Dear Editor,
The in vitro propagation (IVP) of animal spermatogonial stem cells (SSCs) provides materials for studying the mechanism of spermatogenesis and for developing novel animal transgenesis and human therapy. The IVP of SSCs is reported to be promoted by exogenous growth factors, among which the glial cell-derived neurotrophic factor (GDNF) has been recognized as the single essential one 1, 2. FGF2, a prototypical member of the FGF family, has been reported to be required for the IVP of SSCs from some mouse strains 2. The single-copy FGF2 gene generates one low-molecular weight (LMW) and several high-molecular weight (HMW) protein isoforms as a result of alternative translation initiations. While the HMW isoforms predominantly localize to the nucleus and signal independently of FGFR, the LMW isoform (also known as sFGF2) is predominantly found in the cytoplasm and released from the cells to execute its function through binding to membrane FGF receptors (FGFR1-4), activating the kinase activity of FGFRs and downstream signaling molecules such as PI3K-AKT, RAS-RAF-MAPK 3. In the present study, using cultured mouse spermatogonia (mSPG) that possess stem cell activity, we investigated the role, the source, the mechanism, and the target genes of FGF2 in the IVP and stem cell activity of cultured mSPG.
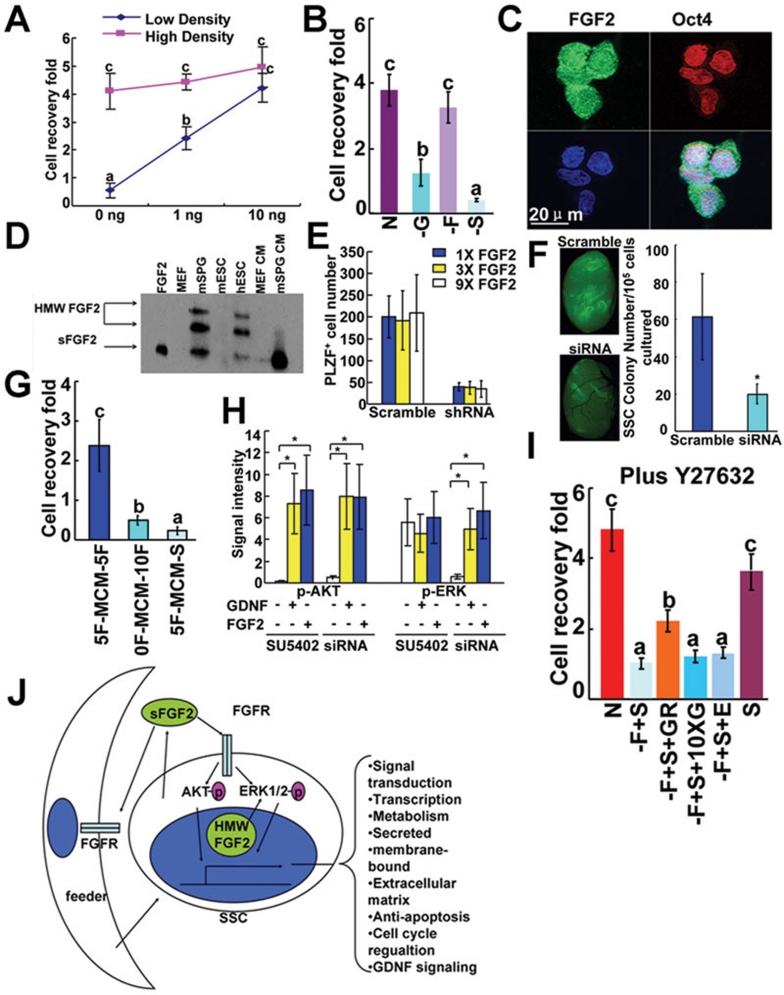
Culture and characterization of mSPG that contained SSCs was described in detail in Supplementary information, Data S1. The SSC activity of mSPG was evident as the transplanted cells colonized the recipient seminiferous tubules and gave birth to live offsprings (Supplementary information, Figure S1). We found that the IVP of high-density culture of mSPG (5 × 104 cells/cm2) was independent of exogenous sFGF2, while the low-density culture (1 × 104 cells/cm2) required 10 ng/ml exogenous sFGF2 (Figure 1A). However, addition of 10 μM FGFR inhibitor SU5402 4 (Supplementary information, Figure S2A) into the exogenous sFGF2-devoid medium (referred to as sFGF2 signaling blockade or the –F+S condition hereafter) of the high-density culture significantly reduced the IVP of mSPG (Figure 1B). As indicated by TUNEL labeling and BrdU incorporation assays, the reduction in IVP induced by sFGF2 signaling blockade was caused by a significant decrease in mitotic division and an increase in apoptosis of mSPG (Supplementary information, Figure S3A-S3F).
Figure 1.
FGF2 is produced by mSPG and is essential for their in vitro propagation (IVP) and stem cell activity. To assess the IVP of mSPG, mSPG were collected by pipetting and trypsinized into single cells and cultured under different conditions for 5 days. The IVP was evaluated in terms of cell recovery fold (RF) values, which were the ratios of the numbers of mSPG at the end of different treatments over those at the beginning. Values with different superscripts were significantly different (P < 0.05). (A) Low-density but not high-density culture of mSPG required the supply of exogenous sFGF2. n = 5. (B) Blocking sFGF2 signaling by FGFR inhibitor SU5402 in exogenous sFGF2-devoid medium downregulated the IVP of high-density mSPG (–F+S) compared with the normal culture (N), while withdrawal of exogenous sFGF2 only (–F) had no significant effect on the high-density culture of mSPG. As a control, withdrawal of GDNF (–G) also reduced the IVP of mSPG significantly. n = 3. (C) mSPG cultured on laminin-coated slide were positively stained for FGF2 both in the cytosol and the nucleus, while stained positively only in the nucleus for the stem cell marker OCT4. (D) sFGF2 and its HMW isoforms were detected in cell lysate of mSPG and hESCs but not of MEFs or mESCs. sFGF2 was detected in conditioned medium (CM) of mSPG but not of MEFs. Recombinant sFGF2 was used as a positive control. (E) FACS analysis revealed that the number of the undifferentiated PLZF+ mSPG was significantly reduced after FGF2 shRNA was introduced into mSPG by lentivirus and that the effect of shRNA could not be rescued by excess amount of sFGF2. n = 5. (F) Colonization of recipient testes by transplanted EGFP-mSSCs that were transiently transfected with FGF2 siRNA or scrambled siRNA. n = 3. (G) Quantitative evaluation of IVP of mSPG cultured on feeder-free laminin-coated dishes in three types of MCM (See text for the preparation of the three types of MEF-conditioned medium). n = 3. (H) Assessment of phosphorylation of AKT and ERK1/2 by FGF2 and GDNF in mSPG in which sFGF2 signaling was blocked by SU5402 or endogenous FGF2 was knocked down by siRNA in advance. mSPG were cultured for 18 h in the absence of both GDNF and sFGF2 but in the presence of SU5402 or siRNA, and GDNF or sFGF2 was then added back. *Represents significant difference from controls with no growth factors (P < 0.05). n = 3. (I) The adverse effect of sFGF2 signaling blockade could not be rescued by GDNF and other factors except for sFGF2 itself in the presence of apoptosis inhibitor Y27632. n = 7. (J) The schematic summary of the source, the signaling pathways and the categories of activated genes of FGF2 as detected by microarray analysis.
These results suggested that endogenous sFGF2 produced by mSPG themselves and/or feeder cells may support the IVP of mSPG. Indeed, immunostaining indicated that FGF2 protein was present in both the cytoplasm and the nucleus of mSPG (Figure 1C). FGF2 was highly expressed in pre-meiotic germ cells of both pup and adult mice, including undifferentiated spermatogonia as indicated by its colocalization with PLZF 5 (Supplementary information, Figure S4A and S4B). Western blotting assays showed that sFGF2 and its HMW isoforms were both produced by cultured mSPG but not by MEFs or mouse ESCs (Figure 1D). Moreover, 0.2 ng/ml sFGF2 was secreted into the medium by 105 mSPG during 5 days in culture estimated based on the ELISA results (Supplementary information, Figure S4C). The four receptors of FGF2 (FGFR1-4) 6 were detected by real-time RT-PCR in both mSPG and MEFs (Supplementary information, Figure S4D).
To further examine the role of FGF2 in mSPG IVP and their stem cell activity, we knocked down endogenous FGF2 in mSPG. The siRNA effectiveness was confirmed by the reduction of all the FGF2 isoforms by western blot (Supplementary information, Figure S3G). After the shRNA targeting the same sequence as the siRNA was delivered into mSPG by lentiviral infection, the number of PLZF+ undifferentiated mSPG 5 was significantly lower than that of the scrambled control (Figure 1E). Similar to the effect of –F+S treatment, the RNAi of FGF2 resulted in reduced mitosis and increased apoptosis (Supplementary information, Figure S3H and S3I). Interestingly, even 9-fold more exogenous sFGF2 could not reverse the effect of FGF2 shRNA, suggesting that endogenous HMW isoforms are required for the IVP of mSPG. Most importantly, the number of green colonies established by the siRNA-treated SSCs from the GFP-transgenic mice in recipient testes was significantly lower than that of the scrambled siRNA control (Figure 1F). Therefore, RNAi of endogenous FGF2 reduced not only the IVP of mSPG but also their stem cell activity.
To examine whether the mitogenic effect of sFGF2 on mSPG is mediated by receptors on either mSPG or MEFs or both, we cultured mSPG on feeder-free laminin-coated dishes with MEF-conditioned medium (MCM) prepared in the presence or absence of 5 ng/ml sFGF2 (5F-MCM, 0F-MCM). Additional sFGF2 was added to the MCM before use to make the final sFGF2 concentration of 10 ng/ml, resulting in two types of MCM (5F-MCM-5F, 0F-MCM-10F). mSPG cultured in 5F-MCM-5F expanded by 2.4-fold in 5 days, while those in 0F-MCM-10F did not expand, suggesting that some mitogenic substances for mSPG were produced by MEF stimulated by sFGF2 (Figure 1G). Moreover, mSPG did not expand in the 5F-MCM-SU5402 medium, which was prepared from 5F-MCM by supplementing 10 μM SU5402 before use. These results indicated that under normal culture condition the IVP-promoting effect of sFGF2 was mediated by receptors both on mSPG and on MEFs.
We next examined whether sFGF2 induces phosphorylation activation of AKT and ERK1/2 signaling pathways related to cell survival and mitotic division. The AKT phosphorylation of growth factor-starved mSPG was dramatically elevated by either GDNF or sFGF2. In contrast, the elevation of ERK1/2 phosphorylation was not induced by either GDNF or sFGF2 unless endogenous FGF2 was knocked down by siRNA in advance, suggesting that the HMW isoforms were responsible for the high basal-level phosphorylation of ERK1/2 (Figure 1H and Supplementary information, Figure S5A). Based on the similar phosphorylation change induced by GDNF and sFGF2, we wondered whether sFGF2 executed its function by enhancing GDNF signaling. We found that neither GDNF (10-fold excess amount) nor other factors such as soluble GFRα1 and EGF were able to rescue the adverse effect of the sFGF2 signaling blockade (Supplementary information, Figure S5B) even in the presence of an apoptosis inhibitor, the ROCK inhibitor Y26732 7 (Supplementary information, Figure S2B and Figure 1I). Therefore, sFGF2 and GDNF probably promote mSPG IVP through different signaling pathways despite of their similar effect on the phosphorylation of AKT and ERK1/2.
We performed microarray analysis to identify genes regulated by sFGF2 and compared them with those regulated by GDNF reported by Oatley et al 8. Four pairs of independent mSPG samples under the normal and the –F+S culture conditions were analyzed and the data were deposited into NCBI GEO database (accession No: GSE21294). A total of 732 upregulated (set G1, Supplementary information, Table S1) and 854 downregulated (set G2, Supplementary information, Table S1) genes by sFGF2 were identified, and some of them were confirmed by real-time RT-PCR (Supplementary information, Table S2). Functional annotation terms (FATs) enriched in upregulated genes mainly contain terms with keywords such as metabolic, nuclear/nucleus, DNA binding, transcription, regulation and intracellular, while those enriched in downregulated genes mainly contain terms with keywords such as extracellular, secreted, adhesion, glycoprotein and disulfide bond (Supplementary information, Table S3). The up or downregulated sets by sFGF2 and the corresponding enriched FATs were quite different from those by GDNF (Supplementary information, Table S4). For example, the similarity index (SI, defined as the percentage of the intersection of two sets over their union) of genes up and downregulated by the two factors was only 2% and 1%, respectively. The SIs of the FATs enriched in the up and downregulated sets by both factors were only 13% and 0%, respectively. Therefore, sFGF2 and GDNF probably execute their functions by quite different mechanisms, although they are both indispensable for the IVP of mSSCs.
As summarized in Figure 1J, we have shown that sFGF2 and its HMW isoforms are produced by mSPG, and are essential for the IVP of mSPG and their stem cell activity by regulating target genes involved in diverse processes that have little overlap with genes regulated by GDNF. The critical role of FGF2 signaling in spermatogenesis has been substantiated by human genetic studies in which constitutive activation of FGFR genes results in positive selection of spermatogonia 9, 10. Our results suggest that the maintenance of an optimal concentration of sFGF2 is critical for the establishment of SSC culture. It will be interesting to examine whether the expression of FGF2 is different in different mouse stains. We think that the conditional knockout of the FGF2 gene in mouse germ cells will help to elucidate the essential role of FGF2 in the proliferation of SSCs in vivo.
Acknowledgments
We thank Dr Hiroshi Kubota (University of Pennsylvania School of Veterinary Medicine, USA) for his kind help in setting up mSPG culture through E-mail communications. This study was supported by the National Basic Research Program of China (2006CB944004) and the National Natural Science Foundation of China (30300182, 30871406).
Footnotes
(Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary Material
Materials and Methods
Culture and characterization of mSPG.
Dose-dependent inhibition of mSPG mitotic division by SU5402 and apoptosis inhibition by Y27632.
Changes in apoptosis and mitotic division of mSPG induced by sFGF2 signaling blockade and RNAi of FGF2.
Expression of FGF2 and FGFRs.
Signaling pathways activated by FGF2 and GDNF.
Genes up-(set G1) and down-regulated by sFGF2 (set G2)
Expression of sFGF2-regulated genes as detected in microarray analysis and confirmed by real-time PCR.
Functional Annotation Terms (FATs) enriched in genes up- or down-regulated by sFGF2.
Functional annotation of genes regulated by GDNF.
Antibodies used in immunostaining and flow cytometry.
Primer sequences used for real-time PCR
References
- Kanatsu-Shinohara M, Ogonuki N, Inoue K, et al. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69:612–616. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci USA. 2004;101:16489–16494. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu PJ, Ferrari G, Galloway AC, Mignatti P, Pintucci G. Basic fibroblast growth factor (FGF-2): the high molecular weight forms come of age. J Cell Biochem. 2007;100:1100–1108. doi: 10.1002/jcb.21116. [DOI] [PubMed] [Google Scholar]
- Mohammadi M, McMahon G, Sun L, et al. Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science. 1997;276:955–960. doi: 10.1126/science.276.5314.955. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Sharma M, Nabeshima Y, Braun RE, Yoshida S. Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science. 2010;328:62–67. doi: 10.1126/science.1182868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahimi OA, Zhang F, Eliseenkova AV, Linhardt RJ, Mohammadi M. Proline to arginine mutations in FGF receptors 1 and 3 result in Pfeiffer and Muenke craniosynostosis syndromes through enhancement of FGF binding affinity. Hum Mol Genet. 2004;13:69–78. doi: 10.1093/hmg/ddh011. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Ueno M, Kamiya D, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- Oatley JM, Avarbock MR, Telaranta AI, Fearon DT, Brinster RL. Identifying genes important for spermatogonial stem cell self-renewal and survival. Proc Natl Acad Sci USA. 2006;103:9524–9529. doi: 10.1073/pnas.0603332103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goriely A, McVean GA, Rojmyr M, Ingemarsson B, Wilkie AO. Evidence for selective advantage of pathogenic FGFR2 mutations in the male germ line. Science. 2003;301:643–646. doi: 10.1126/science.1085710. [DOI] [PubMed] [Google Scholar]
- Goriely A, Hansen RM, Taylor IB, et al. Activating mutations in FGFR3 and HRAS reveal a shared genetic origin for congenital disorders and testicular tumors. Nat Genet. 2009;41:1247–1252. doi: 10.1038/ng.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Materials and Methods
Culture and characterization of mSPG.
Dose-dependent inhibition of mSPG mitotic division by SU5402 and apoptosis inhibition by Y27632.
Changes in apoptosis and mitotic division of mSPG induced by sFGF2 signaling blockade and RNAi of FGF2.
Expression of FGF2 and FGFRs.
Signaling pathways activated by FGF2 and GDNF.
Genes up-(set G1) and down-regulated by sFGF2 (set G2)
Expression of sFGF2-regulated genes as detected in microarray analysis and confirmed by real-time PCR.
Functional Annotation Terms (FATs) enriched in genes up- or down-regulated by sFGF2.
Functional annotation of genes regulated by GDNF.
Antibodies used in immunostaining and flow cytometry.
Primer sequences used for real-time PCR