EGF-induced CTCF activity affects human corneal epithelial cell migration. As an epigenetic factor, CTCF may acts as a genetic switch to mediate EGF-induced regulation of Pax6 and other gene expression in corneal epithelial wound healing.
Abstract
Purpose.
EGF-induced activation of the epigenetic CCCTC binding factor (CTCF) plays an important role in corneal epithelial cell proliferation by suppressing the Pax6 gene. The present study focused on further understanding the role of CTCF in mediating EGF-induced migration of immortalized human corneal epithelial cells.
Methods.
CTCF activities in human corneal epithelial cells immortalized by telomerase (HTCE cells) and SV-40 (HCE cells) transformation were suppressed and enhanced by CTCF mRNA knockdown and by overexpressing CTCF cDNA, respectively. EGF-induced cell migration was evaluated by linear scratch wound healing, a cell migration assay, and live cell motility GFP-tracking with a fluorescence microscope. Immunochemical analysis was performed for detecting focal adhesion changes in EGF-induced and CTCF activity-altered cells.
Results.
EGF-induced wound closure and cell migration rates of human corneal epithelial cells were significantly suppressed and enhanced by CTCF mRNA knockdown and by overexpression of CTCF, respectively. CTCF mRNA knockdown also markedly suppressed cell motility, determined by using a live-cell–tracking system in GFP-tag–expressed HTCE cells. Finally, alterations of EGF-stimulated focal adhesion were observed in CTCF knockdown HTCE cells by immunostaining of F-actin and vinculin in cytoskeleton reorganization.
Conclusions.
CTCF, an epigenetic regulator and transcription factor, involves EGF-induced increases in cell motility and migration. CTCF plays an essential role in growth factor–regulated human corneal epithelial cell wound healing.
Corneal epithelial self-renewal and wound healing play vital roles in protection of the ocular structures behind the corneal layer. Both the self-renewal and wound-healing processes require stimulation of growth factors to activate cell signaling pathways and transcription factors that switch the stimulatory signals to genetic responses.1–8 It is known that epidermal growth factor (EGF) induces the activation of important signaling pathways, such as the phosphatidylinositol 3-kinase (PI3K/AKT) and mitogen-activated protein kinase (MAPK/Erk) cascades, to regulate cell cycle progression and promote cell migration and proliferation.9–12 Earlier studies have shown that the mitogenic effect of EGF on proliferation of corneal epithelial cells requires suppression of eye-specific Pax6 expression.13 The effect of EGF on suppressing Pax6 expression works through activation of an epigenetic CCCTC binding factor (CTCF).14 However, the effect of increased and decreased activities of CTCF on EGF-induced corneal epithelial cell migration and wound healing is still largely unknown.
CTCF is a transcription factor and zinc finger protein that plays an important role in epigenetic regulation of gene expression. CTCF controls DNA imprinting, and X chromosome inactivation during development and also functions as a methylation-sensitive insulator, a transcription activator, and a repressor.15–17 Recent studies from our laboratory and others demonstrate that exposure of mammalian cells to growth factors causes activation of the transcription factors CTCF, NF-κB, and other immediate early genes.11,18–23 In corneal epithelial cells, CTCF is a downstream target protein of the growth factor–induced pathways, and its expression levels are regulated by EGF, insulin, and stresses through activation of Erk, AKT, and NF-κB signaling cascades.11,12,24,25
Cell migration is involved in significant physiological and pathologic activities, such as the wound-healing process and cancer metastasis. In the corneal surface layer, corneal epithelial wound healing requires proper activities of cell migration that is essential for successful re-epithelialization.26 Thus, an accelerated cell migration is favored for wound healing and tissue repair in the cornea. Recent studies suggested that CTCF is involved in altering cell migration in cancer cell proliferation, tumor suppression, and apoptosis.27–29 However, the results obtained are contradictory and the role of CTCF in the corneal epithelia remains unclear. EGF is one of the important growth factors that facilitates corneal epithelial wound repair by promoting corneal epithelial cell migration and proliferation in both in vivo and in vitro model systems.13,26,30–32 The question that remains is whether CTCF is one of the key factors that switch EGF-induced activation of upstream signals to genetic responses that subsequently change corneal epithelial cell stages and cause an acceleration of migration and wound healing. Previously, we found that EGF activates CTCF to increase cell proliferation in transformed human corneal epithelial (HCE) cell lines.13,14,24 In the present study, we report that CTCF is essential for the EGF-induced alteration of focal adhesion and the increase in cell motility and migration that promote wound healing.
Materials and Methods
Culture of Corneal Epithelial Cells
Human telomerase-immortalized corneal epithelial (HTCE) cells were cultured in a serum-free keratinocyte medium (SFKM) containing 120 μM calcium and supplemented with 0.4% bovine pituitary extract and 0.2 ng/mL EGF (Invitrogen, Carlsbad, CA). For EGF-stimulation experiments, HTCE cells were grown in growth factor–deprived SFKM for 24 hours before EGF stimulation. An SV-40 T-antigen-transformed HCE cell line was grown in DMEM/F-12 (1:1) culture medium containing 10% FBS and 5 μg/mL insulin in an incubator supplied with 95% air and 5% CO2 at 37°C.33 The medium was replaced every 2 days, and the cells were subcultured by treatment with 0.05% trypsin-EDTA.
Construction of CTCF shRNA and Lentiviral Infections
For overexpression of CTCF, full-length cDNA encoding human CTCF was cloned into pcDNA4-to-A vector (Invitrogen) and termed pcDNA4-CTCF. Both the pcDNA4-CTCF construct and pcDNA4-to-A vector (served as control) were transfected into HCE cells by lipofectamine for wound-healing assays. Lentiviral particles, containing shRNA of CTCF tagged with a variant of green fluorescent protein (Turbo-GFP; Sigma-Aldrich, St. Louis, MO), were packaged in HEK293T cells. The viral concentrations in the culture medium were titrated by PCR after co-transfecting the cells with pCMV-VSV-G, psPAX2, and pGIPZ-shRNA-CTCF fused to the GFP (Turbo-GFP; Sigma-Aldrich) for 72 hours (Open Biosystems, Huntsville, AL). The culture medium containing the lentivirus secreted from HEK293T cell was added to HTCE or HCE cells, and infected clones stably expressing the shRNAs were selected by selective culture in the presence of puromycin (2 μg/mL). HTCE and HCE cells infected with a pGIPZ-shRNA-control vector packed in lentivirus were served as the controls. In addition, expression of GFP from the pGIPZ-TurboGFP vector allowed measurement of the efficiency of the viral infection and the distinguishing of green from nongreen cells. The green cells integrating the vectors with shRNA were visualized by a fluorescence microscope (Nikon, Tokyo, Japan).
Migration and Wound-Healing Assays
Cell Migration Assay.
The cell migration assay was performed following the instructions of the manufacturer (Transwell; Corning Inc., Corning NY). The migration chamber culture insert contains a polyethylene terephthalate membrane 6.5 mm in diameter with an 8-mm pore size. HTCE cells (5 × 104) were seeded in the culture insert (upper chamber) with plain medium and incubated for 24 hours. EGF (20 ng/mL) or the sham was added to the culture insert and the cells were incubated for 48 hours. Migrated cells that grew on the culture well (bottom chamber) were counted and photographed with an inverted fluorescence microscope (Nikon). The cells were fixed in 4% paraformaldehyde, stained with 0.3% crystal violet (CV), and photographed. The dye in the cells was then dissolved in 10% acetic acid, and the absorbance of the dissolved dye was measured at 600 nm.
Scratch-Induced Directional Wound-Healing Assay.
Cells were seeded at 3 × 105 cells/well in 12-well plates and grown to 100% confluence. A cross-stripe scratch wound was made on the cell surface with a yellow micropipette tip. The wound area was measured by calculating the average values at multiple points (8–10 points per wound along the edges) by using commercial software (NIS-Element; Nikon, Tokyo, Japan) and was photographed with an inverted microscope (Eclipse Ti; Nikon) during the healing period. The microscope was able to record exactly the same area at each time point, by memorizing the x–y directions through a computer-controlled and motorized head stage. The width of the wounded area was measured, and the rate of wound closure was calculated in micrometers per hour.
Live Cell Imaging and Cell Motility Analysis
The Motility of HTCE cells expressing TurboGFP-tagged CTCF shRNAs and nonsilencing shRNA controls was measured by utilization of the inverted microscope (Eclipse Ti; Nikon) with the following functions: (1) time-lapse videos of the phase-contrast/fluorescent live images; (2) built-in TIRF (total internal reflection fluorescence) and FRET; (3) perfect focus system (PFS); and (4) a digital CCD camera in a time interval of 2 minutes for each photo. The system was equipped with a heated chamber at 37°C and flushed with mixed 5% CO2 that kept cells in a normal culture condition. Live cells were recorded for a period of 0.5 to 3 hours. Cell motility was analyzed by tracking cell movements and distances (micrometers/hour) with the software (Tis NIS-Elements; Nikon).
Immunocytochemistry and Western Blot Analysis
Immunocytochemical analyses were performed according to a published protocol. Briefly, the cells were fixed with 4% paraformaldehyde and perforated with 0.3% Triton-X100 in PBS (PBS-T). After the reaction was blocked with 2% BSA and 5% normal serum in PBS-T, the cells were incubated with primary antibody against vinculin (Invitrogen) in 1% BSA- 0.1% PBS-T for 16 hours at 4°C. Cy3-conjugated secondary antibody was applied in 1% BSA-0.1% PBS-T for 1 hour at room temperature (RT). F-actin stress fibers were detected by using FITC-conjugated phalloidin (Invitrogen) according to the company's instruction. Stained cells were mounted with antifade medium (Vectashield; Vector Laboratories Inc., Burlingame, CA) and photographed with the inverted microscope (Eclipse Ti; Nikon) with 60× oil TIRF lens. Western blot analysis were performed by lysing corneal epithelial cells (2 × 105) in sodium dodecyl sulfate polyacrylamide (SDS) sample buffer, which contained 62.5 mM Tris-HCl (pH 6.8), 2% (wt/vol) SDS, 10% glycerol, 50 mM DTT, 0.01% wt/vol bromophenol blue. Proteins in the lysates were denatured by boiling for 5 minutes, and size fractionated in 8% to 10% polyacrylamide gels. The proteins in the gels were electrotransferred to PVDF membranes (Millipore, Billerica, MA) by using a semidry gel transferring apparatus (Bio-Rad, Hercules, CA). The PVDF membranes were exposed to the blocking buffer containing 5% nonfat milk in TBS and 0.1% Tween 20 (TBS-T) for 1 hour at 22°C and incubated with respective primary antibodies at 4°C overnight. Horseradish peroxidase (HRP)–conjugated secondary antibody was applied in TBS-T buffer for 1 hour at RT. Western blots were developed by chemiluminescence (ECL Plus System; Santa Cruz Biotechnology, Santa Cruz, CA) and visualized by exposure to x-ray film.
Results
Effect of Altered CTCF Activity on Wound Healing in HCE Cells
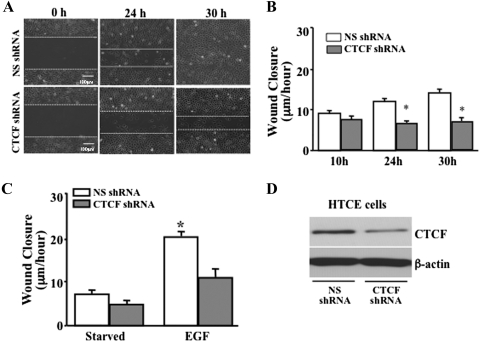
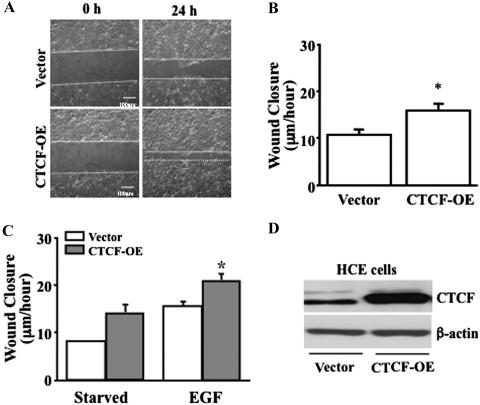
It has been shown that CTCF is upregulated by activation of the Erk signaling pathway to play a role in EGF-induced proliferation of HCE cells.13,24 However, how CTCF promotes cell migration in corneal epithelial wound healing is still unclear. We hypothesize that CTCF plays an important role in promoting EGF-induced corneal epithelial wound healing by increasing migration of the cells. First, the effects of CTCF on the wound-healing rate of HTCE cells were examined by knocking down CTCF mRNA under different culture conditions in the presence and absence of growth factor supplements and with or without EGF stimulation. In the control culture condition (with growth factor supplements), the effect of CTCF knockdown on the wound-healing rate was observed in the HTCE cells after a 30-hour time course (Fig. 1A). There were significant delays of wound-healing rates by lentivirus-mediated infection of CTCF shRNA at the 24- and 30-hour time points (Fig. 1B). The effect on the EGF-induced wound-healing rate of CTCF knockdown was markedly decreased in growth factor–deprived HTCE cells at 24 hours (Fig. 1C). The EGF-induced wound-healing rates were calculated at 20 hours in HTCE cells. There was a significant difference between the control cells at 20.5 ± 1.0 μm/h and CTCF knockdown cells at 10.5 ± 2.7 μm/h (mean ± SD; P < 0.0001). CTCF expression levels were verified by Western blot analysis in HTCE cells infected with nonsilencing (NS) and CTCF-specific shRNAs (Fig. 1D). Second, the effect of CTCF on the wound-healing rate of HCE cells was examined by overexpression of CTCF under various culture conditions and with or without EGF stimulation. In HCE cells overexpressing CTCF, the rate of wound healing was tested in normal culture conditions at 24 hours (Fig. 2A). Overexpression of CTCF in HCE cells significantly increased the wound-healing rate compared with the vector-only–transfected cells (Fig. 2B). In addition, the effect of EGF on the wound-healing rate was enhanced by overexpression of CTCF compared with the vector-transfected controls in the serum-deprived cells (Fig. 2C). EGF-induced wound-healing rates of HCE cells at 24 hours were significantly different at 15.3 ± 1.0 μm/h for the control and 20.7 ± 1.6 μm/h for CTCF knockdown cells (mean ± SD, P < 0.001). CTCF protein levels in transfected HCE cells were verified in both vector and CTCF cDNA-transfected cells (Fig. 2D). These results revealed a functional role that CTCF may play in HCE cells to mediate EGF-induced wound healing.
Figure 1.
Effect of suppressed CTCF expression on wound healing. (A) Effect of knocking down CTCF mRNA on HTCE cell wound closure. (B) Significant suppression of wound closure rate by CTCF shRNA. (C) Effect of suppressing CTCF on EGF-induced wound healing. (D) Expression level of CTCF protein in lentiviral shRNA-infected HTCE cells. HTCE cells were infected with both NS (as a control) and CTCF-specific shRNAs, by using a lentiviral delivery system, and were selected by the puromycin culture method. For the EGF-induced experiments, cells were cultured in a growth factor–deprived condition (starvation) for at least 24 hours. *Significant difference between NS shRNA– and CTCF shRNA–infected cells (P < 0.05, n = 6).
Figure 2.
Effect on wound healing of CTCF overexpression. (A) Effect of overexpressing CTCF on HCE cell wound closures. (B) Significant enhancement of the wound closure rate by overexpression of CTCF. (C) Effect of overexpressed CTCF on EGF-induced wound healing. (D) Expression level of CTCF protein in CTCF cDNA-transfected HCE cells. HCE cells were transfected with both the vector plasmid as a control and the full-length cDNA encoding CTCF by lipofection. For EGF-induced experiments, cells were cultured in a serum-deprived condition (starvation) for at least 24 hours. *Significant difference between vector and CTCF cDNA–transfected cells (P < 0.05, n = 4).
CTCF Promoted Migration in HCE Cells
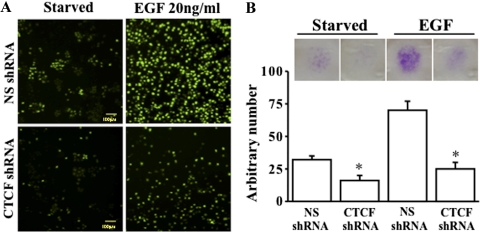
It has known that cell migration plays critical role in corneal epithelial wound healing. To study the effect of CTCF on cell migration, we performed a cell migration assay (Transwell; Corning Inc.) by using puromycin-selected, TurboGFP-tagged and CTCF knockdown HTCE cells. The migrated cells that passed through the filter from the top chamber into the bottom chamber were photographed by both an inverted fluorescence microscope for living cells and digital camera for crystal violet–stained cell after fixation (Fig. 3). The results showed that knockdown of CTCF with CTCF-specific shRNA reduced EGF-stimulated cell migration in growth factor–deprived HCE cells (Fig. 3A). The migration was also reduced in CTCF knockdown HTCE cells in the normal culture condition (data not shown). The number of migrated cells was counted and the absorption of crystal violet staining was measured, and there were significant differences in migration of cells infected with NS shRNA and CTCF shRNA in the presence and absence of EGF (Fig. 3B). These results strongly suggest that CTCF plays a role in promoting the migration of HCE cells.
Figure 3.
Effect of suppressed CTCF expression on migration. (A) Live cell imaging of EGF-induced cell migration measured in TurboGFP-tagged NS and CTCF shRNA-infected HTCE cells. (B) Effect of CTCF mRNA knockdown on EGF-induced migration. Cell migration assays were performed in puromycin-selected HTCE cells after the cells were infected with lentivirus-carried NS and CTCF-specific shRNA. Live cell imaging was captured by an inverted fluorescence microscope. In addition, crystal violet–stained cells in the lower chamber of the cell-migration filters were photographed by a digital camera. *Significant difference between NS shRNA– and CTCF shRNA–infected cells (P < 0.05, n = 4).
CTCF Accelerates Motility in HCE Cells
The ability of cells to migrate depends on cell motility. The effect of CTCF on motility of HCE cells was studied by real-time video-tracking of HTCE cells with an inverted fluorescence microscope (Eclipse Ti; Nikon) equipped with a TIRF lens and perfect focusing system (PFS). Two batches of HTCE cells were individually infected with lentiviral TurboGFP-tagged CTCF shRNA to knock down CTCF mRNA, with TurboGFP-tagged nonsilencing (NS) shRNA used as the control. The motility of CTCF knockdown green cells and NS control green cells was recorded to compare with uninfected cells under white light, UV light, and white+UV light. Random movements of cells in different populations were tracked, and the motility was measured as distance moved in micrometers per hour. Knockdown of CTCF mRNA significantly suppressed HTCE cell motility in the normal culture condition (Figs. 4A, 4B). It was also noted that there were some morphologic changes in CTCF knockdown green cells. These cells were longer than the control cells (nongreen cells in the same views). The effect of CTCF on EGF-stimulated cell motility was also measured in growth factor–deprived cells with and without infections with CTCF shRNA and NS shRNA. The EGF-induced acceleration of cell motility was significantly suppressed by knocking down CTCF mRNA in HTCE cells (Fig. 4C). In contrast, overexpression of CTCF in the CTCF cDNA-transfected HTCE cells resulted in an increased motility compared with that in the vector-transfected cells (Figs. 4D, 4E). These results indicate that CTCF plays an important role in promoting cell motility in EGF-induced HCE cells.
Figure 4.
Effect of altering CTCF expression on cell motility. (A) Measurements of cell motility by tracking control and CTCF-suppressed cells. Live HTCE cells infected with CTCF shRNA were represented in green when exposed to ultraviolet light (UV) and showed no color under white light (WL). Cell bodies were positioned at starting and ending points with cross symbols and their movements traced with red lines. (B) Effect of CTCF knockdown on distance of cell movement. (C) Effect of CTCF knockdown on he distance of EGF-induced cell movement. (D) Effect of overexpressing CTCF on cell movement distance, indicated by colored lines. (E) Enhancement of movement distance in CTCF-overexpressing cells. *Significant difference between control and CTCF-altered cells (P < 0.05, n = 38). Scale bar: (A) 25 μm; (D) 50 μm.
Effect of CTCF on Cytoskeleton Reorganization in HCE Cells
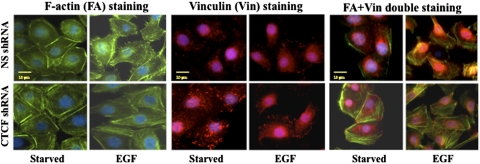
Cell migration is a highly orchestrated process involving rearrangement of F-actin and focal adhesion. To study the effect of CTCF on cell migration, cytoskeleton reorganization was detected in growth factor–deprived and EGF-stimulated HTCE cells by using immunostaining of F-actin and vinculin in both CTCF knockdown and control cells. Figure 5 demonstrates EGF-stimulated increases in cell membrane protrusions (filopodia) and ruffles in growth factor–deprived cells, whereas there were more stress-related F-actin fibers in CTCF knockdown cells. Vinculin, as a marker of focal adhesion, appeared to increase in clusters in CTCF mRNA knockdown cells compared with NS shRNA-infected cells, indicating that CTCF affects the stability of focal adhesion in HCE cells. In addition, increased focal adhesion and decreased membrane protrusion in CTCF mRNA knockdown cells were consistent with a reduced motility in those cells. Thus, these results further support the notion that CTCF requires EGF-induced cytoskeleton protein reorganization to promote motility and migration of HCE cells.
Figure 5.
Effect of suppressed CTCF expression on EGF-induced cytoskeleton reorganization. The effect of CTCF mRNA knockdown on EGF-induced cytoskeleton reorganization was determined in growth factor–deprived HTCE cells infected with NS- and CTCF-specific shRNAs by immunostaining with FA, vinculin, and FA+Vin antibodies. Immunostained cells were imaged by an inverted microscope with a 60× TIRF lens and digital CCD camera. Scale bar, 20 μm.
Discussion
Corneal epithelial wound healing is very important for maintaining the normal function of the cornea during corneal surface self-renewal and wound repair. EGF is one of the major stimulators to promote corneal epithelial wound healing by stimulating cell migration and proliferation.26 Cellular responses in corneal epithelial cells induced by EGF are complex responses at early times that are caused by activation of cell signaling pathways that are distinct from those activated in the nucleus at later times.2,13,24,30,34 In the present study, we explored EGF-induced cellular responses involving CTCF, which is known as an important epigenetic factor that affects corneal epithelial wound healing. EGF activated corneal epithelial wound healing in the HCE monolayer culture system. The effect of EGF on wound healing was dependent on activities of CTCF, since knockdown of CTCF mRNA and overexpression of CTCF cDNA resulted in suppression and enhancement of EGF's effects, respectively (Figs. 1, 2). These results reveal the role of CTCF in growth factor–induced wound healing.
Next, we focused on the effect of CTCF on EGF-induced corneal epithelial cell migration and motility. CTCF was involved in growth factor–induced increases in migration and motilities in corneal epithelial cells, which can have great effects on the wound-healing process. EGF-induced migration and motility were tested in HTCE cells with different approaches in the present study. The results demonstrate that cell migration was impaired significantly as a consequence of CTCF knockdown in all the three testing assays. Data were very consistent indicating that EGF-induced increases in cell migration and motility are dependent on CTCF activity. As has been suggested, CTCF may act as an epigenetic regulator or genetic switch in response to extracellular stimuli. Downstream from EGF-activated pathways, CTCF is activated by EGF-induced heterodimers of p50 and p65 subtypes in the NF-κB family and is suppressed by UV stress that activates p50 and Bcl-3 heterodimers involving of noncanonical NF-κB pathways.35,36
Regulation of CTCF is stimulus-dependent in HCE cells to regulate downstream gene activities affecting cell fate.24 CTCF regulates expressions of many important genes including c-Myc and Pax6. For an example, Pax6 is an important transcription factor in regulations of eye development, and it also determines corneal epithelial terminal differentiation and regulates corneal epithelial specific keratin (K)12 expression.13,37,38 The effect of EGF on corneal epithelial cell proliferation is through upregulation of CTCF, and it subsequently downregulates the Pax6 gene by binding to a repressor element between the EE enhancer and the P0 promoter of the Pax6 gene.13,39 In addition, CTCF's interaction with the Pax6 gene is dependent on methylation modification of the CpG sequences located in the binding sites upstream from the P0 promoter.40 There are reports indicating that Pax6 is expressed at a very low level in HCE cells.41,42 This finding suggests that the effect of CTCF on regulation of cell migration and motility not only involves the Pax6 gene, instead it may involve a complex of many genes in the further downstream events. Since there are multifunctional roles of CTCF in regulating gene expressions, further studies are needed to understand which of the CTCF-regulated genes may be involved in the growth factor-induced processes.
The present study is the first to demonstrate that CTCF activity plays a role in EGF-induced cell migration and motility. We observed the effect of knocking down CTCF mRNA on two essential processes, including increased migration and motility in response to EGF stimulation. Knockdown of CTCF significantly altered cytoskeleton reorganization of F-actin stress fiber and vinculin focal adherence (Fig. 5). The observed cytoskeleton reorganization is expected to occur in the process of changing both corneal epithelial cell migration and motility. As has been shown, F-actin remodeling in a variety of cellular processes, its impacts on cell migration have essential functions, such as cell movement, cell adhesion, and cytokinesis.43 This result further emphasizes that there must be a physiological role for CTCF to play in corneal epithelial wound healing. Our results provide a clear mechanism for understanding how CTCF involves EGF-induced increases in migration and mobility brings insights that will improve corneal epithelial wound healing. In addition, we believe it very likely that EGF-induced CTCF activity affecting cell migration has important physiological and pathologic implications in corneal disease.
Summary
The epigenetic regulator and transcription factor CTCF is found to involve EGF-induced increases in cell motility and migration. As a downstream component in the EGF-induced signaling pathway, CTCF is essential for the EGF-induced alteration of focal adhesion and increases in cell motility and migration that promote wound healing.
Footnotes
Supported by National Institutes of Health Grant R01-EY015282 (LL).
Disclosure: L. Wang, None; S.X. Deng, None; L. Lu, None
References
- 1. Zhang Y, Akhtar RA. Effect of epidermal growth factor on phosphatidylinositol 3-kinase activity in rabbit corneal epithelial cells. Exp Eye Res. 1996;63:265–275 [DOI] [PubMed] [Google Scholar]
- 2. Zhang Y, Akhtar RA. Epidermal growth factor stimulation of phosphatidylinositol 3-kinase during wound closure in rabbit corneal epithelial cells. Invest Ophthalmol Vis Sci. 1997;38:1139–1148 [PubMed] [Google Scholar]
- 3. Zhang Y, Akhtar RA. Epidermal growth factor stimulates phospholipase D independent of phospholipase C, protein kinase C or phosphatidylinositol-3 kinase activation in immortalized rabbit corneal epithelial cells. Curr Eye Res. 1998;17:294–300 [DOI] [PubMed] [Google Scholar]
- 4. Zhang Y, Liou GI, Gulati AK, Akhtar RA. Expression of phosphatidylinositol 3-kinase during EGF-stimulated wound repair in rabbit corneal epithelium. Invest Ophthalmol Vis Sci. 1999;40:2819–2826 [PubMed] [Google Scholar]
- 5. Islam M, Akhtar RA. Epidermal growth factor stimulates phospholipase cgamma1 in cultured rabbit corneal epithelial cells. Exp Eye Res. 2000;70:261–269 [DOI] [PubMed] [Google Scholar]
- 6. Islam M, Akhtar RA. Upregulation of phospholipase Cgamma1 activity during EGF-induced proliferation of corneal epithelial cells: effect of phosphoinositide-3 kinase. Invest Ophthalmol Vis Sci. 2001;42:1472–1478 [PubMed] [Google Scholar]
- 7. Kang SS, Li T, Xu D, Reinach PS, Lu L. Inhibitory effect of PGE2 on EGF-induced MAP kinase activity and rabbit corneal epithelial proliferation. Invest Ophthalmol Vis Sci. 2000;41:2164–2169 [PubMed] [Google Scholar]
- 8. Kang SS, Wang L, Kao WW, Reinach PS, Lu L. Control of SV-40 transformed RCE cell proliferation by growth-factor- induced cell cycle progression. Curr Eye Res. 2001;23:397–405 [DOI] [PubMed] [Google Scholar]
- 9. Madhani HD, Fink GR. The riddle of MAP kinase signaling specificity. Trends Genet. 1998;14:151–155 [DOI] [PubMed] [Google Scholar]
- 10. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70 [DOI] [PubMed] [Google Scholar]
- 11. Li T, Lu L. Functional role of CCCTC binding factor (CTCF) in stress-induced apoptosis. Exp Cell Res. 2007;313:3057–3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou H, Gao J, Lu ZY, Lu L, Dai W, Xu M. Role of c-Fos/JunD in protecting stress-induced cell death. Cell Prolif. 2007;40:431–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li T, Lu L. Epidermal growth factor-induced proliferation requires down-regulation of Pax6 in corneal epithelial cells. J Biol Chem. 2005;280:12988–91295 [DOI] [PubMed] [Google Scholar]
- 14. Li T, Lu Z, Lu L. Regulation of eye development by transcription control of CCCTC binding factor (CTCF). J Biol Chem. 2004;279:27575–27583 [DOI] [PubMed] [Google Scholar]
- 15. Baniahmad A, Steiner C, Kohne AC, Renkawitz R. Modular structure of a chicken lysozyme silencer: involvement of an unusual thyroid hormone receptor binding site. Cell. 1990;61:505–514 [DOI] [PubMed] [Google Scholar]
- 16. Bell KD, Campbell RJ, Bourne WM. Pathology of late endothelial failure: late endothelial failure of penetrating keratoplasty: study with light and electron microscopy. Cornea. 2000;19:40–46 [DOI] [PubMed] [Google Scholar]
- 17. Hark AT, Schoenherr CJ, Katz DJ, Ingram RS, Levorse JM, Tilghman SM. CTCF mediates methylation-sensitive enhancer-blocking activity at the H19/Igf2 locus. Nature. 2000;405:486–489 [DOI] [PubMed] [Google Scholar]
- 18. Herrlich P, Ponta H, Rahmsdorf HJ. DNA damage-induced gene expression: signal transduction and relation to growth factor signaling (review with 222 refs). Rev Physiol Biochem Pharmacol. 1992;119:187–223 [DOI] [PubMed] [Google Scholar]
- 19. Holbrook NJ, Liu Y, Fornace AJ., Jr Signaling events controlling the molecular response to genotoxic stress (review with 72 refs). EXS. 1996;77:273–288 [DOI] [PubMed] [Google Scholar]
- 20. Belandia B, Latasa MJ, Villa A, Pascual A. Thyroid hormone negatively regulates the transcriptional activity of the beta-amyloid precursor protein gene. J Biol Chem. 1998;273:30366–30371 [DOI] [PubMed] [Google Scholar]
- 21. Buscher M, Rahmsdorf HJ, Litfin M, Karin M, Herrlich P. Activation of the c-fos gene by UV and phorbol ester: different signal transduction pathways converge to the same enhancer element. Oncogene. 1988;3:301–311 [PubMed] [Google Scholar]
- 22. Devary Y, Rosette C, DiDonato JA, Karin M. NF-kappa B activation by ultraviolet light not dependent on a nuclear signal. Science. 1993;261:1442–1445 [DOI] [PubMed] [Google Scholar]
- 23. Li T, Dai W, Lu L. Ultraviolet-induced junD activation and apoptosis in myeloblastic leukemia ML-1 cells. J Biol Chem. 2002;277:32668–32676 [DOI] [PubMed] [Google Scholar]
- 24. Lu L, Wang L, Li T, Wang J. NF-kappaB subtypes regulate CCCTC binding factor affecting corneal epithelial cell fate. J Biol Chem. 2010;285:9373–9382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang Y, Lu L. Activation of oxidative stress-regulated Bcl-3 suppresses CTCF in corneal epithelial cells. PLoS ONE. 2011;6:e23984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lu L, Reinach PS, Kao WW. Corneal epithelial wound healing. Exp Biol Med (Maywood). 2001;226:653–664 [DOI] [PubMed] [Google Scholar]
- 27. Qi CF, Martensson A, Mattioli M, Dalla-Favera R, Lobanenkov VV, Morse HC., 3rd CTCF functions as a critical regulator of cell-cycle arrest and death after ligation of the B cell receptor on immature B cells. Proc Natl Acad Sci U S A 2003;100:633–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rasko JE, Klenova EM, Leon J, et al. Cell growth inhibition by the multifunctional multivalent zinc-finger factor CTCF. Cancer Res. 2001;61:6002–6007 [PubMed] [Google Scholar]
- 29. Docquier F, Farrar D, D'Arcy V, et al. Heightened expression of CTCF in breast cancer cells is associated with resistance to apoptosis. Cancer Res. 2005;65:5112–5122 [DOI] [PubMed] [Google Scholar]
- 30. Wang J, Lin A, Lu L. Effect of EGF-induced HDAC6 activation on corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 2010;51:2943–2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu KP, Ding Y, Ling J, Dong Z, Yu FS. Wound-induced HB-EGF ectodomain shedding and EGFR activation in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:813–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yin J, Yu FS. ERK1/2 mediate wounding- and G-protein-coupled receptor ligands-induced EGFR activation via regulating ADAM17 and HB-EGF shedding. Invest Ophthalmol Vis Sci. 2009;50:132–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang L, Dai W, Lu L. Hyperosmotic stress-induced corneal epithelial cell death through activation of polo-like kinase 3 and c-Jun. Invest Ophthalmol Vis Sci. 2011;52:3200–3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wilson SE, Lloyd SA, He YG. EGF, basic FGF, and TGF beta-1 messenger RNA production in rabbit corneal epithelial cells. Invest Ophthalmol Vis Sci. 1992;33:1987–1995 [PubMed] [Google Scholar]
- 35. Kang SM, Tran AC, Grilli M, Lenardo MJ. NF-kappa B subunit regulation in nontransformed CD4+ T lymphocytes. Science. 1992;256:1452–1456 [DOI] [PubMed] [Google Scholar]
- 36. Neumann M, Naumann M. Beyond IkappaBs: alternative regulation of NF-kappaB activity. FASEB J. 2007;21:2642–2654 [DOI] [PubMed] [Google Scholar]
- 37. Ouyang J, Shen YC, Yeh LK, et al. Pax6 overexpression suppresses cell proliferation and retards the cell cycle in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2006;47:2397–2407 [DOI] [PubMed] [Google Scholar]
- 38. Shiraishi A, Converse RL, Liu CY, Zhou F, Kao CW, Kao WW. Identification of the cornea-specific keratin 12 promoter by in vivo particle-mediated gene transfer. Invest Ophthalmol Vis Sci. 1998;39:2554–2561 [PubMed] [Google Scholar]
- 39. Wu D, Li T, Lu Z, Dai W, Xu M, Lu L. Effect of CTCF-binding motif on regulation of PAX6 transcription. Invest Ophthalmol Vis Sci. 2006;47:2422–2429 [DOI] [PubMed] [Google Scholar]
- 40. Gao J, Wang J, Wang Y, Dai W, Lu L. Regulation of Pax6 by CTCF during induction of mouse ES cell differentiation. PLoS ONE. 2011;6:e20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Greco D, Vellonen KS, Turner HC, et al. Gene expression analysis in SV-40 immortalized human corneal epithelial cells cultured with an air-liquid interface. Mol Vis. 2010;16:2109–2120 [PMC free article] [PubMed] [Google Scholar]
- 42. Leiper LJ, Walczysko P, Kucerova R, et al. The roles of calcium signaling and ERK1/2 phosphorylation in a Pax6+/− mouse model of epithelial wound-healing delay. BMC Biol. 2006;4:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bugyi B, Carlier MF. Control of actin filament treadmilling in cell motility. Annu Rev Biophys. 2010;39:449–470 [DOI] [PubMed] [Google Scholar]