Abstract
Dengue is the most important arboviral disease of humans with over half of the world’s population living in areas of risk. The frequency and magnitude of epidemic dengue have increased dramatically in the past 40 years as the viruses and the mosquito vectors have both expanded geographically in the tropical regions of the world. There are many factors that have contributed to this emergence of epidemic dengue, but only three have been the principal drivers: 1) urbanization, 2) globalization and 3) lack of effective mosquito control. The dengue viruses have fully adapted to a human-Aedes aegypti-human transmission cycle, in the large urban centers of the tropics, where crowded human populations live in intimate association with equally large mosquito populations. This setting provides the ideal home for maintenance of the viruses and the periodic generation of epidemic strains. These cities all have modern airports through which 10s of millions of passengers pass each year, providing the ideal mechanism for transportation of viruses to new cities, regions and continents where there is little or no effective mosquito control. The result is epidemic dengue. This paper discusses this unholy trinity of drivers, along with disease burden, prevention and control and prospects for the future.
Keywords: Dengue, urbanization, globalization, aedes aegypti
Introduction
The last half of the 20th century has taught us many lessons about infectious diseases and their resilience in the face of defeat. The heady victories of the 1950s, 1960s and 1970s over smallpox, malaria, yellow fever, dengue, cholera, tuberculosis, plague and other major infectious diseases culminated with the war on infectious diseases being declared won in 1969! [1] This declaration contributed to the initation of a period of increasing apathy and complacency in the last three decades of the 20th century, during which time the public health infrastructure and hygiene deteriorated to the point where they provided little protection against infectious diseases. A new paradigm of surveillance and emergency response was introduced as the recommended method of controlling epidemic infectious diseases. Resources were redirected from infectious diseases to other noncommunicable public health priorities, and the Silent Spring era of increased environmental awareness made it increasingly more difficult to control mosquitoes and other disease vectors [2–5].
Coincident with this period of apathy, and unrecognized by public health and infectious disease experts, however, was the emergence of global trends that would ultimately drive the re-emergence of many of the diseases that had been effectively controlled. These trends included unprecedented population growth, unplanned urbanization and modern air transport of people, animals and commodities (globalization), the latter two being driven by economic growth in the post World War II era [2–6]. The jet airplane provided the ideal mechanism to transport pathogens to new geographic regions, and as globalization increased, so too did the movement of pathogens among urban centers of the world. Thus, beginning in the 1970s, and accelerating in the 1980s and 1990s, a global re-emergence of epidemic infectious diseases occurred and continues as we enter the second decade of the 21st century [3, 5]. Dengue fever provides an ideal case study of how urbanization and globalization have influenced infectious disease emergence.
History and Current Status
Prior to World War II, dengue viruses had a global distribution in the tropics, but because urban populations were relatively small, and the viruses and mosquito vectors depended primarily on ocean going vessels for transportation among regions, epidemics were sporadic, with long intervals between them [7]. As a result, most tropical regions had only one or at most, two viruses circulating at any one time; dengue fever was not considered a major public health problem, except when sporadic epidemics occurred.
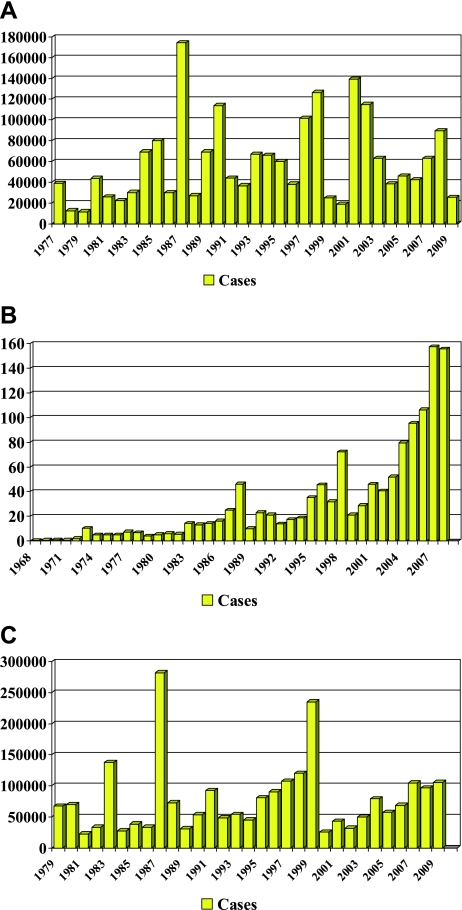
It was World War II that planted the seed for the current dengue pandemic [7]. Dengue fever (DF) and malaria were two of the major diseases that plagued both the Japanese and Allied forces in the Pacific and Asian theatres, with thousands of cases occurring among soldiers [7–9]. The movement of troops and war materials transported the viruses and the principal mosquito vector, Aedes aegypti, to most areas of both regions. By the end of the war, many countries of Asia were hyperendemic with the co-circulation of all four virus serotypes. The end of the war brought a surge in economic growth in many Southeast Asian countries and this was the principal catalyst for the unprecedented urban growth that began in the 1950s and continues today (Fig. 1). It was during this period that the first documented epidemics of dengue hemorrhagic fever (DHF) occurred, first in the Philippines (1953–1954) and Thailand (1958), followed in the 1960s by Singapore, Malaysia and Vietnam, and Indonesia and Burma (Myanmar) in the 1970s [10, 11]. As urbanization and commerce grew, the frequency and magnitude of epidemic disease continued to increase (Fig. 2). By the 1980s, DHF had become a leading cause of hospitalization and death among children in many countries of Southeast Asia [11].
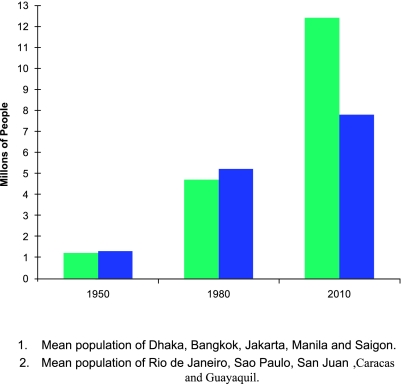
Fig. 1.
Urban growth in Asian and American cities—1950–2010.
Fig. 2.
Reported dengue/DHF cases in Thailand (A), Indonesia (B) and Vietnam (C), by year.
After an absence of 25 years, dengue was re-introduced into the Pacific Islands in 1964 and again in 1971 from the Americas (DENV-3 and DENV-2, respectively) [12–17]. The DENV-3 was limited to relatively small outbreaks in Tahiti, but the DENV-2 spread throughout the islands. The same American genotype of DENV-2 caused severe disease on some islands and only mild disease on others [14, 17–19]. Over the next 10 years, all four serotypes were introduced from Asia and caused epidemics in most islands [7]. Epidemic dengue accompanied by severe and fatal hemorrhagic disease has been a regular occurrence in the Pacific islands since that time.
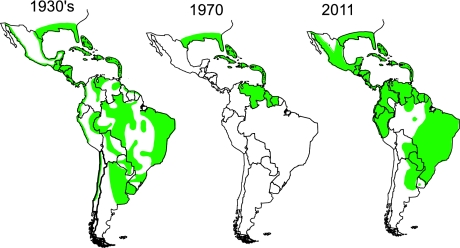
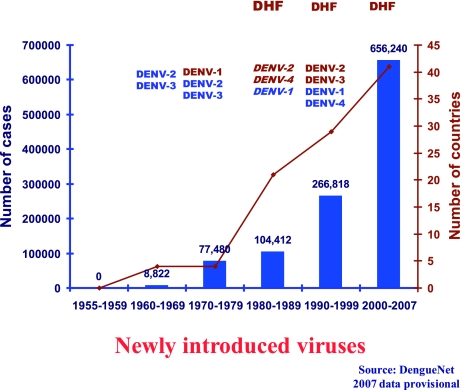
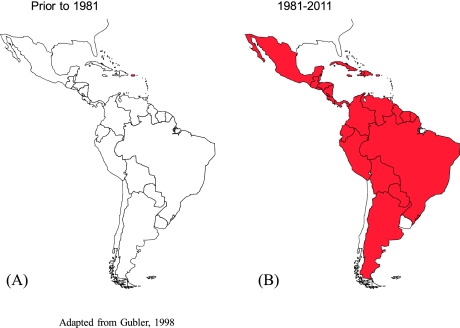
In the Americas, epidemic dengue was effectively controlled along with epidemic yellow fever in most of the region by the Ae aegypti eradication program that eliminated the mosquito from 23 countries during the 1950s and 1960s [2, 20]. When this program was terminated in the early 1970s, Ae aegypti began to reinfest tropical countries in the region (Fig. 3). This reinvasion by Ae aegypti coincided with dramatic urban growth in the American tropics, and by the late 1970s, dengue viruses were beginning to be introduced from Asia [21, 22]. DENV-1 was introduced in 1977, followed by DENV-2 and -4 in 1981, and DENV -3 in 1994 [7]. Most likely, multiple strains of each serotype were introduced, and all four became endemic. As hyperendemicity developed, epidemic DHF emerged and currently affects most of the region (Fig. 4) [23].
Fig. 3.
Reinfestation of tropical America by Aedes aegypti, 1930–2011.
Fig. 4.
The emergence of dengue hemorrhagic fever in the Americas associated with introduction of new virus serotypes and the development of hyperendemicity.
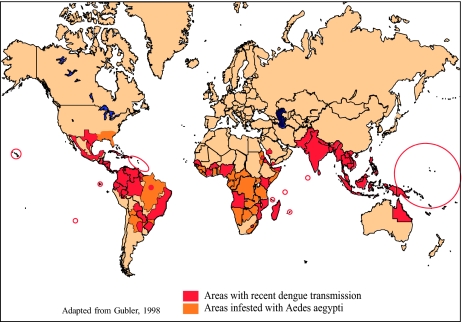
The dramatic global geographic expansion and the increased incidence of epidemic dengue coincided exactly with urban growth and globalization [6]. Currently, an estimated 3.6 billion people in 124 countries live in areas of risk for this primarily urban disease [24]. It is not known how many dengue infections occur each year, but the estimates range from 50 to over 200 million, many of these being cases of mild febrile illness that are not reported as dengue. An estimated 34 million cases of clinical DF, 2 million cases of DHF and over 20,000 deaths occur each year [24]. In addition to the cases of classical DHF (vascular leak syndrome), there are many cases of severe dengue disease that are not reported as dengue because the clinical presentation is atypical [25–31]. These include patients with massive hemorrhage and organ failure, neurologic disease, myocardiopathy and hepatic and renal failure [31]. Dengue is currently the most important vector-borne virus disease affecting humans. If the global trends of population growth, urbanization and globalization continue as projected, we can expect to see continued increases in the frequency, magnitude and severity of epidemic dengue. The current global distribution of Ae aegypti, areas at risk and areas with recent epidemic dengue activity are shown in Fig. 5.
Fig. 5.
The global distribution of epidemic dengue and the principal vector mosquito, Aedes aegypti, 2011.
Burden of Disease
The actual burden of dengue disease is difficult to measure because of the low case fatality rate, misdiagnosis, poor surveillance, and lack of cooperation by the tourism and other industries [24, 32–35]. Many dengue infections are misdiagnosed as other tropical diseases such as malaria [36]. However, dengue is an insidious disease that is always present in large tropical urban centers, lurking in the shadows out of sight and out of mind of physicians and public health officials during interepidemic periods, waiting for the opportunity to strike, either by mutating to a genetic subtype that has greater epidemic potential and virulence, or by catching a ride to another city where the population is more susceptible to that particular serotype. Often times both of these mechanisms come into play as epidemic strains of dengue virus spread in infected humans via modern transportation [5, 6, 37].
Epidemics of dengue often cause chaos in the communities where they occur, resulting in considerable social upheaval and economic loss, both of which are difficult to measure; thus the paucity of reliable disease burden data [38]. Unfortunately, the World Health Organization consistently reports unrealistically low estimates of disease burden for dengue [39]. Moreover, most dengue endemic countries have only passive surveillance systems that consistently underestimate the amount of dengue disease, especially mild illness, atypical severe disease and deaths [40, 25–30]. Recent studies, however, have tried to more accurately measure the actual disease burden and cost of dengue [24, 32, 33]. In the Americas, for example, it was estimated that between 3 to 16 times more cases occurred than were reported in Colombia, Nicaragua, and Puerto Rico [32]. In Thailand, Cambodia and Indonesia, however, the underestimates were smaller, only between 3 and 6 times. These are most likely due to under reporting, however, because Asian countries routinely fail to report mild dengue or even dengue fever. This is underscored by a number of cohort and active surveillance studies in Asia and the Americas showing that the true incidence of dengue infection was underestimated by 11 to 250 times [24].
The actual cost of dengue to a community is also difficult to measure. A recent study in the Americas estimated that dengue cost the region US$2.1 billion per year [32]. However, that figure did not include the cost of vector control, nor did it include any of the costs associated with loss of tourist dollars, which can be considerable. For example, epidemic dengue cost an estimated US$90 million in the state of Gujarat, India in 2007, and US$133 million in Malaysia and US$127 million in Thailand in 2006 (DS Shepard, personal communication, 2010). Most of these dollar estimates grossly underestimate the real cost of the disease to a community. Even so, the burden of dengue exceeds many other infectious diseases, including viral diseases like human papillomavirus and rotavirus [32], and reaches the same order of magnitude as many other infectious diseases [34].
Factors Influencing Incidence and Geographic Spread
There are many factors that have influenced the incidence and geographic spread of dengue disease, including apathy, decay in public health infrastructure, changing life styles, evolutionary changes in the viruses, and misguided mosquito control, among others. However, four factors can be cited as principal drivers of the increased incidence and spread (Box 1). The failure to control Ae aegypti mosquitoes in urban environments is closely linked to changing lifestyles [2]. In the 40 years since 1970, two things have exacerbated our failure to control the mosquito vectors: 1) lack of political will and thus resources, and 2) too much emphasis on high technology such as space spraying of insecticides. The apathy and complacency that set in after the success of the Ae aegypti eradication program in the Americas resulted in a redirection of resources away from successful mosquito control programs, which ultimately resulted in the deterioration of mosquito control infrastructure in much of the world. Successful mosquito control programs were replaced by emergency space spraying of nonresidual insecticides in response to reported cases of dengue [2, 41]. This method had high visibility and was very popular politically, but it simply did not work because it targeted adult mosquitoes which are normally sequestered in resting places inside houses where the insecticides do not reach. In addition, because the passive surveillance relied on physicians to report cases, the spraying was always too late and too limited geographically to interrupt transmission. Changing lifestyles in the past 30 years have also played an important role in our inability to control dengue. The global automobile population has exploded during this time, and used automobile and truck tires provide ideal oviposition sites and larval habitats for the mosquito vectors. As a result, they also served as the principal mechanism for the geographic spread of mosquitoes [42]. In addition, most consumer goods are packaged in nonbiodegradable plastic containers, most of which are discarded into the environment and provide another ideal larval habitat for the mosquito vectors of dengue [2]. Thus, most of our mosquito control efforts were focused on controlling adult mosquitoes using an expensive method that did not work, while our changing lifestyles were providing an increasing number of larval habitats for the mosquitoes. The result has been increased mosquito population densities, increased geographic spread and increased dengue/epidemics.
Box 1.
| Major Drivers of the increased Incidence and Geographic Spread of Dengue |
|---|
| - Lack of effective mosquito control |
| - Changing life styles |
| - Unplanned urbanization |
| - Globalization |
Two other major drivers of increased incidence and geographic spread of epidemic dengue were urbanization and globalization. Aedes aegypti is a highly domesticated urban mosquito that prefers to live with humans in their homes, feed on humans and lay eggs in artificial containers made by humans [7]. Automobile tires and plastic containers are but two of these types of containers found in the crowded urban environment and implicated in the high mosquito population densities. The lack of adequate water supply in many urban areas makes it necessary to store water in large containers such as clay jars and cisterns, another major contribution to increased mosquito densities. The dramatic urban growth that has occurred in the past 40 years has thus provided the ecological conditions that allow large populations of Ae aegypti to thrive in intimate association with large and crowded human populations in tropical cities, creating conditions that are ideal for epidemic dengue transmission.
In Asia, unprecedented urbanization began in the years following World War II and coincided with a remarkable economic boom. Cities like Bangkok, Manila, and Jakarta exploded in population growth, most of it unplanned (Fig. 1). The result was millions of susceptible people moving to the cities and living in shanty towns with inadequate housing and few or no basic services such as water, sewer and waste management. The resulting crowded human communities and large mosquito populations created ideal conditions for dengue transmission. As noted above, it was in this setting that epidemic DHF emerged, first recognized in Manila in 1953–1954, followed by Bangkok in 1958, although retrospectively, sporadic cases were identified early in the decade [7, 10]. Epidemics of the severe disease began to occur in the 1960s and 1970s in Asia and the Pacific Islands (see above). Incidence and geographic spread accelerated in the 1980s and 1990s, with epidemic DF/DHF moving east into Taiwan and China, south into Australia and west to India, Sri Lanka, the Maldives, Saudi Arabia and Pakistan [43].
The American region became highly urbanized in the 1970s and today, over 75% of the population live in urban areas, nearly all of which have been reinfested with Ae aegypti (Fig. 3) [7, 44, 45]. This re-infestation coincided with the re-emergence of epidemic dengue beginning in the 1970s and accelerating in the 1980s and 1990s [7, 23]. Since 1977, when only a few countries reported dengue, most of the region has become hyperendemic with multiple virus serotypes co-circulating; 28 countries have now reported the severe forms of dengue disease (Fig. 6).
Fig. 6.
Geographic spread of DHF in the Americas, 1981 (A) and 2011 (B).
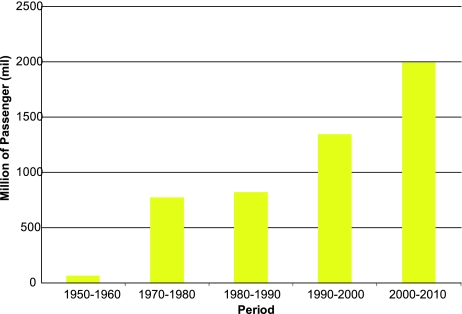
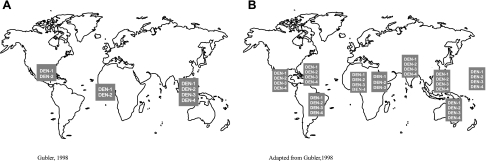
Globalization has been a direct result of economic growth. In the past 30 years, globalization has been the main driver of an integrated global economic system that includes a transnational flow of knowledge, capital, commodities, people and animals. This globalized system has been closely tied to the increased use of the jet airplane as the principal mode of transport over the past four decades. Most large cities in the world have modern airports through which millions of people pass every year (Fig. 7). Thus, the global number of passengers traveling by air increased from an average of 68.5 million per year in the 1950s to over 2 billion per year in the first decade of the 21st century [46]. In 2011, an estimated 2.75 billion people will travel by airplane, many of them to and from tropical urban centers where dengue is endemic. The movement of people infected with dengue viruses has been the principal driver in the global expansion of this disease. In 2011, the entire tropical world is hyperendemic with multiple virus serotypes co-circulating in most large urban centers (Fig. 8). In these cities, dengue viruses are maintained by low level silent transmission associated with mild illness during interepidemic periods; periodic epidemics usually occur every 3 to 5 years [7, 43, 47]. These urban centers are the breeding ground of epidemic dengue, producing viruses with better resilience and epidemic potential that are then transported to other cities via infected people traveling by jet airplane [48–50]. The dengue viruses have fully adapted to humans and no longer require the sylvatic cycle for survival [37]. They have thus found a perfect environment for survival: large crowded tropical urban centers where mosquito control is lacking all of which have modern airports through which tens of millions of people pass each year.
Fig. 7.
Mean annual number of global airline passengers by decade, 1965–2010.
Fig. 8.
The global distribution of dengue virus serotypes, 1970 (A) and 2011 (B).
Prevention and Control
Prevention and control of epidemic dengue has become increasingly problematic. The disease exists in large tropical cities where the mosquito vectors live in intimate contact with the crowded human host. Many of these cities have populations of 15 to 20 million people. To effectively control the mosquito vector using current tools, every house and office in the city must be visited on a weekly basis, a near impossibility without the help of the community. Unfortunately, efforts to develop effective community outreach programs and ownership have largely failed [51]. Clearly, new tools for mosquito control as well as vaccines and antiviral drugs are needed to control this disease. Fortunately, progress is being made on all of these fronts. For mosquito control, new residual insecticides are on the horizon and new biological and genetic methods of control are emerging (Box 2) [52–56]. A discussion of these tools is beyond the scope of this paper, but it should be noted that no single tool or method is likely to be effective in controlling Ae aeqgypti alone. A successful long-term sustainable control program will require a combination top down-bottom up approach [2], with the top down component involving the integration of several new tools and preferably managed over the longterm by the community [51].
Box 2.
| New Tools for Control of Aedes aegypti |
|---|
| New residual insecticides |
| Insecticide treated materials |
| Biocontrol |
| Copepods |
| Wolbachia |
| Genetic Control |
| Sterile male release |
| Genetically resistant mosquitoes |
Good progress has also been made in recent years in the development of antiviral drugs, vaccines and therapeutic antibodies for dengue viruses [57–62]. It is anticipated that all may be available within 5 years. The progress with vaccines has been especially promising, with six tetravalent candidate vaccines currently in clinical trials, including three live attenuated candidate vaccines (one in phase III efficacy trial), one inactivated, one subunit and one DNA vaccine candidate (Table 1). There are thus great expectations about the use of vaccines as the principal means of controlling dengue, but, it must be emphasized that vaccines are not likely to be a panacea.
Table 1.
Dengue Vaccines Currently in Clinical Trial
| Manufacturer/Licensee | Type | Clinical Trial |
| Sanofi Pasteur | *LAV/Chimeric | Phase III |
| NIH | LAV/Chimeric | Phase I |
| Inviragen | LAV/Chimeric | Phase I |
| Glaxo Smith Kline | Inactivated | Phase I |
| Hawaii Biotech/Merk | Subunit | Phase I |
| NAMRI | DNA | Phase I |
* LAV = live attenuated
A good vaccine for prevention of dengue in endemic countries must be 1) effective against at least three of the four serotypes (preferably all 4 and with one dose), 2) safe, 3) long-lasting (preferably up to 10 years), and 4) economical. However, lessons learned from yellow fever show that even if we have such a vaccine for dengue, it may not be the only answer. There is such a vaccine for yellow fever, but it is not used effectively for prevention. In many yellow fever endemic countries, it is used as an emergency response tool after an epidemic has been declared. Unfortunately, it is rarely effective when used in this manner because the response is almost always implemented too late to impact epidemic transmission.
It should be remembered that there are a number of other important diseases that can also be transmitted by the mosquito vectors of dengue (Box 3). Thus, the most effective prevention and control will be an integrated approach that combines enhanced mosquito control using the new tools described above, with vaccines and improved clinical management including the use of antivirals and therapeutic antibodies [63].
Box 3.
| Diseases transmitted by Aedes aegypti |
|---|
| Dengue |
| Yellow fever |
| Chikungunya |
| Epidemic polyarthritis |
| Zika |
Prospects for the Future
The global trends discussed above are projected to continue for the indefinite future. Moreover, it is not anticipated that effective methods of prevention and control will impact disease incidence and spread in the near term. Therefore, it is expected that dengue and perhaps other diseases transmitted by Ae aegypti in urban centers of the tropics will continue to increase in incidence and geographic spread. To reverse these trends, several things must be done (Table 2). First, the movement of viruses and vectors via people traveling by air must be prevented. This problem has been totally ignored by public health agencies because of its complex economic and political implications. Second, vector biologists, epidemiologists, and laboratorians must be trained and supported to improve capacity in endemic countries. Third, laboratory-based surveillance must be developed and implemented in all endemic countries. This too has been ignored. Fourth, integrated Ae aegypti prevention and control programs must be developed and implemented as regional, not national, programs. Fifth, vaccines, when they do become available, must be used for prevention as opposed to emergency response. Finally, policy makers must develop the political will and provide the support needed to develop, implement and maintain these programs.
Table 2.
Requirement for Reversing the Trends of Increased Incidence and Geographic Spread of Dengue
| 1 | Prevent the movement of vectors and viruses via globalization |
| 2 | Build entomologic, epidemiologic and laboratory capacity in dengue endemic countries |
| 3 | Develop laboratory-based, active disease surveillance in dengue endemic countries |
| 4 | Develop regional Ae aegypti control programs |
| 5 | Use vaccines for prevention (as opposed to emergency response) |
| 6 | Obtain political will and resources to develop and support these programs long term |
References
- 1.Henderson DA. Surveillance Systems and Intergovernmental Cooperation. In: Stephen S. Morse, ed. Emerging Viruses. Oxford University Press; pp. 283–289, 1993
- 2.Gubler DJ. Aedes aegypti and Aedes aegypti-borne disease control in the 1990s: Top Down or Bottom Up. Am J Trop Med Hyg 1989; 40: 571–578 [DOI] [PubMed] [Google Scholar]
- 3.IOM (Institute of Medicine). 1992. Emerging infections: microbial threats to health in the United States. Washington, DC: National Academy Press. [PubMed]
- 4.Gubler DJ. The President’s Address: Prevention and control of tropical diseases in the 21st century: Back to the field. Am J Trop Med Hyg 2001; 65(1): v–xi [PubMed] [Google Scholar]
- 5.Gubler DJ. The global threat of emergent/reemergent vector-borne diseases. In: Vector-Borne Diseases: understanding the environmental, human health, and ecological connections; Institute of Medicine. Washington, D.C.: The National Academies Press; 2008, pp. 43–64 [PubMed]
- 6.Gubler DJ. Population growth, urbanization, automobiles and airplanes: The dengue connection. In: Greenwood B, De Cock K, eds. New and Resurgent Infections: Prediction, Detection and Management of Tomorrow’s Epidemics. London: London School of Hygiene and Tropical Medicine; 1998. pp. 117–129
- 7.Gubler DJ. Dengue and Dengue Hemorrhagic Fever: Its History and Resurgence as a Global Public Health Problem. In: Gubler DJ, Kuno G, eds. Dengue and Dengue Hemorrhagic Fever. London: CAB International; 1997. pp. 1–22
- 8.Kimura R, Hotta S. Studies on dengue fever (VI). On the inoculation of dengue virus into mice. (In Japanese.) Nippon Igaku 1944; 3379: 629–633 [Google Scholar]
- 9.Sabin AB. Research on dengue during World War II. Am J Trop Med Hyg 1952; 1: 30–50 [DOI] [PubMed] [Google Scholar]
- 10.Hammon WM. Dengue hemorrhagic fever—Do we know its cause? Am J Trop Med Hyg 1973; 22: 82–91 [DOI] [PubMed] [Google Scholar]
- 11.Technical Advisory Committee on Dengue Haemorrhagic Fever for the South East Asian and Western Pacific Regions. Guide for Diagnosis, Treatment and Control of Dengue Haemorrhagic Fever. 2nd ed. Geneva: World Health Organization; 1980. pp. 1–30
- 12.Laigret J, Rosen L, Schollhammer G. Sur une epidemie de dengue survenue a Tahiti en 1964. Relations avec les feivres hemorragiques du sud-est asiatique. Bulletin de la Societe de Pathologie Exotique 1967; 60: 339–353 [PubMed] [Google Scholar]
- 13.Moreau J-P, Rosen L, Saugrain J, Lagraulet J. An epidemic of dengue on Tahiti associated with hemrrohagic manifestations. Am J Trop Med Hyg 1973; 22: 237–241 [DOI] [PubMed] [Google Scholar]
- 14.Barns WJS, Rosen L. Fatal hemorrhagic disease and shock associated with primary dengue infection on a Pacific island. Am J Trop Med Hyg 1974; 23: 495–506 [DOI] [PubMed] [Google Scholar]
- 15.Loison G, Rosen L, Papillaud J, Tomasini J, Vaujany J, Chanalet G. La dengue en Nouvelle-Caledonie (1971–1972). Bulletin de la Societe de Pathologie Exotique 1973; 66: 511–519 [Google Scholar]
- 16.Maguire T, Miles JAR, MacNamara FN, Wilkinson PJ, Austin FJ, Mataila JU. Mosquito-borne infections in Fiji V. The 1971–72 dengue epidemic. J Hyg (Cambridge) 1974; 73: 263–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gubler DJ, Reed D, Rosen L, Hitchcock JC. Epidemiologic, clinical and virologic observations on dengue in the Kingdom of Tonga. Am J Trop Med Hyg 1978; 27: 581–589 [DOI] [PubMed] [Google Scholar]
- 18.Rosen L. The pathogenesis of dengue haemorrhagic fever. A critical appraisal of current hypothese. S Afr Med J 1986; Suppl: 40–42 [PubMed] [Google Scholar]
- 19.Steel A, Gubler DJ, Bennett SN. Natural attenuation of dengue virus type-2 after a series of island outbreaks: A retrospective phylogenetic study of events in the South Pacific three decades ago. Virology 2010. Sep 30; 405(2): 505–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schliessman DJ, Calheiros LB. A review of the status of yellow fever and Aedes aegypti eradication programs in the Americans. Mosquito News 1974; 34: 1–9 [Google Scholar]
- 21.Dengue in the Caribbean, 1977. Scientific Publication No. 375. Pan American Health Organization, 1979, p. 186
- 22.PAHO 1994. Dengue and Dengue Hemorrhagic Fever in the Americas: Guidelines for Prevention and Control. Scientific Publication No 548.
- 23.Gubler DJ, Trent DW. Emergence of Epidemic dengue/Dengue Hemorrhagic Fever as a public health problem in the Americas. Infect Agents Dis 1994; 2: 383–393 [PubMed] [Google Scholar]
- 24.Beatty ME, Letson GW, Margolis HS. Estimating the Global Burden of Dengue. In: Abstract Book: Dengue 2008. The Second International Conference on Dengue and Dengue Haemorrhagic Fever. Phuket, Thailand
- 25.Sumarmo Wulur H, Jahya E, Gubler DJ, Sutomenggolo TS, Sulianti Saroso J. Encephalopathy associated with dengue infection. Lancet 1977; 8061: 449–450 [DOI] [PubMed] [Google Scholar]
- 26.Sumarmo Wulur H, Jahya E, Gubler DJ, Sorensen K. Clinical observations virologically confirmed fatal Dengue Hemorrhagic Fever in Jakarta, Indonesia. Bull World Health Organ 1983; 61: 693–701 [PMC free article] [PubMed] [Google Scholar]
- 27.Kho LH, Sumarmo Wulur H, Jahya EC, Gubler DJ. Dengue Hemorrhagic Fever accompanied by encephalopathy in Jakarta. Southeast Asian J Trop Med Public Health 1981; 12: 83–86 [PubMed] [Google Scholar]
- 28.Phuong CXT, et al. Evaluation of the World Health Organization standard tourniquet test in the diagnosis of dengue infection in Vietnam. Trop Med Int Health 2002; 7: 125–132 [DOI] [PubMed] [Google Scholar]
- 29.Balmaseda A, et al. Assessment of the World Health Organization scheme for classification of dengue severity in Nicaragua. Am J Trop Med Hyg 2005; 73: 1058–1062 [PubMed] [Google Scholar]
- 30.Nimmannitya S, Thisyakorn U. Dengue Hemorrhagic Fever with Unusual Manifestations. In: Proceedings of the International Conference on Dengue/Dengue Haemorrhagic Fever. Kuala Lumpur, Malaysia; 1983
- 31.WHO. 2009. Dengue. Guidelines for Diagnosis, Treatment, Prevention and Control. World Health Organization. Geneva, Switzerland. [PubMed]
- 32.Shepard DS, Coudeville L, Halasa YA, Zambrano B, Dayan GH. Economic impact of dengue illness in the Americas. Am J Trop Med Hyg 2011; 84: 200–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suaya JA, Shepard DS, Beatty M, Farrar J. Disease burden of dengue fever and dengue hemorrhagic ffever. Preedy VR, Watson RR, eds. Handbook of Disease Burdens and Quality of Life Measures. New York: Springer; 2010, pp. 1263–1279
- 34.Gubler DJ, Meltzer M. The Impact of Dengue/Dengue Hemorrhagic Fever on the Developing World. Adv Virus Res 1999; 53: 35–70 [DOI] [PubMed] [Google Scholar]
- 35.Dechant EJ, Riagu-Perez JG. Hospitalizations for suspected dengue in Puerto Rico, 1991-1995: estimation by capture-recapture methods. The Puerto Rico Association of Epidemiologists. Am J Trop Med Hyg 1999; 61: 574–578 [DOI] [PubMed] [Google Scholar]
- 36.Gubler DJ, Sather GE, Kuno G, Cabral AJR. Dengue 3 transmission in Africa. Am J Trop Med Hyg 1986; 35: 1280–1284 [DOI] [PubMed] [Google Scholar]
- 37.Gubler DJ. Dengue and Dengue Hemorrhagic Fever. Clin Microbiol Rev 1998; 11(3): 480–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gubler DJ. Epidemic dengue/Dengue Hemorrhagic Fever as a public health, social and economic problem in the 21st century. Trends Microbiol 2002; 10: 100–103 [DOI] [PubMed] [Google Scholar]
- 39.WHO. Dengue and dengue haemorrhagic fever. Factsheet N° 117, revised May 2008. Geneva, World Health Organization, 2008. (http://www.who.int/mediacentre/factsheets/fs117/en/).
- 40.Gubler DJ. How effectively is epidemiological surveillance used for dengue programme planning and epidemic response? Dengue Bulletin 2002; 26: 96–106 [Google Scholar]
- 41.Newton EAC, Reiter P. A model of the transmission of dengue fever with an evaluation of the impact of ultra-low volume (ULV) insecticide applications on dengue epidemics. Am J Trop Med Hyg 1992; 47: 709–720 [DOI] [PubMed] [Google Scholar]
- 42.Reiter P. Aedes alpobictus and the world trade in used tires, 1988-1995: The shape of things to come? J Am Mosq Cont Assoc 1998; 14: 83–94 [PubMed] [Google Scholar]
- 43.Gubler DJ. Dengue/dengue haemorrhagic fever: history and current status. In: Novartis Foundation Symposium 277: New Treatment Strategies for Dengue and Other Flaviviral Diseases. John Wiley & Sons, Ltd.; 2006, pp. 3–22 [DOI] [PubMed]
- 44.Alirol E, Getaz L, Stoll B, Chappuis F, Loutan L. Urbanisation and infectious diseases in a globalised world. Lancet Infect Dis 2011; 11: 131–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilcox BA, Gubler DJ, Pizer HF. Urbanization and the Social Ecology of Emerging Infectious Diseases. Chapter 4. In: Mayer K, Pizer HF, eds. Social Ecology of Infectious Diseases. London: Academic Press (Elsevier, Inc); 2008
- 46.IATA, 2011, http://www.iata.org/ps/intelligence_statistics/Pages/index.aspx
- 47.WHO. Dengue haemorrhagic fever: diagnosis, treatment, prevention and control. 2nd ed. Geneva: World Health Organization; 1997
- 48.Gubler DJ, Suharyono W, Sumarmo, Wulur H, Jahya E, Sulianti Saroso J. Virological surveillance for Dengue Hemorrhagic Fever in Indonesia using the mosquito inoculation technique. Bull World Health Organ 1979; 57: 931–936 [PMC free article] [PubMed] [Google Scholar]
- 49.Cummings DA, et al. Travelling waves in the occurrence of dengue haemorrhagic fever in Thailand. Nature 2004; 427: 344–347 [DOI] [PubMed] [Google Scholar]
- 50.Gubler DJ. Cities spawn epidemic dengue viruses. Nat Med 2004; 10: 129–130 [DOI] [PubMed] [Google Scholar]
- 51.Gubler DJ. The emergence of epidemic dengue fever and dengue hemorrhagic fever in the Americas: a case of failed public health policy. Pan Am J Public Health 2005; 17(4): 221–224 [DOI] [PubMed] [Google Scholar]
- 52.McCall PJ, Kittayapong K. (2007) Control of dengue vectors: tools and strategies. Report of the Scientific Working Group meeting on Dengue, Geneva, 1-5 October 2006. pp. 110–119
- 53.Alphey L, Benedict MQ, Bellini R, Clark GG, Dame D, Service M, Dobson S. Sterile-insect methods for control of mosquito-borne diseases—an analysis. Vector Borne and Zoonotic Diseases 2010; 10: 295–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kay B, Vu SN. New strategy against Adedes aegypti in Vietnam. Lancet 2005; 365: 613–617 [DOI] [PubMed] [Google Scholar]
- 55.McMeniman CJ, O’Neil SL. A virulent Wolbachia infection decreases the viability of the dengue vector. Aedes aegypti during period of embryonic quiescence. PLoS Negl Trop Dis 2010; 4: e748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wise de Valdez MR, Nimmo D, Betz J, Gong H, James AA, Alphey L, Black WC., IV Genetic elimination of dengue vector mosquitoes. Proc Nat Acad Sci (U S A) 2011; 108: 4772–4775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keller TH, Chen YL, Know JE, Lim SP, Ma NL, Patel SJ, Sampath A, Wang QY, Yin Z, Vasudevan SG. Finding new medicines for flaviviral targets. In: Novartis Foundation Symposium 277: New Treatment Strategies for Dengue and Other Flaviviral Diseases. John Wiley & Sons, Ltd; 2006, pp. 41–56 [PubMed]
- 58.Patkar CG, Kuhn RJ. Development of novel antivirals against flaviviruses. In: Novartis Foundation Symposium 277: New Treatment Strategies for Dengue and Other Flaviviral Diseases. John Wiley & Sons, Ltd; 2006, pp. 102–119 [PubMed]
- 59.Whitehead SS, Blaney JE, Durbin AP, Murphy BR. Prospects for a dengue virus vaccine. Nat Rev Microbiol 2007; 5: 518–528 [DOI] [PubMed] [Google Scholar]
- 60.Wilder-Smith A, Ooi EE, Vasudevan SG, Gubler DJ. Update on dengue: epidemiology, virus evolution, antiviral drugs, and vaccine development. Curr Infect Dis Rep 2010; 12: 157–164 [DOI] [PubMed] [Google Scholar]
- 61.Tien NT, Luxemburger C, Toan NT, Pollissard-Gadroy L, Huong VT, Van Be P, Rang NN, Wartel TA, Lang J. A prospective cohort study of dengue infection in schoolchildren in Long Xuyen, Viet Nam. Trans R Soc Trop Med Hyg 2010; 104: 592–600 [DOI] [PubMed] [Google Scholar]
- 62.Swaminathan S, Batra G, Khanna N. Dengue vaccines: state of the art. Expert Opin Ther Pat 2010; 20(6): 819–835 [DOI] [PubMed] [Google Scholar]
- 63.Gubler DJ. Emerging vector-borne flavivirus diseases: are vaccines the solution? Expert Rev Vaccines 2011; 10: 563–565 [DOI] [PubMed] [Google Scholar]