Abstract
Protein phosphorylation and dephosphorylation has been recognized as an essential mechanism in the regulation of cellular metabolism and function in various tissues. Serine and threonine protein phosphatases (PP) are divided into four categories: PP1, PP2A, PP2B, and PP2C. At least four isoforms of PP1 catalytic subunit in rat, PP1α, PP1γ1, PP1γ2, and PP1δ, were isolated. In the present study, we examined the localization and expression of PP1δ in human osteoblastic Saos-2 cells. Anti-PP1δ antibody recognized a protein present in the nucleolar regions in Saos-2 cells. Cellular fractionation revealed that PP1δ is a 37 kDa protein localized in the nucleolus. Nucleophosmin is a nucleolar phosphoprotein and located mainly in the nucleolus. Staining pattern of nucleophosmin in Saos-2 cells was similar to that of PP1δ. PP1δ and nucleophosmin were specifically stained as dots in the nucleus. Dual fluorescence images revealed that PP1δ and nucleophosmin were localized in the same regions in the nucleolus. Similar distribution patterns of PP1δ and nucleophosmin were observed in osteoblastic MG63 cells. The interaction of PP1δ and nucleophosmin was also shown by immunoprecipitation and Western analysis. These results indicated that PP1δ associate with nucleophosmin directly in the nucleolus and suggested that nucleophosmin is one of the candidate substrate for PP1δ.
Keywords: protein phosphatase, nucleophosmin, osteoblast
I. Introduction
Protein dephosphorylation and phosphorylation is a major regulatory mechanism of signal transduction cascades in eukaryotic cells [3, 30]. More than 90% of the protein phosphatase activity in eukaryotes is due to protein phosphatase-1 (PP1) and -2A (PP2A), which belong to the phosphoprotein phosphatase (PPP) superfamily of serine and threonine protein phosphatases [3, 30]. cDNA cloning revealed that at least four isoforms of PP1 catalytic subunit, PP1α, PP1δ, PP1γ1, and PP1γ2, exist in rat [23]. PP1s are involved in cellular processes including glycogen metabolism, muscle contraction, protein synthesis, intracellular transport, and Ca2+ cycling [2, 5]. The substrates identified for PP1s include glycogen synthase, glycogen phosphorylase, myosin light chain, and ribosomal protein S6 [24]. However, for many other phosphoproteins the contribution of PP1s to their dephosphorylation has yet to be clarified.
The expression of PP1s was previously investigated at the mRNA and protein levels in various tissues [25]. Immunocytochemistry was also carried out in rat cerebellum and salivary glands for PP1γ1 [8, 27] and in testis for PP1γ2 [26]. In previous studies, we reported the subcellular localization of PP1 isoforms in mouse osteoblastic MC3T3-E1 cells and human osteoblastic MG63 cells [6, 17]. PP1 targeting subunits are thought to direct PP1 to specific subcellular components, and to modulate the activity of the enzyme at these sites.
Nucleophosmin, also known as B23, has a molecular mass of 38 kDa and is an abundantly expressed phosphoprotein located mainly in the fibrillar components of nucleoli, where it associates with nascent preribosomal RNA [15, 21]. Nucleophosmin is involved in the regulation of ribosome biogenesis, cell proliferation and growth, embryogenesis, cytokinesis, and nucleogenesis [15, 21]. In the present study, we provide evidence showing that nucleophosmin is associated with PP1δ and is a candidate substrate for this enzyme.
II. Materials and Methods
Materials
Fetal bovine serum (FBS) was obtained from Equitech-Bio (Kerrville, TX, USA). Alpha-modified Eagle’s minimal essential medium (α-MEM) was purchased from Gibco BRL (Grand Island, NY, USA). Plastic dishes were from Iwaki (Chiba, Japan). Protein Assay Reagent and precision plus protein standards were from Bio-Rad (Hercules, CA, USA). Polyvinylidene difluoride (PVDF) transfer membrane was from Millipore (Bedford, MA, USA). Protein A/G PLUS agarose and immunoglobulin G fraction of an anti-nucleophosmin antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The antisera against PP1s were those described previously [26]. Anti-PP1δ antibody was also developed in our laboratory. Other materials used were of the highest grade commercially available.
Cell culture
Human osteoblastic cell line Saos-2 [22] was purchased from the American Type Culture Collection (Rockville, MD, USA). The cells were cultured in α-MEM containing 10% (v/v) FBS, 2 mM glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin and were maintained at 37°C in a humidified atmosphere of 5% CO2 and 95% air. Cell modification was monitored by an Olympus IMT-2 phase-contrast microscope. For immunocytochemistry, the cells were plated on 18-mm round coverslips in 60-mm plastic dishes. For cellular fractionation, Saos-2 cells growing in 90 mm plastic dishes were washed twice with Ca2+-, Mg2+-free phosphate-buffered saline (PBS), scraped into PBS, collected at 3,000 g, and resuspended in hypotonic buffer (20 mM Hepes, pH 7.2, 10 mM KCl, 1 mM MgCl2, 1 mM DTT, and 0.5 mM EDTA). The cells were allowed to swell for 10 min on ice before lysis by addition of 0.1% NP-40 and 100 mM potassium acetate. After 5 min in ice and vortexing, nuclei were pelleted by centrifugation for 10 min at 8,000 g, resuspended in lysate buffer containing 1 mM DTT, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 µg/ml leupeptin, 2 µg/ml aprotinin, and 5 mM EGTA in PBS, and termed the nuclear fraction, whereas the supernatant was termed the cytosolic fraction. The nucleolar fraction was prepared from the purified nuclei according to the method of Muramatsu et al. [18]. The protein concentration of each fraction was evaluated by using Protein Assay Reagent (Bio-Rad) and diluted to a protein concentration of 1 mg/ml with lysate buffer before the addition of Laemmli’s 5× sample buffer.
Immunoprecipitation
Cells cultured in 90-mm plastic dishes were washed twice with PBS, scraped into PBS, pelleted at 3,000 g, and resuspended in 500 µl of lysis buffer (150 mM NaCl, 1.0% NP-40, 50 mM Tris-HCl [pH 8.0], 50 mM NaF, and 1 mM Na3VO4). The lysate was pre-treated for 60 min with protein A/G PLUS agarose at 4°C and then incubated with 2 µl of anti-PP1δ or anti-nucleophosmin antibodies. The reaction mixture was incubated for overnight at 4°C with 10 µl of protein A/G PLUS agarose. The immunocomplexes were washed 5 times with lysis buffer and resuspended in 40 µl of SDS electrophoresis sample buffer. The samples were boiled for 5 min and the supernatant was analyzed by SDS-PAGE and Western blotting using the anti-nucleophosmin or anti-PP1δ antibodies.
SDS-PAGE and Western analysis
Ten µg of each sample and pre-stained protein markers were separated by SDS-PAGE and transferred to PVDF membranes. The membranes were blocked in a solution containing 5% nonfat skim milk in PBS-Tween for 2 hr at ambient temperature. They were washed briefly in PBS containing 0.05% Tween-20 (PBS-Tween) and incubated overnight at 4°C in a blocking solution containing anti-PP1δ antibody diluted 1:2,000 or anti-nucleophosmin antibody at 1:1,000 dilutions. The membranes were washed four times within 30 min in PBS-Tween on a rotary shaker at ambient temperature. The washed membranes were incubated for 2 hr with horseradish peroxidase (HRP)-conjugated anti-rabbit IgG for PP1δ or anti-goat IgG for nucleophosmin (both diluted 1:5,000 in a blocking solution) at ambient temperature. The membranes were washed as described, and the proteins recognized by the antibodies were visualized by using an ECL detection kit according to the manufacturer’s directions.
Immunocytochemistry
The cells on coverslips were washed three times with PBS and fixed with 3.7% formaldehyde for 10 min at ambient temperature followed by methanol-permeabilization for an additional 20 min at −20°C. Non-specific binding sites were blocked with 4% BSA in PBS for 10 min at ambient temperature. Having been rinsed with cold PBS, the coverslips were incubated simultaneously with anti-PP1δ antibody diluted 1:200 and 5 µg/ml of the IgG fraction of anti-nucleophosmin antibody in 4% BSA for 45 min at ambient temperature. After three washes with 0.1% BSA in PBS-Tween over a 15-min period at ambient temperature, the cells were incubated with a mixture of tetramethylrhodamine isothiocyanate (TRITC, rhodamine)-conjugated sheep anti-rabbit IgG (Chemicon International, Temecula, CA, USA) and fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (Cappel-Organon Teknika, Turnhout, Belgium), both diluted 1:300 in 4% BSA in PBS for another 45 min at ambient temperature. The coverslips were washed as described above and mounted while wet with PermaFluor aqueous mounting medium (Lipshow, Pittsburgh, PA, USA). The samples were examined under an Olympus BX50 microscope equipped with epifluorescence illumination (BX-FLA) with a U-MWIG filter for rhodamine and a U-MNIBA filter for FITC. The U-MNIBA filter separates FITC from rhodamine or Texas Red. The staining reaction was not observed when FITC-labeled cells were examined with a filter for rhodamine (the U-MWIG filter). Rhodamine-labeled cells were not detected with a filter for FITC (the U-MNIBA filter). Microphotographs were recorded on a computer (Olympus, DP70-WPCXP).
III. Results
Localization of PP1 isotypes in Saos-2 cells
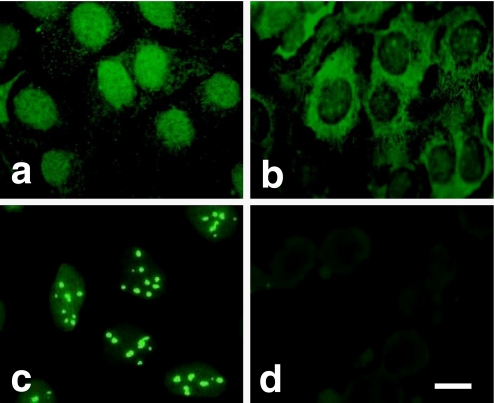
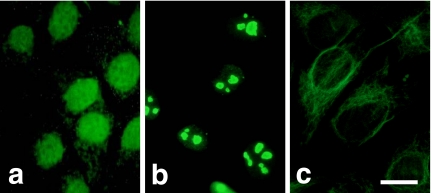
To examine the cytolocalization of PP1 isotypes in human osteoblastic cells, Saos-2 cells at monolayer were fixed, permeabilized, and stained with the rabbit polyclonal antibodies against the catalytic subunits of PP1α, PP1γ1, PP1γ2, and PP1δ. The immunereactivity of each isotype showed quite different cellular distributions. Although a weak staining was observed in the cytoplasm, the distribution of PP1α was mainly in the nucleus (Fig. 1a). PP1γ1 was localized in both the nucleus and the cytoplasm. The punctuated distribution of PP1γ1 in cytoplasm was especially distinct in the perinuclear region. The staining reaction in the cytoplasm was much stronger than that which occurred in the nucleus (Fig. 1b). Intense staining occurred in the nucleus with the anti-PP1δ antibody. PP1δ was specifically stained as dots in the nucleus that may represent nucleoli (Fig. 1c). The immunoreaction was not observed with anti-PP1γ2 antibody (Fig. 1d) and the normal rabbit serum (data not shown). That the distribution of PP1s is not limited to Saos-2 cells was confirmed by the fact that they had an identical distribution in MG63 human osteoblastic cells (Fig. 2) or mouse osteoblastic MC3T3-E1 cells (data not shown).
Fig. 1.
Localization of PP1 isotypes in Saos-2 cells. Saos-2 cells at monolayer were fixed, permeabilized, and stained with the rabbit polyclonal antibodies against the catalytic subunits of PP1α (a), PP1γ1 (b), PP1δ (c), and PP1γ2 (d). Bar=10 µm.
Fig. 2.
Localization of PP1 isotypes in MG63 cells. MG63 cells were stained with antibodies against PP1α (a), PP1δ (b), and PP1γ1 (c). Bar=10 µm.
Detection of PP1 isoforms in subcellular fractions
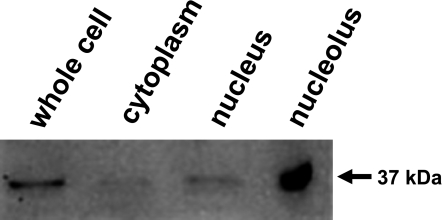
Because PP1δ showed specific localization in Saos-2 cells as described above, we determined the subcellular localization of PP1δ protein by using cellular fractions including cytoplasmic, nuclear, and nucleolar fractions prepared from cultured Saos-2 cells. Twenty µg of proteins obtained from each fraction were subjected to SDS-PAGE and followed by Western analysis. Figure 3 shows that the antibody reacted with a major band corresponding to the estimated molecular weight of 37 kDa. Although the reaction was observed in the nuclear fraction, the strongest interaction was observed in the nucleolar fraction. Very little immunoreactive proteins were detected in the cytosolic fraction. Normal rabbit serum did not recognize any proteins prepared from cultured Saos-2 cells (data not shown).
Fig. 3.
Detection of PP1δ in subcellular fractions of Saos-2 cells. Cytoplasmic, nuclear, and nucleolar fractions were prepared from Saos-2 cells. Whole cell lysate and proteins obtained from each fraction were subjected to Western analysis. The intense positive reaction was detected in the nucleolar fraction.
Co-localization of PP1δ and nucleophosmin in Saos-2 cells
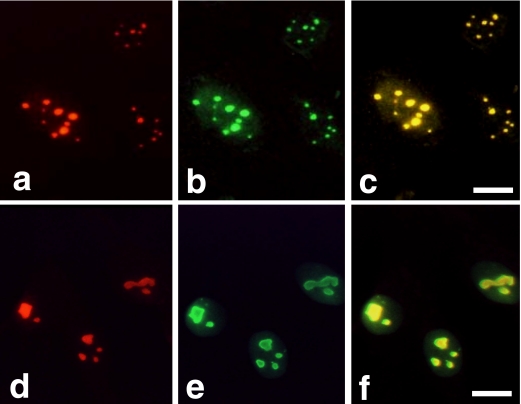
In the previous reports, we and others demonstrated that PP1δ localized in nucleolus in mouse osteoblastic MC3T3-E1 cells and Swiss 3T3 cells [6, 14]. This distribution pattern was similar to that of nucleolin, nucleophosmin, or AgNORs. To examine whether PP1δ and nucleophosmin would be localized at the same site in the nucleolus, we fixed, permeabilized, and stained the cultured Saos-2 cells with the anti-PP1δ antibody and anti-nucleophosmin antibody. Figure 4 shows the distribution of PP1δ and nucleophosmin in Saos-2 cells. With anti-PP1δ antibody, nucleolus-like bodies were intensely stained and visible as red fluorescence from due to the rhodamine-conjugated second antibody (Fig. 4a). An anti-nucleophosmin antibody stained the same sites of the PP1δ-positive regions, which gave a green fluorescence because FITC-conjugated second antibody was used (Fig. 4b). The merged view confirmed that PP1δ and nucleophosmin were localized in the same sites in Saos-2 cells because the reaction was visible as yellow (Fig. 4c). Figure 4 also shows that anti-PP1δ antibody and anti-nucleophosmin antibody stained the nucleolus-like materials in MG63 cells (Fig. 4d and 4e). The positive sites were stained as yellow in the merged view indicating that PP1δ and nucleophosmin were also localized in the same sites in MG63 cells (Fig. 4f). The staining reaction was not observed when normal rabbit serum or normal goat serum was used as a primary antibody both in Saos-2 and MG63 cells (data not shown).
Fig. 4.
Co-localization of PP1δ and nucleophosmin in Saos-2 and MG63 cells. Saos-2 cells (upper panel) and MG63 cells (lower panel) were stained with anti-PP1δ antibody followed by the rhodamine-conjugated second antibody (a and d). The same cultures were also stained with the anti-nucleophosmin antibody followed by the FITC-conjugated second antibody (b and e). The merged view of Figures a and b and Figures d and e are shown in Figure c and Figure f, respectively. Bars=10 µm.
PP1δ associates with nucleophosmin
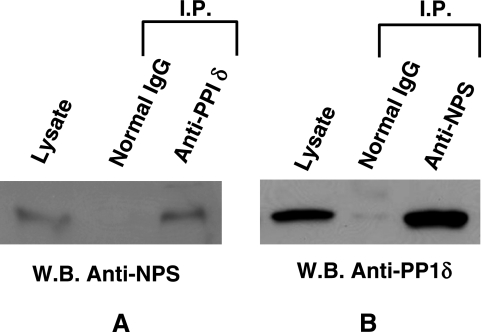
To determine whether PP1δ could associate with nucleophosmin, whole cell lysate prepared from cultured Saos-2 cells was immunoprecipitated with the anti-PP1δ antibody or with the anti-nucleophosmin antibody. The immunoprecipitants were analysed by Western blotting using the anti-nucleophosmin antibody (Fig. 5A) or anti-PP1δ antibody (Fig. 5B). Anti-nucleophosmin antibody interacted with a protein precipitated with the anti-PP1δ antibody. However, this antibody did not recognize any proteins precipitated with the normal goat IgG. The molecular weight of the interacting protein was estimated as 38 kDa which corresponded to the molecular weight of nucleophosmin. Anti-PP1δ antibody interacted with a protein precipitated with the anti-nucleophosmin antibody. The molecular weight of the interacting protein was estimated as 37 kDa which corresponded to PP1δ. Anti-PP1δ antibody did not interact with the proteins precipitated with the normal rabbit IgG. As positive controls, the results of whole cell lysate stained with the anti-nucleophosmin antibody and anti-PP1δ antibody are also shown in Figure 5A and 5B, respectively.
Fig. 5.
PP1δ interacts with nucleophosmin. Whole cell lysate prepared from Saos-2 cells was immunoprecipitated with the anti-PP1δ antibody and normal rabbit IgG (A) or with the anti-nucleophosmin (NPS) antibody and normal goat IgG (B). The immunoprecipitants were analysed by Western blotting using the anti-NPS antibody (A) or anti-PP1δ antibody (B). As a positive control, whole cell lysate was used as input.
IV. Discussion
The specific localization of the catalytic subunit of PP1δ in nucleoli in Saos-2 and MG63 cells indicates that the nucleolus is the site where this enzyme functions. These findings confirm our previous results [6] and those of others [14, 19]. The antibody used in the latter reports was raised against the peptide with additional three amino acids to the peptide that we had used to raise the antibody. However, other studies reported that PP1γ1 rather than PP1δ localized within the nucleolus [1, 29]. The differences of the results obtained from the independent laboratory may be explained in part by the specificity of the antibody used and different conditions used in the independent experiment. The process regulating the nucleolar location of PP1δ is quite interesting. It should be noted that PP1δ does not exhibit any evidence of nuclear localization signals in its sequence [23, 25]. However, proteins the sizes of catalytic subunit of PP1δ have been shown to freely diffuse into the nucleus without the necessity for specific translocation sequences. The noncatalytic subunits of PP1s have a targeting function, enabling the phosphatases to associate with a particular cellular fraction. Once in the nuclei, PP1s bind to immobile components to maintain their nuclear localization. The highest concentration of PP1s is found in the nucleus, where the enzymes are both in the nucleoplasm and associated with heterochromatin [12]. PP1 activity increased in the nuclei of hepatocyte primary cultures stimulated by EGF, whereas no isoforms showed any changes in concentration under these conditions [13]. The nucleolar activity of PP1s including PP1δ is not known at present.
We paid much attention to the relationship between PP1δ and nucleophosmin in the present study. We demonstrated that PP1δ was localized in the nucleolus in the cultured Saos-2 cells and that this enzyme was associated with nucleophosmin. The function of PP1s depends on the localization of these enzymes in cells. As with protein kinases and other enzymes, protein phosphatases should be associated with or located near to their substrates [9, 11, 20], hence the substrates of PP1δ should be present in the nucleolus. PP1 binding proteins are thought to have an R/KV/IXF, R/KXV/IF [4] or IKGI [10, 28] motif; however, such motifs are not identified in nucleophosmin. The association between PP1δ and nucleophosmin should be indirect and some regulatory subunits could exist that allow PP1δ to bind to nucleophosmin. However, there still remains the possibility that nucleophosmin binds directly to PP1δ by some unknown binding domains or mechanisms. The interaction between PP1δ and nucleophosmin demonstrated by immunoprecipitation and Western analysis suggests the possibility that PP1δ is the enzyme that dephosphorylates nucleophosmin. It was recently reported that PP1β (δ) dephosphorylated nucleophosmin both in the genotoxic stress and growth conditions that facilitated DNA repair [16]. Although it is not clear whether or not the 38-kDa protein is a dephosphorylated form of nucleophosmin, the amount of a 38-kDa protein decreased by the protein phosphatase inhibitor okadaic acid-treated Saos-2 cells (unpublished data). These findings suggest that nucleophosmin could be partially dephosphorylated into a 38-kDa protein detected with an anti-nucleophosmin antibody. This does not rule out the possibility that nucleophosmin is partially proteolyzed into a 38-kDa protein. It was also reported that localization of PP1δ is similar to that of nucleolin (C23) and that nucleolin could be a candidate substrate for PP1δ [7, 17].
V. Acknowledgments
We wish to thank Ms. Eiko Sasaki for her skillful technical assistance. This study was supported in part by grants from the Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan (TH and HM).
VI. References
- 1.Andreassen P. R., Lacroix F. B., Villa-Moruzzi E., Margolis R. L. Differential subcellular localization of protein phosphatase-1α, γ1, and δ isoforms during both interphase and mitosis in mammalian cells. J. Cell Biol. 1998;141:1207–1215. doi: 10.1083/jcb.141.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aoyama H., Ikeda Y., Miyazaki Y., Yoshimura K., Nishio S., Yamamoto T., Yano M., Inui M., Aoki H., Matsuzaki M. Isoform-specific roles of protein phosphatase 1 catalytic subunits in sarcoplasmic reticulum-mediated Ca2+ cycling. Cardiovasc. Res. 2010;89:79–88. doi: 10.1093/cvr/cvq252. [DOI] [PubMed] [Google Scholar]
- 3.Bollen M., Peti W., Ragusa M. J., Beullens M. The extended PP1 toolkit: designed to create specificity. Trends Biochem. Sci. 2010;35:450–458. doi: 10.1016/j.tibs.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egloff M. P., Johnson D. F., Moorhead G., Cohen P. T., Cohen P., Barford D. Structural basis of the recognition of regulatory subunits by the catalytic subunit of protein phosphatase 1. EMBO J. 1997;16:1876–1887. doi: 10.1093/emboj/16.8.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fardilha M., Esteves S. L., Korrodi-Gregório L., da Cruz e Silva O. A., da Cruz e Silva F. F. The physiological relevance of protein phosphatase 1 and its interacting proteins to health and disease. Curr. Med. Chem. 2010;17:3996–4017. doi: 10.2174/092986710793205363. [DOI] [PubMed] [Google Scholar]
- 6.Haneji T., Morimoto H., Morimoto Y., Shirakawa S., Kobayashi S., Kaneda C., Shima H., Nagao M. Subcellular localization of protein phosphatase type 1 isotypes in mouse osteoblastic cells. Biochem. Biophys. Res. Commun. 1998;248:39–43. doi: 10.1006/bbrc.1998.8913. [DOI] [PubMed] [Google Scholar]
- 7.Haneji T. Association of protein phosphatase 1 delta with nucleolin in osteoblastic cells and cleavage of nucleolin in apoptosis-induced osteoblastic cells. Acta Histochem. Cytochem. 2005;38:1–8. [Google Scholar]
- 8.Hashikawa T., Nakazawa K., Mikawa S., Shima H., Nagao M. Immunohistochemical localization of protein phosphatase isoforms in the rat cerebellum. Neurosci. Res. 1993;22:133–136. doi: 10.1016/0168-0102(95)00886-x. [DOI] [PubMed] [Google Scholar]
- 9.Hata M., Amano I., Tsuruga E., Kojima H., Sawa Y. Immunoelectron microscopic study of podoplanin localization in mouse salivary gland myoepithelium. Acta Histochem. Cytochem. 2010;43:77–82. doi: 10.1267/ahc.10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang H. B., Horiuchi A., Watanabe T., Shih S. R., Tsay H. J., Li H. C., Greengard P., Nairn A. C. Characterization of the inhibition of protein phosphatase-1 by DARPP-32 and inhibitor-2. J. Biol. Chem. 1999;274:7870–7878. doi: 10.1074/jbc.274.12.7870. [DOI] [PubMed] [Google Scholar]
- 11.Inagaki N., Ito M., Nakano T., Inagaki M. Spatiotemporal distribution of protein kinase and phosphatase activities. Trends Biochem. Sci. 1994;19:448–452. doi: 10.1016/0968-0004(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 12.Jagiello I., Beullens M., Stalmans W., Bollen M. Subunit structure and regulation of protein phosphatase-1 in rat liver nuclei. J. Biol. Chem. 1995;270:17257–17263. doi: 10.1074/jbc.270.29.17257. [DOI] [PubMed] [Google Scholar]
- 13.Kakinoki Y., Mizuno Y., Takizawa N., Imai Y., Miyazaki T., Kikuchi K. TGF1 suppresses EGF-induced increase in nuclear type 1 protein phosphatase activity at the G1/S transition of hepatocyte proliferation. FEBS Lett. 1994;352:356–360. doi: 10.1016/0014-5793(94)00993-7. [DOI] [PubMed] [Google Scholar]
- 14.Kotani H., Ito M., Hamaguchi T., Ichikawa K., Nakano T., Shima H., Nagao M., Ohta N., Furuichi Y., Takahashi T., Umekawa H. The δ isoform of protein phosphatase type 1 is localized in nucleolus and dephosphorylates nucleolar phosphoproteins. Biochem. Biophys. Res. Commun. 1998;249:292–296. doi: 10.1006/bbrc.1998.9126. [DOI] [PubMed] [Google Scholar]
- 15.Lim M. J., Wang X. W. Nucleophosmin and human cancer. Cancer Detect. Prev. 2006;30:481–490. doi: 10.1016/j.cdp.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin C. Y., Tan B. C. M., Liu H., Shih C. J., Chien K. Y., Lin C. L., Yung B. Y. M. Dephosphorylation of nucleophosmin by PP1β facilitates pRB binding and consequent E2F1-dependent DNA repair. Mol. Biol. Cell. 2010;21:4409–4417. doi: 10.1091/mbc.E10-03-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morimoto H., Okamura H., Haneji T. Interaction of protein phosphatase 1 delta with nucleolin in osteoblastic cells. J. Histochem. Cytochem. 2002;50:1187–1193. doi: 10.1177/002215540205000905. [DOI] [PubMed] [Google Scholar]
- 18.Muramatsu M., Hayashi Y., Onishi T., Sakai M., Takai K. Rapid isolation of nucleoli from detergent purified nuclei of various tumor and tissue culture cells. Exp. Cell Res. 1974;88:245–251. doi: 10.1016/0014-4827(74)90250-x. [DOI] [PubMed] [Google Scholar]
- 19.Murányi A., Erdodi F., Ito M., Gergely P., Hartshorne D. J. Identification and localization of myosin phosphatase in human platelets. Biochem. J. 1998;330:225–231. doi: 10.1042/bj3300225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noda Y., Amano I., Hata M., Kojima H., Sawa Y. Immunohistochemical examination on the distribution of cells expressed lymphatic endothelial marker podoplanin and LYVE-1 in the mouse tongue tissue. Acta Histochem. Cytochem. 2010;43:61–68. doi: 10.1267/ahc.10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okuwaki M. The structure and functions of MPM1/Nucleophosmin/B23, a multifunctional nucleolar acidic protein. J. Biochem. 2008;143:441–448. doi: 10.1093/jb/mvm222. [DOI] [PubMed] [Google Scholar]
- 22.Rodan S. B., Imai Y., Thiede M. A., Wesolowski G., Thompson D., Bar-Shavit Z., Shull S., Mann K., Rodan G. A. Characterization of human osteosarcoma cell line (Saos-2) with osteoblastic properties. Cancer Res. 1987;47:4961–4966. [PubMed] [Google Scholar]
- 23.Sasaki K., Shima H., Kitagawa Y., Irino S., Sugimura T., Nagao M. Identification of members of the protein phosphatase 1 gene family in the rat and enhanced expression of protein phosphatase 1α gene in rat hepatocellular carcinomas. Jpn. J. Cancer Res. 1990;81:1272–1280. doi: 10.1111/j.1349-7006.1990.tb02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi Y. Serine/threonine phosphatases: mechanism through structure. Cell. 2009;139:468–484. doi: 10.1016/j.cell.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Shima H., Hatano Y., Chun Y. S., Sugimura T., Zang Z., Lee E. Y. C., Nagao M. Identification of PP1 catalytic subunit isotypes PP1γ1, PP1δ and PP1α in various rat tissues. Biochem. Biophys. Res. Commun. 1993;192:1289–1296. doi: 10.1006/bbrc.1993.1556. [DOI] [PubMed] [Google Scholar]
- 26.Shima H., Haneji T., Hatano Y., Kasugai I., Sugimura T., Nagao M. Protein phosphatase 1γ2 is associated with nuclei of meiotic cells in rat testis. Biochem. Biophys. Res. Commun. 1993;194:930–937. doi: 10.1006/bbrc.1993.1910. [DOI] [PubMed] [Google Scholar]
- 27.Shirakawa S., Mochizuki H., Kobayashi S., Takehara T., Shima H., Nagao M., Haneji T. Immunohistochemical and immunoblotting identification of protein phosphatase 1γ1 in rat salivary glands. FEBS Lett. 1996;393:57–59. doi: 10.1016/0014-5793(96)00821-6. [DOI] [PubMed] [Google Scholar]
- 28.Shirato H., Shima H., Sakashita G., Nakano T., Ito M., Lee E. Y. C., Kikuchi K. Identification and characterization of a novel protein inhibitor of type 1 protein phosphatase. Biochemistry. 2000;39:13848–13855. doi: 10.1021/bi001326n. [DOI] [PubMed] [Google Scholar]
- 29.Trinkle-Mulcahy L., Sleeman J. E., Lamond A. I. Dynamic targeting of protein phosphatase 1 within the nuclei of living mammalian cells. J. Cell Sci. 2001;114:4219–4228. doi: 10.1242/jcs.114.23.4219. [DOI] [PubMed] [Google Scholar]
- 30.Virshup D. M., Shenolikar S. From promiscuity to precision: protein phosphatases get a makeover. Mol. Cell. 2009;33:537–545. doi: 10.1016/j.molcel.2009.02.015. [DOI] [PubMed] [Google Scholar]