In a mouse model of denervated cornea, the relationship between corneal progenitor/stem cells and trigeminal innervation was investigated. This study could help understanding corneal diseases when nerve death/dysfunction is known to play a role (e.g. herpetic or diabetic keratopathy).
Abstract
Purpose.
Neurotrophic keratopathy (NK) is a corneal degeneration associated with corneal nerve dysfunction. It can cause corneal epithelial defects, stromal thinning, and perforation. However, it is not clear if and to which extent epithelial stem cells are affected in NK. The purpose of this study was to identify the relationship between corneolimbal epithelial progenitor/stem cells and sensory nerves using a denervated mouse model of NK.
Methods.
NK was induced in mice by electrocoagulation of the ophthalmic branch of the trigeminal nerve. The absence of corneal nerves was confirmed with β-III tubulin immunostaining and blink reflex test after 7 days. ATP-binding cassette subfamily G member 2 (ABCG2), p63, and hairy enhancer of split 1 (Hes1) were chosen as corneolimbal stem/progenitor cell markers and assessed in denervated mice versus controls by immunofluorescent microscopy and real-time PCR. In addition, corneolimbal stem/progenitor cells were detected as side population cells using flow cytometry, and colony-forming efficiency assay was performed to assess their function.
Results.
ABCG2, p63, and Hes1 immunostaining were significantly decreased in denervated eyes after 7 days. Similarly, the expression levels of ABCG2, p63, K15, Hes1, and N-cadherin transcripts were also significantly decreased in denervated eyes. Stem/progenitor cells measured as side population from NK mice were decreased by approximately 75% compared with normals. In addition, the authors found a significant (P = 0.038) reduction in colony-forming efficiency of stem/progenitor cells harvested from denervated eyes.
Conclusions.
Corneolimbal stem/progenitor cells are significantly reduced after depletion of sensory nerves. The data suggest a critical role of innervation in maintaining stem cells and/or the stem cell niche.
Corneolimbal epithelial stem/progenitor cells are a small subpopulation of oligopotent cells located primarily in the basal epithelial layer of the limbus. They produce undifferentiated progeny with limited proliferative potential that migrate centripetally from the periphery of the cornea to replace cells desquamating during normal life.1–7 Corneal limbal stem cells reside primarily in the palisades of Vogt in a niche which maintains their “stemness” by producing a unique anatomic and functional milieu.8,9 Although the exact anatomic location of the niche is thought to be the limbus in humans, it has recently been proposed that epithelial stem/progenitor cells of equal potency are distributed throughout the entire ocular surface in other mammals.10
Detection of corneal epithelial stem cells is the object of controversy between many groups, as there is still no universal consensus over which marker(s) should be used. Side population (SP) cell detection is a commonly used method to quantify these cells. Stem cells express the SP phenotype based on the ability to efflux the DNA-binding dye Hoechst 33342.11–13 SP cells have also been identified in the hematopoietic compartments of different species, and have been isolated from various other adult tissues.12,13 These findings suggest that the SP phenotype represents a common feature of adult tissue-specific stem cells, and the same method has been used to isolate stem cells from human, rabbit, rat, and mouse limbi.14–17
The cornea is the most densely innervated tissue in the body, being 400 times more sensitive than the skin.18 Corneal nerves, in addition to their well known sensory function, help maintain the integrity of the ocular surface by releasing epitheliotrophic substances that promote corneal surface health.19 The limbus, where stem cells reside, is densely innervated; however, the role of these nerves is poorly understood.20 It has been suggested that various nerve-secreted factors and neuropeptides such as nerve growth factor (NGF), substance P, acetylcholine, and brain-derived neurotrophic factor (BDNF) could influence corneal stem cells, and play a role in maintaining epithelial integrity, and promote epithelial proliferation.19 It has also been shown that human corneal stem cells express TrkA which is a high affinity receptor for NGF.21–23 Moreover, corneal limbal stem cells grown in the presence of both epidermal growth factor (EGF) and nerve-secreted factors show the highest rate of colony expansion in vitro compared with EGF alone.24 However, the influence of corneal denervation on epithelial stem/progenitor cells has not yet been studied in vivo.
The purpose of the present study was to elucidate the relationship between corneal stem/progenitor cells and trigeminal nerves in vivo using a mouse model of denervated cornea. Herein, we hypothesize that corneal stem/progenitor cell survival and/or function is dependent on intact corneal innervation. Our data demonstrate that sensory nerve deprivation of cornea affects stem cell homeostasis and lead to a significant decrease in both the frequency and the function of corneolimbal stem/progenitor cells.
Methods
Animals
Six- to 8-week-old male C57BL/6 mice (Taconic Farms, Germantown, NY) were used for this study. The research protocol was approved by the Schepens Eye Research Institute Animal Care and Use Committee, and it conformed to the standards of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Experimental Procedure
Neurotrophic keratopathy (NK) was induced in mice by electrocoagulation of the ophthalmic branch of the trigeminal nerve, as previously described by Ferrari et al.25 Briefly, animals were anesthetized with a ketamine (100 mg/mL)-xylazine (20 mg/mL)-acepromazine (15 mg/mL) mixture. The animal was then mounted in the stereotactic frame and a median incision was made on the skull. The bregma (the point of conjunction of coronal and sagittal suture) was identified and chosen as a point of reference. The skull was opened with a dental drill, and a conductive electrode was lowered at 3 different locations on the ophthalmic trigeminal nerve and a 2 mA current was passed.
After removal of the electrode, the skin of the skull was sutured. In addition, a tarsorrhaphy (lid closure) was performed to reduce the risk of infection, and to assure that the effect on stem/progenitor cells is primarily due to the direct denervation but not secondary to the dryness caused by abolishment of blink reflex and loss of tears. Tarsorrhaphy was kept on for the entire length of the study until Day 7. Finally antibiotic ointment was placed on the suture and buprenorphine (0.1 mg/kg body weight every 8 to 12 hours for 72 hours) and 1 mL of saline were injected subcutaneously. Corneal sensitivity using a cotton filament was recorded pre- and postoperatively comparing the blinking of the treated eye (left) with the control eye (right). The effectiveness of the procedure was confirmed by biomicroscopy of the cornea, testing blink reflex and immunostaining with β tubulin III (neural marker) at 7 days after the procedure. All the animals used for the experiments showed an abolished blink reflex. All animals were euthanized by carbon dioxide overdose followed by cervical dislocation. All the procedures were performed in the Schepens Eye Research Institute Animal Facility, following an approved protocol.
Isolation of Corneal Epithelial Cells
On postexperimental procedure Day 7, corneas from normal and denervated animals (n = 8 per group) were obtained by scissor dissection under an operating microscope. Corneal epithelial cells were freshly isolated from the normal and denervated eyes after EDTA (Sigma, St. Louis, MO)-mediated removal of the corneal stroma and incubated for 60 minutes at 37°C. The corneal epithelium was subjected to digestion with DNase I (Roche Diagnostics, Basel, Switzerland)/trypsin (Gibco, Grand Island, NY)-EDTA for 45 minutes at 37°C. After incubation, the harvested cell suspension was filtrated through nylon mesh and centrifuged for 10 minutes at 1250 rpm. The pellet was resuspended in 1.0 mL of Dulbecco's Modified Eagle's Medium (DMEM) with 10% fetal calf serum (FCS), and the number of cells was determined by hemocytometry.
Immunohistochemistry
Cryostat sections of 5 μm thickness were prepared from tissue embedded in compound (Tissue-Tek OCT; Sakura Finetek, Torrance, CA). After fixation with cold acetone for 10 minutes, the tissues were incubated with 10% goat serum containing 0.3% Triton X-100 (room temperature [RT], 1 hour) for ATP-binding cassette subfamily G member 2 (ABCG2), and with 2% bovine serum albumin and goat serum (RT, 30 minutes) for hairy enhancer of split 1 (Hes1).26 For ABCG2 staining, overnight incubation with a rat monoclonal antibody anti-BCRP/ABCG2 (20×) (Abcam, Cambridge, MA) was performed. For Hes1 staining, rabbit anti-Hes1 polyclonal antibody (1000×) was used (a gift from Tetsuo Sudo, Toray Industries, Japan). The sections were then incubated (RT, 1 hour) with the appropriate primary antibody and washed three times in phosphate-buffered saline (PBS) containing 0.15% Triton X-100 for 15 minutes. Antibody binding was detected by Alexa 488-conjugated secondary antibodies (Invitrogen-Molecular Probes, Grand Island, NY). The slides were washed (three times) in PBS containing 0.15% Triton X-100 for 15 minutes. Nuclear counterstaining was performed with 4, 6-diamidino-2-phenylindole (DAPI; Vectashield; Vector Laboratories, Burlingame, CA). For p63 staining, after fixation with a cold mixture of methanol and acetone for 10 minutes, we incubated with MOM mouse IgG blocking reagent (Vectashield; Vector Laboratories) when we used mouse mAbs (50×) (Monoclonal Mouse Anti-Human p63; Dako; RT, 1 hour). The samples were incubated overnight with the appropriate primary antibody, and were treated with biotinylated second antibody and finally with Cy3-conjugated streptavidin (Jackson Immuno Research, West Grove, PA). The slides were washed three times in PBS for 15 minutes, coverslipped using antifading mounting medium containing DAPI and examined under a confocal microscope (Leica TCS 4D; Lasertechnik, Heidelberg, Germany). For β tubulin III staining, the cornea was harvested and fixed in paraformaldehyde 4%, stained with anti-β tubulin III antibody (200×) (Rabbit anti-β tubulin III polyclonal antibody; Chemicon, Grand Island, NY) and with a secondary conjugated antibody (200×) (Donkey anti rabbit IgG FITC, Santa Cruz Biotech, Santa Cruz, CA). Corneal whole mounts were prepared using a mounting medium. Images were taken at magnification ×400. Control incubations were done with the appropriate normal mouse, rat, and rabbit IgG at the same concentration as the primary antibody.
Real-Time PCR
RNA was isolated (RNeasy Micro Kit; Qiagen, Valencia, CA) and reverse transcribed (Superscript III Kit; Invitrogen, Carlsbad, CA). Real-time PCR was performed using a PCR mix (TaqMan Universal PCR Mastermix; Invitrogen) and preformulated primers for ABCG2 (assay ID Mm00496364_m1), p63 (assay ID Mm00495788_m1), Hes1 (assay ID Mm00468601_m1), Keratin15 (assay ID Mm00492972_m1), N-cadherin (assay ID Mm00483213_m1), p75 (assay ID Mm01309635_m1), TrkA (assay ID Mm01219407_m1), NGF (assay ID Mm00443039_m1), and GAPDH (assay ID Mm99999915_gl) (all from Applied Biosystems, Austin, TX). Results were analyzed by the comparative threshold cycle method and normalized to GAPDH as an internal control.
Hoechst 33342 Exclusion Assay
Freshly isolated corneal epithelial cells were resuspended at a concentration of 1 × 106 cells/mL in DMEM containing 2% FCS and incubated with 5 μg/mL Hoechst 33342 (Sigma-Aldrich, St. Louis, MO) dye. To determine the effect of verapamil on the Hoechst 33342 efflux, the cells were preincubated with verapamil (50 μM; Sigma-Aldrich) before the addition of Hoechst 33342 dye. After the incubation for 60 minutes at 37°C, propidium iodide (2 μg/mL) was added to exclude dead cells from the analysis, and the cells were then analyzed on a flow cytometer (LSR II; BD Biosciences, Franklin Lakes, NJ). Hoechst 33342 was excited at 350 nm with a UV laser and fluorescence emission was detected through 450-nm band-pass (Hoechst blue) and 660-nm long-pass (Hoechst red) filters.
Colony-Forming Efficiency Assay
3T3 fibroblasts in DMEM containing 10% FCS were treated with MMC for 2.5 hours at 37°C and then treated with trypsin-EDTA and plated at a density of 3 × 104cells per well into 12 mm culture plates. Each well was then seeded with 1 × 103 corneal cells in defined keratinocyte media with 5% FCS. The cell cultures were incubated at 37°C under 5% CO2 and 95% humidity. The cultures were incubated for 10 days. After 10 days, cultured cells were then stained with rhodamine B for 30 minutes. Colony-forming efficiency (CFE) was calculated as the percentage of colonies per number of inoculated cells.
Statistical Analysis
Student's t-test was used for comparison of mean between the groups. Data are presented as mean ± SEM and considered significant at P < 0.05.
Results
Establishment of a Denervated Model Using Trigeminal Stereotactic Electrolysis (TSE)
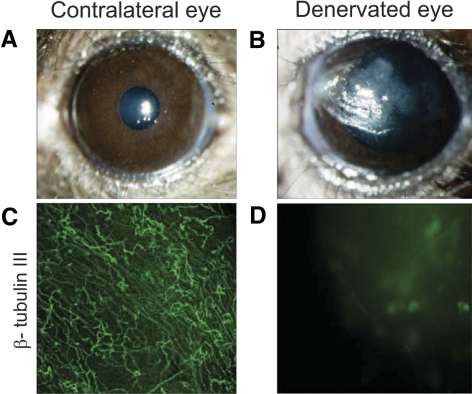
We induced experimental NK in mice by means of TSE. All the animals used in the experiments showed complete absence of blink reflex after 7 days. The TSE-treated animals developed epithelial defects (Figs. 1A and 1B) similar to patients affected by NK. The denervation was confirmed with β tubulin III immunostaining which revealed massive disruption of the subbasal nerve plexus after TSE (Figs. 1C, 1D). In addition, we compared the tear volume between denervated and normal contralateral eyes using a modified phenol red thread test. At Day 7, denervated eyes (0.34 ± 0.1 mm) showed significantly reduced phenol thread wetting compared with the contralateral eyes (2.1 ± 0.09 mm) (data not shown). Therefore, in the present study to prevent the epithelial damage due to the dry eye like conditions, a tarsorrhaphy was performed immediately after denervation and kept all the time until Day 7.
Figure 1.
NK induced in a mouse model by means of TSE. Representative biomicroscopy of a normal cornea (A), and of a denervated cornea (B) after 7 days. (B) Beta III tubulin immunostaining of normal and denervated corneas. (C) Normal cornea, note regular subbasal and epithelial nerve plexus. (D) Denervated cornea, note massive disruption of the subbasal nerve plexus.
Immunostaining and mRNA Expression of Stem Cell Markers
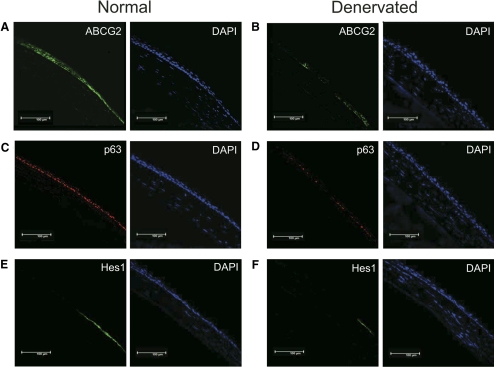
Expression of ABCG2, p63, and Hes1 were observed in the normal corneal limbal cells (Figs. 2A, 2C, 2E). All these stem/progenitor cell markers were decreased in the denervated eyes after 7 days (Figs. 2B, 2D, 2F). To further compare and quantify the levels of expression of these markers, we performed real-time PCR.
Figure 2.
Decreased expression of stem cell markers after denervation. Representative immunostained micrographs of cross section of normal (A, C, E) and denervated (B, D, F) corneas showing expression of stem cell markers. ABCG2 (B), p63 (D), and Hes1 (F) expression were decreased in denervated eyes after 7days compared with those in normal eyes (A, C, E).
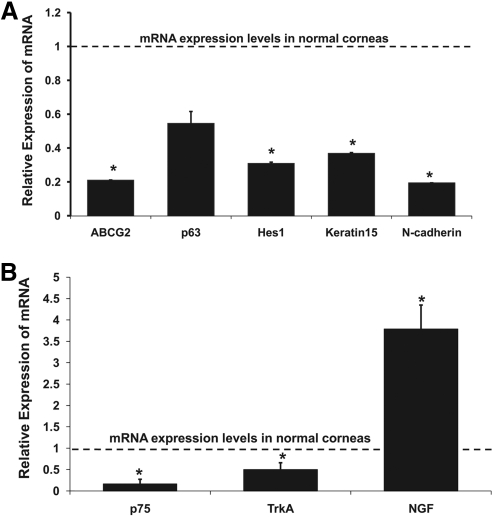
We analyzed the expression levels of ABCG2, Hes1, p63, Keratin15, and N-cadherin in the normal and denervated corneas by real-time PCR (Fig. 3A). Denervated corneas showed decreased mRNA expression levels of ABCG2 (5-fold reduction; P = 0.001), p63 (2-fold reduction; P = 0.059), Hes1 (3-fold reduction; P = 0.006), Keratin15 (3-fold reduction; P = 0.003), and N-cadherin (5-fold reduction; P = 0.0001) compared with those in normal corneas. Moreover, we examined the expression levels of NGF, the low-affinity NGF receptors (p75) and the high-affinity NGF receptors (TrkA) in the normal and denervated corneas (Fig. 3B). Denervated corneas showed decreased mRNA expression of p75 (5-fold reduction; P = 0.008) and TrkA (2-fold reduction; P = 0.032), and increased mRNA expression of NGF (3-fold increase; P = 0.005) compared with those in normal corneas.
Figure 3.
Diminished mRNA expression levels of stem cell markers after denervation. (A) ABCG2, Hes1, Keratin15, and N-cadherin were significantly decreased in denervated corneas. An approximately twofold reduction of p63 was also observed, although this was not statistically significant. (B) Denervated corneas showed decreased expression of p75 and TrkA, and increased expression of NGF compared with normal corneas. The dotted line represents expression of mRNA in normal corneas. Bars represent mean ± SEM. *P < 0.01.
Quantification of Stem Cells by Means of Side Population Cells
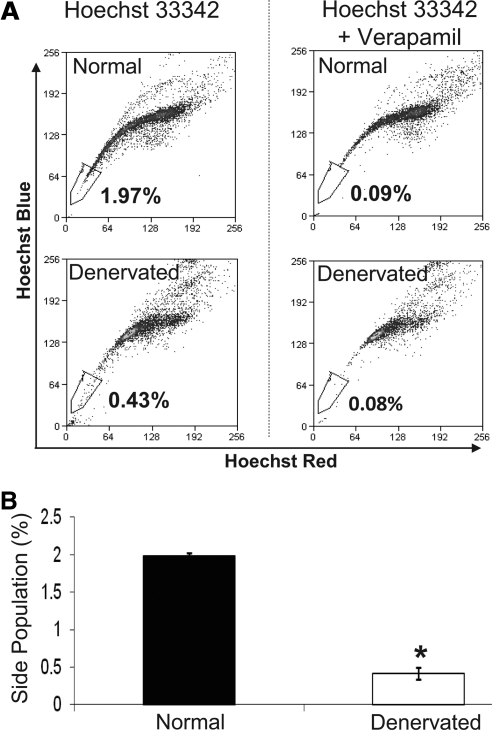
A distinct population of cells with a characteristic tail of low Hoechst 33342 blue-red fluorescence was gated using normal fresh mouse cornea epithelial cells. Side population cells could be detected by verapamil-sensitive disappearance of the tail. We compared the quantification of corneal epithelial stem cells in normal versus denervated corneas (Fig. 4A). We repeated the experiment three times (8 eyes per experiment), and found that this population comprised only 0.42 ± 0.08% of cells in denervated corneas, compared with 1.99 ± 0.03% of cells in the normal eyes (Fig. 4B). As shown in Figure 4B, an approximately 75% reduction of SP cells was observed 7 days after denervation compared with the healthy eyes (P < 0.001).
Figure 4.
Reduced SP cells in denervated eyes. (A) Representative flow cytometric dot plots showing analyses of the frequencies of corneal stem cells by means of SP cells. Note the disappearance of the low Hoechst 33342 blue-red fluorescence population, which represents stem cells, after verapamil addition. (B) Mean SP cells in normal and denervated corneas from three different experiments. SP cell frequencies were 1.99 ± 0.03% and 0.42 ± 0.08% of total epithelial cells in normal and denervated corneas, respectively. Bars represent mean ± SEM. *P < 0.001.
Analysis of Stem Cell Colony- Forming Efficiency (CFE)
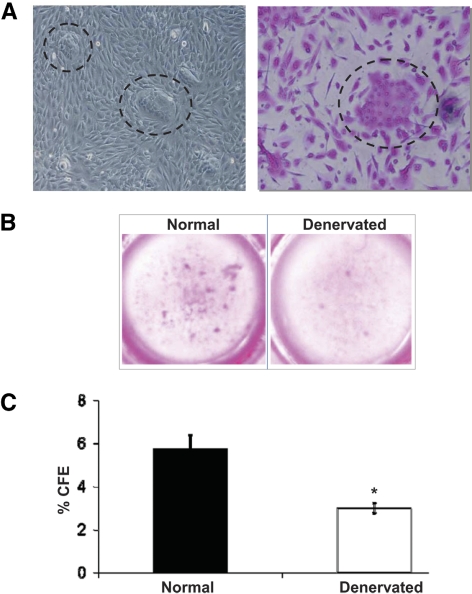
We investigated the proliferative potential of corneal epithelial stem cells using the CFE assay (Figs. 5A, 5B). After 10 days of culture, the colony-forming efficiency (number of colonies/plated cells using a 3T3 feeder layer) of epithelial cells from denervated corneas (3.0 ± 0.4%) was significantly reduced (approximately 50%) compared with CFE from normal corneas (5.73 ± 1.10%; P < 0.05).
Figure 5.
Decreased stem cell function measured by CFE assay after denervation. Representative micrographs of CFE colonies (A) as seen under phase contrast microcopy, and after rhodamine staining at magnification ×200 (dotted circles), and (B) in culture wells from normal and denervated corneal epithelial cells. (C) Quantification of CFE. CFE of normal corneal epithelial cells were 5.73 ± 1.10%, whereas CFE of denervated corneal epithelial cells were 3.0 ± 0.4%. Bars represent mean ± SEM. *P < 0.05.
Discussion
In this study, we used TSE to induce NK in mice. The effectiveness of this technique was confirmed by the development of characteristic neurotrophic epithelial defects, absence of blink reflex, and massive loss of corneal nerves 7 days after TSE. Using this model, we found a significant decrease in corneal epithelial stem/progenitor cell number and function. To the best of our knowledge, this is the first report showing that sensory deprivation affects stem/progenitor cells homeostasis in vivo.
Mouse corneal stem cells have been characterized as SP cells by Krulova et al.17 In the present study using the similar method of quantitative flow cytometry, we observed a significant (75%) decrease in SP cells in denervated eyes. To further characterize and quantify these cell populations, we studied the expression of stem/progenitor cell markers, and found 80% reduction in ABCG2 expression in denervated corneas. In addition to expression by stem cells, ABCG2 and p63 may be expressed by the transient amplifying and basal epithelial cells,26,27 and that could be the reason for their detection in both the peripheral cornea and limbal area. On the other hand, Hes1 is known to regulate corneal development and the homeostatic function of corneal epithelial stem/progenitor cells and is expressed predominantly in the corneal limbal zone.28 Taken together, all these putative stem/progenitor cell markers were decreased in the denervated eyes. Finally, we demonstrated a 50% decrease in stem/progenitor cell function as tested with the colony-forming efficiency assay. In summary, our data demonstrate that stem/progenitor cells are affected by denervation both quantitatively and qualitatively.
Recent studies in various animal models have shown that many nerve-secreted peptides such as substance P are important for epithelial regeneration and wound healing, and are significantly reduced in NK, which is associated with defective wound healing.29–32 Amniotic membrane is commonly used in ocular surgery and in the cases of NK as a biological bandage to improve corneal wound healing. It has been proposed that these therapeutic effects are, at least in part, due to neurotrophic factors contained in the amniotic membrane.33–35 Corneal stem/progenitor cells have an important role in wound healing. In healthy innervated rabbit, corneal stem cells have been shown to increase fivefold; 1 day after a scrape injury, and return to normal levels on Day two.36 In contrast, the denervated eyes used in our experiments exhibited evident epithelial defects 7 days after TSE (Fig. 1A) and no increase, but rather a decrease of stem cells (75% reduction). This, together with the diminished function reflected by the CFE assay, could provide an important insight into why NK eyes are so prone even to minor injuries and develop unhealing ulcers.
Interestingly, a marker for corneal stem/progenitor cells, ABCG2, is also expressed by neural progenitor cells, suggesting a potential close relationship between nerves and epithelial stem/progenitor cells.37 Although no literature exists regarding the impact of peripheral sensory innervation on epithelial stem/progenitor cells, there is anecdotal evidence of defective mobilization of bone marrow stem cells in conditions such as diabetes, where peripheral nerves are severely impaired.38 Similarly, neuroneoplasmic synapses have also been shown to be associated with tumor stem cell migration and proliferation.39 Regarding the eye, diabetic keratopathy may represent a paradigm for the dysfunctional interaction between corneal epithelial stem/progenitor cells and nerves. Diabetic patients suffer severe peripheral neuropathy which is associated with the development of persistent corneal epithelial defects.40–43 Interestingly, Saghizadeh et al. (IOVS 2010;51:ARVO E-Abstract 2954) have recently demonstrated decreased limbal stem cells in human diabetic corneas, which supports our findings that neuropathic states are associated with stem cell dysfunction. However, the high tear glucose concentration and the protean complications of diabetes make it difficult to fully dissociate the contribution of nerve depletion versus other pathologic aspects of the disease, to stem cells reduction. We suggest that our model, which selectively removes sensory innervation from the cornea, represents an excellent way to study these interactions.
In conclusion, our results suggest that corneal stem/progenitor cells are significantly reduced in number and function in our animal model of ocular denervation. We provide novel evidence for the critical role of innervation in maintaining corneal epithelial cells and/or the stem cell niche. It is provocative to speculate that the functional relationship between nerve and stem cells described in this article could apply to other epithelial/nonepithelial stem cells in the body. These findings contribute to our understanding of factors relevant for maintaining the stem cell niche, and suggest new ways of manipulating stem cell number and function, as more sophisticated neuroprotective strategies are developed.
Footnotes
Supported by NIH/NEI Grants K24 EY019098 and R01-EY-20889.
Disclosure: H. Ueno, None; G. Ferrari, None; T. Hattori, None; D.R. Saban, None; K.R. Katikireddy, None; S.K. Chauhan, None; R. Dana, None
References
- 1. Kinoshita S, Friend J, Thoft RA. Sex chromatin of donor corneal epithelium in rabbits. Invest Ophthalmol Vis Sci. 1981;21:434–441 [PubMed] [Google Scholar]
- 2. Buck RC. Measurement of centripetal migration of normal corneal epithelial cells in the mouse. Invest Ophthalmol Vis Sci. 1985;26:1296–1299 [PubMed] [Google Scholar]
- 3. Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103:49–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM. Existence of slow cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–209 [DOI] [PubMed] [Google Scholar]
- 5. Kruse FE. Stem cells and corneal epithelial regeneration. Eye. 1994;8:170–183 [DOI] [PubMed] [Google Scholar]
- 6. Beebe DC, Masters BR. Cell lineage and the differentiation of corneal epithelial cells. Invest Ophthalmol Vis Sci. 1996;37:1815–1825 [PubMed] [Google Scholar]
- 7. Nagasaki T, Zhao J. Centripetal migration of corneal epithelial cells in the normal adult mouse. Invest Ophthalmol Vis Sci. 2003;44:558–566 [DOI] [PubMed] [Google Scholar]
- 8. Stepp MA, Zieske JD. The corneal epithelial stem cell niche. Ocul Surf. 2005;3:15–26 [DOI] [PubMed] [Google Scholar]
- 9. Davies SB, Chui J, Madigan MC, et al. Stem cell activity in the developing human cornea. Stem Cells. 2009;27:2781–2792 [DOI] [PubMed] [Google Scholar]
- 10. Majo F, Rochat A, Nicolas M, Jaoudé GA, Barrandon Y. Oligopotent stem cells are distributed throughout the mammalian ocular surface. Nature. 2008. 13;456:250–254 [DOI] [PubMed] [Google Scholar]
- 11. Budak MT, Alpdogan OS, Zhou M, et al. Ocular surface epithelia contain ABCG2-dependent side population cells exhibiting features associated with stem cells. J Cell Sci. 2005;118:1715–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goodell MA, Rosenzweig M, Kim H, et al. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med. 1997;3:1337–1345 [DOI] [PubMed] [Google Scholar]
- 13. Storms RW, Goodell MA, Fisher A, Mulligan RC, Smith C. Hoechst dye efflux reveals a novel CD7(+)CD34(-) lymphoid progenitor in human umbilical cord blood. Blood. 2000;96:2125–2133 [PubMed] [Google Scholar]
- 14. Watanabe K, Nishida K, Yamato M, et al. Human limbal epithelium contains side population cells expressing the ATP-binding cassette transporter ABCG2. FEBS Lett. 2004;565:6–10 [DOI] [PubMed] [Google Scholar]
- 15. Umemoto T, Yamato M, Nishida K, et al. Rat limbal epithelial side population cells exhibit a distinct expression of stem cell markers that are lacking in side population cells from the central cornea. FEBS Lett. 2005;579:6569–6574 [DOI] [PubMed] [Google Scholar]
- 16. Umemoto T, Yamato M, Nishida K, et al. Limbal epithelial side-population cells have stem cell-like properties, including quiescent state. Stem Cells. 2006;24:86–94 [DOI] [PubMed] [Google Scholar]
- 17. Krulova M, Pokorna K, Lencova A, et al. A rapid separation of two distinct populations of mouse corneal epithelial cells with limbal stem cell characteristics by centrifugation on percoll gradient. Invest Ophthalmol Vis Sci. 2008;49:3903–3908 [DOI] [PubMed] [Google Scholar]
- 18. Nishida T. Neurotrophic mediators and corneal wound healing. Ocul Surf. 2005;3:194–202 [DOI] [PubMed] [Google Scholar]
- 19. Goins KM. New insights into the diagnosis and treatment of neurotrophic keratopathy. Ocul Surf. 2005;3:96–110 [DOI] [PubMed] [Google Scholar]
- 20. Li W, Hayashida Y, Chen YT, Tseng SC. Niche regulation of corneal epithelial stem cells at the limbus. Cell Res. 2007;17:26–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Touhami A, Grueterich M, Tseng SC. The role of NGF signaling in human limbal epithelium expanded by amniotic membrane culture. Invest Ophthalmol Vis Sci. 2002;43:987–994 [PubMed] [Google Scholar]
- 22. Qi H, Chuang EY, Yoon KC, et al. Patterned expression of neurotrophic factors and receptors in human limbal and corneal regions. Mol Vis. 2007;13:1934–1941 [PMC free article] [PubMed] [Google Scholar]
- 23. Qi H, Li DQ, Shine HD, et al. Nerve growth factor and its receptor TrkA serve as potential markers for human corneal epithelial progenitor cells. Exp Eye Res. 2008;86:34–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meyer-Blazejewska EA, Kruse FE, Bitterer K, et al. Preservation of the limbal stem cell phenotype by appropriate culture techniques. Invest Ophthalmol Vis Sci. 2010;51:765–774 [DOI] [PubMed] [Google Scholar]
- 25. Ferrari G, Chauhan SK, Ueno H, et al. A novel mouse model for neurotrophic keratopathy: trigeminal nerve stereotactic electrolysis through the brain. Invest Ophthalmol Vis Sci. 2011;52:2532–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chang CY, Green CR, McGhee CN, et al. Acute wound healing in the human central corneal epithelium appears to be independent of limbal stem cell influence. Invest Ophthalmol Vis Sci. 2008;49:5279–5286 [DOI] [PubMed] [Google Scholar]
- 27. Kawasaki S, Tanioka H, Yamasaki K, et al. Expression and tissue distribution of p63 isoforms in human ocular surface epithelia. Exp Eye Res. 2006;82:293–299 [DOI] [PubMed] [Google Scholar]
- 28. Nakamura T, Ohtsuka T, Sekiyama E, et al. Hes1 regulates corneal development and the function of corneal epithelial stem/progenitor cells. Stem Cells. 2008;26:1265–1274 [DOI] [PubMed] [Google Scholar]
- 29. Morishige N, Komatsubara T, Chikama T, Nishida T. Direct observation of corneal nerve fibres in neurotrophic keratopathy by confocal biomicroscopy. Lancet. 1999;354:1613–1614 [DOI] [PubMed] [Google Scholar]
- 30. Chikama T, Fukuda K, Morishige N, Nishida T. Treatment of neurotrophic keratopathy with substance-P-derived peptide (FGLM) and insulin-like growth factor I. Lancet. 1998;351:1783–1784 [DOI] [PubMed] [Google Scholar]
- 31. Nishida T, Chikama T, Morishige N, et al. Persistent epithelial defects due to neurotrophic keratopathy treated with a substance p-derived peptide and insulin-like growth factor 1. Jpn J Ophthalmol. 2007;51:442–447 [DOI] [PubMed] [Google Scholar]
- 32. Araki-Sasaki K, Aizawa S, Hiramoto M, et al. Substance P-induced cadherin expression and its signal transduction in a cloned human corneal epithelial cell line. J Cell Physiol. 2000;182:189–195 [DOI] [PubMed] [Google Scholar]
- 33. Solomon A, Meller D, Prabhasawat P, et al. Amniotic membrane grafts for nontraumatic corneal perforations, descemetoceles, and deep ulcers. Ophthalmology. 2002;109:694–703 [DOI] [PubMed] [Google Scholar]
- 34. Chen HJ, Pires RT, Tseng SC. Amniotic membrane transplantation for severe neurotrophic corneal ulcers. Br J Ophthalmol. 2000;84:826–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Coassin M, Lambiase A, Micera A, et al. Nerve growth factor modulates in vitro the expression and release of TGF-beta1 by amniotic membrane. Graefes Arch Clin Exp Ophthalmol. 2006;244:485–491 [DOI] [PubMed] [Google Scholar]
- 36. Park KS, Lim CH, Min BM, et al. The side population cells in the rabbit limbus sensitively increased in response to the central cornea wounding. Invest Ophthalmol Vis Sci. 2006;47:892–900 [DOI] [PubMed] [Google Scholar]
- 37. Yoshida S, Shimmura S, Nagoshi N, et al. Isolation of multipotent neural crest-derived stem cells from the adult mouse cornea. Stem Cells. 2006;24:2714–2722 [DOI] [PubMed] [Google Scholar]
- 38. Kang L, Chen Q, Wang L, et al. Decreased mobilization of endothelial progenitor cells contributes to impaired neovascularization in diabetes. Clin Exp Pharmacol Physiol. 2009;36:47–56 [DOI] [PubMed] [Google Scholar]
- 39. Zänker KS. The neuro-neoplastic synapse: does it exist? Prog Exp Tumor Res. 2007;39:154–161 [DOI] [PubMed] [Google Scholar]
- 40. De Cillà S, Ranno S, Carini E, et al. Corneal subbasal nerves changes in patients with diabetic retinopathy: an in vivo confocal study. Invest Ophthalmol Vis Sci. 2009;50:5155–5158 [DOI] [PubMed] [Google Scholar]
- 41. Rosenberg ME, Tervo TM, Immonen IJ, et al. Corneal structure and sensitivity in type 1 diabetes mellitus. Invest Ophthalmol Vis Sci. 2000;41:2915–2921 [PubMed] [Google Scholar]
- 42. Hyndiuk RA, Kazarian EL, Schultz RO, Seideman S. Neurotrophic corneal ulcers in diabetes mellitus. Arch Ophthalmol. 1977;95:2193–2196 [DOI] [PubMed] [Google Scholar]
- 43. Lockwood A, Hope-Ross M, Chell P. Neurotrophic keratopathy and diabetes mellitus. Eye. 2006;20:837–839 [DOI] [PubMed] [Google Scholar]