Abstract
The TRK-fused gene (TFG in human, Tfg in rat) was originally identified in human papillary thyroid cancer as a chimeric form of the NTRK1 gene. It has been reported that the gene product (TFG) plays a role in regulating phosphotyrosine-specific phosphatase-1 activity. However, no information regarding the localization of Tfg in rat tissues is available. In this study, we investigated the expression of Tfg mRNA in normal rat tissues using reverse transcription-polymerase chain reaction (RT-PCR). We also produced an antibody against Tfg gene products and examined the localization of TFG in the rat brain and retina. The RT-PCR experiments demonstrated that two types of Tfg mRNA were expressed in rat tissues: the conventional form of Tfg (cTfg) and a novel variant form, retinal Tfg (rTfg). RT-PCR analyses demonstrated that cTfg was ubiquitously expressed in rat tissues, while rTfg was predominantly expressed in the brain and retina. Western blot analysis demonstrated two bands with molecular weights of about 30 kDa and 50 kDa in the rat brain. Immunohistochemistry indicated that TFG proteins were predominantly expressed by neurons in the brain. In the rat retina, intense TFG-immunoreactivity was detected in the layer of rods and cones and the outer plexiform layer.
Keywords: TFG, retina, brain, alternative splicing, oncogene
I. Introduction
The TRK-fused gene (TFG in human, Tfg in rat) was first identified in human papillary thyroid carcinoma as a fusion partner of the NTRK1 gene, which encodes a tyrosine kinase receptor for nerve growth factor [11, 12]. As a result of chromosomal rearrangements, TFG is fused to the 3' end of the NTRK1 gene, generating the TRK-T3 oncogene [5]. In addition to thyroid cancer, TFG gene is expressed as a fusion partner of various cancer oncogenes, such as anaplastic large cell lymphoma [8] and myxoid chondrosarcoma [9].
The TFG gene is highly conserved among mammals such as human, pig, and mouse, and also in C. elegans [16]. The TFG protein (TFG) contains a coiled-coil sequence in the N-terminal region, an N-glycosylation site, phosphorylation sites for casein kinase 2 and protein kinase C (PKC), and SH2- and SH3-binding motifs [15]. These structures are important for oncogenic activation in carcinoma; however, the function of TFG protein is unclear. In 2005, Roccato et al. reported that TFG protein interacts with and negatively regulates SH2 domain-containing phosphotyrosine-specific phosphatase-1 (SHP-1) [21]. SHP-1 is expressed in the hematopoietic system [19, 22], epithelial cells [18] and the nervous system [10, 14]. Therefore, TFG protein may play an important role in these tissues by regulating SHP-1. In addition, the analogue of TFG gene in C. elegans, tfg-1, suppresses apoptosis and is essential for normal cell size [3].
Recently, we cloned a novel variant of Tfg from a rat retinal cDNA library and named it retinal Tfg (rTfg). While Northern blot analyses have demonstrated that TFG is widely expressed in various fetal and adult tissues of human [5] and mouse [16], there is no information about the localization of Tfg gene and TFG protein in the rat. In this study, therefore, we examined the expression patterns of Tfg mRNAs in normal rat tissues using quantitative reverse transcription-polymerase chain reaction (RT-PCR), and used immunohistochemistry to localize TFG proteins in rat brain and retina using an antibody against the common parts of the conventional and variant forms of TFG proteins.
II. Materials and Methods
Animals
This study was performed in accordance with the PHS Policy on Humane Care and Use of Laboratory Animals, the NIH Guide for the Care and Use of Laboratory Animals (NIH publication No. 85-23, revised 1985) and the Animal Welfare Act (7 U.S.C. et seq.). The animal use protocol was approved by the Institutional Animal Care and Use Committee of Shiga University of Medical Science. Ten Wistar rats weighing 200–300 g were used in this study. The animals were housed under a 12:12 hr light-dark schedule and had food and water available ad libitum.
For the production of antisera against the common region of TFG proteins, three Japanese white rabbits (Kiwa Lab., Wakayama, Japan), weighing 2.0–2.5 kg, were used. The animals were injected every 2 weeks with the fusion protein (200 µg/rabbit) emulsified with Freund’s adjuvant (Life Technologies Inc.). Blood samples were collected 4 days after every booster injection. The specificity of the antiserum was examined by Western blot analysis and immunoabsorption tests as described below.
Immunoscreening of cDNA library from rat retina
The rTfg cDNA was cloned from a rat retinal cDNA library using an antiserum against deltorphin [1, 2]. A rat L Zap cDNA library (Clontech Laboratories, Mountain View, CA, USA) was used. The final library containing 1×106 plaques was screened with the deltorphin antiserum. Clones detected by this screening were applied to a second screening, and screening was repeated until a monoclonal colony was obtained.
The inserted cDNA of a phage from each colony was amplified by polymerase chain reaction (PCR) using AmpliTaq Gold and universal primers. The amplified cDNA was subcloned into a pCR2 vector using a TA cloning kit (Invitrogen Corp., Carlsbad, CA, USA), and the cDNA sequence was determined using an ABI 3100 DNA sequencer (Applied Biosystems, Foster City, CA, USA).
Tissue preparations
Ten male Wistar rats (two for mRNA analysis, two for Western blots and six for immunohistochemistry) weighing 200–300 g, were used. Rats were purchased from Clea Japan (Osaka, Japan). Tissue preparation was performed essentially as reported before [7, 13, 23]. In brief, under pentobarbital anesthesia (80 mg/kg), four animals were perfused via the ascending aorta with 10 mM phosphate buffer containing 0.9% NaCl (PBS; pH 7.4) to remove blood. Two rats were used for each of Western blot analysis and RT-PCR experiments. For immunohistochemistry, the other six rats were transcardially perfused with 10 mM PBS followed by an ice-cold fixative of 0.1 M phosphate buffer (PB; pH 7.4) with 4% formaldehyde (FA). The brain and eyes were removed from each rat and postfixed for 24 hr in 0.1 M PB containing 4% FA at 4°C. The tissues were then immersed for at least 48 hr in 0.1 M PB containing 15% sucrose and 0.1% sodium azide for cryoprotection. The tissues were cut into 20 µm thick sections using a cryostat. Some sections were mounted onto gelatin-chrome-coated glass slides and other sections were used in a free-floating state.
RT-PCR experiments
Total RNA was isolated from the retina, brain, liver, kidney, heart and lung using the acid guanidium thiocyanate-phenol method [4]. Prior to reverse transcription, the total RNA was incubated for 1 hr with 10 units of RNase-free DNase I (Amersham Biosciences Corp.) and 20 units of recombinant RNase inhibitor (Wako Pure Chemicals, Osaka, Japan) at 37°C, to eliminate any trace DNA contamination. RNA (5 µg) was then reverse transcribed for first strand cDNA synthesis using 80 units of SuperScript II (Gibco BRL, Gaithersburg, MD, USA) and 500 pmol of oligo dT12–18 (Amersham Biosciences Corp) as the primers.
In addition, β-actin mRNA was amplified to assess the variability of mRNA contents. The primers for β-actin PCR were designed to encompass different exons, and were expected to yield a 266 base pair (bp) PCR fragment. The reaction mixture for PCR consisted of 2 ng/µl of the template cDNA, 0.8 µM of each of the primers, 0.2 mM of each of four deoxynucleotide triphosphates and 2.0 U Taq polymerase (AmpliTaq Gold, PerkinElmer Japan Co, Tokyo, Japan) dissolved in 1×PCR buffer containing 1.5 mM MgCl2. After heat activation for 10 min at 95°C, the sample was amplified using the following profile of thermal cycling: (1) denaturation at 95°C for 30 sec; (2) annealing at 56°C for 30 sec; and (3) extension at 72°C for 60 sec. We performed the PCR for 28–30 cycles. The PCR products obtained were electrophoresed on a 1–3% agarose gel and stained with ethidium bromide. The nucleotide sequences of the PCR products were determined using an ABI 3100 DNA sequencer (Applied Biosystems).
The quantification of rTfg mRNA in various tissues was analyzed by real-time PCR using a Light Cycler (Roche Applied Science, Mannheim, Germany). The sense primer was 5'-AGATTGAAGGTCAGATGTAC-3' and the antisense primer was 5'-GGAAGTTGGTTGTTGGCCAT-3'. β-actin was used as an internal control to normalize the data. The reaction mixture consisted of 1 X LC-FastStart mixture (Roche Applied Science, Mannheim, Germany), 4 mM MgCl2, 0.5 µM primers and 10 ng/µl cDNA. Cycling conditions involved an initial 10 min incubation at 95°C followed by 1–50 cycles of denaturation at 95°C for 15 sec; annealing at 56°C for 8 sec for rTfg, and at 58°C for 5 sec for β-actin; and extension at 72°C for 15 sec. The expression levels of the rTfg mRNA in the tissues were normalized to that of β-actin mRNA.
Production of antibody against TFG gene products
Antisera were raised using a synthetic peptide corresponding to the common region of TFG protein and its variant as an antigen (SGPPSAPTEDRSGTP: amino acid number 194–208, Accession number BC078947 on GenBank). The peptide was conjugated to bovine serum albumin (BSA) with glutaraldehyde. Three Japanese white rabbits (Kiwa Lab., Wakayama, Japan) received an initial subcutaneous injection of 200 µg of the hapten antigen mixed with equal volume of Freund’s complete adjuvant. The animals received subsequent injections of the hapten antigen mixed with incomplete adjuvant every two weeks. The antisera were collected 4–7 days after each boost. The specificity of the antisera was examined by Western blot and an immunoabsorption test.
Purification of antibody specific to TFG gene products using affinity chromatography
An antiserum was purified by affinity chromatography using CNBr activated sepharose gel bounded with the antigenic peptide (SGPPSAPTEDRSGT). One ml of the antiserum was applied to the affinity chromatograph. After washing the unbound protein with 10 mM PBS (pH 7.4, unbound fraction), the binding IgG was eluted by 0.1 M glycine HCl (pH 2.3). The purified IgG was neutralized with 1 M Tris HCl (pH 8.0). The eluted fraction was dialyzed with 10 mM PBS, and then stored in 50% glycerol/PBS. The immunoactivity was confirmed by ELISA assay and an immunospot test. The purification was done by Medical & Biological Laboratories, Co. Ltd. (Ina, Japan).
Immunospot test
The antigenic peptide (SGPPSAPTEDRSGT) was purchased from Medical & Biological Laboratories, Co. Ltd. (Ina, Japan). Small drops (~1 µl) of the peptide were spotted on the nitrocellulose membrane at doses of 1000, 250, 63, 16, 4 and 1 ng/µl. After drying in air, the membrane was incubated with 2% gelatin in 25 mM Tris HCl-buffered saline (pH 7.5) containing 0.1% Tween 20 (TBST) to block non-specific protein binding (Sigma). The membrane was incubated for 1 hr with the pre-purified antibody (1:10,000), the unbound fraction (0.5 µg/ml) or the purified antibody (0.5 µg/ml) in TBST. After washing with TBST, the membrane was incubated for 1 hr with a peroxidase-coupled anti-rabbit IgG Fab’ fragment (Histofine; Nichirei Corp., Tokyo, Japan; diluted 1:10). Peroxidase labeling was detected with 0.02% 3,3'-diaminobenzidine in 50 mM Tris-HCl buffer (pH 7.6) containing 0.3% nickel ammonium sulfate.
Western blot analysis
The brains were homogenized in 5 volumes of ice-cold 500 mM Tris-HCl (pH 7.4) containing 1% Triton-X100, 1 mM EDTA, 1 mM EGTA, 1 µg/ml pepstatin and Complete Mini protease inhibitor cocktail tablets (Roche Diagnostics, Mannheim, Germany; 1 tablet/10 ml). The homogenates were centrifuged at 12,000 g for 20 min at 4°C. The supernatants were collected as a crude protein fraction. Approximately 50 µg of the crude protein and pre-stained Precision Protein Standards (Bio-Rad Laboratories, Hercules, CA, USA) were electrophoresed on a 5–20% sodium dodecyl sulfate-polyacrylamide gel (Wako Pure Chemicals, Osaka, Japan) under reducing conditions and then transferred to a polyvinyliden difluoride membrane (Immobilon-P, Millipore Japan, Osaka, Japan). The membrane was incubated with 5% skim milk in 25 mM TBS for 1 hr at room temperature (RT), and further incubated with the purified TFG antibody (0.5 µg/ml) in 25 mM TBS containing 1% skim milk overnight at 4°C. After washing with 25 mM TBST, the membrane was reacted for 1 hr with a peroxidase-coupled anti-rabbit IgG (Jackson Laboratories; diluted 1:20,000) at RT. Chemiluminescence signals were obtained by using West Pico Substrate (Pierce Biotechnology, Rockford, IL).
Immunohistochemistry
Immunohistochemical staining for TFG was performed as previously described [15, 20, 23]. In brief, the sections were kept at 4°C for at least 4 days in 0.1 M PBS containing 0.3% Triton X-100 (PBST; pH 7.4) before staining. Sections were incubated for 20 min with PBST containing 0.3% H2O2 at room temperature to eliminate endogenous peroxidase. After washing with PBST, sections were incubated for 30 min with PBST containing 2% BSA to inhibit non-specific protein binding. The sections were incubated in sequence with: the rabbit anti-TFG antibody (1:20,000) or the purified TFG antibody (0.1 µg/ml) at 4°C for 2–3 days; biotinylated anti-rabbit IgG (1:1,000; Vector Laboratories, Burlingame, CA, USA) for 1 hr at RT; and avidin-biotin-peroxidase complex (1:4,000; Vector Laboratories) for 1 hr at RT. PBST containing 0.2% BSA was used to dilute the antibodies and PBST was used to wash the sections between each step. A purple color was developed with 0.02% 3,3'-diaminobenzidine and 0.3% nickel ammonium sulfate in 50 mM Tris-HCl buffer (pH 7.6). The free-floating sections were mounted on gelatin-chrome-coated glass slides and air-dried.
In addition, some rat retinal sections were used for immunofluorescent histochemistry [17]. The sections were incubated with the purified TFG antibody (0.1 µg/ml) at 4°C for 2–3 days. After washing with PBST, the sections were incubated in the dark for 2 hr with Alexa Fluor 647 conjugated anti-rabbit IgG (1:500; Molecular Probes, Eugene, OR, USA) at RT. After three rinses with PBST sections were mounted on silane-coated glass slides and examined under a confocal microscope (Nikon C1si, TE2000-E, Nikon, Tokyo, Japan).
For the immunoabsorption test, the purified antibody was preincubated with the TFG peptide (100 µg/ml) overnight at 4°C, and the sections were stained as described above. All staining was abolished following pre-incubation of the antibody with the TFG peptide.
III. Results
Cloning and sequencing of a variant of the Tfg gene
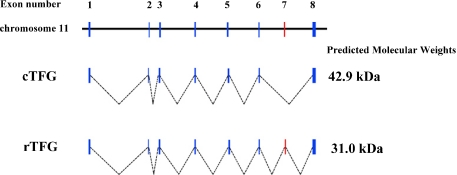
We cloned a variant of the Tfg gene from rat retinal tissue that contains an open reading frame of 837 bp, and encodes 282 amino acids plus a stop codon, a polyadenylation-signal and a poly A tail. The sequence had 98% similarity to cTfg mRNA, and we named it retinal Tfg (rTfg). According to the rat genome, the rTfg contained one additional exon (exon 7, red in Fig. 1) in comparison to the cTfg. The cTfg encodes a 42.9 kDa protein consisting of 396 amino acids, while rTfg encodes a 31.0 kDa protein consisting of 282 amino acids (Fig. 1).
Fig. 1.
Comparison of cTfg (upper) and rTfg (lower) cDNA structures. The rTfg contains additional one exon (exon 7 indicated by red).
Figure 2 shows a comparison of amino acid sequences between rat cTFG and rTFG proteins. Both proteins are rich in glutamine (Q). Eleven amino acids in the C-terminus of the rTGF protein are different from those of the cTFG protein. A putative coiled-coil domain (underlined, italicized characters), myristylation sites (double underline), SH2-binding site (wave line), and phosphorylation sites for PKC and casein kinase 2 (dashed underline) are preserved in the rTFG protein. However, three SH3-binding sites (bold), three myristylation site (double underline) and one glycosylation site (single underline) are lost in rTFG protein.
Fig. 2.
Amino acid sequence of cTFG and rTFG protein. A putative coiled-coil domain is underlined and italicized. N-glycosylation sites are underlined. Putative phosphorylation sites for protein kinase C and casein kinase 2 are indicated by dashed underlining. Myristylation sites are indicated by double underlining. SH2-binding sites are indicated by wavy line. SH3-binding sites are indicated in bold. Note that three SH3-binding sites of cTFG protein are lost in rTFG protein.
Expression of cTfg and rTfg mRNAs in adult rat tissues
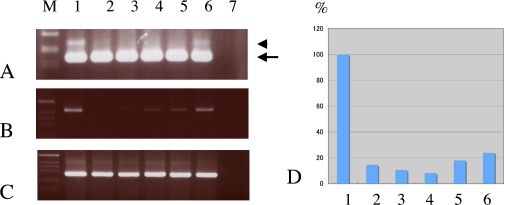
Figures 3A, B and C show the results of RT-PCR experiments using primer pairs to amplify both cTfg and rTFfg, rTfg and β-actin transcripts, respectively. The cTfg mRNA was detected in all tissues examined (arrow in Fig. 3A), while the rTfg mRNA (arrowhead in Fig. 3A) was only detected in the brain (lane 1 in Fig. 3A) and retina (lane 6 in Fig. 3A). When we used the primer pair specific to rTfg, the expression level of rTfg mRNA was found to be relatively high in the brain (lane 1 in Fig. 3B) and retina (lane 6 in Fig. 3B). β-actin mRNA was evenly expressed in all tissues examined (Fig. 3C). The real-time PCR demonstrated high expression levels of rTfg mRNA in the brain, and this was normalized to 100% (Fig. 3D). The expression level of rTfg mRNA in the retina was 24.7% of that in the brain, while in all other tissues, the mRNA level of rTfg was less than 20% of that in the brain.
Fig. 3.
RT-PCR (A, B, C) and real time-PCR (D) analyses of Tfg mRNAs and proteins in the rat retina. A: RT-PCR experiment using a primer set for the common region of cTfg and rTfg. A strong band of 170 bp (arrowhead) and a faint band of 211 bp (arrow) correspond well to the sizes of cTfg and rTfg, respectively. B: RT-PCR experiment using a primer set specific for rTfg. A single band of 426 bp is seen. C: RT-PCR experiment using a primer set specific for β-actin. β-actin mRNA was evenly expressed in all tissues examined. D: Real-time PCR demonstrated high expression levels of rTfg mRNA in the brain, and this was normalized to 100%. M indicates a marker. 1; brain, 2; heart, 3; lung, 4; colon, 5; liver, 6; retina.
Characterization of the antiserum against TFG proteins
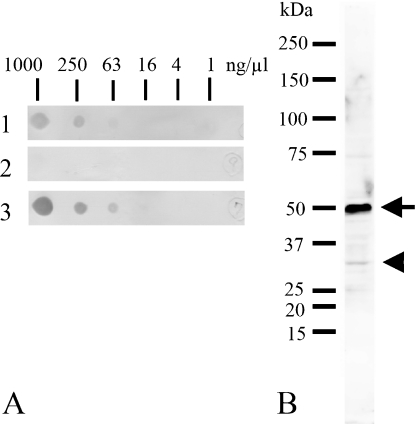
The characterization of the antiserum against TFG proteins was examined by an immunospot test, Western blots and an immunoabsorption test. Figure 4A shows the immunospot test of the antigenic peptide probed with the antiserum (lane 1, diluted at 1:10,000), unbounded fraction (lane 2, diluted at 1 µg/ml) and purified antibody (lane 3, diluted at 1 µg/ml). The antiserum (lane 1) and affinity-purified antibody (lane 3) detected the peptide at an amount of 250 ng/µl and 63 ng/µl, respectively. Unbound fraction failed to detect the antigen (lane 2). Figure 4B demonstrates the result of Western blot analysis of adult rat brain probed with the purified antibody. The antibody detected an intense band of about 50 kDa (arrow) and a weak band of about 30 kDa (arrowhead) in the brain homogenate.
Fig. 4.
Immunospot test (A) of antigenic peptide detected by the antiserum (1, diluted at 1:10,000), unbounded fraction (2, diluted at 0.5 µg/ml) and purified antibody (3, diluted at 0.5 µg/ml), and Western blot analysis (B) of rat brain homogenates probed with the purified antibody (diluted at 0.5 µg/ml). A: Antibodies pre- (1) and post- (3) purification using affinity chromatography of antigenic peptide detect the peptide at a concentration of 25 ng/µl. Unbound fraction cannot detect the antigen (2). B: The purified antibody detected two bands with molecular weights of approximately 30 kDa (arrowhead) and 50 kDa (arrow).
Localization of TFG proteins in adult rat brain and retina
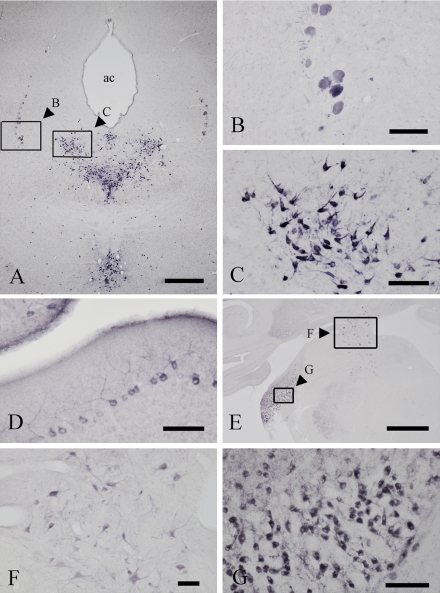
In the brain, TFG immunostaining was localized to neuronal structures including neuronal somata and proximal processes (Fig. 5). Positive neurons were mainly distributed in the hindbrain area. In the midbrain (Fig. 5A–C), intensely stained neurons were observed in the mesencephalic nucleus of trigeminal nerve (Fig. 5B) and the raphe dorsalis (Fig. 5C). In the cerebellum, Purkinje cells with dendrites were clearly stained (Fig. 5D). In the pons (Fig. 5E), intensely stained neurons were seen in the lateral vestibular nucleus (boxed area F in Fig. 5E) and cochlear nucleus (boxed area G in Fig. 5E). In the lateral vestibular nucleus, large neurons with proximal processes were positive for TFG (Fig. 5F).
Fig. 5.
Immunohistochemical localization of TFG proteins in the midbrain (A, B, C), cerebellum (D), and the pons (E, F, G) using the antibody against the common region of cTFG and rTFG proteins. A–C: In the midbrain, intensely stained neurons were seen in the mesencephalic nucleus of the trigeminal nerve (B) and dorsal raphe nucleus (C). as; the aqueductus cerebri. D: In the cerebellum, Purkinje cells with dendritic processes were clearly stained. E–G: In the pons, neurons in the lateral vestibular nucleus (F) and cochlear nucleus were strongly positive for TFG. Bars=1 mm (A, E); 100 µm (B, C, D, F and G).
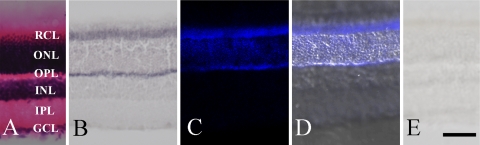
In the retina, TFG was present in the layer of rod and cone cells (RCL) and the outer plexiform layer (OPL) (Fig. 6), and the outer nuclear layer (ONL) was also weakly stained, as demonstrated by immunohistochemical staining (Fig. 6B) and immunofluorescent histochemistry (Fig. 6C and 6D) using the purified antibody against the common region of cTFG and rTFG. Pre-incubation of the antibody with a TFG peptide confirmed all staining observed is specific for TFG proteins (Fig. 6E).
Fig. 6.
Immunohistochemical staining using the purified antibody against the common region of cTFG and rTFG proteins. A: Hematoxylin and eosin staining shows the layers of the rat retina. RCL, layer of rod and cone cells; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. B: Immunohistochemistry shows intense TFG staining in the RCL and OPL and weak staining in the ONL. C: Immunofluorescent histochemistry shows TFG-immunopositivity in the RCL, ONL and OPL. D: Merged photographs of the immunofluorescent histochemistry and bright field photographs. E: Staining is abolished following preincubation of the antibody with the TFG peptide (100 µg/ml). Bar=50 µm.
IV. Discussion
Structure of cTFG and rTFG
In the present study, we investigated the expression and localization of a splice variant of Tfg cDNA, which we have named retinal Tfg (rTfg), in the adult rat brain and retina. The amino acid sequence showed that rTFG protein is a glutamine (Q)-rich protein, and compared with the conventional cTfg cDNA, rTfg cDNA carries the insertion of one exon (exon 7 in Fig. 1). Thus, rTfg mRNA is generated by alternative splicing. Since the amino acid sequence of the rTFG exon 7 contains a stop codon, rTfg mRNA encodes a 31.0 kDa protein, meaning that the molecular weight of rTFG protein is smaller than that of the cTFG protein (42.9 kDa). The rTFG protein preserves the regions that are predicted to be important for transformation and oncoprotein activation, including the coiled-coil domain [5, 6] and SH2-binding motif [20], suggesting that rTFG may have a similar role to cTFG protein. However, three SH3-binding sites (bold), three myristylation site (double underline) and one glycosylation site (single underline) are lost in rTFG protein. Therefore, it cannot be ruled out that cTFG and rTFG have different functions. For example, a previous study suggested that the SH3-binding motifs are not related to oncoprotein activation [20]. In order to clarify the issue, further studies will be needed.
The expression patterns of cTfg and rTfg
The RT-PCR experiments demonstrated the ubiquitous expression of cTfg mRNA, and confirms previous reports [5, 16]. In contrast, rTfg mRNA was preferentially expressed in the brain and less characteristically in the retina. Western blot analysis of brain homogenates using the purifed antibody against the common region of cTFG and rTFG proteins showed two bands with molecular weights of about 30 kDa and 50 kD. The results thus indicated that both types of Tfg are expressed in the brain and retina at both the mRNA and protein level. The intensity of 50 kDa band was much stronger than that of 30 kDa band. The results suggest that the cTFG is the main form of TFG proteins in the brain.
Interestingly, the molecular weight of 50 kDa is larger than predicted based upon the amino acid sequences of the cTFG protein, suggesting that cTFG protein has undergone post-translational modifications such as glycosylation and myristylation, because they have glycosylation and myristylation sites (Fig. 2).
Localization of TFG proteins in the brain and retina
Immunohistochemistry using the antibody against the common region of cTFG and rTFG proteins indicated that TFG proteins are localized to neurons in specific brain regions. Interestingly, positive neurons are richly distributed in the hindbrain area. The results suggest that TFG proteins may play a role in specific neuronal functions. To clarify this, we are now trying to map the distribution of TFG-positive neurons in the rat brain.
In the retina, TFG staining patterns differed across the cell layers. In particular, TFG immunostaining was observed in the RCL and the OPL. The ONL was also weakly stained. In these three layers, there are cell bodies and processes of rod and cone cells. Therefore, TFG protein may play an important role of rod and cone cells. Further studies will be needed to clarify this issue.
Conclusion
We cloned a TFG-like gene cDNA from a rat retinal cDNA library, and named it retinal Tfg (rTfg). Compared with the structure of conventional cTfg, rTfg contains one additional exon (exon 7) and preserves the regions that are important for oncogene activation. RT-PCR and Western blot analyses demonstrated that both cTfg and rTfg are expressed at the mRNA and protein levels in the brain and retina of adult rats. Immunohistochemistry using the purified antibody against the common region of the cTFG and rTFG proteins revealed that TFG proteins are localized to some neurons in specific brain regions. In the retina, TFG immunostaining was observed in the RCL and OPL, and the ONL was also weakly stained. The purified antibody should be a useful tool for further investigating TFG proteins.
V. Acknowledgments
We wish to thank M. Suzaki, N. Urushiyama and T. Yamamoto of the Central Research Laboratory at Shiga University of Medical Science for their technical assistance. This study was supported in part by grants-in-aid from the Ministry of Education, Culture, Science, Sports and Technology of Japan.
VI. References
- 1.Abe H., Tooyama I., Reanda T., Erspamer V., Kimura H. Production of antiserum to [D-Ala2]deltorphin I and its immunohistochemical application to the mouse brain. Neuroreport. 1992;3:669–672. doi: 10.1097/00001756-199208000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Abe H., Tooyama I., Reanda T., Erspamer V., Kimura H. Immunohistochemical localization of [D-Ala2]deltorphin-I in amacrine cells of rat retina. Peptides. 1994;15:49–54. doi: 10.1016/0196-9781(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 3.Chen L., McCloskey T., Joshi P. M., Rothman J. H. ced-4 and proto-oncogene tfg-1 antagonistically regulate cell size and apoptosis in C. elegans. Curr. Biol. 2008;18:1025–1033. doi: 10.1016/j.cub.2008.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 5.Greco A., Mariani C., Miranda C., Lupas A., Pagiliardini S., Pomati M., Pierotti M. The DNA rearrangement that generates the TRK-T3 oncogene involves a novel gene on chromosome 3 whose product has a potential coiled coil domain. Mol. Cell. Biol. 1995;15:6118–6127. doi: 10.1128/mcb.15.11.6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greco A., Fusetti L., Miranda C., Villa R., Zanotti S., Pagliardini S., Pierotti M. A. Role of the TFG n-terminus and coiled coil domain in the transforming activity of the thyroid TRK-T3 oncogene. Oncogene. 1998;16:809–816. doi: 10.1038/sj.onc.1201596. [DOI] [PubMed] [Google Scholar]
- 7.Han L., Itoh K., Yaoi T., Moriwaki S., Kato S., Nakamura K., Fushiki S. Prenatal and lactational exposure to bisphenol A in mice alters expression of genes involved in cortical barrel development without morphological changes. Acta Histochem. Cytochem. 2011;44:25–33. doi: 10.1267/ahc.10042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernandez L., Pinyol M., Hernandez S., Bea S., Pulford K., Rosenwald A., Lamant L., Falini B., Ott G., Mason D. Y., Delsol G., Campo E. TRK-fused gene (TFG) is a new partner of ALK in anaplastic large cell lymphoma producing two structurally different TFG-ALK translocations. Blood. 1999;94:3265–3268. [PubMed] [Google Scholar]
- 9.Hisaoka M., Ishida T., Imamura T., Hashimoto H. TFG is a novel fusion partner of NOR1 in extraskeletal myxoid chondrosarcoma. Genes Chromosomes Cancer. 2004;40:325–328. doi: 10.1002/gcc.20044. [DOI] [PubMed] [Google Scholar]
- 10.Horvat A., Schwaiger F., Hager G., Brocker F., Streif R., Knyazev P., Ullrich A., Kreutzberg G. W. A novel role for protein tyrosine phosphatase shp1 in controlling glial activation in the normal and injured nervous system. J. Neurosci. 2001;21:865–874. doi: 10.1523/JNEUROSCI.21-03-00865.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan D. R., Hampstead B. L., Martin-Zanca D., Chao M. V., Parada L. F. The TRK proto-oncogene product: a signal transducing receptor for the nerve growth factor. Science. 1991;252:554–558. doi: 10.1126/science.1850549. [DOI] [PubMed] [Google Scholar]
- 12.Klein R., Jing S., Namduri V., O’Rourke E., Barbacid M. The TRK proto-oncogene encodes a receptor for nerve growth factor. Cell. 1991;65:189–197. doi: 10.1016/0092-8674(91)90419-y. [DOI] [PubMed] [Google Scholar]
- 13.Kondo Y., Saruta J., To M., Shiiki N., Sato C., Tsukinoki K. Expression and role of the BDNF receptor-TrkB in rat adrenal gland under acute immobilization stress. Acta Histochem. Cytochem. 2010;43:139–147. doi: 10.1267/ahc.10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massa P. T., Saha S., Wu C., Jarosinski K. W. Expression and function of the protein tyrosine phosphatase SHP-1 in oligodendrocytes. Glia. 2000;29:376–385. [PubMed] [Google Scholar]
- 15.Mencinger M., Panagopoulos I., Andreasson P., Lassen C., Mitelman F., Aman P. Characterization and chromosomal mapping of the human TFG gene involved in thyroid carcinoma. Genomics. 1997;41:327–331. doi: 10.1006/geno.1997.4625. [DOI] [PubMed] [Google Scholar]
- 16.Mencinger M., Aman P. Characterization of TFG in Mus musculus and Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 1999;257:67–73. doi: 10.1006/bbrc.1999.0417. [DOI] [PubMed] [Google Scholar]
- 17.Nakahara H., Konishi Y., Beach T. G., Yamada N., Makino S., Tooyama I. Infiltration of T-lymphocytes and expression of ICAM-1 in the hippocampus of patients with hippocampal sclerosis. Acta Histochem. Cytochem. 2010;43:157–162. doi: 10.1267/ahc.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okano H., Bamba H., Hisa Y., Makino S., Ando S., Tamiya G., Goto S., Kaji R., Tooyama I. Distribution of TAFII250 in the rat laryngeal nervous system. Histol. Histopathol. 2005;20:1029–1035. doi: 10.14670/HH-20.1029. [DOI] [PubMed] [Google Scholar]
- 19.Plutzky J., Neel B. G., Rosenberg R. D. Isolation of a src homology 2-containing tyrosine phosphatase. Proc. Natl. Acad. Sci. U S A. 1992;89:1123–1127. doi: 10.1073/pnas.89.3.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roccato E., Pagliardini S., Cleris L., Canevari S., Formelli F., Pierotti M. A., Greco A. Role of TFG sequences outside the coiled coil domain in TRK-T3 oncogene. Oncogene. 2003;22:807–818. doi: 10.1038/sj.onc.1206189. [DOI] [PubMed] [Google Scholar]
- 21.Roccato E., Miranda C., Raho G., Pagliardini S., Pierotti M. A., Greco A. Analysis of SHP-1-mediated down-regulation of the TRK-T3 oncoprotein identifies TRK-fused gene (TFG) as a novel SHP-1-interacting protein. J. Biol. Chem. 2005;280:3382–3389. doi: 10.1074/jbc.M407522200. [DOI] [PubMed] [Google Scholar]
- 22.Wu C., Sun M., Liu L., Zhou G. W. The function of the protein tyrosine phosphatase SHP-1 in cancer. Gene. 2003;306:1–12. doi: 10.1016/s0378-1119(03)00400-1. [DOI] [PubMed] [Google Scholar]
- 23.Yasuhara O., Tooyama I., Aimi Y., Bellier J. P., Hisano T., Matsuo A., Park M., Kimura H. Demonstration of cholinergic ganglion cells in rat retina: expression of an alternative splice variant of choline acetyltransferase. J. Neurosci. 2003;23:2872–2881. doi: 10.1523/JNEUROSCI.23-07-02872.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]