Substratum stiffness impacts HTM matrix gene and protein expression and modulates the impact of Lat-B treatment on the expression of these matrix proteins.
Abstract
Purpose.
To determine the impact of substratum stiffness and latrunculin-B (Lat-B), on the expression of several matrix proteins that are associated with glaucoma.
Methods.
Human trabecular meshwork (HTM) cells were cultured on hydrogels possessing stiffness values mimicking those found in normal (5 kPa) and glaucomatous meshworks (75 kPa), or tissue culture polystyrene (TCP; >1 GPa). Cells were treated with 2.0 μM Lat-B in dimethyl sulfoxide (DMSO) or DMSO alone. RT-PCR was used to determine the impact of substratum stiffness and/or Lat-B treatment on the expression of secreted protein, acidic, cysteine rich (SPARC), myocilin, angiopoietin-like factor (ANGPTL)-7, and transglutaminase (TGM)-2. Immunofluorescence was used to assess changes in protein expression.
Results.
SPARC and myocilin mRNA expression were dramatically increased on the 75 kPa hydrogels and decreased on the 5 kPa hydrogels in comparison to TCP. In contrast, ANGPTL-7 mRNA and TGM-2 mRNA was decreased on the 75 kPa and 5 kPa hydrogels, respectively, in comparison with TCP. Treatment with Lat-B dramatically downregulated both SPARC and myocilin on 75 kPa hydrogels. In contrast, cells grown on TCP produced greater or similar amounts of SPARC and myocilin mRNA after Lat-B treatment. SPARC and myocilin protein expression paralleled changes in mRNA expression.
Conclusions.
Substratum stiffness impacts HTM matrix gene and protein expression and modulates the impact of Lat-B treatment on the expression of these matrix proteins. Integrating the use of biologically relevant substratum stiffness in the conduction of in vitro experiments gives important insights into HTM cell response to drugs that may more accurately predict responses observed in vivo.
Glaucoma is a common cause of blindness worldwide and is associated with irreversible degeneration of the optic nerve head. Although the mechanisms contributing to the onset of most glaucomas are multifactorial, a primary risk factor is increased intraocular pressure (IOP).1 IOP is likely elevated because of an abnormal increase in resistance to aqueous outflow in the human trabecular meshwork (HTM).1–3 The HTM is an intricate, three-dimensional structure comprised of extracellular matrix (ECM) possessing intrinsic complex topography and stiffness that supports the overlying trabecular meshwork cells. Many ECM proteins have been implicated in glaucoma including myocilin, transglutaminase (TGM)-2, fibronectin, and angiopoietin-like factor (ANGPTL)-7.4,5
HTM cells are provided with thousands of biophysical cues through cell-cell interactions, cell-ECM interactions, and fluctuations in IOP. It has been previously demonstrated that nano- to submicron scale topography with features similar to native ECMs and basement membranes dramatically altered HTM cell behavior and expression of the ECM proteins, myocilin and versican.6 Mechanically stretching HTM cells, which occurs with increased IOP, has also been shown to upregulate more than 100 genes including secreted protein, acidic, cysteine rich (SPARC), fibronectin, and myocilin.7,8 In situ, HTM cells are exposed to dynamic, compliant substrates that markedly differ from flat, rigid substrates such as glass or tissue culture polystyrene (TCP) which are typically used in laboratory investigations of HTM cellular behavior. A recent study demonstrated that the HTM elastic modulus, a measure of stiffness, is markedly increased in the glaucomatous HTM.3 Substratum elastic modulus has been shown to modulate a variety of fundamental cell behaviors.3,9–13 Hydrogels mimicking the elastic modulus of normal and glaucomatous HTM have been shown to dramatically alter cytoskeletal structure and dynamics, protein expression patterns, cell stiffness, cell behavior, and the cellular response to therapeutic agents such as latrunculin-B (Lat-B).2,14,15
Lat-B is an actin cytoskeleton disruptor that is currently in human clinical trials as a novel glaucoma treatment directed specifically at the trabecular meshwork. The only clinically validated treatments for glaucoma act by lowering IOP by decreasing aqueous humor (AH) production or by decreasing the resistance to AH outflow. With the exception of Lat-B and Rho-kinase inhibitors, these therapeutic approaches do not target the HTM.16 Lat-B is thought to decrease IOP by decreasing the resistance to AH outflow through the HTM.17–21 Recently, it has been demonstrated that HTM cells adhered to stiffer substrates were significantly more responsive to Lat-B due to the increased number of actin stress fibers suggesting that the effects of Lat-B treatment would be most pronounced in glaucomatous eyes with a stiffer HTM.2 In addition, Lat-B treatment significantly diminished HTM cell migration on stiffer substrates.15 However, the effect of substratum stiffness on the expression of ECM proteins before and after Lat-B exposure has not been reported. The purpose of this investigation was to determine the impact of Lat-B treatment in the context of substratum stiffness on the expression of several ECM proteins, myocilin, SPARC, ANGPTL-7, fibronectin, and TGM-2 that have been implicated in glaucoma.4,22,23 A better understanding of the impact that clinically relevant substratum stiffness has on ECM gene and protein expression will inform the design of improved in vitro testing methodologies especially with regard to identification and development of novel glaucoma therapeutics.
Methods
Hydrogel Fabrication
Polyacrylamide hydrogels that mimic the stiffness of the normal (5 kPa) and glaucomatous (75 kPa) HTM as well as an intermediate stiffness (25 kPa) were prepared as previously described.2,9 Briefly, hydrogels were sterilized with ultraviolet light and rinsed every 24 hours for at least 72 hours in Dulbecco's phosphate buffered solution (DPBS; Hyclone, Logan, UT) to fully hydrate the hydrogels and to ensure removal of monomeric acrylamide. Free acrylamide has been previously shown to disrupt cytoskeletal structure.24 After hydration, the hydrogels (10 mm) were adhered to TCP, stored in HTM cell medium for 24 hours, and coated with a proprietary fibronectin/collagen (FNC) mixture (Athena ES, Baltimore, MD) for 20 minutes. Atomic force microscopy was used to validate the stiffness (elastic modulus) of the fully hydrated hydrogels, and the elastic modulus of the hydrogels used were 4 ± 2 kPa, 28 ± 4 kPa, and 71 ± 5 kPa for 5, 25, and 75 kPa, respectively.2,25
HTM Cell Isolation and Culture
Primary HTM cells were isolated from corneal buttons considered unsuitable for transplant from the Heartland Lions Eye Bank (St. Louis, MO) from donors with no prior history of ophthalmic disease as previously described.2,9,26 All experiments complied with the tenets of the Declaration of Helsinki. Isolated cells were cultured in DME/F-12 medium containing 2.5 mM l-glutamine supplemented with 10% fetal bovine serum (Atlanta Biologicals, Lawrence, GA) and 1% penicillin-streptomycin with amphotericin b (Lonza, Walkerville, MD). All studies were conducted using cells before the eighth passage. HTM cells were plated at a density of 1.5 × 105 on FNC-coated TCP, 25 and 75 kPa hydrogels and 2.5 × 105 on FNC-coated 5 kPa hydrogels for 24 hours before Lat-B treatment. The 5 kPa hydrogels were plated at a higher density than the other substrates because HTM cells proliferate more slowly on the 5 kPa hydrogels.15
Latrunculin-B Treatment
Lyophilized Lat-B (Cal Biochem, La Jolla, CA) was resuspended to a final concentration of 2.5 mM in dimethyl sulfoxide (DMSO) (Fisher, Pittsburgh, PA). Fresh solutions of 2.0 μM Lat-B were prepared in serum free DPBS for all experiments. Control samples were treated with an equivalent concentration of DMSO in serum free DPBS. Treatment with DMSO was compared with serum free DPBS only to ensure DMSO did not have an additional effect on HTM cells. After 30 minutes of exposure to Lat-B, DMSO, or DPBS, all samples were rinsed twice with serum-containing medium and then placed in 3 to 5 mL of DME/F-12 with 10% serum and 1% penicillin-streptomycin with amphotericin b. Serum has been shown to neutralize Lat-B.27
RNA Extraction and Quantitative Real-Time PCR
Seven hours after treatment with Lat-B or DMSO, RNA was extracted using a kit (Qiagen RNeasy; Qiagen, Valencia, CA) following the manufacturer's protocol. A 7-hour recovery period after treatment with Lat-B was chosen to ensure that the HTM cellular area and elastic modulus had returned to pretreatment values.2,15 Semiquantitative real-time PCR (qPCR) was performed with 60 ng RNA per sample using a one-step kit (TaqMan) and commercially available primers for 18 S (Hs99999901_s1), SPARC (Hs00277762_m1), myocilin (Hs00165345_m1), ANGPTL-7 (Hs00221727_m1), TGM-2 (Hs01096681_m1), and fibronectin (Hs01549958_m1) in total volumes of 10 μL per reaction (Applied Biosystems, Carlsbad, CA). The reverse transcription reaction was performed for 20 minutes at 50°C followed by PCR enzyme activation for 10 minutes at 95°C and 40 cycles of 60°C for 1 minute followed by 95°C for 15 seconds. The cycle threshold (Ct) ranges were 10 to 20 for 18S, 19 to 21 for SPARC, 20 to 22 for fibronectin, 28 to 33 for myocilin, 29 to 33 for TGM-2, and 34 to 37 for ANGPTL-7. The 18S ribosomal RNA served as a reference. At least three reactions were run for each sample, and the experimental setup was performed for HTM cells from three individuals. Gene expression was normalized relative to the expression of mRNA from HTM cells on TCP treated with vehicle (DMSO), which was given a value of 1.0. Specifically, variations in cell density were controlled by loaded equal amounts of RNA into all PCR reactions. To control for slight variations in the amount of RNA loaded into the PCR reactions, the difference in Ct (ΔCt) between the gene of interest (e.g., SPARC) and the average Ct of the housekeeping gene, 18S (ran in triplicate) were calculated. By calculating the ΔCt between an experimental condition and the control condition (TCP DMSO), the experimental data were normalized to the control data. Because the control condition was normalized within each experiment, and the cycle thresholds represent logarithmic changes in gene expression, all calculations of the control samples were equal to 1.0 and all experimental conditions are a relative ratio of this value. Ratios with respect to this control were calculated for all other values.
Immunocytochemistry and Microscopy
After a 30-minute treatment in Lat-B or vehicle (DMSO), cells were washed 2 times in serum-containing medium and allowed to recover for 7 hours in serum-containing medium. Cells were then fixed with 10% formaldehyde in DPBS for 20 minutes. After an additional DPBS wash, blocking was performed with blotting buffer (Superblock; Thermo Scientific, Rockford, IL) for 20 minutes After an additional DPBS wash, the cells were permeabilized with 0.1% Triton X-100 (Fischer Scientific, Pittsburgh, PA) for 5 minutes. The samples were then incubated with 1:100 rabbit anti-SPARC antibody (Abcam, San Francisco, CA) or 1:10 chicken anti-myocilin antibody28 in 10% Superblock at 4°C for 12 hours. Cells were washed twice with 10% Superblock before incubation with Alexa 488-conjugated donkey anti-rabbit antibody or Alexa 488-conjugated goat anti-chicken antibody at 1:300 in 10% Superblock at room temperature in the dark for 1 hour. Cells were washed twice with 10% Superblock and cell nuclei were stained for 5 minutes at room temperature in the dark with 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen, Carlsbad, CA). After a DPBS wash, cells were left in 1 mL of DPBS until immunofluorescent imaging with an upright microscope (Zeiss Axio Scope.A1; Carl Zeiss Inc., Thornwood, NY) with a camera (AxioCam HRc; Carl Zeiss Inc.). For analysis of SPARC protein expression, images were obtained from each substrate and treatment at the same exposure time of 3.0 seconds using the 10 × air objective for Alexa 488 fluorescence. For analysis of myocilin protein expression, images were obtained from each substrate and treatment at the same exposure time of 1.0 second using the 10 × air objective for Alexa 488 fluorescence. A negative control was performed for each substrate and treatment which did not contain any primary antibody.
Statistical Analysis
Data were analyzed using a software package (Sigma Plot 11; Systat Software, Chicago, IL). A one-way repeated measures analysis of variance (RMANOVA) was used to assess the effect of compliance on the expression of ANGPTL-7, TGM-2, and fibronectin with or without Lat-B treatment. If the one-way RMANOVA was significant, Student's t-tests were performed with a sequentially rejective adaptation of the Bonferroni correction for multiple comparisons to compare each compliant hydrogel with other compliant hydrogels or TCP with or without Lat-B treatment. A two-way RMANOVA was used to assess the effects of compliance and donor on the expression of SPARC and myocilin. If the two-way RMANOVA was significant, Student's t-tests were performed with a sequentially rejective adaptation of the Bonferroni correction for multiple comparisons to compare each compliant hydrogel with other compliant hydrogels or TCP with or without Lat-B treatment. A Student's t-test was used to compare the effect of Lat-B versus DMSO treatment for each compliance. Significance was set at P < 0.05 for all analyses. All data are presented as mean ± SEM.
Results
Substratum Stiffness and Lat-B Alters SPARC and Myocilin Expression
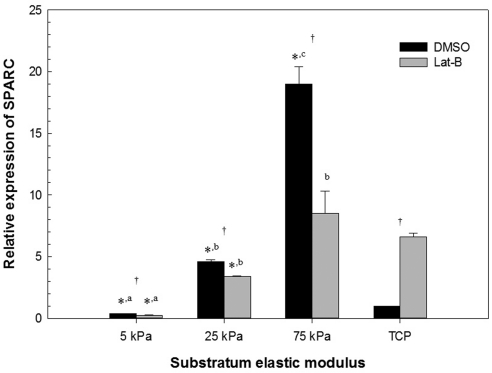
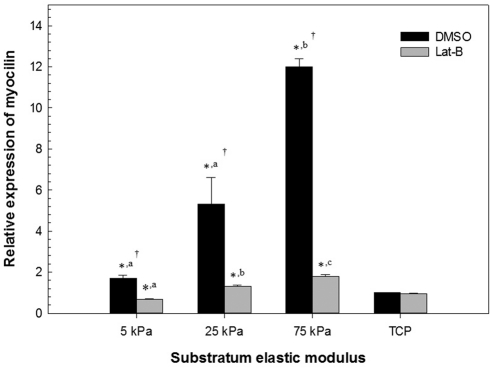
Substratum stiffness by itself dramatically altered the relative amount of both SPARC and myocilin mRNA (Table 1, Figs. 1, 2). SPARC and myocilin mRNA was significantly increased on the 75 kPa hydrogels in comparison with TCP for all three donors. Even in the least responsive HTM cells (HTM 211) from a 21-year old donor, the 75 kPa hydrogel resulted in a 2.7- and 11.9-fold increase in SPARC and myocilin mRNA in comparison with TCP, respectively. In contrast, SPARC and myocilin mRNA was significantly lower on the 5 kPa hydrogels in comparison with TCP in two of three donors and all three donors, respectively. In addition, SPARC and myocilin mRNA on the 5 kPa hydrogels was significantly less than the 75 kPa hydrogels for all three donors. SPARC and myocilin mRNA on the 25 kPa hydrogels was typically intermediate between the 5 and 75 kPa hydogels. To ensure that vehicle (DMSO) treatment did not have an appreciable effect on the SPARC and myocilin mRNA expression, HTM cells were treated with an equal volume of DPBS and results did not differ significantly between the two groups (data not shown).
Table 1.
Relative Quantitative PCR for SPARC and Myocilin mRNA
| DMSO |
Lat-B |
|||||
|---|---|---|---|---|---|---|
| 211 | 424 | 431 | 211 | 424 | 431 | |
| SPARC | ||||||
| 5 kPa | 0.74 ± 0.08a | 0.36 ± 0.01a* | 1.7 ± 0.30a | 0.45 ± 0.05a*† | 0.25 ± 0.01a*† | 2.0 ± 0.60a |
| 25 kPa | 4.7 ± 1.7 | 4.6 ± 0.13b* | 1.5 ± 0.21a | 1.3 ± 0.07b* | 3.4 ± 0.03b*† | 0.81 ± 0.04a† |
| 75 kPa | 2.7 ± 0.45b | 19 ± 1.4c* | 140 ± 34b* | 0.85 ± 0.10c† | 8.5 ± 1.8b† | 0.95 ± 0.08a† |
| TCP (>1 GPa) | 1.0 | 1.0 | 1.0 | 0.9 ± 0.03 | 6.6 ± 0.3† | 340 ± 140 |
| Myocilin | ||||||
| 5 kPa | 1.7 ± 0.15a* | 0.29 ± 0.05a* | 0.54 ± 0.02a* | 0.67 ± 0.03a*† | 0.25 ± 0.05a* | 0.32 ± 0.02a*† |
| 25 kPa | 5.3 ± 1.3a* | 0.97 ± 0.12b | 1.1 ± 0.03b | 1.3 ± 0.08b*† | 1.0 ± 0.04b* | 0.97 ± 0.06b* |
| 75 kPa | 11.9 ± 0.39b* | 170 ± 27c* | 93 ± 1.7c* | 1.8 ± 0.08c*† | 19 ± 7.6† | 1.3 ± 0.09c*† |
| TCP (>1 GPa) | 1.0 | 1.0 | 1.0 | 0.95 ± 0.03 | 4.3 ± 0.6† | 140 ± 13† |
Relative quantification of mean ± SEM SPARC and myocilin mRNA for the HTM 211, 424, and 431 cells when grown on 5, 25, or 75 kPa hydrogels or TCP under control conditions (DMSO) or with 2 μM Lat-B. For each variable, different superscript letters mean significant effect between hydrogels (P < 0.05).
Statistically significant difference from the SPARC or myocilin mRNA on a hydrogel versus TCP (P < 0.05).
Statistically significant difference from the SPARC or myocilin mRNA following DMSO or Lat-B treatment (P < 0.05).
Figure 1.
Substratum stiffness impacts SPARC mRNA and cellular response to Lat-B treatment in HTM cells. Cells grown on stiff hydrogels and treated with DMSO had significantly different SPARC mRNA expression in comparison with DMSO-treated cells on TCP (>1 GPa) or more compliant hydrogels. Cells grown on hydrogels and treated with Lat-B had significantly less SPARC mRNA expression versus treatment with DMSO. In contrast, cells grown on TCP and treated with Lat-B had significantly greater SPARC mRNA expression versus treatment with DMSO. Data are mean ± SEM for HTM 424 donor. (*P < 0.05 for hydrogel versus TCP; a,b,cP < 0.05 between the different hydrogels; †P < 0.05 for DMSO versus Lat-B).
Figure 2.
Substratum stiffness impacts myocilin mRNA and cellular response to Lat-B treatment in HTM cells. Cells grown on stiff hydrogels and treated with DMSO had significantly different myocilin mRNA expression in comparison with DMSO-treated cells on TCP (>1 GPa) or more compliant hydrogels. Cells grown on hydrogels and treated with Lat-B had significantly less myocilin mRNA expression versus treatment with DMSO. In contrast, cells grown on TCP and treated with Lat-B had no significant difference in myocilin mRNA expression versus treatment with DMSO. Data are mean ± SEM for HTM 211 donor. (*P < 0.05 for hydrogel versus TCP; a,b,cP < 0.05 between the different hydrogels; †P < 0.05 for DMSO versus Lat-B).
After treatment with Lat-B, cells on 75 kPa hydrogels produced significantly less SPARC and myocilin mRNA (P < 0.05) in all three donors' cells (Table 1). In contrast, HTM cells cultured on TCP did not appreciably modulate their expression of SPARC and myocilin in response to Lat-B treatment. HTM cellular response to Lat-B was less marked on the 5 and 25 kPa hydrogels in comparison with the 75 kPa hydogels. As previously reported, variations in response of HTM cells from different donors can occur6,29 and this is observed in the present study of SPARC and myocilin with cells from three donors (HTM 211, 424, and 431) that were aged 21, 42, and 43 years, respectively. While there were variations in the magnitude of SPARC and myocilin expression between the three donors, trends were similar with regard to effects of substratum stiffness and treatment with Lat-B.
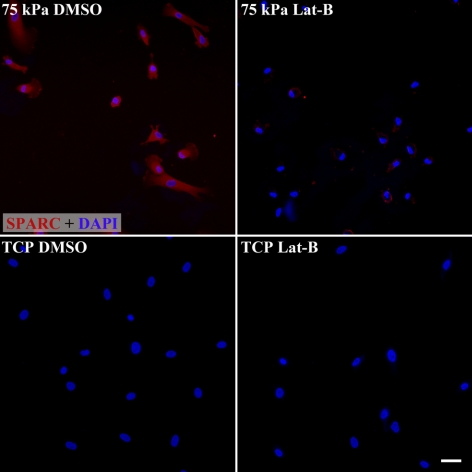
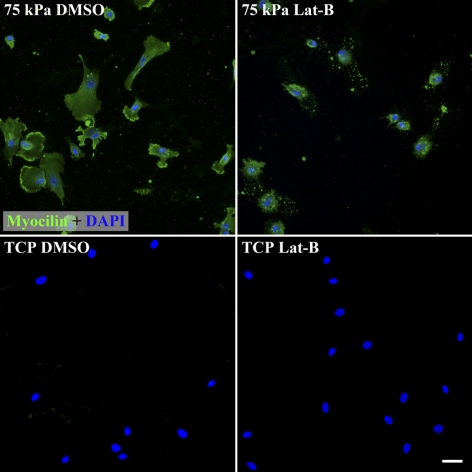
Because the response of the cells to Lat-B was so different between the 75 kPa hydrogels that mimic glaucomatous HTM and TCP, we investigated protein expression in the HTM cells under these two conditions. Consistent with the mRNA data, SPARC protein expression was markedly increased on 75 kPa hydrogels in comparison with TCP while Lat-B decreased SPARC protein expression on 75 kPa hydrogels (Fig. 3). Myocilin protein expression was also markedly increased on 75 kPa hydrogels in comparison with TCP (Fig. 4). However, Lat-B did not dramatically alter the amount or distribution of myocilin protein expression within the HTM cells on 75 kPa hydrogels.
Figure 3.
Substratum stiffness modulates the expression of SPARC protein as well as the impact of Lat-B treatment on SPARC protein expression. HTM cells on 75 kPa hydrogels (mimicking the stiffness of the glaucomatous HTM) have markedly greater SPARC expression than on the much stiffer (>1 GPa) TCP substrates. Treatment of HTM cells on 75 kPa hydrogels with 2 μM Lat-B dramatically decreases SPARC protein expression on the 75 kPa hydrogels. Red, SPARC staining. Blue, DAPI staining of nuclei. Exposure time for each image was 3.0 seconds. Scale bar, 50 μm.
Figure 4.
Substratum compliance modulates the expression of myocilin as well as the impact of Lat-B treatment on myocilin protein expression. HTM cells on 75 kPa hydrogels have greater myocilin expression than on TCP substrates. Treatment of HTM cells on 75 kPa hydrogels with 2 μM Lat-B did not dramatically impact myocilin protein expression. Green, myocilin staining. Blue, DAPI staining of nuclei. Exposure time for each image was 1.0 seconds. Scale bar, 50 μm.
Substratum Stiffness Alters ANGPTL-7, Fibronectin, and TGM-2 Gene Expression
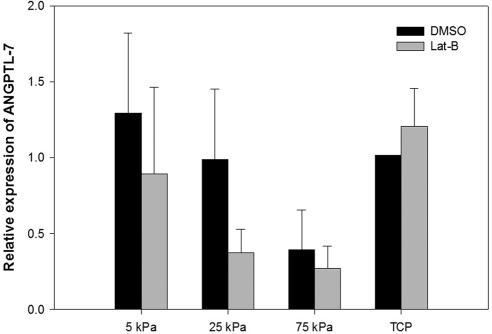
Substratum stiffness significantly altered the relative amount of fibronectin and TGM-2 mRNA. There was much lower variation in gene expression for ANGPTL-7, fibronectin, and TGM-2 in comparison with SPARC and myocilin between HTM donors. There was a trend toward cells grown on the stiffer hydrogels (75 kPa) and treated with DMSO having less ANGPTL-7 mRNA expression in comparison with DMSO-treated cells on TCP opposite to the results obtained with SPARC and myocilin. Cells grown on 25 and 75 kPa hydrogels and treated with Lat-B also tended to have less ANGPTL-7 mRNA expression in comparison with cells treated with Lat-B on TCP (Fig. 5).
Figure 5.
Substratum compliance impacts ANGPTL-7 mRNA in HTM cells. Cells grown on 75 kPa hydrogels and treated with DMSO had less ANGPTL-7 mRNA expression in comparison with DMSO-treated cells on TCP (>1 GPa). Cells grown on 25 and 75 kPa hydrogels and treated with Lat-B had less ANGPTL-7 mRNA expression in comparison with Lat-B-treated cells on TCP. Data are mean ± SEM for three HTM donors.
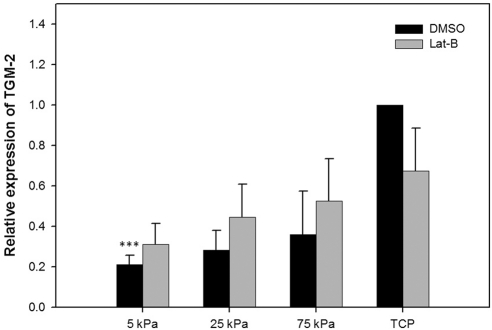
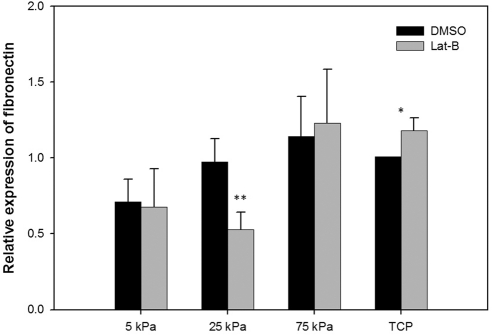
Cells grown on 5 kPa hydrogels had significantly less TGM-2 mRNA expression in comparison with TCP (Fig. 6). Cells grown on 25 kPa hydrogels had significantly less fibronectin mRNA expression in comparison with TCP (Fig. 7). In addition, treatment with 2 μM Lat-B significantly increased fibronectin mRNA expression on TCP.
Figure 6.
Substratum compliance impacts TGM-2 mRNA in HTM cells. Cells grown on 5 kPa hydrogels had significantly less (P < 0.001) TGM-2 mRNA expression in comparison with TCP (>1 GPa). Treatment with 2 μM Lat-B did not significantly impact TGM-2 expression on any of the tested substrates. Data are mean ± SEM for three HTM donors. (***P < 0.001).
Figure 7.
Substratum compliance and Lat-B impacts fibronectin mRNA in HTM cells. Cells grown on 25 kPa hydrogels and treated with Lat-B had significantly less (P = 0.006) fibronectin mRNA expression in comparison with Lat-B treated cells on TCP (>1 GPa). Treatment with 2 μM Lat-B significantly increased (P = 0.045) fibronectin mRNA expression on TCP. Data are mean ± SEM from three HTM donors. **P < 0.01; *P < 0.05.
Discussion
In primary open-angle glaucoma (POAG), increased resistance to AH outflow through the HTM results in elevated IOP.30 In the normal HTM, there is a dynamic balance between ECM protein production and degradation. Expression of many ECM genes are relatively high within the HTM.7 ECM turnover is a primary mechanism by which resistance may be altered within the HTM. In POAG, there is an accumulation of cross-linked ECM proteins in the HTM that likely contributes to increased outflow resistance and elevated IOP.31,32 A recent study demonstrated that the elastic modulus of HTM increases with glaucoma which could increase the resistance to outflow.3
SPARC is a matricellular protein that is widely expressed in ocular tissues including the HTM.33 The expression of SPARC is significantly increased in the iris with primary angle closure glaucoma (PACG), and SPARC-null mice exhibit significantly lower IOPs suggesting that SPARC is important in outflow resistance.22,34 As a matricellular glycoprotein, SPARC is essential in ECM remodeling and has been implicated in disease states especially tissue fibrosis.35–38 Mechanical stretching, a mechanism by which HTM cells respond to changes in IOP, also upregulates SPARC expression in HTM cells.7 Our results show substratum stiffness markedly altered SPARC mRNA and protein expression in HTM cells. Specifically, SPARC mRNA expression was dramatically increased on substrates mimicking the glaucomatous HTM and decreased on substrates mimicking the normal HTM in comparison with rigid TCP. Cells in vivo never interact with matrices possessing stiffness values that approximate TCP (>1 GPa). Culturing HTM cells on hydrogels represents an advantageous method to more closely determine the expression of proteins and cellular behaviors that define cells in tissues.
Myocilin is a secreted glyocoprotein for which a consensus has not been reached as to its function. Myocilin is mutated in some forms of POAG with certain mutations associated with more severe phenotypes.39–42 SPARC and myocilin have been shown to interact together through their C-terminal domains and proteolytic processing of myocilin modulates its interaction with SPARC which may explain the similarities in expression between SPARC and myocilin in the present study.43 It has been previously demonstrated that myocilin expression is upregulated with glaucoma.44 Cells on hydrogels mimicking the stiffness of the glaucomatous HTM also markedly increased the mRNA and protein expression of myocilin, and is more consistent with in vivo conditions than are cells cultured on TCP.
The findings from this study also show that ANGPTL-7 expression tended to decrease on substrates that imitate the glaucomatous HTM. ANGPTL-7 belongs to a family of proteins that are structurally related to the angiopoeitins and plays an important role in ECM turnover. It has been shown that ANGPTL-7 is moderately expressed in the HTM45 and is dramatically upregulated in HTM cells after dexamethasone treatment.46 Overexpression of ANGPTL-7 in HTM cells decreased the expression of multiple ECM proteins including myocilin and fibronectin and silencing ANGPTL-7 during dexamethasone treatment altered the expression of several steroid-induced proteins.23 These findings suggest that ANGPTL-7 is important in modulating the HTM ECM and its response to glucocorticoids23 Interestingly, ANGPTL-7 mRNA was decreased by 2.5-fold on the stiffest hydrogel (75 kPa) in comparison with TCP and this altered ANGPTL-7 expression may have contributed to the upregulation of myocilin that was observed in this study. Utilization of the 75 kPa hydrogels may represent an alternative to ANGPTL-7 silencing RNA when investigating this protein's role in glaucoma progression and treatment.
In the present study, TGM-2 expression was significantly decreased on hydrogels that mimic the normal HTM (5 kPa). TGM-2 belongs to a family of calcium-dependent enzymes that are important in posttranslational modification of the ECM via protein cross-linkage. HTM cells from glaucomatous donors showed significantly greater TGM-2 protein expression and enzyme activity.5 In addition, there was increased colocalization of fibronectin and cross-linked ε-(γ-glutamyl) lysine proteins in glaucomatous donors suggesting that TGM-2 may be instrumental in altering ECM degradation by cross-linking ECM proteins.5 Similar to TGM-2, fibronectin expression is increased in the HTM with aging, POAG, and in steroid-responsive glaucoma.47,48 In the present study, substrate stiffness also affected fibronectin expression after Lat-B treatment.
In addition to investigating the effects of substratum stiffness, this study also demonstrated that Lat-B treatment of HTM cells dramatically decreased SPARC mRNA and protein expression and myocilin mRNA expression on hydrogels imitating the stiffness of the glaucomatous HTM but had a much different effect on cells cultured on TCP. However myocilin protein expression was relatively unchanged on the 75 kPa hydrogels after Lat-B treatment which is not surprising given that there is variable correlation between mRNA and protein expression.49 Lat-B in combination with substrate stiffness also had effects on expression of fibronectin and ANGPTL-7 but not TGM-2 in HTM cells. To the authors' knowledge, this is the first investigation of the effects of Lat-B on the ECM of HTM cells but it has been reported that cytoskeletal disruption of chondrocytes with latrunculin A caused ECM uncoupling and structural changes.50 Lat-B modulation of the ECM may represent an additional mechanism by which this cytoskeletal disruptor decreases resistance to aqueous humor outflow within the HTM and thus lowers IOP. Given that Lat-B is currently in clinical trials as a novel glaucoma therapy, further investigations are warranted to determine the effects of Lat-B and other cytoskeletal disruptors on the structure and composition of the ECM. These studies could provide new insights into the mechanisms by which HTM cells regulate ECM turnover and outflow resistance.
Recently, it was demonstrated that substratum stiffness altered the HTM cellular response to Lat-B with regard to cell morphology and elastic modulus.2 Lat-B treatment of HTM cells on stiff substrates caused dramatic alterations in morphology and elastic modulus while on more compliant substrates Lat-B caused minimal effects in these two variables.2 These observations are consistent with findings in the present study which demonstrate Lat-B treatment to have a minimal effect on SPARC and myocilin expression on hydrogels possessing values for stiffness that mimic the normal HTM.
In conclusion, substratum stiffness had a profound influence on HTM cell expression of ECM proteins that are associated with glaucoma in humans. We also demonstrated that substratum stiffness modulated the response of HTM cells to treatment with Lat-B with regard to expression of these ECM proteins. There is increasing evidence that biophysical cues such as nano- to microscale topography, mechanical stretch, and substratum stiffness profoundly alter HTM cell behaviors and their response to therapeutic agents.2,6–8,15 The inclusion of biomimetic biophysical attributes of the substratum in vitro is necessary to better reflect many HTM cell behaviors observed in normal and glaucomatous tissue in vivo. By integrating biomimetic biophysical cues as well as soluble signaling factors, the predictive value of in vitro systems will likely increase and accelerate our understanding of glaucoma initiation and progression as well as the development of novel glaucoma therapeutics.
Acknowledgments
The authors thank Marion E. Fischer and Marissa L. Hughbanks for hydrogel production.
Footnotes
Supported by National Institutes of Health Grants K08 EY021142, R01 EY019475, and P30 EY12576; a grant from National Glaucoma Research; a program of the American Health Assistance Foundation; and an unrestricted grant from Research to Prevent Blindness.
Disclosure: S.M. Thomasy, None; J.A. Wood, None; P.H. Kass, None; C.J. Murphy, None; P. Russell, None
References
- 1. Rhee DJ, Haddadin RI, Kang MH, Oh DJ. Matricellular proteins in the trabecular meshwork. Exp Eye Res. 2009;88:694–703 [DOI] [PubMed] [Google Scholar]
- 2. McKee CT, Wood JA, Shah NM, et al. The effect of biophysical attributes of the ocular trabecular meshwork associated with glaucoma on the cell response to therapeutic agents. Biomaterials. 2011;32:2417–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Last JA, Pan T, Ding Y, et al. Elastic modulus determination of normal and glaucomatous human trabecular meshwork. Invest Ophthalmol Vis Sci. 2011;52:2147–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liton PB, Luna C, Challa P, Epstein DL, Gonzalez P. Genome-wide expression profile of human trabecular meshwork cultured cells, nonglaucomatous and primary open angle glaucoma tissue. Mol Vis. 2006;12:774–790 [PMC free article] [PubMed] [Google Scholar]
- 5. Tovar-Vidales T, Roque R, Clark AF, Wordinger RJ. Tissue transglutaminase expression and activity in normal and glaucomatous human trabecular meshwork cells and tissues. Invest Ophthalmol Vis Sci. 2008;49:622–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Russell P, Gasiorowski JZ, Nealy PF, Murphy CJ. Response of human trabecular meshwork cells to topographic cues on the nanoscale level. Invest Ophthalmol Vis Sci. 2008;49:629–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vittal V, Rose A, Gregory KE, Kelley MJ, Acott TS. Changes in gene expression by trabecular meshwork cells in response to mechanical stretching. Invest Ophthalmol Vis Sci. 2005;46:2857–2868 [DOI] [PubMed] [Google Scholar]
- 8. Sato Y, Matsuo T, Ohtsuki H. A novel gene (oculomedin) induced by mechanical stretching in human trabecular cells of the eye. Biochem Biophys Res Commun. 1999;259:349–351 [DOI] [PubMed] [Google Scholar]
- 9. Wood JA, Shah NM, McKee CT, et al. The role of substratum compliance of hydrogels on vascular endothelial cell behavior. Biomaterials. 2011;32:5056–5064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94:13661–13665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143 [DOI] [PubMed] [Google Scholar]
- 12. Engler A, Bacakova L, Newman C, Hategan A, Griffin M, Discher D. Substrate compliance versus ligand density in cell on gel responses. Biophys J. 2004;86:617–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689 [DOI] [PubMed] [Google Scholar]
- 14. Schlunck G, Han H, Wecker T, Kampik D, Meyer-ter-Vehn T, Grehn F. Substrate rigidity modulates cell matrix interactions and protein expression in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2008;49:262–269 [DOI] [PubMed] [Google Scholar]
- 15. Wood JA, McKee CT, Thomasy SM, et al. Substratum compliance regulates human trabecular meshwork cell behaviors and response to latrunculin B. Invest Ophthalmol Vis Sci. 2011;52:9298–9303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heijl A, Leske MC, Bengtsson B, Hyman L, Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–1279 [DOI] [PubMed] [Google Scholar]
- 17. Kaufman PL. Enhancing trabecular outflow by disrupting the actin cytoskeleton, increasing uveoscleral outflow with prostaglandins, and understanding the pathophysiology of presbyopia interrogating Mother Nature: asking why, asking how, recognizing the signs, following the trail. Exp Eye Res. 2008;86:3–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rao PV, Deng P, Sasaki Y, Epstein DL. Regulation of myosin light chain phosphorylation in the trabecular meshwork: role in aqueous humour outflow facility. Exp Eye Res. 2005;80:197–206 [DOI] [PubMed] [Google Scholar]
- 19. Rao PV, Deng PF, Kumar J, Epstein DL. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Invest Ophthalmol Vis Sci. 2001;42:1029–1037 [PubMed] [Google Scholar]
- 20. Ethier CR, Read AT, Chan DW. Effects of latrunculin-B on outflow facility and trabecular meshwork structure in human eyes. Invest Ophthalmol Vis Sci. 2006;47:1991–1998 [DOI] [PubMed] [Google Scholar]
- 21. Okka M, Tian B, Kaufman PL. Effects of latrunculin B on outflow facility, intraocular pressure, corneal thickness, and miotic and accommodative responses to pilocarpine in monkeys. Trans Am Ophthalmol Soc. 2004;102:251–257; discussion 257–259 [PMC free article] [PubMed] [Google Scholar]
- 22. Chua J, Seet LF, Jiang Y, et al. Increased SPARC expression in primary angle closure glaucoma iris. Mol Vis. 2008;14:1886–1892 [PMC free article] [PubMed] [Google Scholar]
- 23. Comes N, Buie LK, Borras T. Evidence for a role of angiopoietin-like 7 (ANGPTL7) in extracellular matrix formation of the human trabecular meshwork: implications for glaucoma. Genes Cells. 2011;16:243–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arocena M. Effect of acrylamide on the cytoskeleton and apoptosis of bovine lens epithelial cells. Cell Biol Int. 2006;30:1007–1012 [DOI] [PubMed] [Google Scholar]
- 25. Radmacher M, Tillamnn RW, Fritz M, Gaub HE. From molecules to cells: imaging soft samples with the atomic force microscope. Science. 1992;257:1900–1905 [DOI] [PubMed] [Google Scholar]
- 26. Rhee DJ, Tamm ER, Russell P. Donor corneoscleral buttons: a new source of trabecular meshwork for research. Exp Eye Res. 2003;77:749–756 [DOI] [PubMed] [Google Scholar]
- 27. Spector I, Shochet NR, Blasberger D, Kashman Y. Latrunculins–novel marine macrolides that disrupt microfilament organization and affect cell growth: I. Comparison with cytochalasin D. Cell Motil Cytoskeleton. 1989;13:127–144 [DOI] [PubMed] [Google Scholar]
- 28. Zillig M, Wurm A, Grehn FJ, Russell P, Tamm ER. Overexpression and properties of wild-type and Tyr437His mutated myocilin in the eyes of transgenic mice. Invest Ophthalmol Vis Sci. 2005;46:223–234 [DOI] [PubMed] [Google Scholar]
- 29. Oh DJ, Martin JL, Williams AJ, Russell P, Birk DE, Rhee DJ. Effect of latanoprost on the expression of matrix metalloproteinases and their tissue inhibitors in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2006;47:3887–3895 [DOI] [PubMed] [Google Scholar]
- 30. Grant WM. Experimental aqueous perfusion in enucleated human eyes. Arch Ophthalmol. 1963;69:783–801 [DOI] [PubMed] [Google Scholar]
- 31. Rohen JW, Witmer R. Electron microscopic studies on the trabecular meshwork in glaucoma simplex. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1972;183:251–266 [DOI] [PubMed] [Google Scholar]
- 32. Lutjen-Drecoll E. Functional morphology of the trabecular meshwork in primate eyes. Prog Retin Eye Res. 1999;18:91–119 [DOI] [PubMed] [Google Scholar]
- 33. Tomarev SI, Wistow G, Raymond V, Dubois S, Malyukova I. Gene expression profile of the human trabecular meshwork: NEIBank sequence tag analysis. Invest Ophthalmol Vis Sci. 2003;44:2588–2596 [DOI] [PubMed] [Google Scholar]
- 34. Haddadin RI, Oh DJ, Kang MH, et al. SPARC-null mice exhibit lower intraocular pressures. Invest Ophthalmol Vis Sci. 2009;50:3771–3777 [DOI] [PubMed] [Google Scholar]
- 35. Brekken RA, Sage EH. SPARC, a matricellular protein: at the crossroads of cell-matrix communication. Matrix Biol. 2001;19:816–827 [DOI] [PubMed] [Google Scholar]
- 36. Frizell E, Liu SL, Abraham A, et al. Expression of SPARC in normal and fibrotic livers. Hepatology. 1995;21:847–854 [PubMed] [Google Scholar]
- 37. Kuhn C, Mason RJ. Immunolocalization of SPARC, tenascin, and thrombospondin in pulmonary fibrosis. Am J Pathol. 1995;147:1759–1769 [PMC free article] [PubMed] [Google Scholar]
- 38. Pichler RH, Hugo C, Shankland SJ, et al. SPARC is expressed in renal interstitial fibrosis and in renal vascular injury. Kidney Int. 1996;50:1978–1989 [DOI] [PubMed] [Google Scholar]
- 39. Stone EM, Fingert JH, Alward WL, et al. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668–670 [DOI] [PubMed] [Google Scholar]
- 40. Fingert JH, Heon E, Liebmann JM, et al. Analysis of myocilin mutations in 1703 glaucoma patients from five different populations. Hum Mol Genet. 1999;8:899–905 [DOI] [PubMed] [Google Scholar]
- 41. Tamm ER. Myocilin and glaucoma: facts and ideas. Prog Retin Eye Res. 2002;21:395–428 [DOI] [PubMed] [Google Scholar]
- 42. Fingert JH, Stone EM, Sheffield VC, Alward WL. Myocilin glaucoma. Surv Ophthalmol. 2002;47:547–561 [DOI] [PubMed] [Google Scholar]
- 43. Aroca-Aguilar JD, Sanchez-Sanchez F, Ghosh S, Fernandez-Navarro A, Coca-Prados M, Escribano J. Interaction of recombinant myocilin with the matricellular protein SPARC: functional implications. Invest Ophthalmol Vis Sci. 2011;52:179–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lutjen-Drecoll E, May CA, Polansky JR, Johnson DH, Bloemendal H, Nguyen TD. Localization of the stress proteins alpha B-crystallin and trabecular meshwork inducible glucocorticoid response protein in normal and glaucomatous trabecular meshwork. Invest Ophthalmol Vis Sci. 1998;39:517–525 [PubMed] [Google Scholar]
- 45. Gonzalez P, Epstein DL, Borras T. Characterization of gene expression in human trabecular meshwork using single-pass sequencing of 1060 clones. Invest Ophthalmol Vis Sci. 2000;41:3678–3693 [PubMed] [Google Scholar]
- 46. Lo WR, Rowlette LL, Caballero M, Yang P, Hernandez MR, Borras T. Tissue differential microarray analysis of dexamethasone induction reveals potential mechanisms of steroid glaucoma. Invest Ophthalmol Vis Sci. 2003;44:473–485 [DOI] [PubMed] [Google Scholar]
- 47. Babizhayev MA, Brodskaya MW. Fibronectin detection in drainage outflow system of human eyes in ageing and progression of open-angle glaucoma. Mech Ageing Dev. 1989;47:145–157 [DOI] [PubMed] [Google Scholar]
- 48. Tawara A, Tou N, Kubota T, Harada Y, Yokota K. Immunohistochemical evaluation of the extracellular matrix in trabecular meshwork in steroid-induced glaucoma. Graefes Arch Clin Exp Ophthalmol. 2008;246:1021–1028 [DOI] [PubMed] [Google Scholar]
- 49. Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol. 1999;19:1720–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nofal GA, Knudson CB. Latrunculin and cytochalasin decrease chondrocyte matrix retention. J Histochem Cytochem. 2002;50:1313–1324 [DOI] [PubMed] [Google Scholar]