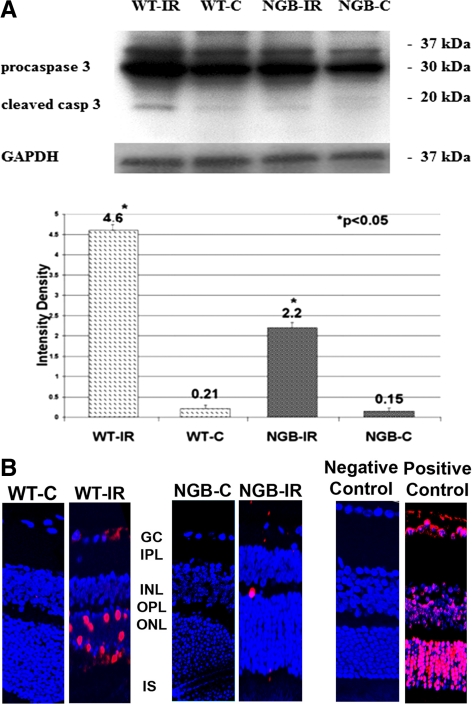
Western blot analysis revealed high protein levels of activated caspase 3 in the WT mice with IR compared with the control WT eyes. However the activation of the caspase 3 in the Ngb-Tg mice with IR was significantly lower compared with the WT- IR mice (Fig. 4A).
Figure 4.
(A) Activated caspase 3 protein expression was found to be significantly less activated in eyes with IR in Ngb-TG mice at day 7 of reperfusion (2.2 densitometric unit, *P < 0.05) when compared with eyes with IR in WT mice at Day 7 of reperfusion. (B) Decreased apoptosis in Ngb-Tg mice with IR. At day 7 of reperfusion the TUNEL apoptosis assay reveals numerous TUNEL-positive cells in the retinas of WT mice with ischemia (WT-IR) in comparison with only occasional TUNEL-positive cells in Ngb-Tg mice with ischemia (NGB-IR). No TUNEL-positive cells were detected in the negative control and eyes without ischemia (WT-C and NGB-C). Retinas treated with DNase I served as positive control retinas and demonstrated abundant apoptotic cells.
In WT mice with ischemia, numerous TUNEL positive cells were detected whereas in the Ngb-Tg mice with IR only a few apoptotic cells were present. Neither the negative control nor nonischemic WT (WT-C) and Ngb controls (Ngb-C) showed TUNEL-positive cells (Fig. 4B).
Discussion
The present study reveals that the Ngb-Tg mice represent a unique model to study the effect of Ngb overexpression in vivo during retinal ischemia. When neuroglobin is overexpressed in the Ngb-Tg mice using a chicken β-actin promotor, this overexpression occurs at the gene and protein level.20 Using real time-polymerase chain reaction analysis, we found the overexpression of Ngb mRNA in the retinas of Ngb-Tg mice to be 11.3-fold (SE ± 0.457, P < 0.05) higher than in the WT- mice during nonischemic conditions (Fig. 2A). Furthermore, the retinal overexpression of Ngb in the transgenic animals was also confirmed at a protein level by the larger Ngb band detected on immunoblot in comparison with WT animals (Fig. 2B). The retinal distribution of Ngb overexpression in the photoreceptor inner segments, IPL, OPL, and GC layers is consistent with the physiological distribution of Ngb in WT mice (Fig. 2C) and correlates with previously reported Ngb distribution in the murine and human retina.5,27
The role of Ngb neuroprotection in retinal ischemia is not known. Since its discovery in 2000, studies on Ngb focus on its role in cerebral ischemia where it has been implicated as an endogenous neuroprotectant in vitro and in vivo using Ngb overexpression cortical neurons as well as in Ngb-overexpressing transgenic (Ngb-Tg) mice.9,10,28–31 By using a well-established model of retinal IR, we investigated the changes of Ngb expression in response to retinal ischemia in WT and Ngb-Tg mice.
In our study, we observed significant differences in the regulation of the gene and protein expression of Ngb between WT and Ngb-Tg mice after ischemic injury. In the WT mice there was a significant transient upregulation of the Ngb protein after 12 hours (Fig. 2C) of reperfusion followed by significant downregulation of the mRNA and protein expression from Day 1 and Day 7 post IR (Fig. 2B). This expression profile is similar to those found during acute retinal ischemia in rats.32 The data of the Ngb expression under hypoxic conditions are conflicting. Several studies have shown the upregulation of the Ngb expression in the cell cultures under hypoxia.33,34 In some species of animals like zebrafish and turtles, significant upregulation of Ngb has been observed under ischemic conditions whereas several reports in rodents have shown downregulation of Ngb expression after brain ischemia.6,35,36
A significant upregulation of the Ngb protein expression were also observed on Day 7 after reperfusion in Ngb-Tg mice correlating with the mRNA expression (Fig. 2B) similar to other reports.6 After Day 7 of reperfusion, the retinas of Ngb-Tg mice with ischemia showed transient increase of retinal thickness due to edema which was later resolved and after 45 days there was no significant difference in retinal thickness and morphologic structures compared with control eyes (Fig. 1). However, in WT-IR mice, on Day 7 and Day 45 of reperfusion there was marked retinal thinning and histologic degenerative changes in the GC, IPL, and INL (Fig. 1), which are consistent with retinal changes during ischemia described in previous studies.37–39 These data demonstrate that Ngb overexpression in these transgenic mice prevents retinal damage from IR injury.
At a subcellular level, Ngb is found in close association with mitochondria as seen by the colocalization of Ngb with COX IV in the photoreceptor inner segments, the IPL, OPL, and GC layers (Fig. 3A). These areas correspond to sites of high oxygen consumption and suggest that Ngb may play a role in oxygen sensing and mitochondrial metabolism. A similar finding has also been reported in other studies, where Ngb was colocalized and comigrated with mitochondria in neuronal processes.40–43
In our study, we used immunohistochemistry and qPCR to demonstrate the presence of mitochondrial oxidative stress during retinal ischemic injury. During oxidative stress, free radical and reactive oxygen species (ROS) release in the nuclear and mitochondrial DNA result in oxidative lesions. One of these lesions, 8-hydroxy-2′ -deoxyguanosine (8-OHdG) has been widely used as a marker for oxidative stress.44,45 Increased 8-OHDG immunoreactivity in the retinas of WT-mice with ischemia was found to colocalize with a mitochondrial marker, COX IV in the ganglion cells, INL, and ONL of the WT-IR mice retinas after Day 7 of reperfusion (Fig. 3B). In contrast, mitochondrial oxidative stress was absent in the ganglion cells, INL, and ONL of Ngb-Tg mice with ischemia suggesting that the neuroprotective effect of Ngb overexpression is through prevention of mitochondrial oxidative stress (Fig. 3B). To further quantify the mitochondrial DNA damage and confirm this protective role of neuroglobin in preventing mitochondrial DNA damage, we measured the mitochondrial DNA damage using a well-established quantitative PCR assay.25,26 The Ngb-Tg mice demonstrated a statistically significant decrease in mitochondrial DNA damage after Day 7 of reperfusion when compared with WT mice with ischemia (Fig. 3C). A comparison of the mitochondrial DNA damage between the nonischemic controls of Ngb-Tg and WT-mice revealed no statistical significance (P = 0.12), indicating Ngb overexpression prevents mitochondrial DNA damage during retinal ischemia. This further confirms that Ngb mediates neuroprotection through prevention of mitochondrial oxidative stress.
As mitochondrial oxidative stress and DNA damage ultimately lead to the activation of caspase 3 and thus apoptosis, we also studied the role of Ngb on apoptosis during retinal ischemia. Our study revealed that a statistically significant reduction in the levels of activated caspase-3 in Ngb-Tg mice with ischemia on immunoblot when compared with WT mice with ischemia (Fig. 4A) suggesting that Ngb further mediates its neuroprotective effects by preventing the activation of caspase 3. To further confirm this, we performed a TUNEL apoptosis assay and found a decreased number of apoptotic cells in the retinas of Ngb-Tg mice subjected to ischemia when compared with the WT mice with apoptosis during retinal ischemia (Fig. 4B).
The molecular mechanism of Ngb has not been clearly elucidated. When Ngb was first discovered, it was believed to act as an oxygen transporter or buffer supplying oxygen during anoxia, similar to hemoglobin.43,46 However, this has been found to be unlikely due to its low concentration in the brain and its relatively low oxygen affinity.6,46 Studies have demonstrated that Ngb binds to cytochrome c (Cyt c) and membrane bound G-protein coupled receptor (GPCR) and thus the proposed mechanisms of action are closely related to these proteins.46–48 One possible mechanism of action is the role of Ngb in a redox reaction with Cyt c.46,49 During oxidative stress, Cyt c is released and triggers apoptosis through activation of proapoptotic caspase 3. This proposed redox interaction between Cyt c and Ngb suggests that Cyt c in converted by Ngb to a reduced ferrous form that is now unable to trigger apoptosis.46,49 Furthermore, in vitro studies have demonstrated that oxidative stress only occurs in neuroglobin overexpressing transgenic cells when the level of exogenous Cyt c is elevated beyond the usual level required to induce apoptosis.46 These findings suggest that Ngb resets the trigger level for apoptosis. Other studies, however, reveal that Ngb maintains the mitochondrial membrane potential and mitochondrial function during oxidative stress through activation of phosphatidylinositol 3-kinases (PI3) and anti-apoptotic AKT pathways.43,46,50,51 Neuroglobin is also believed to couple with the membrane-bound GPCR.43,46 This binding leads to the suppression of elevated calcium levels through the inositol trisphosphate (IP3) pathway and prevents apoptosis.2,46,52 Putting these theories together, it may be postulated that during retinal ischemia, Ngb binds to the released cytochrome c during the initial mitochondrial oxidative stress. This leads to the inactivation of cytochrome c and the prevention of apoptosis as we found in our Ngb-Tg mice with ischemia. Moreover, the prevention of further mitochondrial oxidative damage in retinal ischemia after the initial release of Cyt c may be explained by the coupling of Ngb with GPCR. During retinal ischemia, there is an increase in calcium levels that lead to apoptosis and neuronal cell death.14,53 Studies have demonstrated that Ngb is associated with decreased calcium levels and it can be postulated that by activating the IP3 pathway through GPCR, this rise in calcium levels during retinal ischemia is suppressed and thus preventing further mitochondrial oxidative stress and apoptosis.10,53 In addition, Ngb may prevent further mitochondrial oxidative stress by coupling with GPCR to activate PI3 and trigger antiapoptotic events through AKT. However, further studies will be necessary to confirm our postulation and confirm the definitive molecular mechanisms behind this neuroprotection in retinal ischemia.
The neuroprotective role of Ngb in retina ischemia has not been elucidated. Our study reveals for the first time that Ngb overexpression is neuroprotective during retinal ischemia and that it mediates this protection by inhibiting mitochondrial oxidative stress and caspase-3 activation leading to decreased apoptosis. In addition, we demonstrate that the Ngb-Tg mice provide a unique model to study the putative mechanisms for Ngb-mediated neuroprotection in vivo. Although further work is necessary to confirm the molecular mechanisms behind Ngb neuroprotection, the identification of Ngb as a potential endogenous neuroprotectant in the retina is important as it raises the possibility of its therapeutic use for various ophthalmic conditions such as retinal ischemia and glaucoma which share the underlying pathogenesis of ischemic injury.
Footnotes
Supported by National Institutes of Health Grants EY03040, EY019506, and EY017347 and Research to Prevent Blindness.
Disclosure: A.S.Y. Chan, None; S. Saraswathy, None; M. Rehak, None; M. Ueki, None; N.A. Rao, None
References
- 1. Burmester T, Weich B, Reinhardt S, Hankeln T. A vertebrate globin expressed in the brain. Nature. 2000;407:520–523 [DOI] [PubMed] [Google Scholar]
- 2. Dewilde S, Kiger L, Burmester T, et al. Biochemical characterization and ligand binding properties of neuroglobin, a novel member of the globin family. J Biol Chem. 2001;276:38949–38955 [DOI] [PubMed] [Google Scholar]
- 3. Burmester T, Hankeln T. Neuroglobin: a respiratory protein of the nervous system. News Physiol Sci. 2004;19:110–113 [DOI] [PubMed] [Google Scholar]
- 4. Brunori M, Vallone B. Neuroglobin, seven years after. Cell Mol Life Sci. 2007;64:1259–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schmidt M, Giessl A, Laufs T, Hankeln T, Wolfrum U, Burmester T. How does the eye breathe? Evidence for neuroglobin-mediated oxygen supply in the mammalian retina. J Biol Chem. 2003;278:1932–1935 [DOI] [PubMed] [Google Scholar]
- 6. Burmester T, Hankeln T. What is the function of neuroglobin? J Exp Biol. 2009;212:1423–1428 [DOI] [PubMed] [Google Scholar]
- 7. Bentmann A, Schmidt M, Reuss S, Wolfrum U, Hankeln T, Burmester T. Divergent distribution in vascular and avascular mammalian retinae links neuroglobin to cellular respiration. J Biol Chem. 2005;27:280:20660–20665 [DOI] [PubMed] [Google Scholar]
- 8. Wang X, Liu J, Zhu H, et al. Effects of neuroglobin overexpression on acute brain injury and long-term outcomes after focal cerebral ischemia. Stroke. 2008;39:1869–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khan AA, Wang Y, Sun Y, et al. Neuroglobin-overexpressing transgenic mice are resistant to cerebral and myocardial ischemia. Proc Natl Acad Sci U S A. 2006;103:17944–17948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu J, Yu Z, Guo S, et al. Effects of neuroglobin overexpression on mitochondrial function and oxidative stress following hypoxia/reoxygenation in cultured neurons. J Neurosci Res. 2009;87:164–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ostojić J, Grozdanić SD, Syed NA, et al. Patterns of distribution of oxygen-binding globins, neuroglobin and cytoglobin in human retina. Arch Ophthalmol. 2008;126:1530–1536 [DOI] [PubMed] [Google Scholar]
- 12. Dong Y, Zhao R, Chen XQ, Yu AC. 14-3-3gamma and neuroglobin are new intrinsic protective factors for cerebral ischemia. Mol Neurobiol. 2010;41:218–231 [DOI] [PubMed] [Google Scholar]
- 13. Ostojić J, Sakaguchi DS, de Lathouder Y, et al. Neuroglobin and cytoglobin: oxygen-binding proteins in retinal neurons. Invest Ophthalmol Vis Sci. 2006;47:1016–1023 [DOI] [PubMed] [Google Scholar]
- 14. Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res. 2004;23:91–147 [DOI] [PubMed] [Google Scholar]
- 15. Lam TT, Abler AS, Tso MO. Apoptosis and caspases after ischemia-reperfusion injury in rat retina. Invest Ophthalmol Vis Sci. 1999;40:967–975 [PubMed] [Google Scholar]
- 16. Singh N, Sun Y, Nakamura K, Smith MR, Colburn NH. C-JUN/AP-1 as possible mediators of tumor necrosis factor-alpha-induced apoptotic response in mouse JB6 tumor cells. Oncol Res. 1995;7:353–362 [PubMed] [Google Scholar]
- 17. Bonne C, Muller A, Villain M. Free radicals in retinal ischemia. Gen Pharmacol. 1998;30:275–280 [DOI] [PubMed] [Google Scholar]
- 18. Flaherty JT, Weisfeldt ML. Reperfusion injury. Free Radic Biol Med. 1988;5:409–419 [DOI] [PubMed] [Google Scholar]
- 19. Buchi ER, Suivaizdis I, Fu J. Pressure-induced retinal ischemia in rats: an experimental model for quantitative study. Ophthalmologica. 1991;203:138–147 [DOI] [PubMed] [Google Scholar]
- 20. Khan AA, Sun Y, Jin K, et al. A neuroglobin-overexpressing transgenic mouse. Gene. 2007;398:172–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen YG, Zhang C, Chiang SK, Wu T, Tso MO. Increased nuclear factor-kappa B p65 immunoreactivity following retinal ischemia and reperfusion injury in mice. J Neurosci Res. 2003;72:125–131 [DOI] [PubMed] [Google Scholar]
- 22. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408 [DOI] [PubMed] [Google Scholar]
- 23. Saraswathy S, Nguyen AM, Rao NA. The role of TLR4 in photoreceptor αA crystallin upregulation during early experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2010;51:3680–3686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khurana RN, Parikh JG, Saraswathy S, Wu G-S, Rao NA. Mitochondrial oxidative DNA damage in experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2008;49:3299–3304 [DOI] [PubMed] [Google Scholar]
- 25. Santos JH, Meyer JN, Mandavilli BS, Van Houten B. Quantitative PCR-based measurement of nuclear and mitochondrial DNA damage and repair in mammalian cells. Methods Mol Biol. 2006;314:183–199 [DOI] [PubMed] [Google Scholar]
- 26. Santos JH, Mandavilli BS, Van Houten B. Measuring oxidative mtDNA damage and repair using quantitative PCR. Methods Mol Biol. 2002;197:159–176 [DOI] [PubMed] [Google Scholar]
- 27. Rajendram R, Rao NA. Neuroglobin in normal retina and retina from eyes with advanced glaucoma. Br J Ophthalmol. 2007;91:663–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hundahl C, Stoltenberg M, Fago A, et al. Effects of short-term hypoxia on neuroglobin levels and localization in mouse brain tissues. Neuropathol Appl Neurobiol. 2005;31:610–617 [DOI] [PubMed] [Google Scholar]
- 29. Jin K, Mao Y, Mao X, Xie L, Greenberg DA. Neuroglobin expression in ischemic stroke. Stroke. 2010;41:557–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shang A, Zhou D, Wang L, et al. Increased neuroglobin levels in the cerebral cortex and serum after ischemia-reperfusion insults. Brain Res. 2006;1078:219–226 [DOI] [PubMed] [Google Scholar]
- 31. Sun Y, Jin K, Peel A, Mao XO, Xie L, Greenberg DA. Neuroglobin protects the brain from experimental stroke in vivo. Proc Natl Acad Sci U S A. 2003;100:3497–3500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ding GP, Yan ZG, He T, et al. The neuroglobin expression when retinal ganglion cells death in acute rats' retina ischemia [in Chinese]. Zhonghua Yan Ke Za Zhi. 2010;46:590–596 [PubMed] [Google Scholar]
- 33. Schmidt-Kastner R, Haberkamp M, Schmitz C, Hankeln T, Burmester T. Neuroglobin mRNA expression after transient global brain ischemia and prolonged hypoxia in cell culture. Brain Res. 2006;1103:173–180 [DOI] [PubMed] [Google Scholar]
- 34. Sun Y, Jin K, Mao XO, Zhu Y, Greenberg DA. Neuroglobin is up-regulated by and protects neurons from hypoxic-ischemic injury. Proc Natl Acad Sci U S A. 2001;98:15306–15311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Roesner A, Hankeln T, Burmester T. Hypoxia induces a complex response of globin expression in zebrafish (Danio rerio). J Exp Biol. 2006;209:2129–2137 [DOI] [PubMed] [Google Scholar]
- 36. Milton SL, Nayak G, Lutz PL, Prentice HM. Gene transcription of neuroglobin is upregulated by hypoxia and anoxia in the brain of the anoxia-tolerant turtle Trachemys scripta. J Biomed Sci. 2006;13:509–514 [DOI] [PubMed] [Google Scholar]
- 37. Szabo ME, Droy-Lefaix MT, Doly M, Carre C, Braquet P. Ischemia and reperfusion-induced histologic changes in the rat retina. Demonstration of a free radical-mediated mechanism. Invest Ophthalmol Vis Sci. 1991;32:1471–1478 [PubMed] [Google Scholar]
- 38. Hughes WF. Quantitation of ischemic damage in the rat retina. Exp Eye Res. 1991;53:573–582 [DOI] [PubMed] [Google Scholar]
- 39. Sakamoto K, Ohki K, Saito M, Nakahara T, Ishii K. Histological protection by donepezil against neurodegeneration induced by ischemia-reperfusion in the rat retina. J Pharmacol Sci. 2010;112:327–335 [DOI] [PubMed] [Google Scholar]
- 40. Greenberg DA, Jin K, Khan AA. Neuroglobin: an endogenous neuroprotectant. Curr Opin Pharmacol. 2008;8:20–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bonding SH, Henty K, Dingley AJ, Brittain T. The binding of cytochrome c to neuroglobin: a docking and surface plasmon resonance study. Int J Biol Macromol. 2008;43:295–299 [DOI] [PubMed] [Google Scholar]
- 42. Chen XQ, Qin LY, Zhang CG, et al. Presence of neuroglobin in cultured astrocytes. Glia. 2005;50:182–186 [DOI] [PubMed] [Google Scholar]
- 43. Yu Z, Fan X, Lo EH, Wang X. Neuroprotective roles and mechanisms of neuroglobin. Neurol Res. 2009;31:122–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2′ -deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27:120–139 [DOI] [PubMed] [Google Scholar]
- 45. Ohtaki H, Takeda T, Dohi K, et al. Increased mitochondrial DNA oxidative damage after transient middle cerebral artery occlusion in mice. Neurosci Res. 2007;58:349–355 [DOI] [PubMed] [Google Scholar]
- 46. Brittain T, Skommer J, Henty K, Birch N, Raychaudhuri S. A role for human neuroglobin in apoptosis. IUBMB Life. 2010;62:878–885 [DOI] [PubMed] [Google Scholar]
- 47. Raychaudhuri S, Skommer J, Henty K, Birch N, Brittain T. Neuroglobin protects nerve cells from apoptosis by inhibiting the intrinsic pathway of cell death. Apoptosis. 2010;15:401–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wakasugi K, Morishima I. Identification of residues in human neuroglobin crucial for Guanine nucleotide dissociation inhibitor activity. Biochemistry. 2005;44:2943–2948 [DOI] [PubMed] [Google Scholar]
- 49. Fago A, Mathews AJ, Brittain T. A role for neuroglobin: resetting the trigger level for apoptosis in neuronal and retinal cells. IUBMB Life. 2008;60:398–401 [DOI] [PubMed] [Google Scholar]
- 50. Li RC, Morris MW, Lee SK, Pouranfar F, Wang Y, Gozal D. Neuroglobin protects PC12 cells against oxidative stress. Brain Res. 2008;1190:159–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Antao ST, Duong TT, Aran R, Witting PK. Neuroglobin over-expression in cultured human neuronal cells protects against hydrogen peroxide insult via activating phosphoinositide-3 kinase and opening the mitochondrial KATP channel. Antioxid Redox Signal. 2010;13:769–781 [DOI] [PubMed] [Google Scholar]
- 52. Kitatsuji C, Kurogochi M, Nishimura S, Ishimori K, Wakasugi K. Molecular basis of guanine nucleotide dissociation inhibitor activity of human neuroglobin by chemical cross-linking and mass spectrometry. J Mol Biol. 2007;368:150–160 [DOI] [PubMed] [Google Scholar]
- 53. Kuriyama H, Nakagawa M, Tsuda M. Intracellular Ca(2+) changes induced by in vitro ischemia in rat retinal slices. Exp Eye Res. 2001;73:365–374 [DOI] [PubMed] [Google Scholar]