Abstract
Background
T-helper (Th) 22 is involved in the pathogenesis of inflammatory diseases. The roles of Th22 cells in the pathophysiological of ankylosing spondylitis (AS) and rheumatoid arthritis (RA) remain unsettled. So we examined the frequencies of Th22 cells, Th17 cells and Th1 cells in peripheral blood (PB) from patients with AS and patients with RA compared with both healthy controls as well as patients with osteoarthritis.
Design and Methods
We studied 32 AS patients, 20 RA patients, 10 OA patients and 20 healthy controls. The expression of IL-22, IL-17 and IFN-γ were examined in AS, RA, OA patients and healthy controls by flow cytometry. Plasma IL-22 and IL-17 levels were examined by enzyme-linked immunosorbent assay.
Results
Th22 cells, Th17 cells and interleukin-22 were significantly elevated in AS and RA patients compared with OA patients and healthy controls. Moreover, Th22 cells showed positive correlation with Th17 cells as well as interleukin-22 in AS and RA patients. However, positive correlation between IL-22 and Th17 cells was only found in AS patients not in RA patients. In addition, the percentages of both Th22 cells and Th17 cells correlated positively with disease activity only in RA patients not in AS patients.
Conclusions
The frequencies of both Th22 cells and Th17 cells were elevated in PB from patients with AS and patients with RA. These findings suggest that Th22 cells and Th17 cells may be implicated in the pathogenesis of AS and RA, and Th22 cells and Th17 cells may be reasonable cellular targets for therapeutic intervention.
Introduction
Ankylosing spondylitis (AS) is a chronic inammatory disease that is characterized by mainly involving bilateral sacroiliitis and axial joints, but sometimes peripheral joints and extra-articular organs are also involved [1]. Rheumatoid arthritis (RA), which represents an example of autoimmunity disease, is another form of arthritis. The abnormality of T cells is implicated in the pathogenesis of many autoimmune diseases, and many autoimmune diseases, especially arthritis, were considered to be mainly driven by Th1 cells [1]–[3]. A new IL-17-producing T cell subset, termed Th17 cells, has been described in recent years [4]–[7]. It has been established that Th17 cells play critical roles in several animal models of autoimmunity, such as experimental allergic encephalomyelitis (EAE) [8] and murine arthritis models [9], [10]. Besides, Th17 cells are considered to be involved in many human inflammatory diseases, including multiple sclerosis, psoriasis and inflammatory arthritis [11]–[15]. As to AS and RA, increased Th17 cells were found in PBMC from patients with AS and RA [16]. IL-17, secreted mainly by Th17 cells, is a cytokine shown to stimulate RA synovial fibroblast (RASF) to release several mediators of joint inflammation including IL-6, IL-8, GM-CSF and PGE2 [17]–[19]. Moreover, elevated serum levels of IL-17 and IL-23 has been reported in AS which is one of the forms of arthritis [20].
IL-22, a member of IL-10 cytokine family, exerts its effects via a heterodimeric transmembrane receptor complex consisting of IL-10R2 and IL-22R1 [21]. IL-22 has been believed as an important player in regulating inflammatory responses associated with many inflammatory diseases. Higher expression of IL-22 mRNA was observed in psoriatic skin lesion, and elevated serum IL-22 levels were found in patients with psoriasis [22]. In addition, the involvement of IL-22 in other inflammatory diseases such as inflammatory bowel disease [23] also proves its proinflammatory roles. However, diminishing intestinal inflammatory in a mouse model of ulcerative colitis and providing protection to hepatocytes during acute liver inflammation by IL-22 demonstrate its anti-inflammatory properties [24]. The situation of IL-22 was not completely consistent in autoimmune diseases. Consistent with psoriasis, increased IL-22 has also been found in serum samples from RA and Crohn disease patients. On the contrary, decreased plasma IL-22 levels were found in patients with SLE [25]. So, diverse pathogenic mechanisms and tissue microenvironments may result in different contributions of IL-22 in autoimmune disease development. The precise pathophysiologic function of IL-22 remains unclear, and the involvement of IL-22 in AS and RA remains to be established.
Th22 subset is a more recently identified new human T helper subset, which is characterized by abundant secretion of IL-22 but not IL-17 or IFN-γ [26]–[28]. Th22 cells express the chemokine receptors CCR4, CCR6 and CCR10 [26]. Moreover, this newly identified CD4+ T cells clones have low or undetectable expression of Th1 and Th17 transcription factor T-bet and RORγt, and arylhydrocarbon receptor (AHR) has been considered to be the key transcription factor of Th22 subset [26]. In addition, naïve T cells differentiate toward the Th22 phneotype in the presence of IL-6 and TNF-α[26]. All of above provide strong evidence that Th22 cells represent an independent and terminally differentiated T cells subtype. It has been reported that Th22 cells were detected in psoriatic skin lesions. Moreover, the increasing circulating Th22 cells [29] suggest that Th22 cells may be implicated in the pathogenesis of psoriasis which is a chronic inammatory disease. In addition, elevated Th22 cells in peripheral blood of RA patients have also been reported in our previous study. Thus, the involvement of Th22 cells in other chronic inammatory diseases needs to be further investigated.
The roles of both Th22 cells and IL-22 in the pathogenesis of ankylosing spondylitis and rheumatoid arthritis are still unclear and remain to be clarified. Therefore, to investigate their roles in the pathogenesis of AS and RA, we examined the frequencies of Th22 cells in peripheral blood as well as the levels of plasma IL-22 of both AS and RA patients, and assayed their correlations with disease activity in this study.
Materials and Methods
Ethics Statement
Enrollment took place between May, 2010 and April, 2011 in two centers: Qilu Hospital, Shandong University and Shandong Provincial Hospital, Shandong University, China. Our research has been approved by both the Medical Ethical Committee of Qilu Hospital, Shandong University and the Medical Ethical Committee of Shandong Provincial Hospital, Shandong University. A written informed consent document has been obtained from each participant.
Patients and Controls
A total of 32 patients with AS according to the modified New York criteria [30] ere recruited in this study. The Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score [31] were measured for the patients with AS. All patients were HLA-B27-positive. This group consisted of 27 men and 5 women, with mean ± SD disease duration of 8.6±5.9 years. The mean age of the patients was 36.6±10.2 years. A total of 20 patients with active RA according to the criteria of the American College of Rheumatology were included in this study [32]. The DAS28 score [33] were measured for the patients with RA. This group consisted of 16 women and 4 men, with mean ± SD disease duration of 8.9±3.9 years. The mean age of the patients was 47.5±9.2 years. The demographic and key clinical information of AS and RA patients are summarized in Table 1 and Table 2. All of the patients did not receive immunosuppressive or immunomodulatory drugs for at least 2 months when sampling. The major previous treatment of AS and RA patients were shown in Table S1 and Table S2. Ten osteoarthritis (OA) patients (3 females and 7 males; mean age 48.9±10.3 years) as disease controls and twenty healthy controls (5 females and 15 males; mean age 37.9±9.1 years) were also recruited in the study, and all of them did not have any rheumatologic conditions.
Table 1. Characteristics of the patients with Ankylosing Spondylitis.* .
| Characteristics | value |
| Age(y) | 36.6±10.2 |
| Sex(male/female) | 27/5 |
| Disease duration(y) | 8.6±5.9 |
| ESR(mm/h) | 38.2±20.8 |
| ESR(mm/h) of Healthy Control | 9.5±4.2 |
| CRP(mg/L) | 24.9±13.7 |
| CRP(mg/L) of Healthy Control | 3.4±1.6 |
| BASDAI score | 3.2±1.7 |
| HLA-B27,positive member | 32 |
BASDAI = Bath Ankylosing Spondylitis Disease Activity Index (range 0–10); ESR = erythrocyte sedimentation rate; CRP = C-reactive protein.
Table 2. Demographic and clinical characteristics of RA patients.* .
| Characteristics | value |
| No. of patients | 20 |
| Age(y) | 47.5±9.2 |
| Sex(male/female) | 4/16 |
| Disease duration(y) | 8.9±3.9 |
| RF | 14/20(70%) |
| ESR(mm/h) | 35.8±27.9 |
| CRP(mg/L) | 27.1±23.3 |
| No. of swollen joints | 7.3±3.7 |
| No. of tender joints | 8.1±3.8 |
| DAS28 | 5.2±1.4 |
RF = rheumatoid factor; ESR = erythrocyte sedimentation rate; CRP = C-reactive protein; DAS28 = Disease Activity Score in 28 joints.
Flow Cytometric Analysis
Intracellular cytokines were studied by flow cytometry to reflex the cytokine-producing cells. Briefly, heparinized peripheral whole blood (400 µl) with an equal volume of Roswell Park Memorial Institute 1640 medium were incubated for 4 h at 37°C, 5% CO2 in the presence of 25 ng/mL of phorbol myristate acetate (PMA), 1 µg/mL of ionomycin, and 1.7 µg/ml Golgiplug(Monensin; all from Alexis Biochemicals, San Diego, CA, USA). PMA and ionomycin are pharmacological T-cell-activating agents that mimic signals generated by the T-cell receptor (TCR) complex and have the advantage of stimulating T cells of any antigen specificity. Monensin was used to block intracellular transport mechanisms, thereby leading to an accumulation of cytokines in the cells. After incubation, the cells were stained with PE-Cy5-conjugated anti-CD4 monoclonal antibodies (clone: RPA-T4, Cat: 45-0049-42) at room temperature in the dark for 20 min. The cells were next stained with FITC-conjugated anti-interferon (IFN)-γ monoclonal antibodies (clone: 4S-BS, Cat: 11-7319-82), PE-conjugated anti-IL-17A monoclonal antibodies (clone: eBio64DEC17, Cat: 12-7179-42) and APC-conjugated anti-IL22 monoclonal antibodies (clone: 22URTI, Cat: 50-7229-42) after fixation and permeabilization. All the antibodies were from eBioscience, San Diego, CA, USA. Isotype controls were given to enable correct compensation and confirm antibody specificity. Stained cells were analyzed by flow cytometric analysis using a FACScan cytometer equipped with CellQuest software (BD Bioscience PharMingen). Th22, Th17, Th1 and Th1/Th17 cells were defined as CD4+IFNγ–IL17–IL-22+, CD4+IFNγ–IL17+, CD4+IFNγ+ and CD4+IFNγ+IL17+T cells respectively.
IL-22 and IL-17 Enzyme-linked Immunosorbent Assay (ELISA)
Peripheral blood was collected into heparin-anticoagulant vacetainer tubes. Plasma was obtained from all subjects by centrifugation and stored at –80°C for determination of cytokines. Plasma IL-22 (Cat: BMS2047) and IL-17 (Cat: BMS2017) levels were determined with a quantitative sandwich enzyme immunoassay technique in accordance with the manufacturer’s recommendations (lower detection limit 9 pg/ml; eBioscience).
Clinical Assessment
BASDAI score of AS patients and disease activity score in 28-joints (DAS28) of RA patients was calculated in our study. At the time of clinical assessment for disease activity, blood samples were collected for the measurement of levels of C-reactive protein (CRP) and ESR.
Statistical Analysis
Results were expressed as mean ± SD or median (range). Statistical significance of Th22, Th17, Th1 and plasma IL-22 as well as IL-17 among patients with AS, RA, OA and HC was determined by ANOVA, and difference between two groups was determined by Newman–Keuls multiple comparison test (q test) unless the data were not normally distributed, in which case Kruskal - Wallis test (H test) and Nemenyi test were used. The Pearson or Spearman correlation test was used for correlation analysis depending on data distribution. All tests were performed by SPSS 17.0 system. P value less than 0.05 was considered statistically significant.
Results
Elevated Th22 Cells Correlated with Increased Plasma Levels of IL-22 in AS and RA Patients
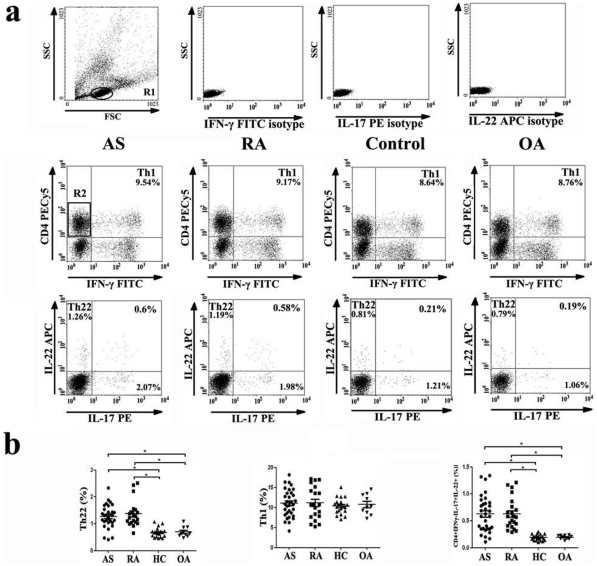
We analyzed the frequency of Th22 cells based on cytokine patterns after in vitro activation by PMA/ionomycin in short-term cultures. The expression of a typical dot-plot of Th22 cells in representative AS, RA as well as OA patients and healthy controls was shown in Fig. 1a. The percentage of Th22 cells was significantly elevated in AS (1.27±0.42%) and RA (1.37±0.49%) patients compared to OA patients (0.70±0.19%) or healthy controls (0.68±0.18%) (Fig. 1b).
Figure 1. Circulating Th22 cells and CD4+IFNγ-IL17+IL-22+ T cells are significantly increased in ankylosing spondylitis (AS) patients and rheumatoid arthritis (RA) patients compared with osteoarthritis (OA) patients and healthy controls.
a, Representative flow cytometry dot plots example of each group. b, The percentages of circulating Th22 cells (left panel), Th1 cells (middle panel) and CD4+IFNγ-IL17+IL-22+ T cells (right panel) from AS, RA, OA patients and healthy controls after stimulation with phorbol myristate acetate, ionomycin, and monensin for 4 h. (* = P<0.05).
In addition, we also quantified the number of Th22 cells per volume (50µL) of peripheral blood. The number of Th22 cells was significantly increased in AS (204±34) and RA (211±43) patients compared with healthy controls (123±23) after stimulation with phorbol myristate acetate, ionomycin, and monensin for 4 h (Fig. S2a).
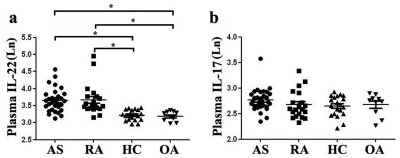
Plasma levels of IL-22 were examined by ELISA. The levels of IL-22 were significantly increased in AS (41.03±16.00pg/ml) and RA (44.53±29.84pg/ml) patients compared to OA patients (24.53±3.45pg/ml) and healthy controls (25.33±3.75pg/ml) (Fig. 2a).
Figure 2. Concentrations of IL-22 and IL-17 in plasma of non-stimulated peripheral blood from AS, RA, OA patients and healthy controls.
a, Concentrations of IL-22 in plasma from AS, RA, OA patients and healthy controls. The levels of IL-22 was significantly increased in AS and RA patients compared with OA patients or healthy controls. b, Concentrations of IL-17 in plasma from AS, RA, OA patients and healthy controls. As to IL-17, there was no significant difference between AS or RA patients and OA as well as healthy controls (* = P<0.05).
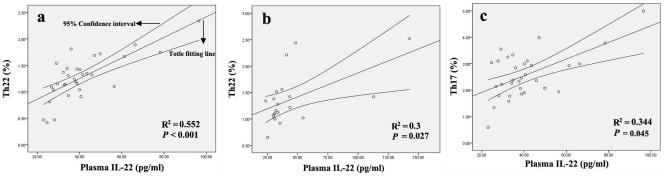
A positive correlation was found between Th22 cells and plasma levels of IL-22 in AS (r = 0.743, P<0.001; Fig. 3a) and RA (r = 0.548, P = 0.027; Fig. 3b) patients. Moreover, positive correlations were also found between IL-22 plasma levels and Th17 cells (r = 0.587, P = 0.045; Fig. 3c) in AS patients. However, no corresponding correlation was found in RA patients (P = 0.801) (Fig. S1l).
Figure 3. Correlation between the percentages of each T cell subset and the plasma IL-22 concentrations in AS or RA patients.
a and b, Positive correlation was found between Th22 cells and IL-22 in AS (a) and RA (b) patients. c, Positive correlation was found between Th17 cells and IL-22 in AS patients.
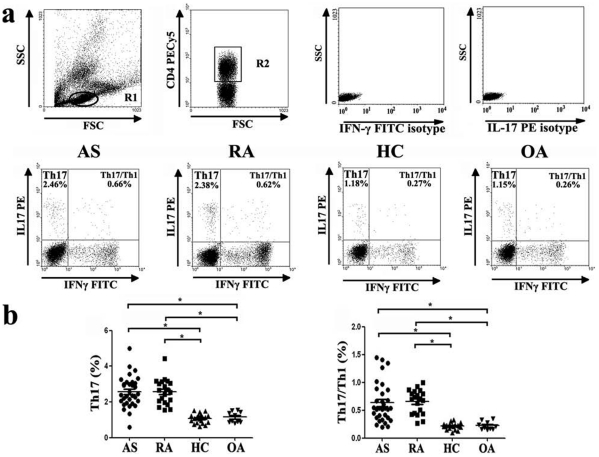
Elevated Th17 Cells in AS and RA Patients
The expression of a typical dot-plot of Th17 cells in representative AS, RA, OA patients and healthy controls was shown in Fig. 4a. We found significantly increased percentage of Th17 cells in AS (2.58±0.86%) and RA (2.57±0.72%) patients compared with OA patients (1.15±0.31%) and healthy controls (1.07±0.26%)(Fig. 4b). Consistently, the number of Th17 cells per volume (50µL) of peripheral blood was significantly increased in AS (320±36) and RA (337±39) patients compared with healthy controls (214±26) after stimulation with phorbol myristate acetate, ionomycin, and monensin for 4 h (Fig. S2b). However, there was no significant difference regarding plasma IL-17 between each group. (AS: 16.28±4.25, P>0.05; RA: 15.02±4.60, P>0.05; OA: 14.80±2.88, P>0.05; HC: 14.39±2.72, P>0.05) (Fig. 2b).
Figure 4. Circulating Th17 cells and Th17/Th1 cells are significantly increased in ankylosing spondylitis (AS) patients and rheumatoid arthritis (RA) patients compared with osteoarthritis (OA) patients and healthy controls.
a, Representative flow cytometry dot plots example of each group. b, The percentages of circulating Th17 cells (left panel) and Th17/Th1 cells (right panel) from AS, RA, OA patients and healthy controls after stimulation with phorbol myristate acetate, ionomycin, and monensin for 4 h (* = P<0.05).
As to Th1 cells, there was no significant difference between each group. (AS: 11.05±3.41%, P>0.05; RA: 11.13±4.09%, P>0.05; OA: 10.73±2.50%, P>0.05; HC: 10.37±2.00, P>0.05) (Fig. 1a, b).
Increased Expression of IL-17 and IL-22 Double-Positive CD4 T Cells as well as IL-17 and IFN-γ Double-Positive CD4 T Cells in AS and RA Patients
For these experiments, we also examined the frequencies of CD4+IFNγ-IL17+IL-22+ T cells and CD4+IFNγ+IL17+ T cells. Though most Th17 cells did not simultaneously express both IL-22 and IL-17, the percentage of CD4+IFNγ-IL17+IL-22+ T cells was significantly increased in AS (0.63±0.34%)) and RA (0.65±0.29%) patients compared with OA patients (0.20±0.04%) and healthy controls (0.19±0.05%)(Fig. 1b). In addition, ratios of Th1/Th17 cells were significantly increased in AS patients (0.63±0.35%) and RA patients (0.66±0.21%) compared to OA patients (0.22±0.07%) and healthy controls (0.22±0.06%). (Fig. 4b).
Correlation between Th22, Th17 and Th1 Cells in AS and RA Patients
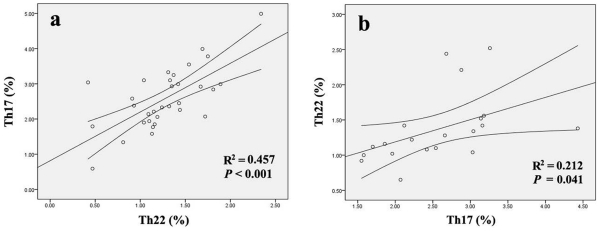
In AS patients, there was a significant positive correlation between Th22 cells and Th17 cells (r = 0.676, P<0.001) (Fig. 5a). Similarly, a positive correlation was also found between Th22 cells and Th17 cells (r = 0.46, P = 0.041) in RA patients (Fig. 5b). However, Th1 cells failed to show a significant correlation with Th22 cells and Th17 cells.
Figure 5. Correlation between the percentages of Th22 cells and the percentages of Th17 cells in AS and RA patients.
a, Positive correlation was found between Th22 cells and Th17 cells in AS patients. b, Positive correlation was found between Th22 cells and Th17 cells in RA patients.
The Correlation of the Frequencies of Th22 Cells and Th17 Cells with Disease Activity or Laboratory Parameters in AS and RA Patients
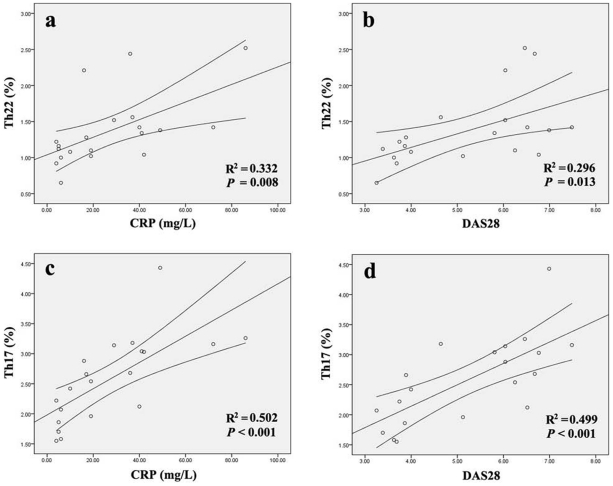
In patients with RA, there were positive correlations between the percentage of Th22 cells and CRP level or DAS28 (r = 0.576, P = 0.008 or r = 0.544, P = 0.013 respectively) (Fig. 6 a, b). Consistently, positive correlations were also found between the percentage of Th17 cells (r = 0.709, P<0.001 or r = 0.706, P<0.001 respectively) (Fig. 6 c, d) and CRP level as well as DAS28. However, the percentage of Th1 cells was not correlated with either CRP level or DAS28 (P = 0.105 or P = 0.205), and plasma level of IL-22 or IL-17 failed to show a statistical correlation with CRP level or DAS28 (P = 0.622 and P = 0.357 or P = 0.317 and P = 0.872) in RA patients (Fig. S1j, k). In patients with AS, there was no correlation between the percentage of Th22 cells and clinical parameters, including ESR (P = 0.964), CRP (P = 0.393) and BASDAI score (P = 0.226) (Fig. S1a, b, c). Consistently, no correlation was found between plasma level of IL-22 and clinical parameters in AS patients, including ESR (P = 0.918), CRP (P = 0.862) and BASDAI score (P = 0.320) (Fig. S1g, h, i). Correlation analysis between the percentage of Th17 cells and clinical parameters also showed no association (P = 0.189, P = 0.852 and P = 0.733 respectively) (Fig. S1d, e, f).
Figure 6. Correlation between the percentages of Th22 cells as well as Th17 cells and CRP levels as well as DAS28.
Discussion
Th22 cells, a recently defined lineage of T cells distinct from Th1, Th2 and Th17 cells, have been believed to play a complicated and important role in inflammatory and autoimmune diseases. The function of Th22 cells is achieved through the signature cytokine they secreted. Th17 cells have already been proposed to be the primary driver of autoimmune diseases, including rheumatoid arthritis. However, the potential role of Th22 cells in inflammatory arthritis such as AS and RA is far from clear.
To determine whether Th22 is involved in AS and RA, the percentages of Th22 cells were examined in the peripheral blood of patients with AS, RA OA, and healthy controls in this study. This is the first study that evaluates the relative abundance of Th22 cells in peripheral blood of RA and AS patients. Our results demonstrated that the percentages of Th22 cells were significantly elevated in the peripheral blood of patients with AS and also in those with RA compared with OA patients and healthy controls, which was consistent with our previous experiments on RA [34]. Moreover, we also quantified the number of Th22 cells in per volume blood. Consistent with the results of the percentage, the number of Th22 cells in per volume of blood was significantly increased in AS and RA patients compared to healthy controls. Likewise, increased Th22 cells has been observed in peripheral blood of patients with psoriasis [29], which implicating the involvement of Th22 cells in the chronic inflammatory skin disorder. These observations are compatible with the idea that Th22 cells may contribute to the pathogenesis of both RA and AS, which are two types of inflammatory arthritis. In the present study, correlation was also found between levels of Th22 cells and disease activity as assessed by DAS28 or the CRP concentration in RA. However, such correlation was not observed in AS. The possible explanation for this inconsistency between AS and RA is that many patients with AS do not have elevated CRP or ESR levels, so it is more difficult to assess disease activity in AS. It is known that Th17 cells are enriched in the joints of RA patients and co-express the chemokine receptor CCR6 and CCR4 [35]. Besides, CCL20 [36] as well as CCL22 [37], the ligand of CCR6 and CCR4 respectively, have already been observed in synovial fluid of human. Thus, all signs indicate that the interaction between receptor and ligand may play an important role in attracting Th17 cells to the joints in RA. Similar to Th17 cells, Th22 cells also co-express the chemokine receptor CCR6 and CCR4. So, we can reasonable believe that Th22 cells might selectively migrate to the joints from peripheral blood in the similar way. Moreover, we also speculated that Th22 cells might be elevated in the joints of RA and AS patients, which could play a pathogenic role in the lesions. Otherwise, what the pathogenic role does the Th22 cells play in AS and RA when migrating to the lesion remain to be elucidated. Then, we would focus our further investigation on the involvement of this new subset in synovial fluid of patients’ joints.
IL-22, a cytokine of IL-10 family, is the most important functional cytokine of Th22 cells. Considerable evidence suggests that IL-22 may be involved in the pathogenesis of many inflammatory diseases, but its pathophysiologic function is not well known. To investigate the potential role of IL-22 in AS and RA patients, we examined the concentrations of plasma IL-22 in our experiments. In line with our previous study, elevated levels of plasma IL-22 were also detected in AS and RA patients in the present study, implicating that IL-22 may be involved in the pathogenesis of both AS and RA. IL-22 has been demonstrated to exert its biological effects via binding to the heterodimeric receptor complex, including IL-22R1 and IL-10R2, and IL-22 activates Jak1 and Tyk2, which further activates STAT-1, STAT-3 and STAT-5 [38]. It has already been reported that IL-22R1 mRNA was expressed in RA synovial tissue. In addition, activation of ERK-1/2 as wells as p38 MAPK by IL-22 has also been reported in RA [39]. In vitro, recombinant IL-22 promoted proliferation of synovial fibroblasts of RA patients (RASF) and increased production of monocyte chemoattractant protein 1 (MCP-1) by RASF. Thus, we speculated the similar effect of IL-22 in AS patients as in RA patients. More recently, IL-22 serum levels were demonstrated to be associated with radiographic progression in rheumatoid arthritis [40], which further improved the importance of IL-22 in chronic inflammatory arthritis. In our study, plasma IL-22 levels correlated positively with Th22 cells in both AS patients and RA patients. In addition, correlation between plasma IL-22 level and Th17 cells were observed in AS patients. By contrast, no association of plasma IL-22 levels with Th17 cells was found in RA patients. Th22 cells, Th17 cells and Th1 cells are the main T cells subsets secreting IL22 [41], [26], [28], [42]. The correlation between Th17 cells and IL-22 in AS supported the idea that Th17 subset was an important T cells subset secreting IL-22 in peripheral blood of AS patients. Otherwise, no correlations in RA patients suggested that Th22 cells may produced much greater portion of total IL-22, and Th17 cells may produced a relatively smaller portion of total IL-22 in peripheral blood of RA patients than AS patients. So far, the pathophysiologic role of IL-22 in both AS and RA is not fully understood and need further investigation.
Considerable evidence suggests Th17 cells and Th1 cells have been involved in the development of autoimmune diseases [43], [44]. Thus, we examined the frequencies of Th17 cells and Th1 cells in each group in this experiment. In consistence with the experiment of Hui et al [16], the percentages of Th17 cells were demonstrated to be significantly increased in the peripheral blood of patients with AS and RA. Consistent with the results of Th22 cells, the number of Th17 cells in per volume of blood was significantly increased in AS and RA patients compared to healthy controls. In contrast to the results for Th17 and Th22 cell, no statistical difference was detected in percentages of Th1 cells between each group. Our observations supported the idea that Th17 cells contributed to the pathogenesis of AS and RA. Furthermore, we also examined the correlation between disease activity of AS or RA and the percentages Th17 cells. Like Th22 cells, there was a positive correlation between disease activity and Th17 in RA patients but not in AS patients. This inconsistent results between T cells and disease activity in AS is unclear, and needs further study. In this study, a positive correlation was found between the frequencies Th22 cells and Th17 cells in peripheral blood of patients with both AS and RA. This co-elevated level of Th22 cells and Th17 cells suggests these two T cell subsets may play a synergistic role in AS and RA, and the specific links between Th22 cells and Th17 cells need further investigation.
IL-17, the main cytokine of Th17 cells, contributes to the pathogenesis of arthritis as has been demonstrated in many experimental arthritis models. Although elevated levels of IL-17 have been observed in synovial fluid of RA patients, the levels of this T cell cytokine in plasma of these patients is hard to detect. Furthermore, the reports on IL-17 levels in plasma of RA or AS patients are inconsistent [35], [20]. The levels of IL-17 in plasma of each group were also measured in this experiment. In line with recent report [35], plasma IL-17 levels were not significantly different between the patients and healthy controls in our study. Moreover, the situation of IL-17 in autoimmune diseases is different. Increased levels of IL-17 have been shown in the serum of systemic sclerosis patients, but not in that of SLE patients or healthy controls.
In humans, it is not unusual to encounter dual +Th1/Th17 cells. It has previously been demonstrated that T cells from synovial fluid of RA can co-express IL-17 and IFN-γ [45], and both IL-17 and IFN-γ can be secreted by T cells derived from the joints [46]. In addition, IL-17 and IFN-γ cytokines have been shown to be co-expressed in human memory CD4+CD45+RO+ T cells from treatment-naïve early RA patients [47]. So we examined the percentages of Th17/Th1 cells. Consistent with a recent report in psoriasis [29], Th17/Th1 cells were increased in peripheral blood of AS and RA compared with OA and healthy controls. However, cells secreting IFN-γ alone were much more than those secreting IL-17 after stimulating with PMA and ionomycin; therefore, the contribution by Th17 cells to total IFN-γ secretion would be very low. Because Th17 cells also produced IL-22, Th17 cells that were positive for IL-22 were examined in this study. It has been shown that IL-22-positive Th17 cells were only observed in human but not in mouse [48]. In this experiment, increasing percentages of CD4+IL-22+IL-17+IFNγ- T cells were detected in peripheral blood from AS and RA patients compared to OA patients and healthy controls. Our results were in line with the study of Colin et al [47], which demonstrated increased percentages of IL-17+ and IL-22+CD4+ T cells in PBMCs from treatment-naïve patients with early RA. IL-22 production by Th17 cells has been demonstrated to be dependent on IL-23 [49], and elevated serum IL-23 level was detected in the patients with AS [20]. Our data suggest that the tendency to develop both CD4+IL-22+IL-17+IFNγ- T cells and Th1/Th17 cells may be a common immunologic characteristic shown in AS and RA patients.
In conclusion, our experiments showed that the frequencies of Th22 cells and Th17 cells were significantly increased in AS and RA patients compared to OA patients and healthy controls. Plasma IL-22 levels, which correlated positively with Th22 cells, were also demonstrated to be elevated in both AS and RA patients. The increasing percentages of Th22 cells and Th17 cells and the elevation of IL-22 may play important roles in the pathogenesis of AS and RA. Thus, Th22 cells and IL-22 as well as Th17 cells may prove to be a promising therapeutic targets for AS and RA. Further studies are awaited to clarify the pathophysiologic role of Th22 in AS and RA and determine the situation of Th22 cells in AS and RA patients’ joints.
Supporting Information
a, b and c, No positive correlations were found between the percentage of Th22 cells and ESR, CRP as well as BASDAI in AS patients. d, e and f, No positive correlations were found between the percentage of Th17 cells and ESR, CRP as well as BASDAI in AS patients. g, h and i, No positive correlations were found between the levels of plasma IL-22 and ESR, CRP as well as BASDAI in AS patients. j and k, No positive correlations were found between the levels of plasma IL-22 and CRP as well as DAS28 in RA patients. i, No positive correlation was found between the levels of plasma IL-22 and the percentage of Th17 cells in RA patients.
(TIF)
The number of Th22 cells and Th17 cells in per volume of peripheral blood in AS patients, RA patients and healthy controls. a, The number of Th22 cells was significantly increased in AS and RA patients compared with healthy controls after stimulation with phorbol myristate acetate, ionomycin, and monensin for 4 h. b, The number of Th17 cells was significantly increased in AS and RA patients compared with healthy controls after stimulation with phorbol myristate acetate, ionomycin, and monensin for 4 h. (* = P<0.05)
(TIF)
Major previous treatment of each patient of AS*
(DOC)
Major previous treatment of each patient of RA*
(DOC)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was partially supported by research funding from the National Natural Science Foundation (30600680, 81070407 and 30973018), the Shandong Technological Development Project (2005BS03022, Q2008C07, and BS2009SW014), and Graduate Independent Innovation Foundation of Shandong University, GIIFSDU (yzc21300071613082, yzc10147). (http://www.nsfc.gov.cn/Portal0/default152.htm) (http://www.art.sdu.edu.cn/2011/0321/931.html). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 2.Dolhain RJ, van der Heiden AN, ter Haar NT, Breedveld FC, Miltenburg AM. Shift toward T lymphocytes with a T helper 1 cytokine-secretion profile in the joints of patients with rheumatoid arthritis. Arthritis Rheum. 1996;39:1961–1969. doi: 10.1002/art.1780391204. [DOI] [PubMed] [Google Scholar]
- 3.Wedderburn LR, Robinson N, Patel A, Varsani H, Woo P. Selective recruitment of polarized T cells expressing CCR5 and CXCR3 to the inflamed joints of children with juvenile idiopathic arthritis. Arthritis Rheum. 2000;43:765–774. doi: 10.1002/1529-0131(200004)43:4<765::AID-ANR7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 4.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 5.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 6.Park H, Li Z, Yang XO, Chang SH, Nurieva R, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 9.Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirota K, Hashimoto M, Yoshitomi H, Tanaka S, Nomura T, et al. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J Exp Med. 2007;204:41–47. doi: 10.1084/jem.20062259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitch E, Harper E, Skorcheva I, Kurtz SE, Blauvelt A. Pathophysiology of psoriasis: recent advances on IL-23 and Th17 cytokines. Curr Rheumatol Rep. 2007;9:461–467. doi: 10.1007/s11926-007-0075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmechel S, Konrad A, Diegelmann J, Glas J, Wetzke M, et al. Linking genetic susceptibility to Crohn’s disease with Th17 cell function: IL-22 serum levels are increased in Crohn’s disease and correlate with disease activity and IL23R genotype status. Inflamm Bowel Dis. 2008;14:204–212. doi: 10.1002/ibd.20315. [DOI] [PubMed] [Google Scholar]
- 14.Miossec P. Interleukin-17 in fashion, at last: ten years after its description, its cellular source has been identified. Arthritis Rheum. 2007;56:2111–2115. doi: 10.1002/art.22733. [DOI] [PubMed] [Google Scholar]
- 15.Gaston JS. Cytokines in arthritis--the ‘big numbers’ move centre stage. Rheumatology (Oxford) 2008;47:8–12. doi: 10.1093/rheumatology/kem203. [DOI] [PubMed] [Google Scholar]
- 16.Shen H, Goodall JC, Hill Gaston JS. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 2009;60:1647–1656. doi: 10.1002/art.24568. [DOI] [PubMed] [Google Scholar]
- 17.Chabaud M, Durand JM, Buchs N, Fossiez F, Page G, et al. Human interleukin-17: A T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 1999;42:963–970. doi: 10.1002/1529-0131(199905)42:5<963::AID-ANR15>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 18.Miossec P. Interleukin-17 in rheumatoid arthritis: if T cells were to contribute to inflammation and destruction through synergy. Arthritis Rheum. 2003;48:594–601. doi: 10.1002/art.10816. [DOI] [PubMed] [Google Scholar]
- 19.Parsonage G, Filer A, Bik M, Hardie D, Lax S, et al. Prolonged, granulocyte-macrophage colony-stimulating factor-dependent, neutrophil survival following rheumatoid synovial fibroblast activation by IL-17 and TNFalpha. Arthritis Res Ther. 2008;10:R47. doi: 10.1186/ar2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mei Y, Pan F, Gao J, Ge R, Duan Z, et al. Increased serum IL-17 and IL-23 in the patient with ankylosing spondylitis. Clin Rheumatol. 2011;30:269–273. doi: 10.1007/s10067-010-1647-4. [DOI] [PubMed] [Google Scholar]
- 21.Renauld JC. Class II cytokine receptors and their ligands: key antiviral and inflammatory modulators. Nat Rev Immunol. 2003;3:667–676. doi: 10.1038/nri1153. [DOI] [PubMed] [Google Scholar]
- 22.Lo YH, Torii K, Saito C, Furuhashi T, Maeda A, et al. Serum IL-22 correlates with psoriatic severity and serum IL-6 correlates with susceptibility to phototherapy. J Dermatol Sci. 2010;58:225–227. doi: 10.1016/j.jdermsci.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 23.Brand S, Beigel F, Olszak T, Zitzmann K, Eichhorst ST, et al. IL-22 is increased in active Crohn’s disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am J Physiol Gastrointest Liver Physiol. 2006;290:G827–838. doi: 10.1152/ajpgi.00513.2005. [DOI] [PubMed] [Google Scholar]
- 24.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, et al. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27:647–659. doi: 10.1016/j.immuni.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng F, Guo Z, Xu H, Yan D, Li Q. Decreased plasma IL22 levels, but not increased IL17 and IL23 levels, correlate with disease activity in patients with systemic lupus erythematosus. Ann Rheum Dis. 2009;68:604–606. doi: 10.1136/ard.2008.097089. [DOI] [PubMed] [Google Scholar]
- 26.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 27.Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573–3585. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10:864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 29.Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010;130:1373–1383. doi: 10.1038/jid.2009.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361–368. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- 31.Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, et al. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol. 1994;21:2286–2291. [PubMed] [Google Scholar]
- 32.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 33.Prevoo ML, van‘t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 34.Zhang L, Li JM, Liu XG, Ma DX, Hu NW, et al. Elevated Th22 cells correlated with Th17 cells in patients with rheumatoid arthritis. J Clin Immunol. 2011;31:606–614. doi: 10.1007/s10875-011-9540-8. [DOI] [PubMed] [Google Scholar]
- 35.Leipe J, Grunke M, Dechant C, Reindl C, Kerzendorf U, et al. Role of Th17 cells in human autoimmune arthritis. Arthritis Rheum. 2010;62:2876–2885. doi: 10.1002/art.27622. [DOI] [PubMed] [Google Scholar]
- 36.Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204:2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flytlie HA, Hvid M, Lindgreen E, Kofod-Olsen E, Petersen EL, et al. Expression of MDC/CCL22 and its receptor CCR4 in rheumatoid arthritis, psoriatic arthritis and osteoarthritis. Cytokine. 2010;49:24–29. doi: 10.1016/j.cyto.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Lejeune D, Dumoutier L, Constantinescu S, Kruijer W, Schuringa JJ, et al. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J Biol Chem. 2002;277:33676–33682. doi: 10.1074/jbc.M204204200. [DOI] [PubMed] [Google Scholar]
- 39.Ikeuchi H, Kuroiwa T, Hiramatsu N, Kaneko Y, Hiromura K, et al. Expression of interleukin-22 in rheumatoid arthritis: potential role as a proinflammatory cytokine. Arthritis Rheum. 2005;52:1037–1046. doi: 10.1002/art.20965. [DOI] [PubMed] [Google Scholar]
- 40.Leipe J, Schramm MA, Grunke M, Baeuerle M, Dechant C, et al. Interleukin 22 serum levels are associated with radiographic progression in rheumatoid arthritis. Ann Rheum Dis. 2011;70:1453–1457. doi: 10.1136/ard.2011.152074. [DOI] [PubMed] [Google Scholar]
- 41.Wolk K, Kunz S, Asadullah K, Sabat R. Cutting edge: immune cells as sources and targets of the IL-10 family members? J Immunol. 2002;168:5397–5402. doi: 10.4049/jimmunol.168.11.5397. [DOI] [PubMed] [Google Scholar]
- 42.Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupe P, et al. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 43.Shahrara S, Huang Q, Mandelin AM, 2nd, Pope RM. TH-17 cells in rheumatoid arthritis. Arthritis Res Ther. 2008;10:R93. doi: 10.1186/ar2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 45.Lubberts E, Joosten LA, Oppers B, van den Bersselaar L, Coenen-de Roo CJ, et al. IL-1-independent role of IL-17 in synovial inflammation and joint destruction during collagen-induced arthritis. J Immunol. 2001;167:1004–1013. doi: 10.4049/jimmunol.167.2.1004. [DOI] [PubMed] [Google Scholar]
- 46.Lubberts E, Koenders MI, Oppers-Walgreen B, van den Bersselaar L, Coenen-de Roo CJ, et al. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 2004;50:650–659. doi: 10.1002/art.20001. [DOI] [PubMed] [Google Scholar]
- 47.Colin EM, Asmawidjaja PS, van Hamburg JP, Mus AM, van Driel M, et al. 1,25-dihydroxyvitamin D3 modulates Th17 polarization and interleukin-22 expression by memory T cells from patients with early rheumatoid arthritis. Arthritis Rheum. 2010;62:132–142. doi: 10.1002/art.25043. [DOI] [PubMed] [Google Scholar]
- 48.Okey AB, Franc MA, Moffat ID, Tijet N, Boutros PC, et al. Toxicological implications of polymorphisms in receptors for xenobiotic chemicals: the case of the aryl hydrocarbon receptor. Toxicol Appl Pharmacol. 2005;207:43–51. doi: 10.1016/j.taap.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 49.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
a, b and c, No positive correlations were found between the percentage of Th22 cells and ESR, CRP as well as BASDAI in AS patients. d, e and f, No positive correlations were found between the percentage of Th17 cells and ESR, CRP as well as BASDAI in AS patients. g, h and i, No positive correlations were found between the levels of plasma IL-22 and ESR, CRP as well as BASDAI in AS patients. j and k, No positive correlations were found between the levels of plasma IL-22 and CRP as well as DAS28 in RA patients. i, No positive correlation was found between the levels of plasma IL-22 and the percentage of Th17 cells in RA patients.
(TIF)
The number of Th22 cells and Th17 cells in per volume of peripheral blood in AS patients, RA patients and healthy controls. a, The number of Th22 cells was significantly increased in AS and RA patients compared with healthy controls after stimulation with phorbol myristate acetate, ionomycin, and monensin for 4 h. b, The number of Th17 cells was significantly increased in AS and RA patients compared with healthy controls after stimulation with phorbol myristate acetate, ionomycin, and monensin for 4 h. (* = P<0.05)
(TIF)
Major previous treatment of each patient of AS*
(DOC)
Major previous treatment of each patient of RA*
(DOC)