Abstract
Presbytis chrysomelas chrysomelas endemic only in Sarawak and Kalimantan was categorized by IUCN as a critically endangered primate that require special attention from research and conservation perspectives. A qualitative study on ranging patterns of P. c. chrysomelas was conducted in the Samunsam Wildlife Sanctuary, Sarawak. The study was conducted over a period of 13 months from December 2004 to December 2005 with 213 days of observation. Behavioural observation covered 17 groups with special emphasis on two main groups and 1 subadult group. Scanning and focal sampling were employed as the observation methods. Results indicated that P. c. chrysomelas had vertical, straight horizontal, and cross-horizontal types of movement patterns. P. c. chrysomelas was recorded to have a short movement distance (31.8–54.3 m). Distribution, abundance types, and food resources might be the factors that shaped the patterns of movement and distance in P. c. chrysomelas.
1. Introduction
Presbytis chrysomelas chrysomelas is classified by the IUCN as a critically endangered species identified as a member of the family Cercopithecinae, subfamily Colobinae [1]. P. c. chrysomelas can only be found in Sarawak and Kalimantan as among the Malaysian Presbytis species along with P. hosei, P. rubicunda, P. frontata, and P. melalophos [2]. Previously, it has been classified as a subspecies of the Presbtis melalophos [3, 4]. However, Groves [1] categorized the species based on morphological data, and his finding was supported by molecular systematic data [5]. Bennett [6] noted that the only three locations available for P. c. chrysomelas in Borneo are the Samunsam Wildlife Sanctuary (SWS), the Similajau National Park in Sarawak, and the Ulu Sg. Kapuas in West Kalimantan, Indonesia. In addition, Ampeng [7] further reported the distribution of this langurs at Tanjung Datu National Park, Sarawak. P. c. chrysomelas is among four species of the genus Presbytis distributed in Borneo [8]. P. c. chrysomelas ecological and behavioral aspects are less known than those of P. frontata [9]. Many studies involving the Borneo colobines focused on P. hosei [10–12] and P. rubicunda [13, 14]. Meanwhile, other primate surveys have been conducted outside Borneo involving other Presbytis species such as P. comata [15], P. melalophos [16, 17], and P. thomasi [18, 19].
Ranging patterns in many primates are influenced by ecological, behavioral, and climate factors such as rainfall [20–22]. The availability and spatial patterning of food resources [23], group size [24–26], mating opportunity [27], sleeping site [28, 29], and parasite avoidance [30] are among the factors affecting the ranging patterns in primates. Many studies have attempted to explain ranging patterns of Southeast Asian colobines [11, 16, 31]. However, the study on ranging patterns in P. c. chrysomelas has yet to be conducted. No intensive study is reported on the behavioural aspect of this species thus causing the lack of understanding in the behavioural ecology of the colobines species in Sarawak. In this paper, qualitative assessments were done on the ranging behavior of the P. c. chrysomelas in the Samunsam Wildlife Sanctuary, Sarawak, by describing types of movement and daily distance travel and discovering the potential factors influencing their ranging behavior.
2. Methods
This study was conducted at the Samunsam Wildlife Sanctuary, Sarawak, Malaysia, (SWS: 1° 78′ N and 109° 36′ E) with the area size of 60.9 km² (6092 ha). The Samunsam Wildlife Sanctuary (Figure 1) was originally established for the conservation of Nasalis larvatus [6, 32]. The sanctuary comprised of five habitats, namely, the riverine forest, mangrove forest, tropical heath forest, secondary forest, and lowland mix dipterocarp forest [6, 33]. However, in this study, the division was expanded into six habitats by differentiating hill mix dipterocarp from the hilly part of lowland mix dipterocarp forest. The division was based on the discovery of Shorea curtisii in the hilly part of the sanctuary. According to Hamid [34], S. curtisii can be used as the main indicator for the occurrence of hill mix dipterocarp forest. The Samunsam Wildlife Sanctuary was under the jurisdiction of the State Government of Sarawak (Sarawak Forest Department). However, since 2003, this sanctuary was supervised by the management of the Sarawak Forestry Corporation Sendirian Berhad (SFCSB).
Figure 1.
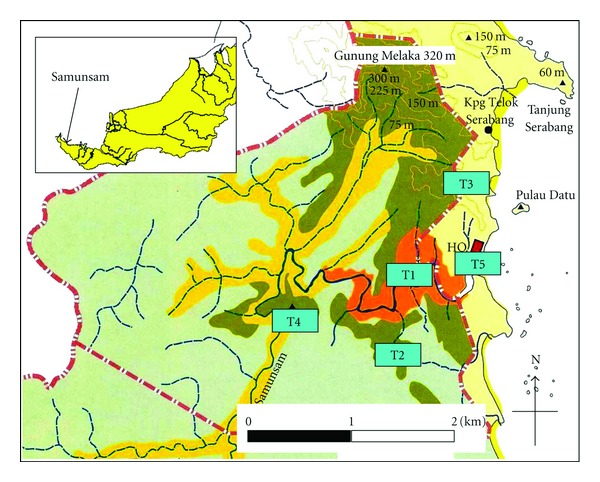
Map of the Samunsam Wildlife Sanctuary to indicate area of line transects (T1–T5).
One transect line of 2 km length has been set in the hill dipterocarp forest, lowland dipterocarp forest, riverine forest, tropical heat forest, and 1 km long in the secondary forest (Figure 1). Transects were surveyed and categorized commonly from time of dawn (06:00–06:30) until dusk (17:00–18:30). Detecting groups were discussed in detail by Ampeng and Md-Zain [35]. Within a 13-month study period (November 2004 to December 2005), 17 groups of P. c. chrysomelas were encountered in the SWS (Table 1). Out of this, nine groups were identified to originate from the main groups (MG) and eight were from the subadult groups (SAG). Main and subadult groups were defined based on age status. All groups were observed but only three groups were constantly followed during the study. The forested habitat and the arboreal habits of the P. c. chrysomelas made the groups extremely difficult to follow. The illegal logging and hunting, weather condition, and the shyness of the monkeys also limited the result of the survey. Three groups were focused on, two main groups (MG) and a subadult group (SAG). MG group and SAG in transect three and MG in transect two were relatively tolerant to the presence of the researchers.
Table 1.
Group composition of P. c. chrysomelas in the Samunsam Wildlife Sanctuary, (December 2004–December 2005) (MG = main group, SAG = subadult group). Three groups (italic) were consistently followed during research period.
| Transect | Group type | Adult/subadult | Juv. 1 | Juv. 2 | Subadult | Individual count, Dec. 04-Jan. 05 | Born Infant Jan-Mar. 2005 | Individual count, Dec. 31 2005 | |
|---|---|---|---|---|---|---|---|---|---|
| MG | SAG | ||||||||
| 1 | MG | 5 | — | 1 | 1 | 2 | 9 | 1 | 9 |
| MG | 6 | — | — | 1 | 2 | 9 | 1 | 9 | |
| SAG | — | 2 | — | — | — | 2 | — | 7 | |
| SAG | — | 5 | — | — | — | 5 | — | 9 | |
|
| |||||||||
| 2 | MG | 7 | — | 1 | 2 | 3 | 13 | 1 | 13 |
| SAG | — | 7 | — | — | — | 7 | — | 8 | |
| SAG | — | 6 | — | — | — | 6 | — | 9 | |
|
| |||||||||
| 3 | MG | 5 | — | 1 | 1 | 2 | 9 | 1 | 9 |
| MG | 6 | — | — | 2 | 2 | 10 | 1 | 10 | |
| MG | 4 | — | 1 | 1 | 2 | 8 | 1 | 8 | |
| MG | 6 | — | 1 | 2 | 2 | 11 | 1 | 11 | |
| SAG | — | 5 | — | — | — | 5 | — | 8 | |
| SAG | — | 7 | — | — | — | 7 | — | 9 | |
| SAG | — | 8 | — | — | — | 8 | — | 9 | |
|
| |||||||||
| 4 | MG | 6 | — | — | — | 3 | 9 | 1 | 9 |
| MG | — | 5 | 1 | 1 | 3 | 9 | 1 | 9 | |
| SAG | — | 8 | — | — | — | 8 | — | 12 | |
Scanning sampling was employed during intensive observation [36–38]. Every sampling subsession was 15 minutes with 10 minutes allocated for scan sampling and 5 minutes for describing and concluding each subsession. Langurs in the Samunsam Wildlife Sanctuary were not habituated in the presence of humans because of poachers who often enter the area [39]. To overcome the problem, focal sampling was also used as an alternative to record langur behavior [40, 41]. Focal sampling was employed each time an individual was seen; only one individual was observed at a time. In this study, quantitative ranging pattern ethogram was scored based on Fashing [22]. Furthermore, in this paper, further details to explain the types of langur movements throughout the study period were provided based on qualitative assessment. Daily distance movement based on Rabinowitz [40] were measured, and potential factors that shape types and distant movement of P. c. chrysomelas in Samunsam Wildlife Sanctuary were explored. The collected data were not analyzed using statistical methods as data acquisition required more detailed qualitative description [42].
3. Results
3.1. Movement Patterns
P. c. chrysomelas was not found to be dispersed or scattered during daily activities, especially when feeding the young leaves or fruits. This movement pattern was used as the main indicator to distinguish the group P. c. chrysomelas with the other diurnal primates found in this sanctuary. Observation showed three types of movement patterns; vertical, straight horizontal, and cross-horizontal movements.
Vertical movement was practiced only by the main groups (Figure 2). It was observed in the morning and not during the afternoon. The main groups moved in the vertical mid-canopy and lower canopy (3–8 m). This movement was consistent with the male leader in the bottom position (1–3 m) and performed only from March to July 2005.
Figure 2.
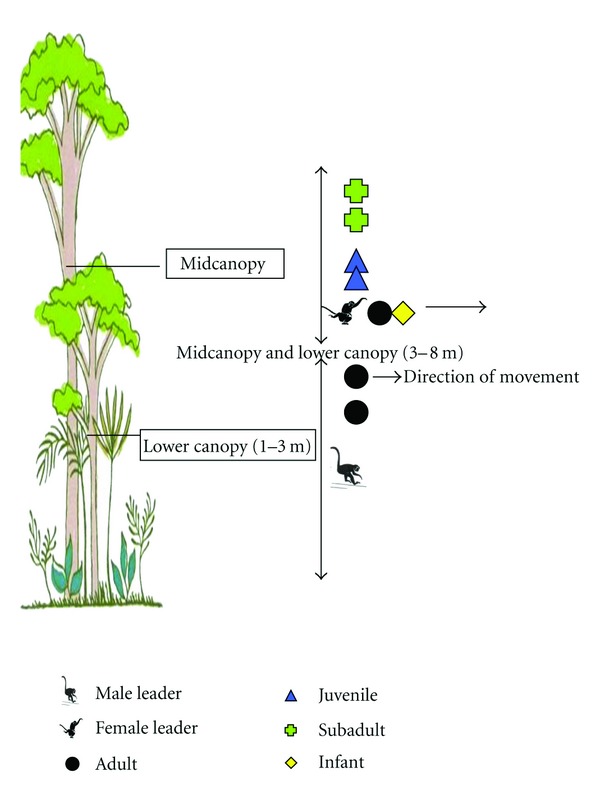
Illustration of vertical movement practiced by the main groups at mid- and lower canopy.
For other months beyond March to July 2005, the pattern of movement of the main groups and subadults was in a horizontal line at midcanopy level. At this time, the young leaves and fruit resources were abundant in the mid-canopy level. This is similar to findings by Bourliere [43] and Napier [44] who found that the pattern of movement was associated with the use of canopy space. The straight horizontal movement pattern was common to both groups during the beginning of their daily activities. For main groups, the adult male was observed to be positioned in front to lead the movement. Adult females were observed to move in the middle of the group with the infant, juvenile 1 and juvenile 2. Subadults moved in the back position. Some adults (1-2 individuals) travelled at the second and third positions (Figure 3). For the sub-adult group, the male leader moved forward once while others followed behind (Figure 3).
Figure 3.
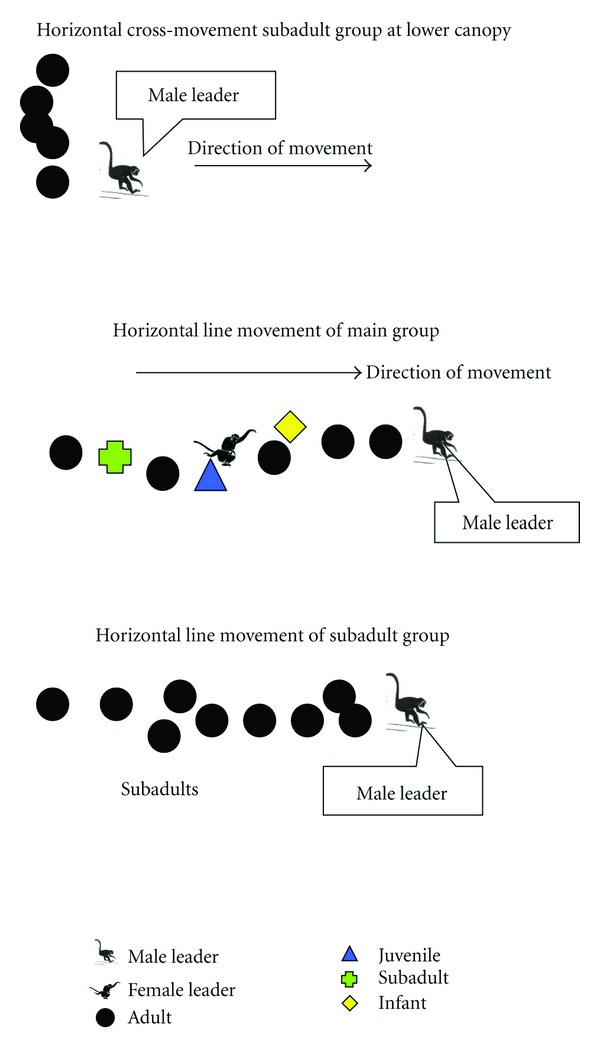
The horizontal cross- and horizontal line movement of P. c. chrysomelas.
Distribution of groups to the smaller units during foraging was not recorded in the main nor sub-adult groups. However, when fruit resources were available (the size of trees ≤30 cm), rotation on trees occurred between individuals of the main groups. Only 2-3 individuals were on the trees at one time consuming fruits. Meanwhile, others waited on adjacent trees (50–10 m), consuming leaves or watching the surrounding. Afterwards, the first group would leave to wait in nearby trees (50–10 m) and be replaced by a group of 2-3 individuals who were yet to eat. It was only after everyone finished eating fruits that all individuals in the group would travel together. This pattern of behavior, however, was only recorded twice in the main group. The results showed that both main and sub-adult groups moved towards the limit of the temporary home range if the group moved in a straight horizontal pattern. The results of this study agreed with studies by Lakim [11] and Fashing [22] in which the movement pattern was determined by the distance of daily movement, finding of food sources, and determination of the home range limit.
During horizontal movement, a low and short vocal tone was produced. A short vowel sound was produced during horizontal movement in determining the size of the home range area. The group would respond to this sound by turning either left or right. The results of this study agreed with those by Boonratana [45] who observed a straight horizontal movement in order to determine the limits of the home range. Straight horizontal movement was used by both groups while moving in the middle of the canopy space. The tree branches in the middle of the canopy space were large enough to accommodate the movement of individuals and also up to 2–5 individuals at a time.
The horizontal cross-movement was observed for the sub-adult group when travelling in the lower canopy (1–3 m) (Figure 3). This movement pattern was recorded only to occur between the months of March to July 2005 and involved 25% of the total movement recorded. This movement pattern only occurred in the morning. For the sub-adult group, the pattern of horizontal movement contradicted the vertical movement pattern of the main group. The space under the canopy tree size was small with limited food resources. This situation made it difficult for individuals to be at one tree at a given time as others may have already occupied it.
3.2. Daily Movement Distance
Daily movement distance for the whole period observation between the main groups and sub-adult group was different. For main groups, the total hours of daily movements spent from morning to late evening was 448.3 hours with a total movement distance of 11,344.7 m. In the morning, the total number of hours of movement observed for the main groups was 243.73 hours with an average of 1.44 ± 0.03 hours. With a total movement distance of 5978.5 m in the morning, the estimated daily movement distance was 35.4 ± 0.7 with a range between 33.9 and 36.8 m and speed of movement of about 27.2 m/hr. In the evening, the total number of hours of movement for the main groups was 204.54 hours with an average of 1.2 ± 0.04. With a total movement distance of 5366.2 m, the estimated range of daily movements in the afternoon was 31.8 ± 0.6 with a range of 30.5–33 m and a speed of about 26.5 m/hr (Table 2).
Table 2.
Daily movement of main and subadult group of P. c. chrysomelas.
| Period | Distance (m) | Standard deviation | Range | Speed (m/hour) |
|---|---|---|---|---|
| Main group | ||||
| Morning | 35.4 | 0.7 | 33.9–36.8 | 27.2 |
| Evening | 31.8 | 0.6 | 30.5–33 | 26.5 |
|
| ||||
| Sub-adult group | ||||
| Morning | 54.3 | 1.9 | 50.3–58.2 | 16 |
| Evening | 44.1 | 1.4 | 41.3–47 | 14 |
For the sub-adult group movement, a total number of 287.23 hours was spent in the morning and evening with a total movement distance of 4329 m. In the morning, the movement for the sub-adult group was observed to be 150.64 hours with an average of 3.42 ± 1.30 hours and a movement distance of 2386.8 m. The average distance of movement in the morning was 54.3 ± 9.1 m with a speed of about 16 m/hr. In the evening, 136.59 hours of the movement was observed for the subadult group, with an average 3.1 ± 0.9 hours. Total distance movement observed in the evening was 1942.2 m. The average distance of movement in the afternoon was 44.1 ± 1.4 m with a speed of about 14 m/hr (Table 2).
4. Discussion
Distribution and abundance of food resources in the Samunsam Wildlife Sanctuary may influence the pattern and range movement of P. c. chrysomelas. Main and sub-adult groups of P. c. chrysomelas entered temporary home range when fruit sources were available. This is in agreement with studies by Fashing and Cords [46] and Fashing [47] which stated that the distribution and abundance of food resources is a major factor influencing the ranging pattern of primates. These findings also concurred with Zhang and Wang [48] who observed that food resources, especially from abundant leaves affected the daily movement range of primates.
When food is scarce, the distance of daily movement may be increased [16, 49]. This contradicted findings in this study; P. c. chrysomelas did not seem to suffer food shortage despite a short daily movement distance. The results of this study supports Fashing [22] who observed a close distance movements of many leaf monkeys.
Ostro et al. [25] found that group size can influence the daily movement distance. Daily movement distance of the big group size is greater than that of the small group size [50]. This was observed in this study since the group size of main group is usually greater than that of sub-adult group. Group size of P. c. chrysomelas in the Samunsam Wildlife Sanctuary is small, between 8 and 13 individuals. With a small group size and food sources abundant in the Samunsam Wildlife Sanctuary, groups do not need to forage in long distance.
The use of different habitats will influence the pattern and range of leaf monkey movement [33]. The use of different habitats in the Rajanathan [33] study was based on the availability of fruit resources. This statement is true for this study because P. c. chrysomelas entered different habitats in the temporary home range when there were fruit resources. P. c. chrysomelas moved far only when fruit sources were available. Fruit trees in the forest habitat of the Samunsam Wildlife Sanctuary were scattered. This caused P. m. chrysomelas to move a little further to get those fruits. This study supports the findings of Zhang and Wang [48] and Fashing [22] which found that the long daily movement distance is influenced by the presence of fruit trees. Observations of P. siamensis melalophos in the Universiti Kebangsaan Malaysia also showed that the daily movement distance was influenced by the availability of fruit resources.
Acknowledgments
The authors would like to thank the State Government of Sarawak, Sarawak Forestry Department, especially Assistant Director Enforcement (Mr. Paul Ng) and Universiti Kebangsaan Malaysia. They also thank the management of the Samunsam Wildlife Sanctuary. They also thank Abu Kanchil, Phillip Ampeng, Ampeng Anggu, Reming Runyed, Ali Ahmad Mat, and Jefery Senan, for their cooperation in the field and financial and moral support while in the SWS. They wish to thank Farhana Shukor, Sri Harminda Hartantyo, and anonymous reviewer for their comments on the paper. This research was made possible under the IRPA 0802020019 EA301 Grant from the Ministry of Science Technology and Innovation, Malaysia. They also thank the State Government of Sarawak that sponsored the study leave scholarship for Mr. Ahmad Ampeng.
References
- 1.Groves CP. Primate Taxonomy. Washington, DC, USA: Smithsonian Institution Press; 2001. [Google Scholar]
- 2.Md-Zain BM, Morales JC, Nordin H, et al. Is Presbytis a distinct monophyletic genus: inference from mitochondrial DNA sequences. Asian Primates Journal. 2008;1(1):26–36. [Google Scholar]
- 3.Oates JF, Davies AG, Delson E. The diversity of living colobines. In: Davies AG, Oates JF, editors. Colobine Monkeys: Their Ecology, Behaviour and Evolution. Cambridge, UK: Cambridge University Press; 1994. pp. 45–73. [Google Scholar]
- 4.Md-Zain BM. Molecular systematics of the genus Presbytis. New York, NY, USA: Columbia University; 2001. Ph.D. dissertation. [Google Scholar]
- 5.Vun VF, Mahani MC, Lakim M, Ampeng A, Md-Zain BM. Phylogenetic relationships of leaf monkeys (Presbytis; Colobinae) based on cytochrome b and 12S rRNA genes. Genetics and Molecular Research. 2011;10(1):368–381. doi: 10.4238/vol10-1gmr1048. [DOI] [PubMed] [Google Scholar]
- 6.Bennett EL. A Wildlife Survey in Sarawak. New York, NY, USA: Wildlife Conservation Society; 1992. [Google Scholar]
- 7.Ampeng A. Densiti dan kepelbagaian primat diurnal di Taman Negara Tanjung, Sematan Sarawak. Universiti Kebangsaan Malaysia; 2003. B.Sc. thesis. [Google Scholar]
- 8.Brandon-Jones D, Eudey AA, Geissmann T, et al. Asian primate classification. International Journal of Primatology. 2004;25(1):97–164. [Google Scholar]
- 9.Meredith ME. A faunal survey of Batang Ai National Park, Sarawak, Malaysia. Sarawak Museum Journal. 1995;48(69):133–155. [Google Scholar]
- 10.Nijman V. Decline of the endemic Hose’s langur Presbytis hosei in Kayan Mentarang National Park, East Borneo. Oryx. 2005;39(2):223–236. [Google Scholar]
- 11.Lakim M. Comparative behavioral ecology of sympatric Presbytis rubicunda and Macaca fascicularis in Tawau Hills Parks, Sabah, Malaysia. Universiti Malaysia Sabah; 2008. Ph.D. dissertation. [Google Scholar]
- 12.Setiawan A, Nugroho TS, Djuwantoko, Pudyatmoko S. A survey of Miller’s grizzled surili, Presbytis Hosei canicrus, in East Kalimantan, Indonesia. Primate Conservation. 2009;24(1):139–143. [Google Scholar]
- 13.Supriatna J, Manullang BO, Soekara E. Group composition, home range, and diet of the maroon leaf monkey (Presbytis rubicunda) at Tanjung Puting Reserve, Central Kalimantan, Indonesia. Primates. 1986;27(2):185–190. [Google Scholar]
- 14.Davies AG, Bennett EL, Waterman PG. Food selection by two South-east Asian colobine monkeys (Presbytis rubicunda and Presbytis melalophos) in relation to plant chemistry. Biological Journal of the Linnean Society. 1988;34(1):33–56. [Google Scholar]
- 15.Nijman V. On the occurrence and distribution of Presbytis comata (Desmarest, 1822) (Mammalia: Primates: Cercopithecidae) in Java, Indonesia. Contributions to Zoology. 1997;66(4):247–256. [Google Scholar]
- 16.Bennett EL. Environmental correlates of ranging behaviour in the banded langur, (Presbytis melalophos) Folia Primatologica. 1986;47(1):26–38. doi: 10.1159/000156261. [DOI] [PubMed] [Google Scholar]
- 17.Aimi M, Bakar A. Distribution and deployment of Presbytis melalophos group in Sumatera, Indonesia. Primates. 1996;37(4):399–409. [Google Scholar]
- 18.Wich SA, Steenbeek R, Sterck EHM, Korstjens AH, Willems EP, van Schaik CP. Demography and life history of Thomas langurs (Presbytis thomasi) American Journal of Primatology. 2007;69(6):641–651. doi: 10.1002/ajp.20386. [DOI] [PubMed] [Google Scholar]
- 19.Wich SA, Schel AM, De Vries H. Geographic variation in Thomas langur (Presbytis thomasi) loud calls. American Journal of Primatology. 2008;70(6):566–574. doi: 10.1002/ajp.20527. [DOI] [PubMed] [Google Scholar]
- 20.Raemaekers JJ. Causes of variation between months in the distance traveled daily of gibbons. Folia Primatologica. 1980;34(1-2):46–60. doi: 10.1159/000155947. [DOI] [PubMed] [Google Scholar]
- 21.Olupot W, Chapman CA, Waser PM, Isabirye-Basuta G. Mangabey (Cercocebus albigena) ranging patterns in relation to fruit availability and the risk of parasite infection in Kibale National Park, Uganda. American Journal of Primatology. 1997;43(1):65–78. doi: 10.1002/(SICI)1098-2345(1997)43:1<65::AID-AJP5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 22.Fashing PJ. Activity and ranging patterns of querezas in the Kakamega forest: intergroup variability and implications for intra group feeding competition. International Journal of Primatology. 2001;22(4):549–577. [Google Scholar]
- 23.Zhang S-Y. Activity and ranging patterns in relation to fruit utilization by brown capuchins (Cebus apella) in French Guiana. International Journal of Primatology. 1995;16(3):489–507. [Google Scholar]
- 24.van Schaik CP, van Noordwijk MA, de Boer RJ, den Tonkelaar I. The effect of group size on time budgets and social behaviour in wild long-tailed macaques (Macaca fascicularis) Behavioral Ecology and Sociobiology. 1983;13(3):173–181. [Google Scholar]
- 25.Ostro LET, Silver SC, Koontz FW, Young TP, Horwich RH. Ranging behavior of translocated and established groups of black howler monkeys Alouatta pigra in Belize, Central America. Biological Conservation. 1999;87(2):181–190. [Google Scholar]
- 26.Li D, Zhou Q, Tang X, Huang H, Huang C. Sleeping site use of the white-headed langur Trachypithecus leucocephalus: the role of predation risk, territorial defense, and proximity to feeding sites. Current Zoology. 2011;57(3):260–268. [Google Scholar]
- 27.Overdorff DJ. Ecological and reproductive correlates to range use in red-bellied lemurs (Eulemur rubriventer) and rufous lemurs (Eulemur fulvus rufus) in Madagascar. In: Kappeler P, Ganzhorn JU, editors. Lemur Social Systems and Their Ecological Basis. New York, NY, USA: Plenum; 1993. pp. 167–192. [Google Scholar]
- 28.Harrison MJS. Pattern of range use by green monkey, Cercopithecus sabeus at Mt. Assirik, Senegal. Folia Primatologica. 1983;41:157–179. [Google Scholar]
- 29.Sigg H, Stolba A. Home range and daily march in a Hamadryas baboon troop. Folia Primatologica. 1981;36(1-2):40–75. doi: 10.1159/000156008. [DOI] [PubMed] [Google Scholar]
- 30.Hausfater G, Meade BJ. Alternation of sleeping groves by yellow baboons (Papio cynocephalus) as a strategy for parasite avoidance. Primates. 1982;23(2):287–297. [Google Scholar]
- 31.Boonratana R. Ranging behavior of proboscis monkeys (Nasalis larvatus) in the lower Kinabatangan, northern Borneo. International Journal of Primatology. 2000;21(3):497–518. [Google Scholar]
- 32. Sarawak Government Gazette Vol. 6 (NS) 28th May 1998 no. 2, Wildlife Protection Ordinance, 1998.
- 33.Rajanathan R. Differential habitat use by primates in Samunsam Wildlife Sanctuary, Sarawak and its application to conservation management. University of Florida; 1992. M.Sc. thesis. [Google Scholar]
- 34.Hamid AMI. Hutan: Pengurusan Dan Penilaian . Kuala Lumpur, Malaysia: Dewan Bahasa Dan Pustaka; 1998. [Google Scholar]
- 35.Ampeng A, Md-Zain BM. A short note on methodology of detecting leaf monkeys (Presbytis melalophos chrysomelas and Trachypithecus cristatus) in Samunsam Wildlife Sanctuary, Sarawak. The Journal of Wildlife and Parks. 2007;24:7–9. [Google Scholar]
- 36.Martin P, Bateson P. Measuring Behaviour: An Introductory Guide. Cambridge, UK: Cambridge University Press; 1993. [Google Scholar]
- 37.Md-Zain BM, Sha’ari NA, Mohd-Zaki M, et al. A comprehensive population survey and daily activity budget on long-tailed macaques of Universiti Kebangsaan Malaysia. Journal of Biological Sciences. 2010;10(7):608–615. [Google Scholar]
- 38.Md-Zain BM, Ch’ng CE. The activity patterns of a group of Cantor’s dusky leaf monkeys (Trachypithecus obscurus halonifer) International Journal of Zoological Research. 2011;7(1):59–67. [Google Scholar]
- 39.Jebron WG. Preliminary survey of wildlife on the baseline transects at Sungai Asam, Samunsam Wildlife Sanctuary, Malaysia. In: Proceedings of the 5th Workshop on Sarawak’s National Parks and Wildlife; 2001; pp. 14–20. [Google Scholar]
- 40.Rabinowitz A. Wildlife field research and conservation training manual. Paul's art, New York, NY, USA, 1995.
- 41.Md-Zain BM, Yen NM, Idris AB. Peruntukan aktiviti harian dan kesan pengkayaan terhadapan Cimpanzi (Pan troglodytes) di dalam kurungan. Sains Malaysiana. 2008;37(1):15–19. [Google Scholar]
- 42.Md-Zain BM, Lee CN. Semi-wild bornean orang utan: some qualitative aspects of unique daily behaviour. The Journal of Wildlife and Parks. 2003;21:57–65. [Google Scholar]
- 43.43. Bourliere F. Primates communities. Their structure and role in tropical ecosystem. International Journal of Primatology. 1985;9:233–255. [Google Scholar]
- 44.Napier JR. Primates and Their Adaptations. Burlington, NC, USA: Carolina Biological Supply; 1987. [Google Scholar]
- 45.Boonratana R. The ecology and behavior of the Proboscis monkey (Nasalis larvatus) in the Lower Kinabatangan, Sabah. Bangkok, Thailand: Mahidol University; 1993. Ph.D. thesis. [Google Scholar]
- 46.Fashing PJ, Cords M. Diurnal primate densities and biomass in the Kakamega Forest: an evaluation census methods and a comparisons with other forest. American Journal of Primatology. 2000;22:549–577. doi: 10.1002/(SICI)1098-2345(200002)50:2<139::AID-AJP4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 47.Fashing PJ. Mortality trends in the African cherry (Prunus africana) and the implications for colobus monkeys (Colobus guereza) in Kakamega Forest, Kenya. Biological Conservation. 2004;120(4):449–459. [Google Scholar]
- 48.Zhang SY, Wang LX. Fruit consumption and seed dispersal of Ziziphus cannamomun (Rhamacea) by two sympatric primates (Cebus paella and Ateles paniscus) in French Guiana. Biotropica. 1995;27(3):397–400. [Google Scholar]
- 49.Boinski S. Habitat use by squirrel monkeys (Saimiri oerstedi) in Costa Rica. Folia Primatologica. 1987;49(3-4):151–167. doi: 10.1159/000156319. [DOI] [PubMed] [Google Scholar]
- 50.Steenbeek R. Tenure related changes in wild Thomas langurs I: between-group interactions. Behaviour. 1999;136(5):595–625. [Google Scholar]


