Abstract
A methodology that utilizes 1H-NMR spectroscopy has been developed to simultaneously analyze toxic terpenes (thujone and camphor), major polyphenolic compounds, the total antioxidant capacity (ORAC) and the Folin-Ciocalteu (FC) index in foods and medicines containing sage. The quantitative determination of rosmarinic acid (limit of detection (LOD) = 10 mg/L) and total thujone (LOD = 0.35 mg/L) was possible using direct integration of the signals. For other parameters (derivatives of rosmarinic acid, carnosol and flavone glycosides, ORAC and FC index), chemometric regression models obtained separately for alcohol-based tinctures (R2 = 0.94–0.98) and aqueous tea infusions (R2 = 0.79–0.99) were suitable for screening analysis. The relative standard deviations for authentic samples were below 10%. The developed methodology was applied for the analysis of a wide variety of sage products (n = 108). The total thujone content in aqueous tea infusions was found to be in the range of not detectable (nd) to 37.5 mg/L (average 9.2 mg/L), while tinctures contained higher levels (range nd—409 mg/L, average 107 mg/L). The camphor content varied from 2.1 to 43.7 mg/L in aqueous infusions and from not detectable to 748 mg/L in tinctures (averages were 14.1 and 206 mg/L, respectively). Phenolic compounds were also detected in the majority of the investigated products. 1H-NMR spectroscopy was proven to have the ability to holistically control all important adverse and beneficial compounds in sage products in a single experiment, considerably saving time, resources and costs as NMR replaces four separate methodologies that were previously needed to analyze the same parameters.
Keywords: sage, Salvia officinalis L., tea infusion, NMR spectroscopy, polyphenols
Introduction
Sage teas and tinctures are used as traditional herbal medicines.1 Sage (Salvia officinalis L.) has been proposed as effective against cardiovascular diseases, brain and nervous disorders, various infections (such as throat infections, dental abscesses, and mouth ulcers) and digestion problems.1 Polyphenolic compounds (phenolic acids, polyphenols, flavonoids, phenolic terpenes) that lead to antioxidative potential could be responsible for these health benefits of sage products. However, on the other side, some investigations pointed out adverse effects of sage caused by the presence of two health relevant terpenoid compounds, thujone and camphor.1,2
Some attempts have been made by means of chromatographic,3–6 capillary electrophoretic7 and flame atomic absorption spectroscopic8 techniques to quantitatively determine selected compounds involved in the quality assessment of sage products. For example, α- and β-thujone and camphor were analyzed by gas chromatography (GC) with mass spectrometric (MS) detection.3 Polyphenols can be measured by ultra high performance liquid chromatography (UHPLC) with diode array and MS detection.4 However, there is currently no single method available that can provide a combined determination of all important compounds found in sage in a single experiment.
It is known that nuclear magnetic resonance (NMR) spectroscopy has excellent selectivity to qualify and quantify main constituents of complex mixtures.9 Therefore, we hypothesized that direct NMR spectroscopy might be applicable instead of complex chromatographic separation techniques. So far, NMR was extensively evaluated for the characterization of different types of tea,10–17 however, we were able to find only one article dealing with the application of 1H-NMR to sage tea and this evaluated only a single parameter (total phenolic content).18
The aim of the study was to develop a method that would allow us to simultaneously quantify the health-relevant compounds in sage tea using NMR spectroscopy. This includes thujone, camphor, rosmarinic acid, flavone glycosides as well as carnosol derivatives. The prediction of sum parameters, such as the Oxygen Radical Absorbance Capacity (ORAC), which characterizes the antioxidant capacity, and the Folin-Ciocalteu (FC) index, which is a measure of total polyphenolic content, was also studied. The procedure was then applied to analyze a large sample collection (n = 108) of sage foods and medicines.
Experimental
Samples and sample preparation
A total of 108 sage samples were analyzed. These included herbal teas (n = 66), instant drinking powders (n = 3), alcohol-based tinctures (n = 38), and one supplement (tablet), which were all purchased in November 2011 in wholesale and retail supermarkets, drug stores, health food shops, and pharmacies. An internet-based market research was conducted to identify all products and manufacturers available in Germany. Samples that were not found through internet searches or were not available in wholesale or in stores were obtained by mail-order from different internet shops. The sampling can be seen as representative for the current German market of sage products.
All solvents and reagents used were in pro analysis quality—rosmarinic acid and luteolin-7-O-glucoside (Sigma-Aldrich, Steinheim, Germany), α-/β-thujone-isomer mixture and camphor (Fluka, Buchs, Switzerland). Stock standard solutions were prepared at a final concentration of about 1000 mg/L in distilled water. For dissolving luteolin-7-O-glucoside, additional ethanol (about 50% v/v) was required. Na2SO3 (about 100 mg/L) was added to the standard solutions of phenolic compounds to prevent their oxidation. Calibration solutions were prepared by diluting the standard solution in water or in ethanol/water mixture (70% v/v). The calibration curves were evaluated by integrating specific resonances of the selected compounds against 3-(trimethylsilyl)-propionate acid-d4 (TSP) as an intensity reference.
For sage tea, infusions were generally prepared according to the standard protocol specified in DIN 10809/ISO 3103.19 A 150-mL white porcelain pot without lid was used. The aqueous infusions were analyzed as this is the form of consumption. Deviating from the standard protocol, we used 1.5 g of herbal tea material (or 1 tea bag containing 1.5 g) instead of 2.0 g per cup as this more realistically conforms to the specification as prescribed by the manufactures on the labeling. In general, the tea material was infused in 150 mL of hot water for 15 min. The instant drinking powders were prepared as prescribed on the package and then analyzed in the same fashion as tea. The sage supplement (3 solid tablets, about 0.6 g) was dissolved in 50 mL of ethanol.
For the aqueous tea infusions, 540 μL was mixed with 60 μL of pH 7.4 buffer (1.5 M KH2PO4 in D2O, 0.1% TSP, 3 mM NaN3). For medicinal sage extracts and other products based on ethanol, 300 μL of sample was mixed with 50 μL of pure ethanol, 190 μL of distilled water and 60 μL of the above mentioned pH 7.4 buffer. All samples have been measured within 5 hours after preparation to ensure their stability. Adding of ethanol along with buffer solution avoids problems with precipitation that could occur in tinctures with high amounts of essential oils, which precipitate if pure water or aqueous buffer is added. A separation of water and ethanol −OH protons is also effectively avoided using this protocol.20,21 In both cases, the mixture is then poured into an NMR tube and is directly measured.
NMR method
All 1H NMR measurements were performed using a Bruker Avance 400 Ultrashield spectrometer (Bruker Biospin, Rheinstetten, Germany) equipped with a 5-mm SEI probe with Z-gradient coils and a Bruker Automatic Sample Changer (B-ACS 120). All spectra were acquired at 300.0 K.
NMR spectra of the aqueous infusions were acquired using Bruker standard water suppression 1D noesygppr1d pulse sequence with 64 scans (NS) and four prior dummy scans (DS). The sweep width (SW) was 19.9914 ppm and the time domain point was set at 65536 (65k). Furthermore, for the acquisition of 2D J-resolved NMR spectra, the Bruker experiment jrespprgf was used. After the application of 16 dummy scans (DS), eight free induction decays (FIDs) (NS = 4) were collected into a time domain of 8192 (8.2k) complex data points using a 16.6595 ppm SW and a receiver gain (RG) of 22.6. For the ethanol-containing medicines (tinctures), we were able to use our previous procedure for alcoholic beverages20,21 without deviations.
The data were acquired automatically under the control of ICON-NMR (Bruker Biospin, Rheinstetten, Germany), requiring about 17 min per sample. All NMR spectra were phased, baseline-corrected and integrated using Topspin 3.1 (Bruker Biospin, Rheinstetten, Germany).
Chemometrics and reference analysis
We tested several spectral regions of 1D spectra for the chemometric calculations: aliphatic (δ 0.25–3.0 ppm), mid-field (δ 3.0–6.0 ppm), aromatic (δ 6.0–10 ppm) as well as the whole spectral region (δ 0.25–10 ppm) with δ 0.01 ppm bucket width. The bucketing was performed using the software Amix version 3.9.4 (Bruker Biospin, Rheinstetten, Germany). The resulting buckets were analyzed using the software package Unscrambler X version 10.0.1 (Camo Software AS, Oslo, Norway). Buckets were scaled with respect to the total spectrum intensity, thus taking into account the different concentrations and composition of samples. The sum of all points was used for integration. Residues of ethanol at δ 1.32–1.08 ppm and δ 3.52–3.79 ppm and water peaks at δ 4.85–4.75 ppm (only for water infusions) were excluded from the data sets when necessary.
Partial Least Squares (PLS) models for separate calibration sets comprising of 27 tea samples and 27 medicines containing ethanol were constructed and validated by means of leave-one-out full cross validation. The NMR ranges for the best fitting PLS model were selected based on the correlation between the reference results for the components in question. The optimal number of PLS factors, indicated by the lowest prediction error, was selected for all models. To additionally check the accuracy of our models, randomly selected five samples (excluded from the calibrations) were quantified.
The NMR spectra were also analyzed by principal component analysis (PCA). Analysis was done separately for sage teas and other products based on ethanol. The technique of full cross-validation was applied to determine the optimal number of principal components (PCs) needed to have robust models: this technique excludes one of the samples, models the remaining samples and tests the models on the left-out sample, so the significant number of components and the expected prediction error can be estimated. Seven and four PCs were found sufficient for the discrimination of tinctures and herbal teas by PCA. Then the data were plotted in a coordinate system defined by 2 PCs in order to detect the key relationships in the data.
Reference data for the PLS calibration sets were obtained using UHPLC-MS/MS4 and GC/MS3 methods. In addition, the Oxygen Radical Absorbance Capacity (ORAC) method was used to determine the antioxidant capacity of the tea samples according to Prior et al.22 The Folin-Ciocalteu (FC) index was determined according to the reference procedure for wine analysis using a commercial FC reagent (Merck, Darmstadt, Germany, No. 1.09001.0100).23 Further details on ORAC and FC determination were previously published.24
Application to authentic samples
The proposed methodology was applied for the determination of the selected parameters in authentic samples from the German market. Total thujone content and rosmarinic acid in tea infusions were analyzed by integrating the doublets at δ 1.16 ppm (thujone) and δ 6.37 ppm (rosmarinic acid) using linear calibration curves constructed with the substance/TSP ratios (2D J-resolved NMR spectra, water suppression). For the other products based on ethanol, singlets in the δ 2.13–3.11 ppm range (thujone) and δ 7.15–5.11 ppm range (rosmarinic acid) were used for direct quantification (2D J-resolved NMR spectra, water and ethanol suppression). Other parameters (camphor, luteolin-7-O-glucuronide, sum of rosmarinic acid and carnosol derivatives, sum of flavone glycosides as well as ORAC and FC index) were quantified using the PLS models.
Validation studies
For the validation, standard solutions as well as authentic sage samples were analyzed several times daily (intraday, n = 5) and for several days (interday, n = 10). The linearity of the calibration curves was evaluated in the range that covers concentrations typically found in sage products. The limits of detection (LOD) and quantification (LOQ) were calculated from the residual standard deviation of the regression line.25 The recovery rates were ascertained by adding standard solution at two different concentrations (within a range of observed concentrations for a particular compound) to a real sample separately for aqueous infusions and tinctures. For all calculations statistical significance was assumed at below the 0.05 probability level. To investigate the stability of aqueous tea infusions on the autosampler tray, two sage tea samples were prepared similar to the other samples and were analyzed for total thujone and rosmarinic acid content over two days (every one hour during the first seven hours and then every eight hours).
Results and Discussion
Method development
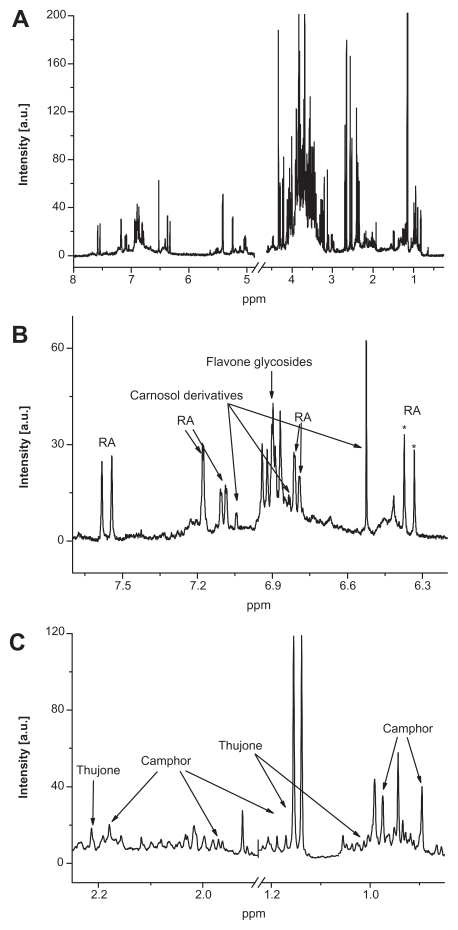
Figure 1 shows a representative 1H NMR spectrum of an aqueous infusion of sage tea. The compounds of interest were assigned by 2D J-resolved NMR experiments, multivariate analysis (loadings plot from PLS regression), spiking and comparison with spectra of standard solutions. Signals present in the high-frequency region at δ 6.0–10.0 ppm were mainly attributable to the polyphenolic acids and flavonoids (Fig. 1B). Similar to our previous experience with absinthe,21 thujone and camphor in sage products were observed in the low-frequency region at δ 0.0–3.0 ppm (Fig. 1C).
Figure 1.
1H NMR spectrum of sage tea in the whole δ 8.0–0.0 ppm range (A), as well as magnifications in the aromatic region (B) and aliphatic region (C) with assignments of compounds of interest. Stars denote the resonances of rosmarinic acid (RA) that were used for quantification (no overlap with other constituents).
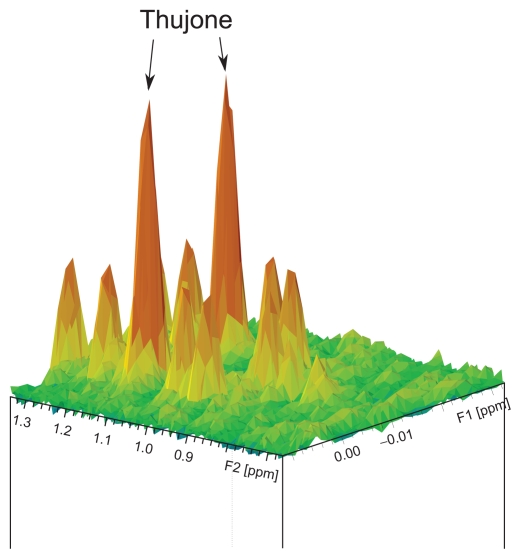
However, due to the high complexity of 1H-NMR spectra of sage products, we encountered some difficulties in developing direct quantification protocols by integration for all our compounds. Indeed, we have observed extensive signal overlap in the targeted regions (Fig. 1B and C). Direct integration is only possible for rosmarinic acid (which is the main representative of rosmarinic acid derivatives in sage products4) and total thujone (sum of α- and β-isomers). For rosmarinic acid, the doublet at δ 6.40–0.35 ppm (aqueous infusions) and the singlet at δ 7.15–5.11 ppm (ethanol-based products) were chosen for direct integration as these were not overlapped with other compounds, including other rosmarinic acid derivatives in the respective product category (Fig. 1B). However, while analyzing real samples, we observed that 2D J-resolved NMR spectra are preferable for quantification regarding to the specific resolution with easy identification and integration of the NMR-Signals. For thujone quantification, the doublet at δ 1.16 ppm (aqueous infusions) and the singlet at δ 2.12 ppm (for products based on ethanol) in 2D J-resolved NMR spectra were selected for the same reasons (Fig. 2).
Figure 2.
2D J-resolved 1H NMR spectrum of an aqueous tea infusion with a total thujone content of 9.4 mg/L (the region of the thujone doublet used for quantification by integration is magnified).
For the other parameters (besides rosmarinic acid and total thujone), chemometric techniques have to be applied for reliable quantification. The most commonly used choice in case of strong spectral overlap or if sum parameters have to be calculated (such as FC index and ORAC) is PLS regression. To perform PLS regression, we correlated different NMR ranges to the data of reference analysis. In our preliminary experiments, we evaluated all NMR spectral regions detailed in the Experimental section (both 1D Nuclear Overhauser Effect
Spectroscopy (NOESY) and 2D J-resolved spectra). Separate PLS models were developed for aqueous tea infusions and ethanol-containing tinctures because the NMR spectra were recorded under different conditions for these product groups (see Experimental section). The parameters of the best-fitting PLS models (the number of PLS factors, reference range, root mean squared error (RMSE) and correlation coefficient (R2)) are listed in Table 1. It turned out that the most informative ranges were in good agreement with the position of the most intensive resonances in the NMR spectra of the analyzed substances.
Table 1.
PLS correlation between data of reference analysis and NMR spectra of sage products (separately for tinctures and teas, 1D NOESY experiments).
| Parameter | Reference range | NMR range | PLS factors | Calibration | Validation | ||
|---|---|---|---|---|---|---|---|
| RMSEa | R2 | RMSE | R2 | ||||
| Ethanol-containing medicines | |||||||
| Camphor (mg/L) | 0–883 | 0–3 | 6 | 16 | 0.98 | 32 | 0.94 |
| FC | 0.6–40 | 0–3 | 5 | 13 | 0.94 | 19 | 0.90 |
| Herbal teas (water infusions) | |||||||
| Camphor (mg/L) | 1.5–55 | 0–3 | 4 | 2.4 | 0.79 | 2.87 | 0.76 |
| FC | 5–15 | 0–3 | 7 | 0.27 | 0.99 | 1.63 | 0.69 |
| ORAC (mmol trolox equivalents/100 mL) | 0.4–1.4 | 0–3 | 4 | 0.03 | 0.98 | 0.11 | 0.76 |
| Luteolin-7-O-glucuronide (mg/L) | 37–100 | 6–10 | 7 | 4.3 | 0.98 | 14 | 0.72 |
| Sum of flavone glycosides (mg/L) | 52–130 | 6–10 | 8 | 0.46 | 0.99 | 13 | 0.86 |
| Sum of rosmarinic acid derivatives (mg/L) | 23–208 | 0–10 | 4 | 9.0 | 0.98 | 15 | 0.91 |
| Sum of carnosol derivatives (mg/L) | 33–53 | 0–10 | 5 | 0.8 | 0.99 | 5.4 | 0.71 |
Note:
Root-mean squared error.
Notably, besides quantification of major constituents of sage-containing products, other important parameters such as FC index and ORAC can be obtained from the low-frequency 1H NMR region (δ 0–3 ppm) due to intensive resonances of methyl and methylene protons of phenolic compounds. A previous method to measure the total phenolic content by 1H NMR was based on the quantification of resonances of phenolic hydroxyl protons in the δ 8–14 ppm range.18 This method, however, cannot be used to directly measure aqueous solutions such as tea because an aprotic solvent (DMSO-d6) has to be used.
Validation
Table 2 summarizes the method validation results for terpenes and polyphenolic compounds calculated either with direct integration (thujone and rosmarinic acid) or with PLS models (all other parameters). The 1H-NMR assays were linear in a broad concentration range, making the analysis of all samples under the same conditions possible (without the need for dilution). The limits of detection for thujone and camphor were found to be below 1 mg/L while for polyphenolic compounds (rosmarinic acid and luteolin-7-O-glucoside) these values were considerably higher. For tinctures, the LOD and LOQ values were higher than for the tea infusions due to the dilution with the necessary ethanol addition (Table 2). As expected, the LODs were higher than what could be reached with chromatographic methods but were sufficient to detect the concentrations occurring in the products studied.
Table 2.
Results of method validation for selected parameters.
| Parameter | Total thujone | Camphor | Rosmarinic acid derivatives | Flavon glycosides derivatives | Total carnosol derivatives | FC index | ORAC | ||
|---|---|---|---|---|---|---|---|---|---|
| Rosmarinic acid | Total rosmarinic acid derivatives | Luteolin-7-Oglucuronide | Total flavon derivativesa | ||||||
| NMR range used for direct integration or for PLS models (δ ppm) | 1.18–1.14 (2.13–2.11) | 1.0–0.8 (2.50–2.40) | 6.40–6.35 (7.15–7.11) | 6–10 | 6–10 | 6.60–6.55 | 0–10 | 0–3 | 0–3 |
| Linear range (mg/L) | 1.0–1000 | 1–1000 | 20–1000 | –g | –g | 20–1000 | –g | –g | –g |
| LODb (mg/L) | 0.35 (1.0)f | 0.92 (2.0) | 10 (17) | –g | –g | 7.4 | –g | –g | –g |
| LOQb (mg/L) | 1.3 (2.5) | 2.2 (5.0) | 19 (35) | –g | –g | 26 | –g | –g | –g |
| Precision intradayc (%) | |||||||||
| Authentic sampled | 9.6 (5.8) | 0.8 (5.3) | 5.6e (8.1) | 12 | 6.3 | 11 | 11 | 6.8 | 3.5 |
| Standard solution | 8.3 (4.0) | 7.3 (4.5) | 6.8e (9.6) | –g | –g | 1.8 | –g | –g | –g |
| Precision interdayc (%) | |||||||||
| Authentic sampled | 11 (6.7) | 3.2 (3.7) | 8.8e (5.7) | 8.2 | 8.1 | 10 | 11 | 4.9 | 5.7 |
| Standard solution | 7.3 (4.9) | 6.5 (5.9) | 7.1e (3.5) | –g | –g | 1.7 | –g | –g | –g |
| Recovery ranged (%) | 91 (95) | 98 (94) | 108e (105) | 108 | 94 | 90 | 89 | 102 | 105 |
Notes:
Measured as luteolin-7-O-glucoside;
Limit of detection (LOD) and quantitation (LOQ) were determined by establishing a separate calibration curve near LODs. The limits were calculated from the residual standard deviation of the regression line;24
Precisions are expressed as relative standard deviation (RSD) (%), intraday (n = 5), interday (n = 10);
Recovery ranges and precision for authentic samples (except total thujone and rosmarinic acid) were calculated with PLS regression models;
Calculated with 2D J-resolved NMR by direct integration;
Values for ethanol solutions are shown in brackets;
Value not evaluated as the parameters can only be indirectly quantified using chemometric PLS models and no pure standard was available.
The NMR method is characterized by high precision and reproducibility. For standard solutions, the relative standard deviations (RSD) were always below 6% intraday and 8% interday (Table 2). For real samples the RSDs were also below 10% for all parameters. For further validation, we have applied our experimental protocol on five sage tea samples for which reference data were available (Table 2). In general, good precision for all parameters (calculated with integration or with PLS) was achieved with ranges between 0.8% and 11%. The recoveries were between 91 and 108%. Therefore, we believe that the proposed methodology is applicable to all sage-containing products with sufficient precision and reliability.
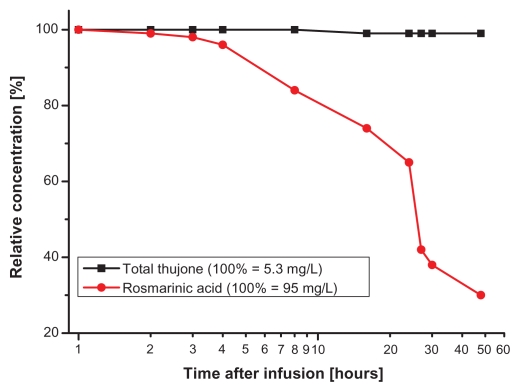
Furthermore, we wanted to evaluate the stability of water infusions of the tea samples while standing on the tray of the autosampler. We, therefore, conducted an experiment in which thujone and rosmarinic acid were analyzed in two days in two selected samples with results for one of them shown in Figure 3 (the other sample showed similar behavior). Up to five hours, the concentrations of thujone and rosmarinic acid found in the infusions remained constant (Fig. 3). After that, the actual amount of rosmarinic acid gradually decreased to 65% of the initial concentration (at 24 hours) and then reached 30% after 48 hours. The total thujone amount remained constant during the whole experiment (48 hours). This result reconfirmed the stability of thujone under storage conditions.26 Our results showed that rosmarinic acid (in contrast to thujone) is only stable in aqueous tea infusions for approximately five hours (which means that about 15 samples can be prepared and measured in one sequence by NMR).
Figure 3.
Experiment about sample stability of a sage tea infusion while standing in the NMR autosampler.
Measurement of authentic samples
We analyzed 64 herbal teas, out of which 24 products were sold as food and 42 products were sold as medicine (Table 3). In general, in both groups, a comparably wide variance of results was detected, confirming our previous study.3 The total thujone content varied between the range of not detectable to 26.6 mg/L (for products sold as food) or to 37.5 mg/L (for medicines).
Table 3.
Results of sage tea (measured in the aqueous tea infusion prepared according to DIN 10809/ISO 3103/1980 in a cup without lid). Values are given in [mg/L] (with the exception of FC-Index (without unit) and ORAC Index [mmol trolox equivalents/100 mL]).
| Samplea | Sold as | Total thujone | Camphor | Rosmarinic acid | Sum of rosmarinic acid derivatives | Luteolin-7-O-glucuronide | Sum of flavone glycosides | Sum of carnosol derivatives | ORAC | FC | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Herbal tea | Food | n.d. | 2.1 | 118 | 139 | 106 | 134 | 10 | 0.80 | 10.0 |
| 2 | Herbal tea | Food | n.d. | 2.2 | 119 | 143 | 106 | 123 | 12 | 0.70 | 10.0 |
| 3 | Herbal tea | Food | 2.9 | 2.2 | 47 | 73 | 78 | 124 | 26 | 0.86 | 8.9 |
| 4 | Herbal tea | Food | 1.9 | 1.9 | 41 | 71 | 78 | 105 | 8 | 0.82 | 8.4 |
| 5 | Herbal tea | Food | 3.0 | 2.1 | 42 | 48 | 67 | 96 | 43 | 1.05 | 8.8 |
| 6 | Herbal tea | Food | 1.6 | 2.1 | 42 | 63 | 71 | 80 | 37 | 1.05 | 8.8 |
| 7 | Herbal tea | Food | 9.2 | 1.6 | 72 | 92 | 91 | 107 | 45 | 0.59 | 8.9 |
| 8 | Tea bag | Food | n.d. | 5.3 | 89 | 112 | 94 | 113 | 41 | 0.76 | 8.4 |
| 9 | Herbal tea | Food | 8.5 | 14.7 | 117 | 191 | 83 | 95 | 44 | 0.79 | 8.4 |
| 10 | Tea bag (sage with honey) | Food | 13.6 | 11.9 | 32 | 86 | 92 | 102 | 31 | 0.26 | 10.3 |
| 11 | Tea bag | Food | 5.2 | 16.1 | 68 | 114 | 81 | 113 | 31 | 0.35 | 11.0 |
| 12 | Tea bag | Food | 4.4 | 12.9 | 62 | 163 | 88 | 101 | 32 | 0.34 | 11.0 |
| 13 | Tea bag | Food | 5.9 | 15.6 | 78 | 111 | 77 | 114 | 42 | 1.32 | 9.8 |
| 14 | Tea bag | Food | 9.0 | 16.1 | 87 | 125 | 98 | 131 | 29 | 1.33 | 10.9 |
| 15 | Tea bag | Food | 8.8 | 15.9 | 93 | 122 | 97 | 130 | 30 | 1.34 | 10.6 |
| 16 | Tea bag | Food | 6.8 | 15.5 | 91 | 107 | 104 | 135 | 31 | 0.53 | 9.8 |
| 17 | Tea bag | Food | 5.3 | 16.3 | 62 | 95 | 82 | 116 | 44 | 0.35 | 8.3 |
| 18 | Tea bag | Food | 6.2 | 12.2 | 194 | 205 | 87 | 115 | 56 | 0.34 | 9.2 |
| 19 | Tea bag | Food | 9.4 | 12.2 | 160 | 198 | 82 | 115 | 65 | 0.34 | 9.2 |
| 20 | Herbal tea | Food | 26.6 | 17.9 | 34 | 70 | 44 | 49 | 36 | 0.45 | 15.7 |
| 21 | Herbal tea | Food | 5.7 | 12.4 | 89 | 215 | 61 | 74 | 43 | 0.30 | 16.9 |
| 22 | Herbal tea | Food | 6.2 | 13.2 | 125 | 187 | 96 | 121 | 37 | 0.35 | 12.3 |
| 23 | Herbal tea | Food | 5.9 | 13.1 | 133 | 183 | 95 | 120 | 37 | 0.35 | 12.2 |
| 24 | Tea bag | Food | 4.1 | 13.9 | 79 | 119 | 86 | 114 | 42 | 0.36 | 9.9 |
| Average | 6.3 | 10.4 | 86 | 126 | 85 | 109 | 35 | 0.66 | 10.3 | ||
| 1 | Herbal tea | Medicine | 7.3 | 11.0 | 54 | 88 | 80 | 109 | 52 | 0.33 | 14.6 |
| 2 | Tea bag | Medicine | 12.4 | 26.3 | 67 | 86 | 90 | 119 | 41 | 0.74 | 12.3 |
| 3 | Tea bag (sage with honey) | Medicine | n.d. | 2.6 | n.d. | 18 | 61 | 70 | 10 | 0.33 | 9.5 |
| 4 | Herbal tea | Medicine | 12.5 | 19.0 | 159 | 201 | 100 | 118 | 27 | 0.86 | 10.4 |
| 5 | Herbal tea | Medicine | 10.2 | 17.5 | 128 | 199 | 92 | 109 | 30 | 1.20 | 9.9 |
| 6 | Tea bag | Medicine | 12.0 | 43.7 | 116 | 121 | 102 | 109 | 33 | 0.63 | 8.5 |
| 7 | Herbal tea | Medicine | 20.7 | 15.9 | 186 | 241 | 90 | 117 | 70 | 0.84 | 8.4 |
| 8 | Herbal tea | Medicine | 6.0 | 23.9 | 24 | 38 | 46 | 59 | 32 | 0.67 | 10.8 |
| 9 | Herbal tea | Medicine | 15.5 | 20.3 | 57 | 97 | 60 | 87 | 42 | 0.89 | 9.4 |
| 10 | Herbal tea | Medicine | 9.4 | 14.3 | 90 | 137 | 77 | 108 | 35 | 1.00 | 8.2 |
| 11 | Herbal tea | Medicine | 8.4 | 14.9 | n.d. | 35 | 61 | 31 | 40 | 0.43 | 8.5 |
| 12 | Herbal tea | Medicine | 8.1 | 13.0 | 83 | 119 | 30 | 39 | 48 | 0.33 | 7.6 |
| 13 | Herbal tea | Medicine | 5.8 | 13.5 | 80 | 125 | 70 | 92 | 37 | 0.44 | 7.7 |
| 14 | Herbal tea | Medicine | 6.0 | 13.3 | 98 | 120 | 50 | 15 | 36 | 0.43 | 7.7 |
| 15 | Herbal tea | Medicine | 6.3 | 22.7 | 27 | 49 | 57 | 62 | 60 | 0.82 | 10.4 |
| 16 | Herbal tea | Medicine | 7.6 | 22.6 | 22 | 73 | 58 | 66 | 45 | 0.80 | 10.3 |
| 17 | Herbal tea | Medicine | 6.5 | 24.6 | 31 | 47 | 69 | 83 | 52 | 0.80 | 11.4 |
| 18 | Herbal tea | Medicine | 16.5 | 16.6 | 229 | 237 | 101 | 114 | 35 | 0.82 | 8.6 |
| 19 | Herbal tea | Medicine | 17.6 | 16.1 | 209 | 243 | 99 | 110 | 47 | 0.92 | 8.3 |
| 20 | Herbal tea | Medicine | 7.2 | 17.7 | 24 | 86 | 63 | 81 | 54 | 0.86 | 10.1 |
| 21 | Herbal tea | Medicine | 9.7 | 14.0 | 111 | 134 | 94 | 126 | 35 | 0.14 | 9.4 |
| 22 | Herbal tea | Medicine | 8.6 | 9.8 | 124 | 181 | 94 | 96 | 34 | 1.29 | 12.7 |
| 23 | Herbal tea | Medicine | 10.7 | 17.1 | 81 | 92 | 105 | 138 | 33 | 0.31 | 9.7 |
| 24 | Herbal tea | Medicine | 26.1 | 12.4 | 248 | 264 | 87 | 113 | 46 | 1.37 | 11.1 |
| 25 | Herbal tea | Medicine | 17.3 | 16.3 | 31 | 67 | 66 | 77 | 36 | 0.22 | 8.8 |
| 26 | Herbal tea | Medicine | 6.9 | 19.9 | 36 | 86 | 63 | 83 | 43 | 0.79 | 10.9 |
| 27 | Herbal tea | Medicine | 10.1 | 12.7 | 160 | 180 | 108 | 127 | 40 | 1.35 | 9.8 |
| 28 | Herbal tea | Medicine | 2.5 | 16.9 | 45 | 72 | 72 | 122 | 39 | 0.76 | 9.6 |
| 29 | Herbal tea | Medicine | 13.2 | 13.5 | 47 | 81 | 62 | 78 | 53 | 0.93 | 11.1 |
| 30 | Herbal tea | Medicine | 9.3 | 13.6 | 120 | 160 | 74 | 109 | 49 | 1.04 | 10.9 |
| 31 | Herbal tea | Medicine | 7.3 | 15.6 | 95 | 114 | 109 | 144 | 30 | 0.54 | 10.2 |
| 32 | Herbal tea | Medicine | 2.7 | 17.8 | 20 | 74 | 32 | 57 | 51 | 0.53 | 12.1 |
| 33 | Herbal tea | Medicine | 5.3 | 16.5 | 69 | 114 | 118 | 146 | 28 | 0.54 | 11.8 |
| 34 | Tea bag | Medicine | 7.1 | 14.3 | 97 | 137 | 105 | 137 | 34 | 0.36 | 11.7 |
| 35 | Herbal tea | Medicine | 24.7 | 19.2 | 57 | 79 | 21 | 48 | 53 | 0.41 | 10.3 |
| 36 | Herbal tea | Medicine | 6.5 | 13.9 | 113 | 156 | 92 | 114 | 37 | 0.36 | 11.0 |
| 37 | Tea bag | Medicine | 9.9 | 14.3 | 95 | 85 | 95 | 120 | 34 | 0.37 | 10.5 |
| 38 | Herbal tea | Medicine | 7.6 | 13.0 | 90 | 127 | 85 | 112 | 54 | 0.37 | 12.8 |
| 39 | Herbal tea | Medicine | 37.5 | 10.9 | 178 | 261 | 75 | 124 | 84 | 0.46 | 8.5 |
| 40 | Herbal tea | Medicine | 27.8 | 9.6 | 165 | 241 | 92 | 106 | 47 | 0.32 | 12.0 |
| 41 | Tea bag | Medicine | 5.9 | 9.7 | 90 | 139 | 113 | 153 | 29 | 0.36 | 11.7 |
| 42 | Tea bag | Medicine | 2.4 | 13.8 | 34 | 92 | 86 | 104 | 34 | 0.30 | 11.3 |
| Average | 11.1 | 16.3 | 95 | 126 | 79 | 98 | 42 | 0.65 | 10.3 |
Note:
Pure sage tea if not otherwise indicated.
With regard to polyphenolic compounds, rosmarinic acid, luteolin-7-O-glucuronide and triterpenes (carnosol derivatives) were detected in all samples (Table 3). The qualitatively dominating compound is either rosmarinic acid or luteolin-7-O-glucuronide. The concentrations ranged from 34 to 194 mg/L (rosmarinic acid) and from 44 to 113 mg/L (luteolin-7-O-glucuronide). We also reconfirmed the previous study that rosmarinic acid and luteolin-7-O-glucuronide are the quantitatively dominating compounds of caffeic acid derivatives or flavone glycosides.4 In all samples the sum of the rosmarinic acid derivatives is higher (or equal) than the sum of the concentrations of the triterpenes.
The products sold as medicine had a tendency to have a higher thujone content (the average value is 11.1 mg/L compared to 6.3 mg/L for foods). Camphor content varied from 2.1 to 17.9 mg/L (average 10.4 mg/L) in foods and from 2.6 to 43.7 mg/L (average 16.3 mg/L) in medicines. The differences between foods and medicines are statistically significant on the 5% level for thujone and camphor (ANOVA: thujone P = 0.011, camphor P = 0.0004) but not significant for polyphenols, ORAC and FC index (ANOVA: rosmarinic acid P = 0.55, sum of rosmarinic acid derivatives P = 0.99, luteolin-7-O-glucuronide P = 0.22, sum of flavone glycosides P = 0.13, sum of carnosol derivatives P = 0.065, ORAC P = 0.94, FC index P = 0.88).
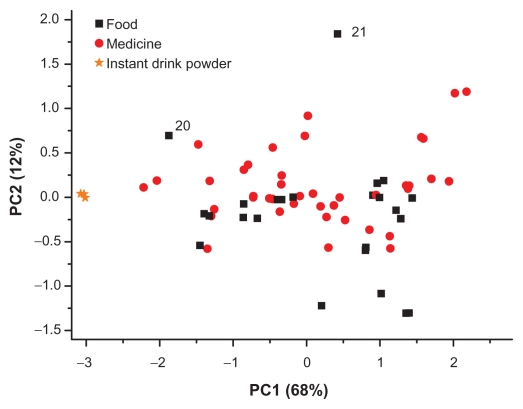
The result of explorative data analysis using PCA is shown in Figure 4 for sage teas. No clear differences or grouping is detectable especially in the products sold as medicines. Food tea samples 20 and 21 are different from the rest of the samples because they had a high content of thujone and rosmarinic acid derivatives (see Table 3). Additionally, the instant drinking powders (n = 3) can be differentiated; they are located in the negative values of PC1.
Figure 4.
Scatter plot of the PCA scores for sage tea (δ 6.0–0.25 ppm).
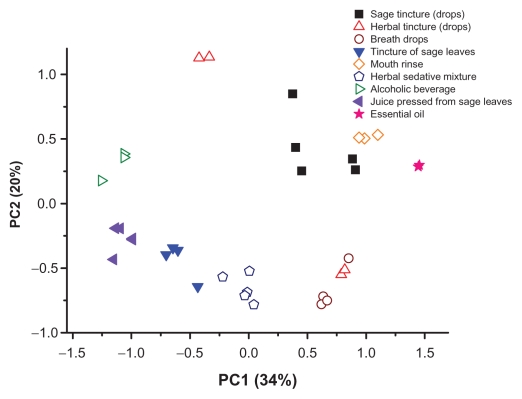
Apart from the teas, we also analyzed various medicines containing sage and other products based on ethanol (Table 4). Overall, they showed thujone content that ranged from not detectable to 409 mg/L. Camphor also occurred in samples in the not detectable to 890 mg/L range. The two essential oil samples showed total thujone and camphor concentrations above 1500 mg/L. We also quantified rosmarinic acid in all samples and found that its content ranged from non detectable to 1474 mg/L. Significant positive linear correlation between rosmarinic acid content and FC index was proved (R = 0.59, P = 0.00015). The best PCA model with regard to classification ability for these products was obtained in the δ 6.0–0.25 ppm region (Fig. 5). All different kinds of products were clearly distinguished from each other (various kinds of tinctures, breath drops, mouth rinse, alcoholic beverages, juices and essential oils). Additionally, the products made from pure sage extract (the markers are filled in the Fig. 5) were located separately from other products that additionally contained extracts from other plants besides sage.
Table 4.
Results of other sage products.
| Sample | Sold as | Sum of thujone isomers [mg/L] | Camphor [mg/L] | FC | Rosmarinic acid [mg/L] | |
|---|---|---|---|---|---|---|
| 1 | Sage tincture (drops) | Medicine | 274 | 748 | 135 | 243 |
| 2 | Herbal tincture (drops) | Medicine | 400 | 505 | 168 | 722 |
| 3 | Herbal tincture (drops) | Medicine | 409 | 523 | 160 | 828 |
| 4 | Herbal tincture (drops) | Medicine | 350 | 393 | 69 | 774 |
| 5 | Herbal tincture (drops) | Medicine | 17 | n.d. | 29 | n.d. |
| 6 | Herbal tincture (drops) | Medicine | 29 | n.d. | 22 | n.d. |
| 7 | Herbal anti-dyspepsia drops | Medicine | 11 | 160 | 47 | n.d. |
| 8 | Herbal tincture (expectorant) | Medicine | 11 | n.d. | 46 | n.d. |
| 9 | Herbal tincture (expectorant) | Medicine | 96 | n.d. | 45 | n.d. |
| 10 | Sage leaves extract | Medicine | 111 | 113 | 99 | 77 |
| 11 | Sage leaves extract | Medicine | 110 | 128 | 98 | 80 |
| 12 | Sage leaves extract | Medicine | 111 | 118 | 87 | 105 |
| 13 | Mouth rinse | Consumer product | 112 | n.d. | 3 | n.d. |
| 14 | Mouth rinse | Consumer product | 112 | n.d. | 2 | n.d. |
| 15 | Mouth rinse | Consumer product | 109 | n.d. | 30 | n.d. |
| 16 | Herbal medicinal products | Medicine | 10 | n.d. | 1 | n.d. |
| 17 | Herbal medicinal products | Medicine | 11 | n.d. | 1 | n.d. |
| 18 | Herbal medicinal products | Medicine | 21 | n.d. | 1 | n.d. |
| 19 | Sage tincture (drops) | Medicine | 304 | 890 | 149 | 773 |
| 20 | Tincture | Medicine | 349 | 414 | 2 | 104 |
| 21 | Tincture | Medicine | 343 | 491 | 2 | 106 |
| 22 | Sage tincture (drops) | Medicine | 78 | 305 | 71 | 386 |
| 23 | Juice pressed from fresh sage leaves | Food | 49 | n.d. | 108 | 1474 |
| 24 | Juice pressed from fresh sage leaves | Food | 51 | n.d. | 103 | 802 |
| 25 | Juice pressed from fresh sage leaves | Food | 50 | n.d. | 108 | 1396 |
| 26 | Sage juice | Food | 45 | n.d. | 115 | 192 |
| 27 | Sage juice | Food | 46 | n.d. | 115 | 182 |
| 28 | Registered homeopathic medicine | Medicine | n.d. | 298 | 88 | 170 |
| 29 | Herbal tincture (expectorant) | Medicine | n.d. | 280 | 50 | n.d. |
| 30 | Sage tincture (drops) | Medicine | n.d. | 330 | 86 | n.d. |
| 31 | Sage tincture (drops) | Medicine | n.d. | n.d. | 105 | 650 |
| 32 | Herbal tincture (expectorant) | Medicine | n.d. | 288 | 69 | n.d. |
| 33 | Herbal tincture (drops) | Medicine | 51 | 297 | 82 | 394 |
| 34 | Sage tincture (drops) | Medicine | n.d. | 347 | 82 | n.d. |
| 35 | Herbal medicinal products | Medicine | 151 | 429 | 27 | 151 |
| 36 | Alcoholic beverage | Food | 12,0 | 343 | 78 | n.d. |
| 37 | Essential oil | Essential oil | 1605 | 1875 | 175 | n.d. |
| 38 | Essential oil | Essential oil | 1950 | 1500 | 104 | n.d. |
| 39 | Hot sage | Food, instant drink | n.d. | 9.7 | 12.7 | 36 |
| 40 | Hot sage | Food, instant drink | n.d. | 10.9 | 12.7 | 52 |
| 41 | Hot sage | Food, instant drink | n.d. | 14.70 | 12.7 | 22 |
| 42 | Herbal Supplementa | Tablets | n.d. | n.d. | 87 | 12.7 |
Note:
For this sample results are expressed in [mg/kg].
Figure 5.
Scatter plot of the PCA scores (δ 6.0–0.25 ppm) for other sage products (the markers are filled in for pure sage products, other products may contain other plants besides sage).
Conclusions
NMR allows considerable chemical information to be obtained in a single experiment. While the previous research was focused on the determination of either polyphenolic compounds4 or terpenes,3 this study is the first to provide a comprehensive overview about commercial sage products (including herbal teas and herbal tinctures). As all important adverse and beneficial compounds can be quantified in a single assay without a sample preparation step, our method offers an opportunity to provide a holistic risk-benefit evaluation of sage products.
By a combination of multivariate methods, it is possible to cope with the matrix effect, which could prevent the accurate quantification of selected compounds in the case of sage tea by spectral overlap. Previously, 1H NMR spectroscopy was only used for the calculation of total phenolic content in crude plant extracts including sage tea leaves.18 This method was based on the process of determining of specific resonances of −OH groups and it required an extraction step with organic solvent prior to the NMR experiment. In this study, we have extended the scope of quantitative NMR to all important compounds in sage-containing products with simplified sample preparation.
In this paper, it is shown that 1H-NMR spectroscopy can provide quantitative information necessary to judge the quality of medicinal and food sage products in a short analysis period. All important health-relevant compounds could be identified and quantified using a single methodology. Furthermore, NMR can be applied to a wide range of sage matrices ranging from medicinal tinctures to herbal teas. All in all, NMR contains the same information as at least four traditional methods (GC/MS for thujone and camphor, UPLC for polyphenols, spectrophotometry for FC index and fluorimetry for ORAC). It therefore helps to save valuable resources, such as time, money and organic solvents (such as environmentally unfriendly 1,1,2-trichloro-1,2,2-trifluoroethane necessary for conventional thujone analysis). According to our price list (including costs for labor), NMR saves about 80% of the costs needed for these four reference methods.
Footnotes
Author Contributions
Conceived and designed the experiments: SGW, YBM, DWL. Analysed the data: SGW, YBM. Wrote the first draft of the manuscript: SGW, YBM. Contributed to the writing of the manuscript: DWL, TK, WS. Agree with manuscript results and conclusions: SGW, DWL, TK, WS, YBM. Jointly developed the structure and arguments for the paper: SGW, DWL, TK, WS, YBM. Made critical revisions and approved final version: SGW, DWL, TK, WS, YBM. All authors reviewed and approved of the final manuscript.
Competing Interests
None.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1.EMA. Community herbal monograph on Salvia officinalis L., folium. London, UK: European Medicines Agency; 2009. [Google Scholar]
- 2.Lachenmeier DW, Uebelacker M. Risk assessment of thujone in foods and medicines containing sage and wormwood—evidence for a need of regulatory changes? Regul Toxicol Pharmacol. 2010;58:437–43. doi: 10.1016/j.yrtph.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Walch SG, Kuballa T, Stühlinger W, Lachenmeier DW. Determination of the biologically active flavour substances thujone and camphor in foods and medicines containing sage (Salvia officinalis L.) Chem Cent J. 2011;(5):44. doi: 10.1186/1752-153X-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zimmermann BF, Walch SG, Tinzoh LN, Stühlinger W, Lachenmeier DW. Rapid UHPLC determination of polyphenols in aqueous infusions of Salvia officinalis L (sage tea) J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:2459–64. doi: 10.1016/j.jchromb.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 5.Sajewicz M, Staszek D, Wojtal L, et al. Binary HPLC-diode array detector and HPLC-evaporative light-scattering detector fingerprints of methanol extracts from the selected sage (Salvia) species. J AOAC Int. 2011;94:71–6. [PubMed] [Google Scholar]
- 6.Grygierczyk G, Sajewicz M, Staszek D, et al. TLC-Based Start-to-End method of analysis of selected biologically active compounds contained in common sage (Salvia officinalis L.) J Liq Chrom Rel Technol. 2009;32:1223–40. [Google Scholar]
- 7.Baskan S, Oztekin N, Erim F. Determination of carnosic acid and rosmarinic acid in sage by capillary electrophoresis. Food Chem. 2007;101:1748–52. [Google Scholar]
- 8.Blagojevic N, Damjanovic-Vratnica B, Vukasinovic-Pesic V, Durovic D. Heavy metals content in leaves and extracts of wild-growing salvia officinalis from montenegro. Pol J Environ Stud. 2009;18:167–73. [Google Scholar]
- 9.Le Gall G, Colquhoun IJ. NMR spectroscopy in food authentication. In: Lees M, editor. Food authenticity and traceability. Cambridge, UK: Woodhead Publishing Ltd; 2003. pp. 131–55. [Google Scholar]
- 10.Ohno A, Oka K, Sakuma C, Okuda H, Fukuhara K. Characterization of tea cultivated at four different altitudes using (1)H NMR analysis coupled with multivariate statistics. J Agric Food Chem. 2011;59:5181–7. doi: 10.1021/jf200204y. [DOI] [PubMed] [Google Scholar]
- 11.Lee JE, Lee BJ, Chung JO, et al. (1)H NMR-based metabolomic characterization during green tea (Camellia sinensis) fermentation. Food Res Int. 2011;44:597–604. [Google Scholar]
- 12.Le Gall G, Colquhoun IJ, Defernez M. Metabolite profiling using H-1 NMR spectroscopy for quality assessment of green tea, Camellia sinensis (L.) J Agric Food Chem. 2004;52:692–700. doi: 10.1021/jf034828r. [DOI] [PubMed] [Google Scholar]
- 13.Tarachiwin L, Ute K, Kobayashi A, Fukusakii E. H-1 NMR based metabolic profiling in the evaluation of Japanese green tea quality. J Agric Food Chem. 2007;55:9330–6. doi: 10.1021/jf071956x. [DOI] [PubMed] [Google Scholar]
- 14.Lu WJ, Zou HB, Zhang XL, Yang GS, Aboul-Enein HY. Study on (1) HNMR Fingerprint Spectra of Tea by Common and Variation Peak Ratio Dual-Index Sequence Analysis Method. Anal Lett. 2009;42:2244–53. [Google Scholar]
- 15.Saito T, Honma D, Tagashira M, Kanda T, Nesumi A, Maeda-Yamamoto M. Anthocyanins from New Red Leaf Tea ‘Sunrouge’. J Agric Food Chem. 2011;59:4779–82. doi: 10.1021/jf200250g. [DOI] [PubMed] [Google Scholar]
- 16.Piccinelli AL, De Simone F, Passi S, Rastrelli L. Phenolic constituents and antioxidant activity of Wendita caiysina leaves (Burrito), a folk Paraguayan tea. J Agric Food Chem. 2004;52:5863–8. doi: 10.1021/jf040100e. [DOI] [PubMed] [Google Scholar]
- 17.Fujiwara M, Ando I, Arifuku K. Multivariate analysis for H-1-NMR spectra of two hundred kinds of tea in the world. Anal Sci. 2006;22:1307–14. doi: 10.2116/analsci.22.1307. [DOI] [PubMed] [Google Scholar]
- 18.Nerantzaki A, Tsiafoulis C, Charisiadis P, Kontogianni V, Gerothanassis I. Novel determination of the total phenolic content in crude plant extracts by the use of (1)H NMR of the -OH spectral region. Anal Chim Acta. 2011;688:54–60. doi: 10.1016/j.aca.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 19.ISO. ISO 3103 Tea—Preparation of liquor for use in sensory tests. Geneva, Switzerland: International Organization for Standardization; 1980. [Google Scholar]
- 20.Monakhova YB, Schäfer H, Humpfer E, Spraul M, Kuballa T, Lachenmeier DW. Application of automated eightfold suppression of water and ethanol signals in 1H NMR to provide sensitivity for analyzing alcoholic beverages. Magn Reson Chem. 2011;49:734–9. doi: 10.1002/mrc.2823. [DOI] [PubMed] [Google Scholar]
- 21.Monakhova YB, Kuballa T, Lachenmeier DW. Rapid determination of total thujone in absinthe using 1H NMR spectroscopy. Int J Spectrosc. 2011;2011:171684. [Google Scholar]
- 22.Prior RL, Hoang H, Gu LW, et al. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORAC(FL))) of plasma and other biological and food samples. J Agric Food Chem. 2003;51:3273–9. doi: 10.1021/jf0262256. [DOI] [PubMed] [Google Scholar]
- 23.European Commission. Commission Regulation (EC) No 2676/90 determining Community methods for the analysis of wines. Off J Europ Comm. 1990;L272:1–192. [Google Scholar]
- 24.Walch SG, Ngaba Tinzoh L, Zimmermann BF, Stühlinger W, Lachenmeier DW. Antioxidant capacity and polyphenolic composition as quality indicators for aqueous infusions of Salvia officinalis L (sage tea) Front Pharmacol. 2011;2:79. doi: 10.3389/fphar.2011.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DIN 32645. Chemische Analytik: Nachweis-, Erfassungs- und Bestim-mungsgrenze, Ermittlung unter Wiederholbedingungen Begriffe, Verfahren, Auswertung. Berlin, Germany: Beuth Verlag; 1994. [Google Scholar]
- 26.Lachenmeier DW, Nathan-Maister D, Breaux TA, Kuballa T. Long-Term Stability of Thujone, Fenchone, and Pinocamphone in Vintage Preban Absinthe. J Agric Food Chem. 2009;57:2782–5. doi: 10.1021/jf803975m. [DOI] [PubMed] [Google Scholar]