Abstract
Circular dichroism (CD) is a useful technique for monitoring changes in the conformation of antimicrobial peptides or gelatin. In this study, interactions between cationic peptides and gelatin were observed without affecting the triple helical content of the gelatin, which was more strongly affected by anionic surfactant. The peptides did not adopt a secondary structure in the presence of aqueous solution or Tween 80, but a peptide secondary structure formed upon the addition of sodium dodecyl sulfate (SDS). The peptides bound to the phosphate group of lipopolysaccharide (LPS) and displayed an alpha-helical conformation while (KW)4 adopted a folded conformation. Further, the peptides did not specifically interact with the fungal cell wall components of mannan or laminarin. Tryptophan blue shift assay indicated that these peptides interacted with SDS, LPS, and gelatin but not with Tween 80, mannan, or laminarin. The peptides also displayed antibacterial activity against P. aeruginosa without cytotoxicity against HaCaT cells at MIC, except for HPA3NT3-analog peptide. In this study, we used a CD spectroscopic method to demonstrate the feasibility of peptide characterization in numerous environments. The CD method can thus be used as a screening method of gelatin-peptide interactions for use in wound healing applications.
Keywords: circular dichroism, antimicrobial peptides, reduced glutathione, gelatin, sodium dodecyl sulfate, Tween 80, cell wall components, lipopolysaccharide
1. Introduction
Circular dichroism (CD) spectroscopy is the most widespread technique used for estimating the secondary structures of proteins and polypeptides in solution [1]. This technique can be used to distinguish between unordered (random coil) and ordered (alpha-helix or beta-sheet) structures [2,3]. CD detects wavelength-dependent differences in the absorption of right and left circularly polarized light by optically active molecules such as peptides and proteins. The CD spectrum of unordered peptides is usually characterized by a single band below 200 nm, whereas alpha-helical structures usually present two negative bands at 208 and 222 nm along with one positive band at 192 nm; beta-sheet structures typically show a negative band at 217 nm and a positive band at 195 nm. Most linear cationic antimicrobial peptides (AMPs) are in an unordered state in aqueous solution. As these molecules are amphipathic, they can adopt folded conformations in both hydrophobic and hydrophilic environments [4]. AMPs are generally variable in length, sequence, and structure (helical, beta-sheet, extended, and looped) [4–6]. Alpha-helical AMPs are one of the most abundant types of AMPs. CD methods are very useful for studying protein-ligand interactions and protein denaturation due to their quantitative nature. Thus, any change in the CD spectrum of a protein upon addition of ligand, denaturant, or heat is directly proportional to the amount of protein perturbed [7].
In this study, we synthesized (KW)4 peptide based on previous data showing that (KW)3 has antimicrobial activity [8]. Another peptide with antimicrobial activity, HPA3NT3-analog, was also used [9]. Furthermore, we used three natural peptides, NRC-16, magainin-II, and reduced glutathione (GSH), in this study. NRC-16 peptide is derived from flatfish genes [10] and shows potent antimicrobial activity. Magainin-II was originally isolated from the skin of African clawed frog, Xenopus laevis [11]. GSH is a water-soluble tripeptide composed of the amino acids cysteine, glutamic acid, and glycine. The biological (antimicrobial or antioxidative) activities of these peptides promote interactions with gelatin, an important connective tissue protein. Here, we used CD spectroscopy to monitor the gelatin response to these peptides, sodium dodecyl sulfate (SDS), or calcium chloride (CaCl2). The structures and organization of the peptides in aqueous solution, SDS, Tween 80, lipopolysaccharide (LPS), mannan, or laminarin were determined using CD spectroscopy. The peptide-dependent interactions in gelatin, SDS, LPS, mannan, and laminarin were investigated by tryptophan (Trp) blue shift assay. Finally, the antibacterial and cytotoxicity activities of the peptides were determined.
2. Results and Discussion
Gelatin is a protein produced by acid and alkaline processing of collagen and is characterized by a three-chain structure in which individual helical chains are stranded in a superhelix about a common molecular axis [12–14]. The triple helical structure of gelatin can be quantified by using CD measurements [15]. In the present study, the CD spectra of gelatin showed two peaks, a negative peak at 205 nm suggesting a random coil conformation, and a positive peak at 222 nm characteristic of the triple-helial conformation of gelatin [16–19]. This positive peak corresponds to the typical maximum peak of collagen at 222 nm [20–27]. The triple helical structure of collagen was established by Ramachandran and Kartha [28], Rich and Crick [29], and Cowan et al. [30].
2.1. Effects of Antimicrobial Peptides on Gelatin Conformation
CD spectroscopy has been used to characterize the interactions between small molecules and collagen with the aim of determining collagen stability [24,25,27,31–33]. We have also characterized the effects of peptides on the structural conformation of gelatin for biomedical applications. We prefer gelatin over collagen since the cost of producing collagen-containing sheets is higher than that of gelatin-containing sheets. Additionally, gelatin is water-soluble compared to the acid and salt solubilility of collagen, which is very important in peptide-based drug development. The CD spectra of gelatin solutions treated with various concentrations of peptides at 25 °C are shown in Figure 1.
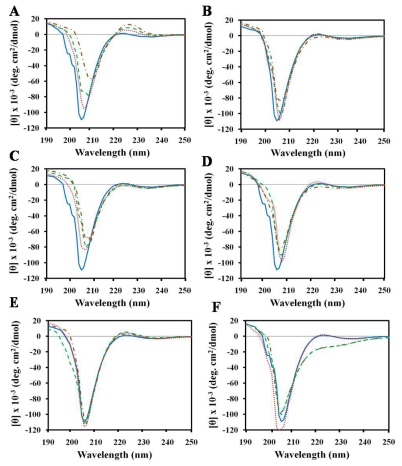
Figure 1.
Effects of peptides on Circular dichroism (CD) spectra of gelatin in the wavelength region from 250–190 nm. Gelatin solution was treated with peptides at an increasing molar ratio of gelatin to peptide from 11:1 to 11:3 and incubated for 10 min at 25 °C. (A) (KW)4; (B) HPA3NT3-analog; (C) NRC-16; (D) Magainin-II; and (E) Reduced glutathione (GSH). CD spectra of 0.1% gelatin in the absence of peptides (solid line). Peptide concentrations were as follows: 50 μM (dotted line), 100 μM (dashed line), and 200 μM (dashed-dotted line). (F) CD spectra of gelatin in the absence (solid line) or presence of 1 mM SDS (dotted line), 5 mM SDS (dashed line), and 10 mM SDS (dashed dotted line).
Compared to gelatin solution alone, the gelatin-peptide mixture displayed a marked decrease in the negative intensity of the dichroic spectrum as well as a slight decrease or increase in the molar ellipticity of the 222 nm bands. In addition, the negative peak shifted to a higher wavelength with increasing concentration of peptides. It has been reported that upon complete denaturation of gelatin, the positive peak at 222 nm disappears completely while the negative band shifts to nearly 230 nm [18]. However, our results showed that the addition of peptides to the gelatin solution did not cause the positive band at 222 nm to disappear, and there was a slight change in the negative band for the gelatin-peptide complex. This shows that peptide brought about a very slight change in the packing of the helices and did not change the triple helical conformation of gelatin. It was reported earlier that the interaction of collagen with glycoprotein [34] or small molecules such as polyphenols [24], curcumin [25], dicarboxylic acids [27], chromium [21], tannins [35], aldehydes [36], and 3,4-dihydroxyphenylalanine [37] does not alter the secondary structural conformation of collagen. Other studies reported that the triple helicity of collagen is not affected by AMP (pexiganan, an analog of magainin [38]) incorporation as confirmed by FT-IR [39]. Maintaining the triple helical conformation of gelatin or collagen-based biomaterials during preparation is important in eliciting the desired biomedical functions of both.
The CD spectra of gelatin with peptides displayed a decrease in negative ellipticity, suggesting that the peptides bound intra-molecularly, i.e., within a gelatin molecule, or promoted aggregation of gelatin. This point is likely correlated with aggregation of collagen-glycoprotein [34] or small molecule interactions [24,27]. On the other hand, the result that GSH did not increase negative ellipticity indicates that the gelatin molecules did not aggregate in the presence of GSH peptide. Thus, peptide promoted the native state of gelatin without affecting the peaks at 222 nm, as confirmed by CD measurements. It has been suggested that the presence of GSH in the gelatin matrix may have antioxidant effects in a wound environment such as, preventing damage to important cellular components caused by reactive oxygen species such as free radicals and peroxides [40]. Moreover, the antioxidant function of biotinylated matrikine peptide has been shown to enhance wound healing in rats [41].
2.2. Effect of SDS on Gelatin Conformation
SDS is an anionic additive frequently used in studies on protein denaturation, and it is a well-known destabilizing agent of biopolymers. To confirm that the peptide does not alter the triple helical content of gelatin, we used SDS to denature gelatin. This procedure can be used to differentiate proteins with a triple helical conformation from others with a non-triple helical conformation [18]. Figure 1F shows the CD spectra of a 0.1% gelatin solution in various concentrations of SDS at 25 °C. The triple helical content was reduced with increasing concentration of SDS. At increasing surfactant concentrations, absence of the 222 nm peak was observed. A previous study also reported that SDS eliminates the 222 nm peak [18]. Denaturation also occurred in 3 M CaCl2 solution, as indicated by the disappearance of the 222 nm peak (data not shown).
2.3. Structures of Peptides in Aqueous and SDS Solution
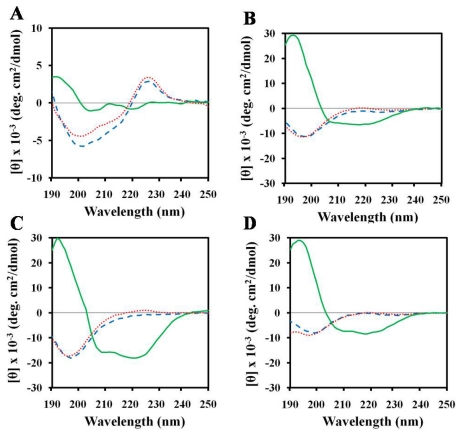
In order to examine the behavior of the AMPs in membrane-mimicking media, the CD spectra of the peptides were recorded in the absence and presence of 30 mM SDS (Figure 2). The peptides showed a random coil conformation in aqueous solution. The conformations of the three peptides magainin-II, HPA3NT3-analog, and NRC-16 in SDS micelles included significant alpha-helical content. The alpha-helical contents of HPA3NT3-analog, magainin-II, and NRC-16 were 19, 29, and 59%, respectively. In the presence of SDS micelles, (KW)4 peptide exhibited a slightly folded conformation in which Trp residues were positioned mostly near the membrane-water interfacial region [42], suggesting interfacial association. Such a structural feature is believed to be important for the antibacterial activity of linear AMPs [43–46].
Figure 2.
CD spectra of peptides in aqueous solution (dashed line) as well as in the presence of 0.1% Tween 80 (dotted line) and 30 mM sodium dodecyl sulfate (SDS) (solid line). (A) (KW)4; (B) HPA3NT3-analog; (C) NRC-16; and (D) Magainin-II.
2.4. Structures of Peptides in Nonionic Surfactant
Tween 80 belongs to a class of nonionic surfactants that are frequently used in membrane protein solubilization, since they generally do not induce protein folding. Its chemical stability and high biocompatibility mean that it can be regarded as a strong candidate for future use in cosmetics and drug delivery. Based on CD, we investigated whether or not Tween 80 causes changes in peptide conformation. To accomplish this, we determined the conformations of peptides in the presence of Tween 80 (Figure 2). According to the results, Tween 80 did not alter peptide conformation. In addition, Trp containing (KW)4 peptide showed a negative band at 200 nm. This band is characteristic of a random coil, whereas the band at 225 nm was related to the Trp side chain in (KW)4, which contributes to the CD signal in this spectral region [47–49]. On the other hand, the Trp side chain peak did not disappear in response to Tween 80, indicating that there was no hydrophobic interaction between the peptide and Tween 80. This is further supported by a previous study that did not find any general structural changes in insulin in the presence of Tween 80, which suggests that only limited interactions, if any, occur between the two species in solution [50]. Clearly, this result shows that Tween 80 had no harmful effects on the peptides, which could be useful for the cosmetic application of these peptides using Tween 80 as a co-surfactant.
2.5. Structures of Peptides in Cell Wall Components
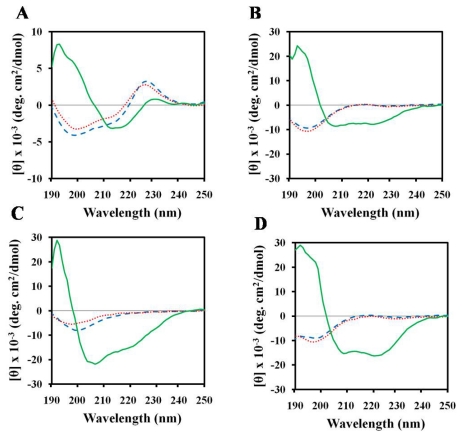
Finally, we used CD to investigate the conformational changes brought on by the interactions between peptides and cell wall components (LPS, mannan, and laminarin). CD spectroscopy showed that (KW)4 peptide displayed major conformational changes in association with LPS (Figure 3), which is a cell wall component of Gram-negative bacteria [51].
Figure 3.
CD spectra of peptides in the presence of 0.1% mannan (dashed line), 0.1% laminarin (dotted line), and 0.1% lipopolysaccharide (LPS) (solid line). (A) (KW)4; (B) HPA3NT3-analog; (C) NRC-16; and (D) Magainin-II.
On the other hand, the peptides magainin-II, NRC-16, and HPA3NT3-analog transitioned from a random coil conformation to an alpha-helix upon interaction with LPS. Examples of other native, synthetic peptides that undergo alpha-helix formation upon LPS binding include melittin and magainin [52,53]. The ability of AMPs to bind LPS is a prerequisite for their antibacterial and endotoxin detoxifying activities [53]. Additionally, we determined whether or not binding of these peptides to fungal surfaces occurs via interaction with mannan or laminarin, which are major components of fungal cell walls. CD spectroscopy showed that these peptides displayed no major conformational changes associated with the fungal cell wall components mannan and laminarin (Figure 3). Therefore, it is clear that cell wall components did not affect the fungicidal activities of these peptides. This point suggests that the antifungal activity of AMPs is not affected by the removal of cell wall components [54,55].
2.6. Characterization of the Trp Environment Using Fluorescence Spectroscopy
The peptide-binding process can also be followed by analysis of the Trp flourescence spectra (Table 1).
Table 1.
Tryptophan (Trp) emission maxima of 2 μM peptides in 10 mM sodium phosphate buffer (pH 7.2), gelatin, sodium dodecyl sulfate (SDS), Tween 80, mannan, laminarin, and lipopolysaccharide (LPS).
| Blue shift (nm) | |||||||
|---|---|---|---|---|---|---|---|
| Peptides | λmax buffer (nm) | Gelatin | SDS | Tween | Mannan | Laminarin | LPS |
| (KW)4 | 355 | 8 | 10 | 0 | 0 | 0 | 10 |
| NRC-16 | 356 | 8 | 25 | 0 | 0 | 0 | 17 |
The (KW)4 and NRC-16 peptides were used as they contain Trp residues in their sequences. In the presence of sodium phosphate (SP) buffer (pH 7.2), (KW)4 and NRC-16 peptides displayed fluorescence emission maxima at 355 and 356 nm, respectively, which corroborates a previous report that the Trp residues of these peptides are located in a hydrophilic environment [56]. When these two peptides bound to gelatin, SDS micelles, and LPS, their fluorescence maxima shifted to shorter wavelengths, suggesting that the Trp side chains partitioned preferentially into more rigid, hydrophobic environments in gelatin, SDS, and LPS. This tendency is consistent with the CD spectra of these peptides in the presence of SDS micelles or LPS, which indicated a much more structured conformation upon binding (Figures 2,3). The recorded blue shift suggests that Trp residues were involved in the interaction with the cationic or hydrophobic domain of gelatin. Cationic peptides are thought to undergo electrostatic, cation-π, and hydrophobic interactions with gelatin or collagen. Gelatin consists of positively and negatively charged as well as hydrophobic domains. A previous study reported that hydrophobic interactions are mainly involved in the interactions between polyphenols and collagen [24]. Bioinformatics studies have also shown that quinone components interact with collagen through hydrophobic interactions [57]. Lysine (Lys) and arginine (Arg) residues mediate electrostatic interactions that attach cationic peptides to negatively charged amino acids in gelatin molecules. It has been suggested that cation-π interactions between a protonated amine (Lys) or guanidine (Arg) side chain and an aromatic ring side-chain (phenylalanine, tyrosine, or Trp) promote peptide-gelatin interactions [58,59]. Therefore, electrostatic, hydrophobic, and cation-π interactions might contribute to peptide-gelatin interactions.
2.7. Antibacterial and Cytotoxicity Activities of Peptides
A high level of microorganisms inhibits the normal wound healing process [60]. Wound infections are most frequently attributed to P. aeruginosa [61]. The MIC values of the peptides against P. aeruginosa under low salt (without NaCl) and high salt (with 135 mM NaCl) conditions are summarized in Table 2.
Table 2.
MICs of the peptides against drug–resistant P. aeruginosa.
| MIC (μM) | ||||
|---|---|---|---|---|
| Resistant strains | (KW)4 | HPA3NT3-analog | NRC-16 | Magainin-II |
| P. aeruginosa 3547 | 16 (32) | 8 (16) | 4 (8) | 32 (64) |
| P. aeruginosa 3592 | 32 (64) | 8 (16) | 8 (16) | 32 (64) |
| P. aeruginosa 4007 | 16 (32) | 4 (16) | 4 (8) | 32 (32) |
| P. aeruginosa 4891 | 8 (16) | 4 (8) | 4 (8) | 16 (32) |
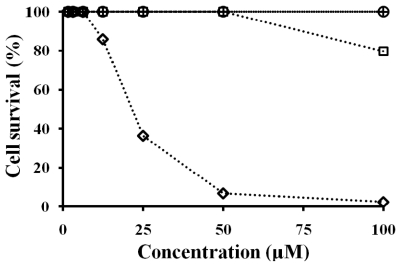
Antibacterial activity was measured in 10 mM sodium phosphate buffer, pH 7.2, and phosphate buffered saline, pH 7.2 (number in the parentheses). NRC-16 peptide showed higher antibacterial activity than the other peptides. However, all of the peptides showed antibacterial activity against P. aeruginosa under both low and high salt conditions. The cytotoxic activities of the four peptides were assessed in HaCaT cells as a measurement of their toxicity towards higher order eukaryotic skin cells (Figure 4). At a concentration of 100 μM, (KW)4 and magainin-II peptides displayed non-cytotoxicity towards HaCaT cells, whereas NRC-16 and HPA3NT3-analog peptides showed cytotoxicity. Therefore, the (KW)4 and magainin-II peptides were selective against bacteria with no effect on HaCaT cells. The ability to localize AMPs to wound sites is important for the effective treatment of bacterial infection at wound sites. Previous studies provide rationale for the application of collagen membranes to AMP delivery in infected wounds [39,41,62]. Other studies have reported that gelatin sheets containing bioactive molecules possess effective wound healing activity [63–66]. Gelatin gel shows non-cytotoxicty towards HaCaT cells [67] and is biodegradable in nature [68]. Additionally, it has good film forming properties and can be used in wound healing by preventing fluid loss due to exudation [69]. Gelatin gel is also effective in peptide delivery and has antibacterial effects at wound sites. Cationic peptides may also possess anticancer activity [70,71] or promote wound healing [72], and several AMPs are currently undergoing clinical trials [73]. Therefore, gelatin-peptides sheets could be useful for the inhibition of microbes at wound sites due to their wound healing properties.
Figure 4.
Activities of the peptides against HaCaT cells. HaCaT cells (2 × 104/well) were incubated for 24 h with the indicated concentrations of (KW)4 (+), HPA3NT3-analog (⋄), NRC-16 (□), and magainin-II (○).
3. Experimental Section
3.1. Materials
Rink amide 4-methylbenzhydrylamine resin, fluoren-9-ylmethoxycarbonyl (Fmoc) amino acids, and other reagents for peptide synthesis were purchased from Calibochem-Novabiochem (La Jolla, CA, USA). LPS from P. aeruginosa, GSH, and Tween-80 were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Granulated gelatin was obtained from DIFCO Laboratories (Detroit, MI, USA). The anionic surfactant, SDS, and CaCl2 were acquired from Calbiochem (La Jolla, CA, USA). All other reagents were of analytical grade. Buffers were prepared using double distilled water (Millipore Co.).
3.2. Peptide Synthesis and Purification
The peptides KWKWKWKW-NH2 (KW)4, FKKLKKLFKKILKLK-NH2 (HPA3NT3-analog), GWKKWLRKGAKHLGQAAIK-NH2 (NRC-16), and NMIEGVFAKGFKKASHLFKGIG (magainin-II) were synthesized by the solid-phase method using Fmoc chemistry on a solid support of Rink amide 4-methylbenzhydrydrylamine resin. Then, 0.1 M N-hydroxy benzotriazole (HOBt) and 0.45 M 2-(1Hbenzotriazole- 1-yil)-1,1,3,3-tetramethyluroniumhexafluorophosphate (HBTU) in dimethylformamide (DMF) along with 2 M N,N-diisopropyl ethylamine (DIEA) in N-methylpyrrolidone (NMP) were used as a coupling reagent, and 10-fold excess Fmoc-amino acid was added during every coupling cycle. Following a final deprotection with a solution of 20% piperidine in DMF and cleavage with a mixture of TFA/water/triisopropylsilane (90:5:5) for 2 h at room temperature [74], the crude peptides were repeatedly extracted with diethyl ether and purified using reverse phase preparative HPLC on a Vydac C18 column (4.6 × 250 mm, 300 Å, 5 nm). The molecular masses of the peptides were confirmed by using a matrix-assisted laser desorption ionization mass spectrometer (data not shown) (MALDI II, Kratos Analytical Ins.).
3.3. Interaction of Peptides with Gelatin by CD Spectroscopy
Gelatin solutions were prepared by weighing the required amount of gelatin flakes and soaking in hot water (~40 °C) with stirring. The concentrations quoted here are expressed in weight percentage of gelatin.
CD spectra were recorded at 25 °C on a Jasco 810 spectropolarimeter (Jasco, Tokyo, Japan) equipped with a temperature control unit using a 0.1-cm path-length quartz cell. The gelatin (0.1%) was scanned in the presence of 10 mM SP buffer. The CD spectra were measured for the 0.1% gelatin samples (dissolved in 10 mM SP buffer (pH 7.2)) containing peptide. CD data represent the average value of three separate recordings with four scans per sample. A reference spectrum containing 10 mM SP buffer was also recorded. The CD spectra of the samples were obtained after subtracting the reference spectrum. Changes in the conformation of gelatin upon addition of peptides were recorded.
3.4. Interaction of Peptides with Surfactants by CD Spectroscopy
The CD spectra of the peptides (50 μM) were obtained in different environments, including 10 mM SP buffer, 30 mM SDS, and 0.1% Tween 80. Ten millimoles of SP buffer was used to prepare 30 mM SDS and 0.1% Tween 80. At least five scans in the 250–190 nm wavelength range were conducted, and the average blank spectra were subtracted from the average of the sample spectra. All CD spectra are presented as the mean residue ellipticity, [θ]MRW, in deg·cm2/dmol. The alpha-helical content was determined from the mean residue ellipticities at 222 nm, as indicated in Equation 1 [75]:
| (1) |
where [θ]obs is the mean-residue ellipticity observed experimentally at 222 nm, [θ]helix is the ellipticity of a peptide of infinite length with a 100% helix population, taken as −39,500 deg·cm2/dmol, and l is the peptide length or, more precisely, the number of peptide bonds.
3.5. Interaction of Peptides with Cell Wall Components by CD Spectroscopy
The peptides were scanned in the presence or absence of LPS (0.1%) dissolved in 10 mM SP buffer. The secondary structures were monitored at a peptide concentration of 50 μM in 10 mM SP buffer in the presence of laminarin from digitata laminarin (0.1%; Sigma-Aldrich, St. Louis, MO, USA) and in the presence of mannan from Saccharomyces cerevisiae (0.1%; Sigma-Aldrich, St. Louis, MO, USA). CD data represent the average value of three separate recordings.
3.6. Trp Fluorescence Assay
The fluorescence emission spectra of the Trp residues in the peptides were monitored in the presence of 10 mM SP buffer, 0.1% gelatin, 30 mM SDS, cell wall components (0.1% LPS, 0.1% laminarin, and 0.1% mannan), and 0.1% Tween 80. The Trp fluorescence measurements were taken using a spectrofluorometer. The final concentration (2 μM) of each peptide was added to 200 μL of the above solutions, and each peptide: environment mixture was allowed to interact at 25 °C for 10 min. Fluorescence was measured at an excitation wavelenth of 280 nm and an emission wavelength from 300 to 400 nm.
3.7. Antibacterial Activity
The antibacterial activities of the peptides against drug-resistant P. aeruginosa (3547, 3592, 4007, 4891) were examined using the microbroth dilution method. Aliquots of bacterial suspensions (50 μL) in mid-log phase at a concentration of 2 × 105 colony forming units (CFUs/mL) in Mueller Hinton Broth (MHB, BD, Sparks, MD, USA) culture medium were added to each well containing 50 μL of the peptide solution that had been serially diluted 2-fold in buffer (10 mM SP buffer, pH 7.2 or phosphate buffered saline (PBS); 1.5 mM KH2PO4, 2.7 mM KCl, 8.1 mM Na2HPO4, 135 mM NaCl, pH 7.2). Several wells were kept untreated as a control in order to monitor bacterial growth. Inhibition of bacterial growth was determined by measuring the absorbance at 620 nm using a Versa-Max microplate Elisa Reader (Molecular Devices, Sunnyvale, CA, USA) after incubation for 18 h at 37 °C. The MIC is defined as the minimal peptide concentration that inhibits bacterial growth. All MIC measurements are the average of three to four independent experiments. The bacterial strains were procured from Chonnam University Hospital, in Gwangju, South Korea. All isolates were stored at −70 °C until required.
3.8. Cell Culture and Cytotoxicity
To examine the cytotoxic effects of the peptides, HaCaT (human keratinocyte cell line) cells were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with antibiotics (100 U/mL of penicillin, 100 μg/mL of streptomycin) and 10% fetal calf serum at 37 °C in a humidified chamber containing 5% CO2. The percentage of growth inhibition was evaluated by MTT (Sigma) assay for the measurement of viable cells. A total of 2 × 104 cells/well was seeded onto a 96-well plate and then incubated for 24 h. Various concentrations of the test peptides were then added to the wells, after which the cells were incubated for an additional 24 h at 37 °C. Subsequently, 10 μL of MTT at a concentration of 5 mg/mL was added to each of the wells, after which the cells were incubated for an additional 4 h. The supernatants were then aspirated, and 100 μL of dimethyl sulfoxide was added to the wells in order to dissolve any remaining precipitate. Absorbance was then measured at a wavelength of 570 nm using an ELx800 reader (Bio-Tek instruments, Inc., Winooski, VT).
4. Conclusion
The native structure of gelatin was not altered upon treatment with peptides. Therefore, changes in the structural properties of gelatin upon interaction with peptides assist the preparation of gelatin-peptide sheets. In this study, the tested peptides showed antimicrobial activity, which suggests that they might be useful in the development of a topical application for wound sites. Among them, (KW)4 possesses a simple composition with microbial selective properties, making it economically viable for many applications, including inhibition of bacterial infection at wound sites.
Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. 2011-0017532) and the Human Resource Training Project for Regional Innovation.
References
- 1.Greenfield N.J. Methods to estimate the conformation of proteins and polypeptides from circular dichroism data. Anal. Biochem. 1996;235:1–10. doi: 10.1006/abio.1996.0084. [DOI] [PubMed] [Google Scholar]
- 2.Johnson W.C. Secondary structure of proteins through circular dichroism spectroscopy. Annu. Rev. Biophys. Biophys. Chem. 1988;17:145–166. doi: 10.1146/annurev.bb.17.060188.001045. [DOI] [PubMed] [Google Scholar]
- 3.Johnson W.C. Analyzing protein circular dichroism spectra for accurate secondary structures. Proteins. 1999;35:307–312. [PubMed] [Google Scholar]
- 4.Yeaman M.R., Yount N.Y. Mechanism of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003;55:27–55. doi: 10.1124/pr.55.1.2. [DOI] [PubMed] [Google Scholar]
- 5.Hancock R.E., Chapple D.S. Peptide antibiotics. Antimicrob. Agents Chemother. 1999;43:1317–1323. doi: 10.1128/aac.43.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powers J.P., Hancock R.E. The relationship between peptide structure and antibacterial activity. Peptides. 2003;24:1681–1691. doi: 10.1016/j.peptides.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 7.Greenfield N.J. Applications of circular dichroism in protein and peptide analysis. Trend Anal. Chem. 1999;18:236–244. [Google Scholar]
- 8.Gopal R., Kim Y.J., Seo C.H., Hahm K.S., Park Y. Reversed sequence enhances antimicrobial activity of a synthetic peptide. J. Peptide Sci. 2011;17:329–334. doi: 10.1002/psc.1369. [DOI] [PubMed] [Google Scholar]
- 9.Gopal R., Park S.C., Ha K.J., Cho S.J., Kim S.W., Song P.I., Nah J.W., Park Y., Hahm K.S. Effect of leucine and lysine substitution on the antimicrobial activity and evaluation of the mechanism of the HPA3NT3 analog peptide. J. Peptide Sci. 2009;15:589–594. doi: 10.1002/psc.1155. [DOI] [PubMed] [Google Scholar]
- 10.Patrzykat A., Gallant J.W., Seo J.K., Pytyck J., Douglas S.E. Novel antimicrobial peptides derived from flatfish genes. Antimicrob. Agents Chemother. 2003;47:2464–2470. doi: 10.1128/AAC.47.8.2464-2470.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: Isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc. Natl. Acad. Sci. USA. 1987;84:5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawanishi N., Christenson H.K., Ninham B.W.J. Measurement of the interaction between adsorbed polyelectrolytes: Gelatin on mica surfaces. J. Phys. Chem. 1990;94:4611–4617. [Google Scholar]
- 13.Likos C.N., Vaynberg K.A., Lowen H., Wagner N.J. Colloidal stabilization by adsorbed gelatin. Langmuir. 2000;16:4100–4108. [Google Scholar]
- 14.Tabata Y., Ikada Y. Protein release from gelatin matrices. Adv. Drug Deliv. Rev. 1998;31:287–301. doi: 10.1016/s0169-409x(97)00125-7. [DOI] [PubMed] [Google Scholar]
- 15.Wetzel R., Buder E., Hermel H., Hütter A. Conformations of different gelatins in solutions and in films an analysis of circular dichroism (CD) measurements. Colloid Polym. Sci. 1987;265:1036–1045. [Google Scholar]
- 16.Gardi A., Nischmann H.S. Intracatenar cross-linking in gelatin with carbodiimide. Helv. Chim. Acta. 1972;55:2468–2486. doi: 10.1002/hlca.19720550724. [DOI] [PubMed] [Google Scholar]
- 17.Wustneck R., Buder E., Wetzel R., Hermel H. The modification of the triple helical structure of gelatin in aqueous solution 3. The influence of cationic surfactants. Colloid Polym. Sci. 1989;267:429–433. [Google Scholar]
- 18.Wustneck R., Buder E., Wetzel R., Hermel H. The modification of the triple helical structure of gelatin in aqueous solution 1. The influence of anionic surfactants, pH-value, and temperature. Colloid Polym. Sci. 1988;266:1061–1067. [Google Scholar]
- 19.Guillen M.C.G., Turnay J., Fernandez-Diaz M.D., Ulmo N., Lizarbe M.A., Montero P. Structural and physical properties of gelatin extracted from different marine species: A comparative study. Food Hydrocoll. 2002;16:25–34. [Google Scholar]
- 20.Engel J, Prockop D.J. The zipper-like folding of collagen triple helices and the effects of mutations that disrupt the zipper. Annu. Rev. Biophys. Biophys. Chem. 1991;20:137–152. doi: 10.1146/annurev.bb.20.060191.001033. [DOI] [PubMed] [Google Scholar]
- 21.Gayatri R., Sharma A.K., Rajaram R., Ramasami T. Chromium (III)-induced structural changes and self-assembly of collagen. Biochem. Biophys. Res. Commun. 2001;283:229–235. doi: 10.1006/bbrc.2001.4713. [DOI] [PubMed] [Google Scholar]
- 22.Leikina E., Mertts M.V., Kuznetsova N., Leikin S. Type 1 collagen is thermally unstable at body temperature. Proc. Natl. Acad. Sci. USA. 2002;99:1314–1318. doi: 10.1073/pnas.032307099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goo H.C., Hwang Y.S., Choi Y.R., Cho H.N., Suh H. Development of collagenase-resistant collagen and its interaction with adult human dermal fibroblasts. Biomaterials. 2003;24:5099–5113. doi: 10.1016/s0142-9612(03)00431-9. [DOI] [PubMed] [Google Scholar]
- 24.Madhan B., Subramanian V., Rao J.R., Nair B.U., Ramasami T. Stabilization of collagen using plant polyphenol: Role of catechin. Int. J. Biol. Macromol. 2005;37:47–53. doi: 10.1016/j.ijbiomac.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Fathima N.N., Devi R.S., Rekha K.B., Dhathathreyan A. Collagen-curcumin interaction—A physico-chemical study. J. Chem. Sci. 2009;121:509–514. [Google Scholar]
- 26.Rosenblum G., Van den Steen P.E., Cohen S.R., Bitler A., Brand D.D., Opdenakker G., Sagi I. Direct visualization of protease action on collagen triple helical structure. Plos One. 2010;5 doi: 10.1371/journal.pone.0011043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitra T., Sailakshmi G., Gnanamani A., Mandal A.B. Di-carboxylic acid cross-linking interactions improves thermal stability and mechanical strength of reconstituted type I collagen. Part I. Oxalic acid. J. Therm. Anal. Calorim. 2011;105:325–330. [Google Scholar]
- 28.Ramachandran G.N., Kartha G. Structure of collagen. Nature. 1955;176:593–595. doi: 10.1038/176593a0. [DOI] [PubMed] [Google Scholar]
- 29.Rich A., Crick F.H. The structure of collagen. Nature. 1955;176:915–916. doi: 10.1038/176915a0. [DOI] [PubMed] [Google Scholar]
- 30.Cowan P.M., McGavin S., North A.C. The polypeptide chain configuration of collagen. Nature. 1955;176:1062–1064. doi: 10.1038/1761062a0. [DOI] [PubMed] [Google Scholar]
- 31.Fathima N.N., Madhan B., Rao J.R., Nair B.U. Effect of zirconium (IV) complexes on the thermal and enzymatic stability of type I collagen. J. Inorg. Biochem. 2003;95:47–54. doi: 10.1016/s0162-0134(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 32.Fathima N.N., Bose M.C., Rao J.R., Nair B.U. Stabilization of type I collagen against collagenases (type I) and thermal degradation using iron complex. J. Inorg. Biochem. 2006;100:1774–1780. doi: 10.1016/j.jinorgbio.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 33.Fathima N.N., Suresh R., Rao J.R., Nair B.U. Role of green tea polyphenols cross linking in alleviating UV radiation effect on collagen. J. Appl. Polym. Sci. 2007;104:3642–3648. [Google Scholar]
- 34.Franzblau C., Schmid K., Faris B., Beldekas J., Garvin P., Kagan H.M., Bruce J., Baum B.J. The interaction of collagen with alpha1-acid glycoprotein. Biochim. Biophys. Acta. 1976;427:302–314. doi: 10.1016/0005-2795(76)90306-8. [DOI] [PubMed] [Google Scholar]
- 35.Madhan B., Muralidharan C., Jayakumar R. Study on the stabilisation of collagen with vegetable tannins in the presence of acrylic polymer. Biomaterials. 2002;23:2841–2847. doi: 10.1016/s0142-9612(01)00410-0. [DOI] [PubMed] [Google Scholar]
- 36.Fathima N.N., Madhan B., Rao R.T., Nair B.U., Ramasami T. Interaction of aldehydes with collagen: Effect on thermal enzymatic and conformational stability. Int. J. Biol. Macromol. 2004;4:241–247. doi: 10.1016/j.ijbiomac.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Usha R., Rajaram A., Ramasami T. Stability of collagen in the presence of 3,4-dihydroxyphenylalanine (DOPA) J. Photochem. Photobiol. B. 2009;97:34–39. doi: 10.1016/j.jphotobiol.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 38.Ge Y., Macdonald D.L., Holroyd K.J., Thornsberry C., Wexler H., Zasloff M. In vitro antibacterial properties of pexiganan, an analog of magainin. Antimicrob. Agents Chemother. 1999;43:782–788. doi: 10.1128/aac.43.4.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gopinath D., Kumar M.S., Selvaraj D., Jayakumar R. Pexiganan-incorported collagen matrices for infected wound-healing processes in rat. J. Biomed. Mater. Res. A. 2005;73:320–331. doi: 10.1002/jbm.a.30303. [DOI] [PubMed] [Google Scholar]
- 40.Townsend D.M., Tew K.D., Tapiero H. The importance of glutathione in human disease. Biomed. Pharmacother. 2003;57:145–155. doi: 10.1016/s0753-3322(03)00043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arul V., Gopinath D., Gomathi K., Jayakumar R. Biotinylated GHK peptide incorporated collagenous matrix: A novel biomaterial for dermal wound healing in rats. J. Biomed. Mater. Res. B Appl. Biomater. 2005;73:383–391. doi: 10.1002/jbm.b.30246. [DOI] [PubMed] [Google Scholar]
- 42.Schibli D.J., Epand R.F., Vogel H.J., Epand R.M. Tryptophan-rich antimicrobial peptides: Comparative properties and membrane interactions. Biochem. Cell Biol. 2002;80:667–677. doi: 10.1139/o02-147. [DOI] [PubMed] [Google Scholar]
- 43.Dathe M., Wieprecht T. Structural features of helical antimicrobial peptides: Their potential to modulate activity on model membranes and biological cells. Biochim. Biophys. Acta. 1999;1462:71–87. doi: 10.1016/s0005-2736(99)00201-1. [DOI] [PubMed] [Google Scholar]
- 44.Tossi A., Sandri L., Giangaspero A. Amphipathic, alpha-helical antimicrobial peptides. Biopolymers. 2000;55:4–30. doi: 10.1002/1097-0282(2000)55:1<4::AID-BIP30>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 45.Powers J.P.S., Hancock R.E.W. The relationship between peptide structure and antibacterial activity. Peptides. 2003;24:1681–1691. doi: 10.1016/j.peptides.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 46.Huang Y., Huang J., Chen Y. Alpha-helical cationic antimicrobial peptides: Relationships of structure and function. Protein Cell. 2010;1:143–152. doi: 10.1007/s13238-010-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woody R.W. Contributions of tryptophan side chains to the far-ultraviolet circular dichroism of proteins. Eur. Biophys. J. 1994;23:253–262. doi: 10.1007/BF00213575. [DOI] [PubMed] [Google Scholar]
- 48.Ladokhin A.S., Selsted M.E., White S.H. Bilayer interactions of indolicidin, a small antimicrobial peptide rich in tryptophan, proline, and basic amino acids. Biophys. J. 1997;72:794–805. doi: 10.1016/s0006-3495(97)78713-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Z., Brady A., Young A., Rasimick B., Chen K., Zhou C., Kallenbach N.R.Length effects in antimicrobial peptides of the (RW) n series Antimicrob. Agents Chemother 2007. 51 597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mollmann S.H., Elofsson U., Bukrinsky J.T., Frokjaer S. Displacement of adsorbed insulin by tween 80 monitored using total internal reflection fluorescence and ellipsometry. Pharm. Res. 2005;22:1931–1941. doi: 10.1007/s11095-005-7249-1. [DOI] [PubMed] [Google Scholar]
- 51.Torrent M., Navarro S., Moussaoui M., Nogues M.V., Boix E. Eosinophil cationic protein high-affinity binding to bacteria-wall lipopolysaccharides and peptidoglycans. Biochemistry. 2008;47:3544–3555. doi: 10.1021/bi702065b. [DOI] [PubMed] [Google Scholar]
- 52.Ding L., Yang L., Weiss T.M., Waring A.J., Lehrer R.I., Huang H.W. Interaction of antimicrobial peptides with lipopolysaccharides. Biochemistry. 2003;42:12251–12259. doi: 10.1021/bi035130+. [DOI] [PubMed] [Google Scholar]
- 53.Rosenfeld Y., Sahl H.G., Shai Y. Parameters involved in antimicrobial and endotoxin detoxification activities of antimicrobial peptides. Biochemistry. 2008;47:6488–6478. doi: 10.1021/bi800450f. [DOI] [PubMed] [Google Scholar]
- 54.Van der Weerden N.L., Hancock R.E., Anderson M.A. Permeabilization of fungal hyphae by the plant defension NAD1 occurs through a cell wall dependent process. J. Biol. Chem. 2010;285:37513–37520. doi: 10.1074/jbc.M110.134882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee D.G., Kim D.H., Park Y., Kim H.K., Shin Y.K., Choi C.H., Hahm K.S. Fungicidal effect of antimicrobial peptides, PMAP-23, isolated from porcine myeloid against Candida albicans. Biochem. Biophys. Res. Commun. 2001;282:570–574. doi: 10.1006/bbrc.2001.4602. [DOI] [PubMed] [Google Scholar]
- 56.Zhao H., Kinnunen P.K.J. Binding of the antimicrobial peptide temporin L to liposomes assessed by Trp fluorescence. J. Biol. Chem. 2002;277:25170–25177. doi: 10.1074/jbc.M203186200. [DOI] [PubMed] [Google Scholar]
- 57.Swamy R.N., Gnanamani A., Shanmugasamy S., Gopal R.K., Mandal A.B. Bioinformatics in crosslinking chemistry of collagen with selective cross linkers. BMC Res. Notes. 2011;4 doi: 10.1186/1756-0500-4-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chan D.I., Prenner E.J., Vogel H.J. Tryptophan- and arginine-rich antimicrobial peptides: Structure and mechanism of action. Biochim. Biophys. Acta. 2006;1758:1184–1202. doi: 10.1016/j.bbamem.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 59.Chen C.C., Hsu W., Hwang K.C., Hwu J.R., Lin C.C., Horng J.C. Contributions of cation-π interactions to the collagen triple helix stability. Arch. Biochem. Biophys. 2011;508:46–53. doi: 10.1016/j.abb.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 60.Edwards R., Harding K.G. Bacteria and wound healing. Curr. Opin. Infect. Dis. 2004;17:91–96. doi: 10.1097/00001432-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 61.Kustos T., Kustos I., Kilar F., Rappai G., Kocsis B. Effect of antibiotics on cell surface hydrophobicity of bacteria using orthopedic wound infections. Chemotherapy. 2003;9:237–242. doi: 10.1159/000072447. [DOI] [PubMed] [Google Scholar]
- 62.Gomathi K., Gopinath D., Rafiuddin Ahmed M., Jayakumar R. Quercetin incorported collagen matrices for dermal wound healing processes in rat. Biomaterials. 2003;24:2767–2772. doi: 10.1016/s0142-9612(03)00059-0. [DOI] [PubMed] [Google Scholar]
- 63.Hima Bindu T.V.L., Vidyavathi M., Kavitha K., Sastry T.P., Suresh Kumar R.V. Preparation and evaluation of chitosan-gelatin composite films for wound healing activity. Trends Biomater. Artif. Organs. 2010;24:123–130. [Google Scholar]
- 64.Hima Bindu T.V.L., Vidyavathi M., Kavitha K., Sastry T.P. Preparation and evaluation of gentamicin loaded chitosan-gelatin composite films for wound healing activity. Int. J. Appl. Biol. Pharm. Technol. 2011;2:453–463. [Google Scholar]
- 65.Iwakura A., Tabata Y., Tamura N., Doi K., Nishimura K., Nakamura T., Shimizu Y., Fujita M., Komeda M. Gelatin sheet incorporating basic fibroblast growth factor enhances healing of devascularized sternum in diabetic rats. Circulation. 2001;104:I325–I329. doi: 10.1161/hc37t1.094544. [DOI] [PubMed] [Google Scholar]
- 66.Hima Bindu T.V.L., Vidyavathi M., Kavitha K., Sastry T.P., Suresh Kumar R.V. Preparation and evaluation of ciprofloxacin loaded chitosan-gelatin composite films for wound healing activity. Int. J. Drug. Deliv. 2010;2:173–182. [Google Scholar]
- 67.Thakur G., Mitra A., Rousseau D., Basak A., Sarkar S., Pal K. Crosslinking of gelatin-based drug carriers by genipin induces changes in drug kinetic profiles in vitro. J. Mater. Sci. Mater. Med. 2011;22:115–123. doi: 10.1007/s10856-010-4185-3. [DOI] [PubMed] [Google Scholar]
- 68.Gomez-Guillen M.C., Perez-Mateos M., Gomez-Estaca J., Lopez-Caballero E., Gimenez B., Montero P. Fish gelatin: A renewable material for developing active biodegradable film. Trends Food Sci. Technol. 2009;20:3–16. [Google Scholar]
- 69.Akane T., Toshiaki N., Hiroshi M. Acceleration of wound healing by gelatin film dressings with epidermal growth factor. J. Vet. Med. Sci. 2005;67:909–913. doi: 10.1292/jvms.67.909. [DOI] [PubMed] [Google Scholar]
- 70.Baker M.A., Maloy W.L., Zasloff M., Jacob L.S. Anticancer efficacy of magainin 2 and analogue peptides. Cancer Res. 1993;53:3052–3057. [PubMed] [Google Scholar]
- 71.Johnstone S.A., Gelmon K., Mayer L.D., Hancock R.E.W., Bally M.B. In vitro characterization of the anticancer activity of membrane-active cationic peptides. I. Peptide-mediated cytotoxicity and peptide-enhanced cytotoxic activity of doxorubicin against wild-type and p-glycoprotein over-expressing tumor cell lines. Anticancer Drug Des. 2000;15:151–160. [PubMed] [Google Scholar]
- 72.Gallo R.L., Ono M., Povsic T., Page C., Eriksson E., Klagsbrun M., Bernfield M. Syndecans, cell surface heparan sulfate proteoglycans, are induced by a proline-rich antimicrobial peptide from wounds. Proc. Natl. Acad. Sci. USA. 1994;91:11035–11039. doi: 10.1073/pnas.91.23.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hancock R.E., Sahl H.G. Antimicrobial and host defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006;24:1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 74.Atherton E., Sheppard R.C. Solid Phase Peptide Synthesis: A Practical Approach. IRL Press; Oxford, UK: 1989. [Google Scholar]
- 75.Chen Y.H., Yang J.T., Chau K.H. Determination of the helix and beta from of proteins in aqueous solution by circular dichroism. Biochemistry. 1974;13:3350–3359. doi: 10.1021/bi00713a027. [DOI] [PubMed] [Google Scholar]